Novel Indigenous Strains and Communities with Copper Bioleaching Potential from the Amolanas Mine, Chile
Abstract
:1. Introduction
2. Materials and Methods
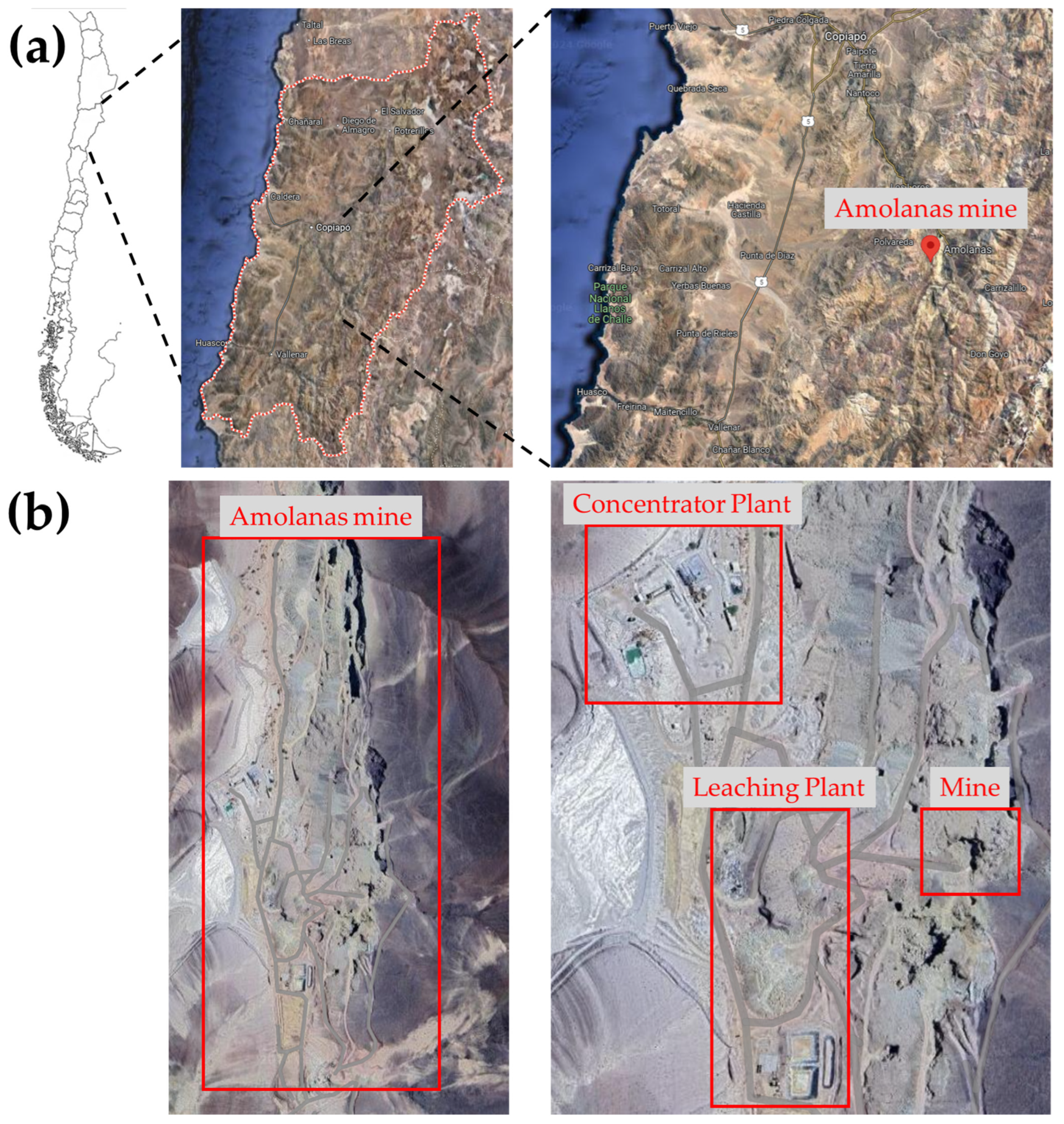
2.1. Mine Sample Collection
2.2. Enrichment and Growth Conditions
2.3. Isolation and Characterization of Iron-Oxidizing Acidophile Bacteria
2.4. Molecular Identification and Characterization
2.5. Microbial Composition Identification
2.6. Mineral Preparation
2.7. Bioleaching Experiments
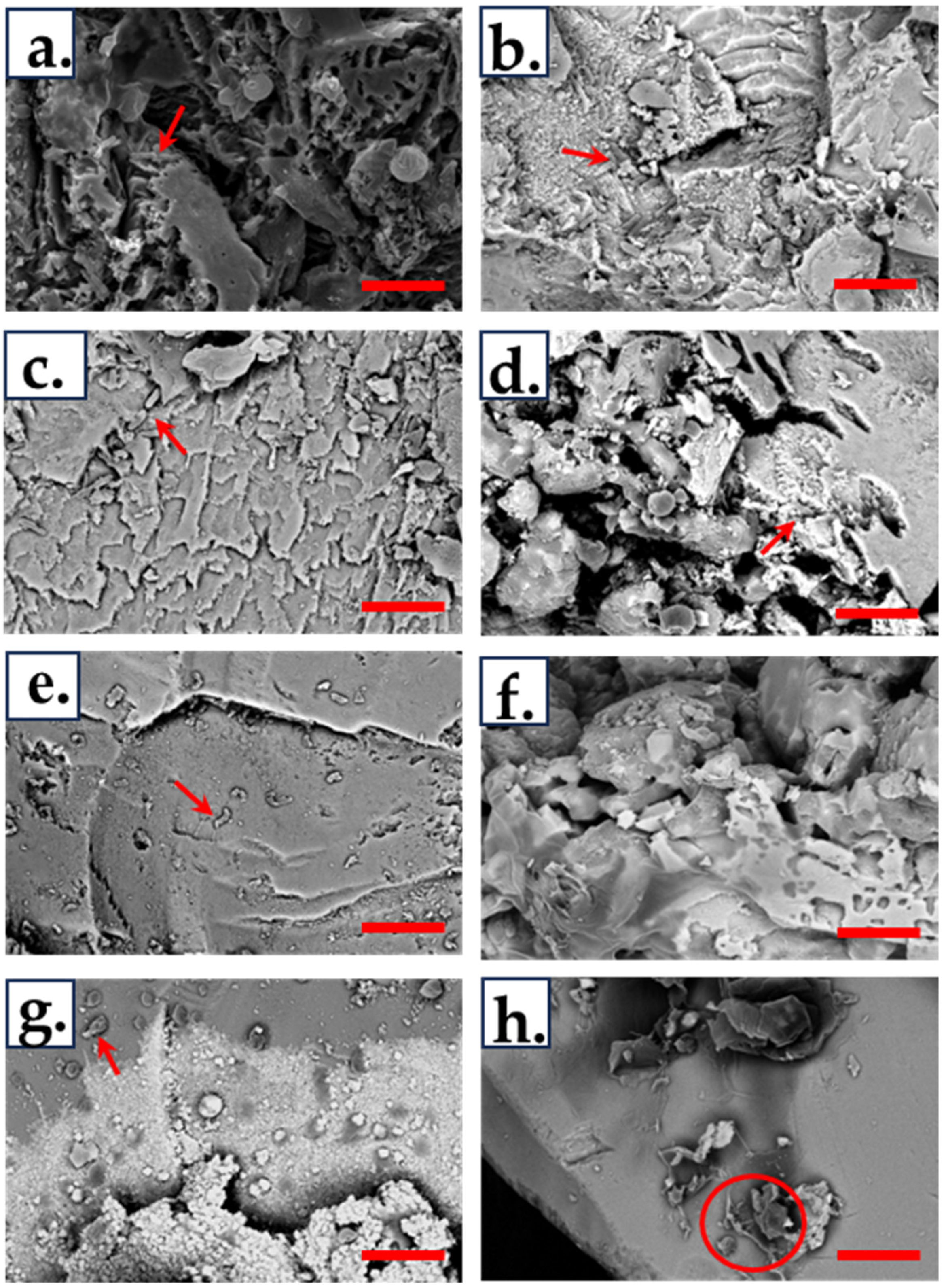
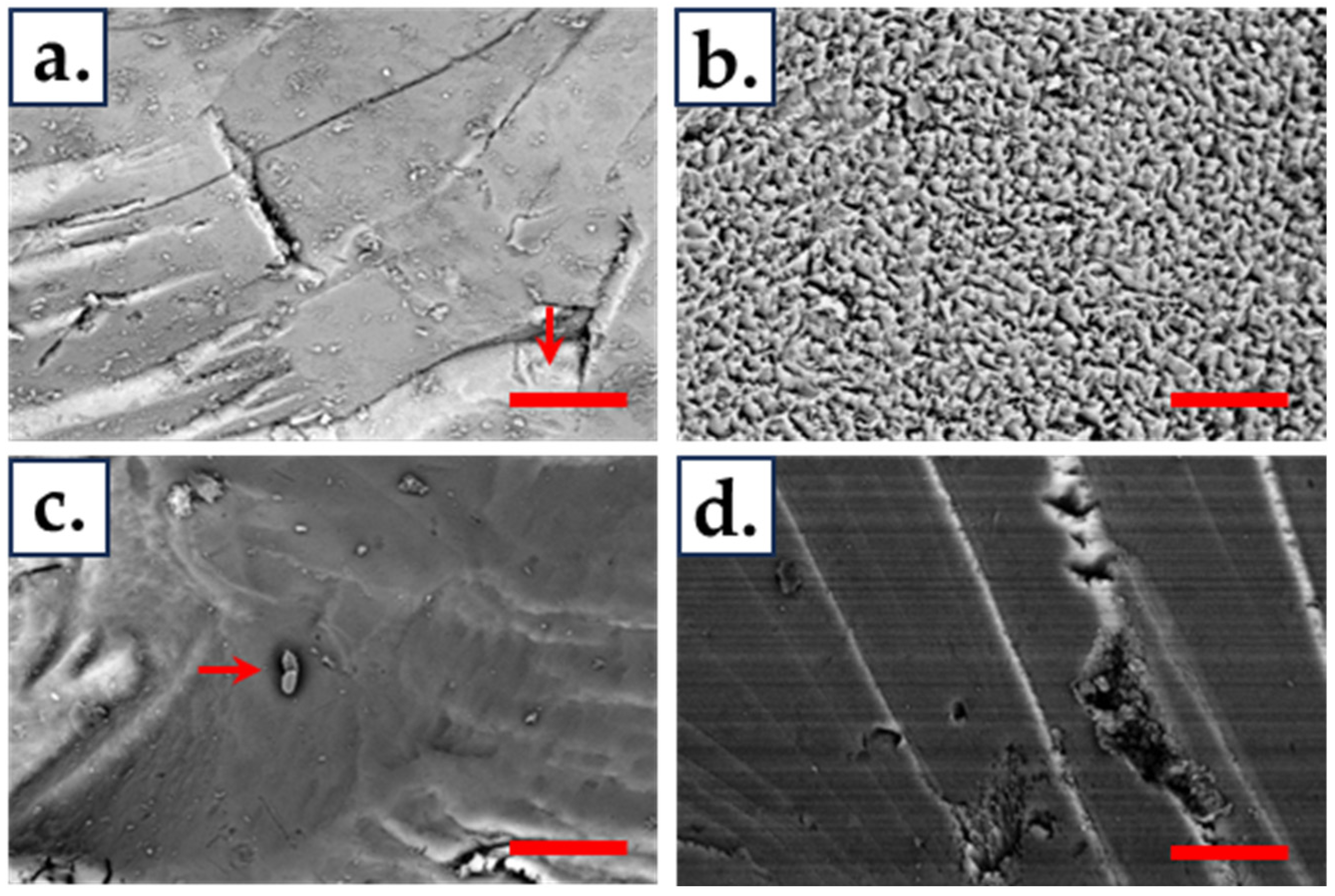
2.8. Scanning Electron Microscopy (SEM)
2.9. Statistical Methods
3. Results
3.1. Strains Molecular Identification and Characterization
3.2. Microbial Diversity
3.2.1. Analysis of Isolates by Next-Generation Sequencing
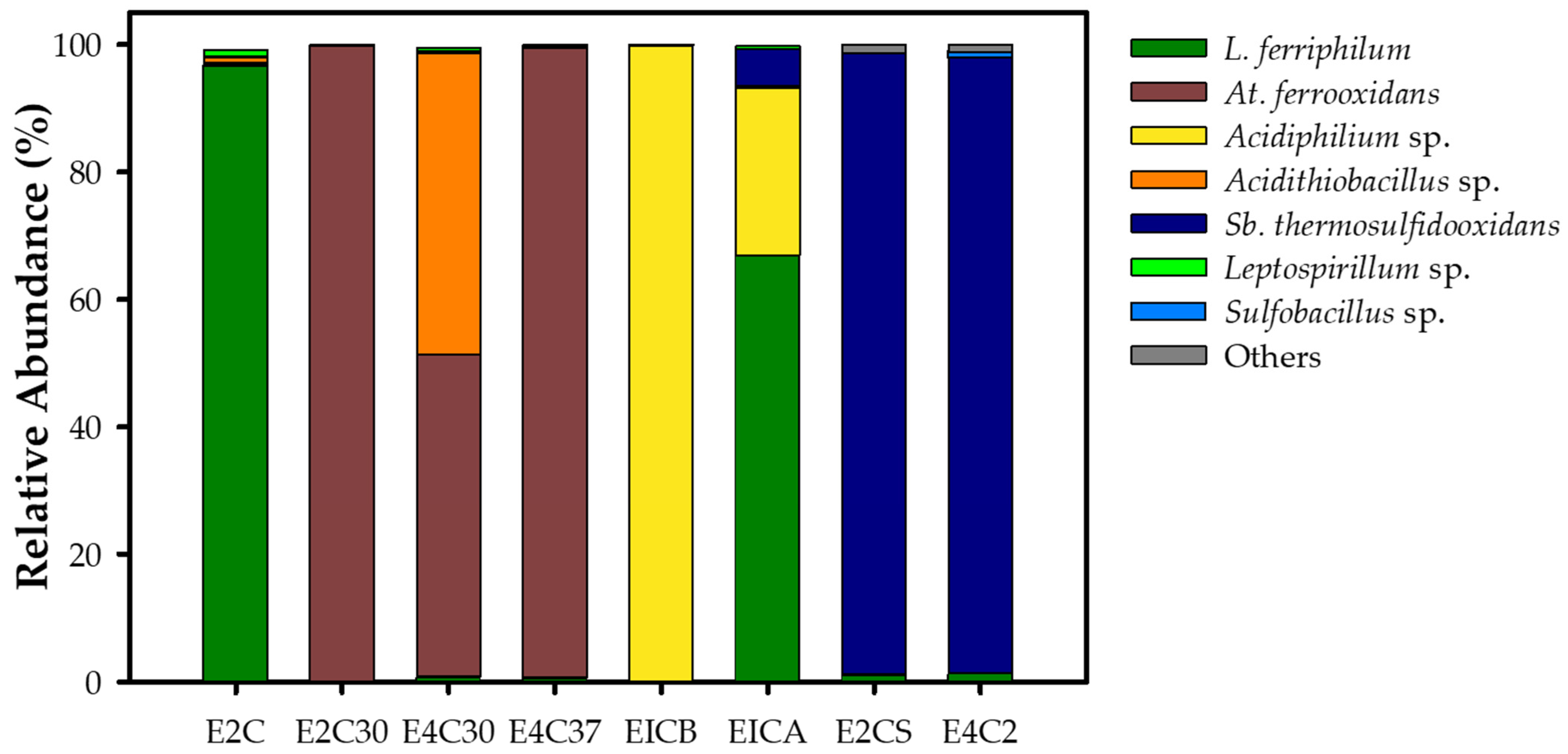
3.2.2. Enrichment Community Composition
3.3. Bioleaching of Pyrite
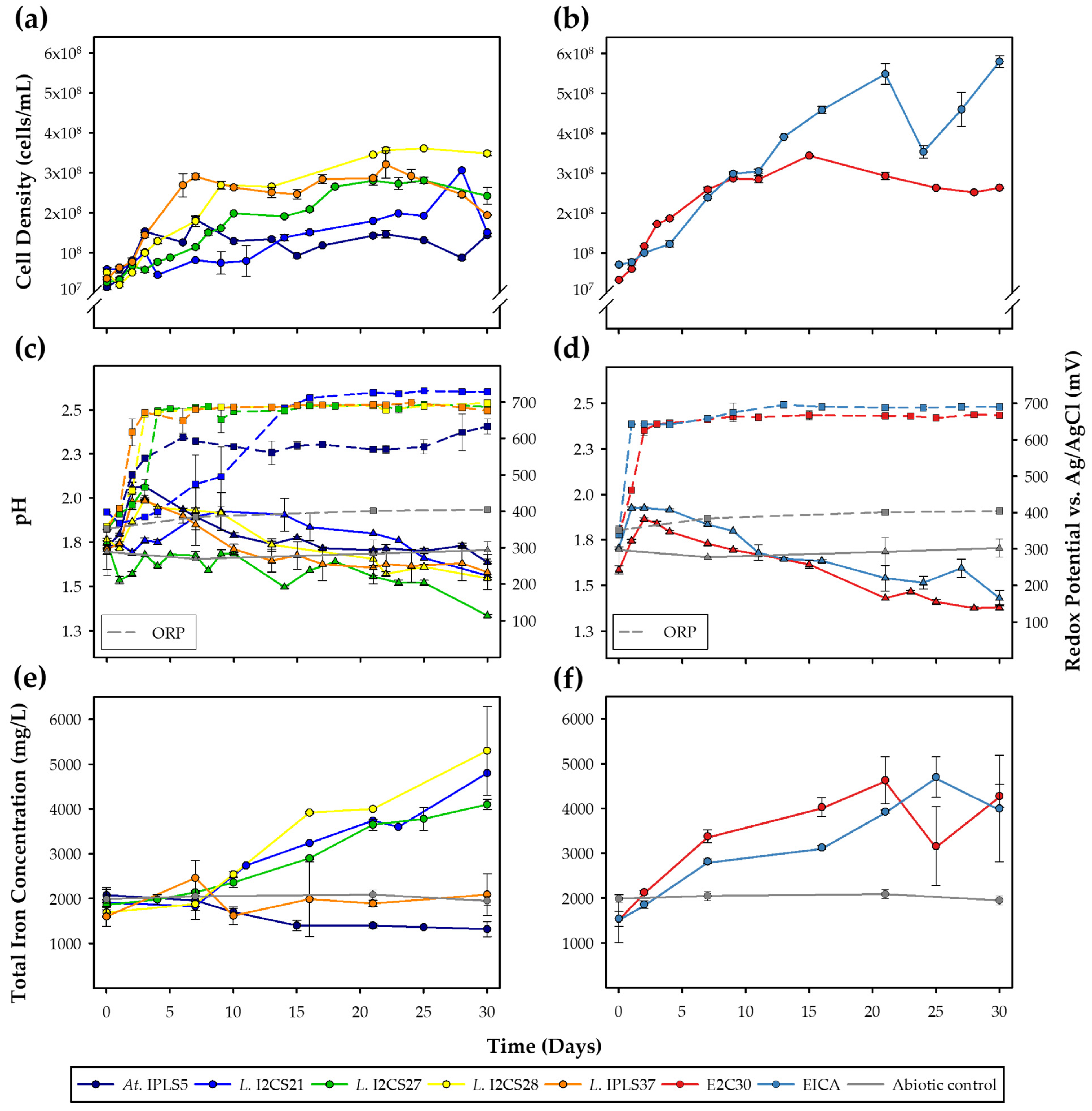
3.3.1. Mesophile Cultures
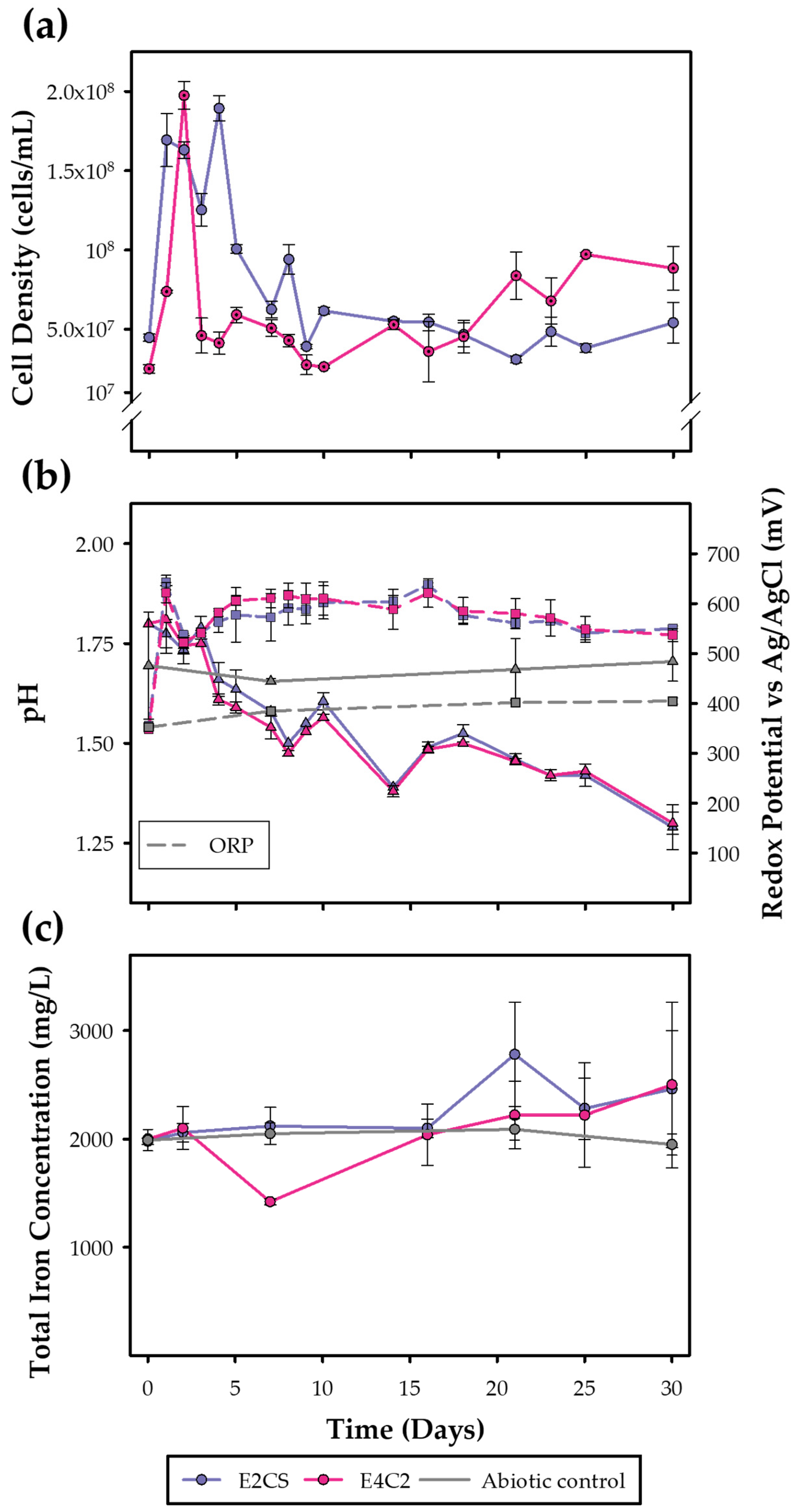
3.3.2. Moderate Thermophile Cultures
3.4. Bioleaching of Chalcopyrite
3.4.1. Mesophile Cultures
3.4.2. Moderate Thermophile Cultures
3.5. Biofilm Formation and Attachment on Chalcopyrite
4. Discussion
4.1. Microbial Biodiversity of the Amolanas Mine
4.2. Bioleaching of Metal Sulfides
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brierley, C.L.; Brierley, J.A. Progress in Bioleaching: Part B: Applications of Microbial Processes by the Minerals Industries. Appl. Microbiol. Biotechnol. 2013, 97, 7543–7552. [Google Scholar] [CrossRef] [PubMed]
- Schippers, A.; Sand, W. Bacterial Leaching of Metal Sulfides Proceeds by Two Indirect Mechanisms via Thiosulfate or via Polysulfides and Sulfur. Appl. Environ. Microbiol. 1999, 65, 319–321. [Google Scholar] [CrossRef]
- Vera, M.; Schippers, A.; Hedrich, S.; Sand, W. Progress in Bioleaching: Fundamentals and Mechanisms of Microbial Metal Sulfide Oxidation—Part A. Appl. Microbiol. Biotechnol. 2022, 106, 6933–6952. [Google Scholar] [CrossRef] [PubMed]
- Buetti-Dinh, A.; Herold, M.; Christel, S.; Hajjami, M.E.; Bellenberg, S.; Ilie, O.; Wilmes, P.; Poetsch, A.; Sand, W.; Vera, M.; et al. Systems Biology of Acidophile Biofilms for Efficient Metal Extraction. Sci. Data 2020, 7, 215. [Google Scholar] [CrossRef]
- Panda, S.; Akcil, A.; Pradhan, N.; Deveci, H. Current Scenario of Chalcopyrite Bioleaching: A Review on the Recent Advances to Its Heap-Leach Technology. Bioresour. Technol. 2015, 196, 694–706. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Tsunekawa, M.; Okamoto, H.; Nakayama, R.; Kuroiwa, S. Improved Chalcopyrite Leaching through Optimization of Redox Potential. Can. Metall. Q. 2008, 47, 253–258. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A Review of the Structure, and Fundamental Mechanisms and Kinetics of the Leaching of Chalcopyrite. Adv. Colloid Interface Sci. 2013, 197–198, 1–32. [Google Scholar] [CrossRef]
- Watling, H.R. Chalcopyrite Hydrometallurgy at Atmospheric Pressure: 1. Review of Acidic Sulfate, Sulfate–Chloride and Sulfate–Nitrate Process Options. Hydrometallurgy 2013, 140, 163–180. [Google Scholar] [CrossRef]
- Zhang, R.; Bellenberg, S.; Neu, T.R.; Sand, W.; Vera, M. The Biofilm Lifestyle of Acidophilic Metal/Sulfur-Oxidizing Microorganisms. In Grand Challenges in Biology and Biotechnology; Rampelotto, P., Ed.; Springer: Cham, Switzerland, 2016; Volume 1, pp. 177–213. ISBN 978-3-319-13521-2. [Google Scholar]
- Debernardi, G.; Carlesi, C. Chemical-Electrochemical Approaches to the Study Passivation of Chalcopyrite. Miner. Process. Extr. Metall. Rev. 2013, 34, 10–41. [Google Scholar] [CrossRef]
- Christel, S.; Herold, M.; Bellenberg, S.; Buetti-Dinh, A.; El Hajjami, M.; Pivkin, I.V.; Sand, W.; Wilmes, P.; Poetsch, A.; Vera, M.; et al. Weak Iron Oxidation by Sulfobacillus thermosulfidooxidans Maintains a Favorable Redox Potential for Chalcopyrite Bioleaching. Front. Microbiol. 2018, 9, 3059. [Google Scholar] [CrossRef]
- Sand, W.; Gehrke, T. Extracellular Polymeric Substances Mediate Bioleaching/Biocorrosion via Interfacial Processes Involving Iron(III) Ions and Acidophilic Bacteria. Res. Microbiol. 2006, 157, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.; Donati, E.R.; Vera, M. Characterization of Extracellular Polymeric Substances Produced by an Acidianus Species and Their Relevance to Bioleaching. Minerals 2023, 13, 310. [Google Scholar] [CrossRef]
- Riekkola-Vanhanen, M. Talvivaara Mining Company—From a Project to a Mine. Miner. Eng. 2013, 48, 2–9. [Google Scholar] [CrossRef]
- Johnson, D.B.; Quatrini, R. Acidophile Microbiology in Space and Time. Curr. Issues Mol. Biol. 2020, 39, 63–76. [Google Scholar] [CrossRef]
- Rossoni, S.; Beard, S.; Segura-Bidermann, M.I.; Duarte-Ramírez, J.; Osorio, F.K.; Varas-Godoy, M.; Martínez-Bellange, P.; Vera, M.; Quatrini, R.; Castro, M. Membrane Vesicles in Acidithiobacillia Class Extreme Acidophiles: Influence on Collective Behaviors of ‘Fervidacidithiobacillus caldus’. Front. Microbiol. 2024, 14, 1331363. [Google Scholar] [CrossRef]
- Okibe, N.; Johnson, D.B. Biooxidation of Pyrite by Defined Mixed Cultures of Moderately Thermophilic Acidophiles in PH-controlled Bioreactors: Significance of Microbial Interactions. Biotechnol. Bioeng. 2004, 87, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Romo, E.; Weinacker, D.F.; Zepeda, A.B.; Figueroa, C.A.; Chavez-Crooker, P.; Farias, J.G. Bacterial Consortium for Copper Extraction from Sulphide Ore Consisting Mainly of Chalcopyrite. Braz. J. Microbiol. 2013, 44, 523–528. [Google Scholar] [CrossRef]
- Watling, H.R. The Bioleaching of Sulphide Minerals with Emphasis on Copper Sulphides—A Review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Mackintosh, M.E. Nitrogen Fixation by Thiobacillus ferrooxidans. J. Gen. Microbiol. 1978, 105, 215–218. [Google Scholar] [CrossRef]
- de Bruyn, J.C.; Boogerd, F.C.; Bos, P.; Kuenen, J.G. Floating Filters, a Novel Technique for Isolation and Enumeration of Fastidious, Acidophilic, Iron-Oxidizing, Autotrophic Bacteria. Appl. Environ. Microbiol. 1990, 56, 2891–2894. [Google Scholar] [CrossRef]
- Natarajan, K.A. Biotechnology of Metals: Principles, Recovery Methods and Environmental Concerns; Elsevier: Amsterdam, The Netherlands, 2018; ISBN-10: 012804022X; ISBN-13: 978-0128040225. [Google Scholar]
- Lin, C.C.; Casida, L.E. GELRITE as a Gelling Agent in Media for the Growth of Thermophilic Microorganisms. Appl. Environ. Microbiol. 1984, 47, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Lindström, E.B.; Sehlin, H.M. High Efficiency of Plating of the Thermophilic Sulfur-Dependent Archaebacterium Sulfolobus acidocaldarius. Appl. Environ. Microbiol. 1989, 55, 3020–3021. [Google Scholar] [CrossRef] [PubMed]
- Giaveno, M.A.; Urbieta, M.S.; Ulloa, J.R.; González Toril, E.; Donati, E.R. Physiologic Versatility and Growth Flexibility as the Main Characteristics of a Novel Thermoacidophilic Acidianus Strain Isolated from Copahue Geothermal Area in Argentina. Microb. Ecol. 2013, 65, 336–346. [Google Scholar] [CrossRef]
- Schut, F.; de Vries, E.J.; Gottschal, J.C.; Robertson, B.R.; Harder, W.; Prins, R.A.; Button, D.K. Isolation of Typical Marine Bacteria by Dilution Culture: Growth, Maintenance, and Characteristics of Isolates under Laboratory Conditions. Appl. Environ. Microbiol. 1993, 59, 2150–2160. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Pan, J.; Wang, F.; Li, M. Perspectives on Cultivation Strategies of Archaea. Microb. Ecol. 2020, 79, 770–784. [Google Scholar] [CrossRef]
- Muñoz-Villagrán, C.; Grossolli-Gálvez, J.; Acevedo-Arbunic, J.; Valenzuela, X.; Ferrer, A.; Díez, B.; Levicán, G. Characterization and Genomic Analysis of Two Novel Psychrotolerant Acidithiobacillus ferrooxidans Strains from Polar and Subpolar Environments. Front. Microbiol. 2022, 13, 960324. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Aguilera, A. Extremophiles and Acidic Environments. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2019; pp. 206–227. [Google Scholar] [CrossRef]
- Latorre, M.; Cortés, M.P.; Travisany, D.; Di Genova, A.; Budinich, M.; Reyes-Jara, A.; Hödar, C.; González, M.; Parada, P.; Bobadilla-Fazzini, R.A.; et al. The Bioleaching Potential of a Bacterial Consortium. Bioresour. Technol. 2016, 218, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Dopson, M. Physiological and Phylogenetic Diversity of Acidophilic Bacteria. In Acidophiles: Life in Extremely Acidic Environments; Quatrini, R., Johnson, D.B., Eds.; Caister Academic Press: Wymondham, UK, 2016; pp. 79–92. [Google Scholar]
- Huynh, D.; Giebner, F.; Kaschabek, S.R.; Rivera-Araya, J.; Levican, G.; Sand, W.; Schlömann, M. Effect of Sodium Chloride on Leptospirillum ferriphilum DSM 14647T and Sulfobacillus thermosulfidooxidans DSM 9293T: Growth, Iron Oxidation Activity and Bioleaching of Sulfidic Metal Ores. Miner. Eng. 2019, 138, 52–59. [Google Scholar] [CrossRef]
- Romero, J.; Yañez, C.; Vásquez, M.; Moore, E.R.B.; Espejo, R.T. Characterization and Identification of an Iron-Oxidizing, Leptospirillum-like Bacterium, Present in the High Sulfate Leaching Solution of a Commercial Bioleaching Plant. Res. Microbiol. 2003, 154, 353–359. [Google Scholar] [CrossRef]
- Watkin, E.; Zammit, C. Adaptation to Extreme Acidity and Osmotic Stress. In Acidophiles: Life in Extremely Acidic Environments; Quatrini, R., Johnson, D.B., Eds.; Caister Academic Press: Wymondham, UK, 2016; pp. 49–62. [Google Scholar]
- Rivera-Araya, J.; Huynh, N.D.; Kaszuba, M.; Chávez, R.; Schlömann, M.; Levicán, G. Mechanisms of NaCl-Tolerance in Acidophilic Iron-Oxidizing Bacteria and Archaea: Comparative Genomic Predictions and Insights. Hydrometallurgy 2020, 194, 105334. [Google Scholar] [CrossRef]
- Neira, A.; Pizarro, D.; Quezada, V.; Velásquez-Yévenes, L. Pretreatment of Copper Sulphide Ores Prior to Heap Leaching: A Review. Metals 2021, 11, 1067. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Torres, D.; Toro, N. Leaching of Chalcopyrite Ore Agglomerated with High Chloride Concentration and High Curing Periods. Hydrometallurgy 2018, 181, 215–220. [Google Scholar] [CrossRef]
- Battaglia, F.; Morin, D.; Garcia, J.L.; Ollivier, P. Isolation and Study of Two Strains of Leptospirillum-like Bacteria from a Natural Mixed Population Cultured on a Cobaltiferous Pyrite Substrate. Antonie Van Leeuwenhoek 1994, 66, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, H.; Moya-Beltrán, A.; Covarrubias, P.C.; Issotta, F.; Cárdenas, J.P.; González, M.; Atavales, J.; Acuña, L.G.; Johnson, D.B.; Quatrini, R. Molecular Systematics of the Genus Acidithiobacillus: Insights into the Phylogenetic Structure and Diversification of the Taxon. Front. Microbiol. 2017, 8, 30. [Google Scholar] [CrossRef]
- Rawlings, D.E. Heavy Metal Mining Using Microbes. Annu. Rev. Microbiol. 2002, 56, 65–91. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, D.E.; Johnson, D.B. Biomining, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-34909-9. [Google Scholar]
- Hedrich, S.; Joulian, C.; Graupner, T.; Schippers, A.; Guézennec, A.-G. Enhanced Chalcopyrite Dissolution in Stirred Tank Reactors by Temperature Increase during Bioleaching. Hydrometallurgy 2018, 179, 125–131. [Google Scholar] [CrossRef]
- Boon, M.; Brasser, H.J.; Hansford, G.S.; Heijnen, J.J. Comparison of the Oxidation Kinetics of Different Pyrites in the Presence of Thiobacillus ferrooxidans or Leptospirillum ferrooxidans. Hydrometallurgy 1999, 53, 57–72. [Google Scholar] [CrossRef]
- Lazaroff, N. Mineral Leaching, Iron Precipitation, and the Sulfate Requirement for Chemolithotrophic Iron Oxidation. In Studies in Environmental Science; Elsevier: Amsterdam, The Netherlands, 1997; Volume 66, pp. 61–75. [Google Scholar] [CrossRef]
- Larsson, L.; Olsson, G.; Holst, O.; Karlsson, H.T. Pyrite Oxidation by Thermophilic Archaebacteria. Appl. Environ. Microbiol. 1990, 56, 697–701. [Google Scholar] [CrossRef]
- Bigham, J.M.; Nordstrom, D.K. Iron and Aluminum Hydroxysulfates from Acid Sulfate Waters. Rev. Mineral. Geochem. 2000, 40, 351–403. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Morris, C.; Rea, S.; Li, J.; Wylie, J.; Usher, K.M.; Ginige, M.P.; Cheng, K.Y.; Hilario, F.; du Plessis, C.A. Biohydrometallurgical Iron Oxidation and Precipitation: Part I—Effect of PH on Process Performance. Hydrometallurgy 2014, 147–148, 255–263. [Google Scholar] [CrossRef]
- Behrad Vakylabad, A.; Nazari, S.; Darezereshki, E. Bioleaching of Copper from Chalcopyrite Ore at Higher NaCl Concentrations. Miner. Eng. 2022, 175, 107281. [Google Scholar] [CrossRef]
- Tao, J.; Liu, X.; Luo, X.; Teng, T.; Jiang, C.; Drewniak, L.; Yang, Z.; Yin, H. An Integrated Insight into Bioleaching Performance of Chalcopyrite Mediated by Microbial Factors: Functional Types and Biodiversity. Bioresour. Technol. 2021, 319, 124219. [Google Scholar] [CrossRef]
- Borilova, S.; Mandl, M.; Zeman, J.; Kucera, J.; Pakostova, E.; Janiczek, O.; Tuovinen, O.H. Can Sulfate Be the First Dominant Aqueous Sulfur Species Formed in the Oxidation of Pyrite by Acidithiobacillus ferrooxidans? Front. Microbiol. 2018, 9, 3134. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Jiao, F.; Qin, W. Leaching of Chalcopyrite: An Emphasis on Effect of Copper and Iron Ions. J. Cent. South Univ. 2018, 25, 2380–2386. [Google Scholar] [CrossRef]
- Ríos, D.; Bellenberg, S.; Christel, S.; Lindblom, P.; Giroux, T.; Dopson, M. Potential of Single and Designed Mixed Cultures to Enhance the Bioleaching of Chalcopyrite by Oxidation-Reduction Potential Control. Hydrometallurgy 2024, 224, 106245. [Google Scholar] [CrossRef]
- Li, Q.; Sand, W.; Zhang, R. Enhancement of Biofilm Formation on Pyrite by Sulfobacillus thermosulfidooxidans. Minerals 2016, 6, 71. [Google Scholar] [CrossRef]
- Zhang, R.; Blanchard, V.; Vera, M.; Sand, W. EPS Characterization of a Cell Wall-Lacking Archaeon Ferroplasma acidiphilum. Solid State Phenom. 2017, 262 SSP, 434–438. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Vera, M.; Bellenberg, S.; Sand, W. Attachment to Minerals and Biofilm Development of Extremely Acidophilic Archaea. Adv. Mater. Res. 2013, 825, 103–106. [Google Scholar] [CrossRef]




| Sample Code | Microorganisms | Collection Site | Isolation Method | Isolation Temperature (°C) |
|---|---|---|---|---|
| IPLS5 | Acidithiobacillus | PLS pond | Extinction dilution | 30 |
| IRPS15 | Leptospirillum | Underground well—rocks | Extinction dilution | 30 |
| IRPS17 | Leptospirillum | Underground well—rocks | Extinction dilution | 37 |
| I2CS21 | Leptospirillum | Oxide leaching heap—surface | Extinction dilution | 30 |
| I2CS22 | Leptospirillum | Oxide leaching heap—surface | Extinction dilution | 30 |
| I2CS27 | Leptospirillum | Oxide leaching heap—surface | Double-layer solid | 30 |
| I2CS28 | Leptospirillum | Oxide leaching heap—surface | Double-layer solid | 30 |
| I2CS29 | Leptospirillum | Oxide leaching heap—surface | Double-layer solid | 30 |
| I2CS30 | Leptospirillum | Oxide leaching heap—surface | Double-layer solid | 30 |
| I2C3031 | Acidithiobacillus | Oxide leaching heap—30 cm deep | Extinction dilution | 30 |
| ISPL37 | Leptospirillum | Oxide leaching heap—outflow | Extinction dilution | 30 |
| E2C | Community | Underground well—water | Enrichment | 30 |
| E2C30 | Community | Oxide leaching heap—30 cm deep | Enrichment | 37 |
| E4C30 | Community | PLS pond | Enrichment | 30 |
| E4C37 | Community | PLS pond | Enrichment | 37 |
| EICB | Community | Underground well—rocks | Enrichment | 30 |
| EICA | Community | Underground well—rocks | Enrichment | 30 |
| E2CS | Community | Oxide leaching heap—surface | Enrichment | 50 |
| E4C2 | Community | PLS pond | Enrichment | 50 |
| Isolate | Temperature (°C) | NaCl 11.7 g/L | NaCl 17.5 g/L | Fe(II) Oxidation | S0 Oxidation | ||
|---|---|---|---|---|---|---|---|
| 30 | 37 | 40 | |||||
| Acidithiobacillus IPLS5 | + | + | - | + | + | + | + |
| Leptospirillum IRPS15 | + | + | + | x | x | + | - |
| Leptospirillum IRPS17 | + | + | + | + | + | + | - |
| Leptospirillum I2CS21 | + | + | + | x | x | + | - |
| Leptospirillum I2CS22 | + | + | - | x | x | + | - |
| Leptospirillum I2CS27 | + | + | + | + | + | + | - |
| Leptospirillum I2CS28 | + | + | - | x | x | + | - |
| Leptospirillum I2CS29 | + | + | - | x | x | + | - |
| Leptospirillum I2CS30 | + | + | + | x | x | + | - |
| Acidithiobacillus I2C3031 | + | + | - | x | x | + | + |
| Leptospirillum ISPL37 | + | + | - | x | x | + | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas-Vargas, J.C.; Martínez-Bussenius, C.; Videla, Á.; Vera, M. Novel Indigenous Strains and Communities with Copper Bioleaching Potential from the Amolanas Mine, Chile. Minerals 2024, 14, 867. https://doi.org/10.3390/min14090867
Casas-Vargas JC, Martínez-Bussenius C, Videla Á, Vera M. Novel Indigenous Strains and Communities with Copper Bioleaching Potential from the Amolanas Mine, Chile. Minerals. 2024; 14(9):867. https://doi.org/10.3390/min14090867
Chicago/Turabian StyleCasas-Vargas, Julián C., Cristóbal Martínez-Bussenius, Álvaro Videla, and Mario Vera. 2024. "Novel Indigenous Strains and Communities with Copper Bioleaching Potential from the Amolanas Mine, Chile" Minerals 14, no. 9: 867. https://doi.org/10.3390/min14090867





