Effect of Fine Particle Content on Solution Flow and Mass Transfer of Ion-Adsorption-Type Rare Earth Ores
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
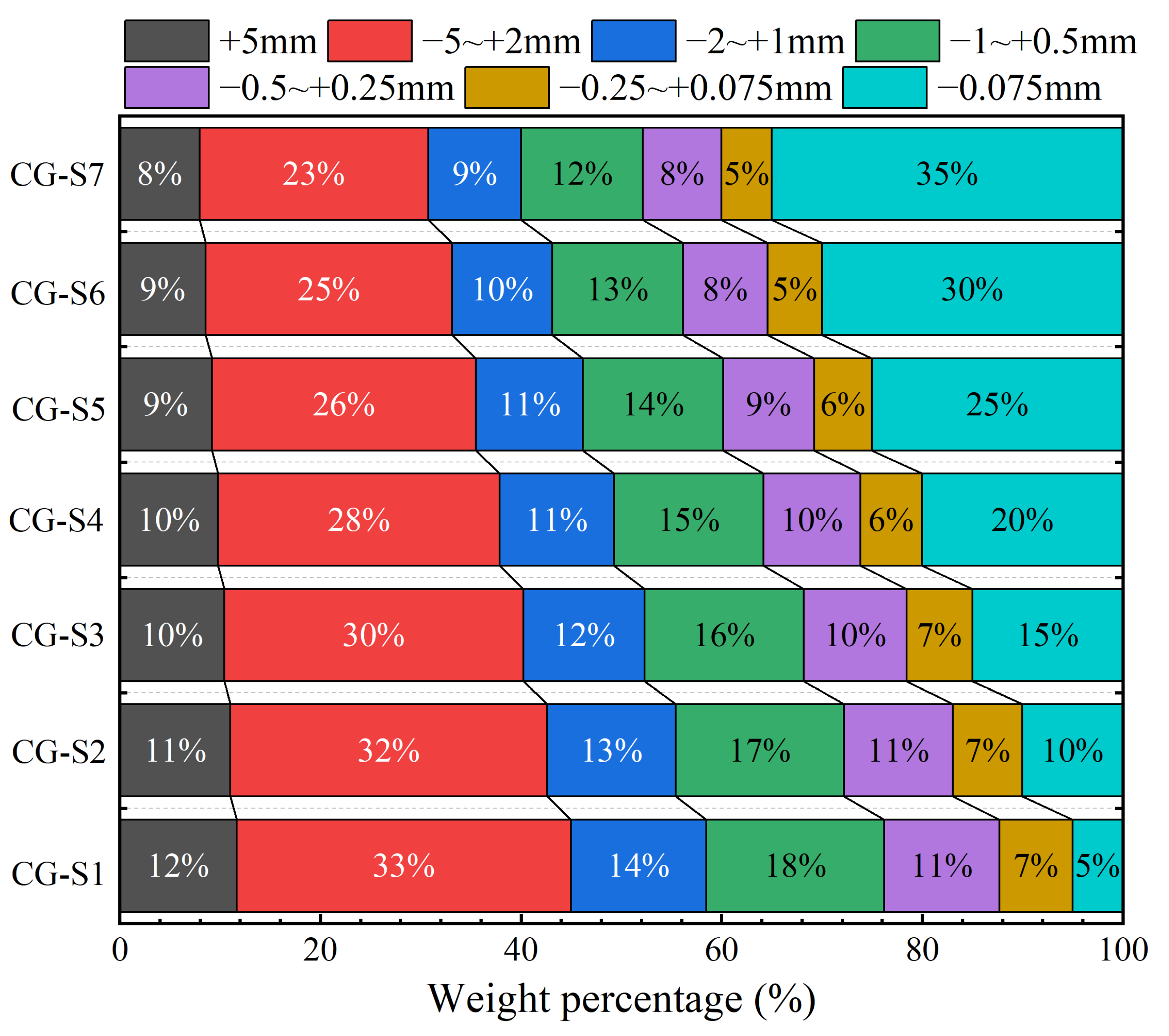
2.1.1. Properties of Rare Earth Raw Ores
2.1.2. Properties of Recombinant Rare Earth Ore
2.2. Experimental Process
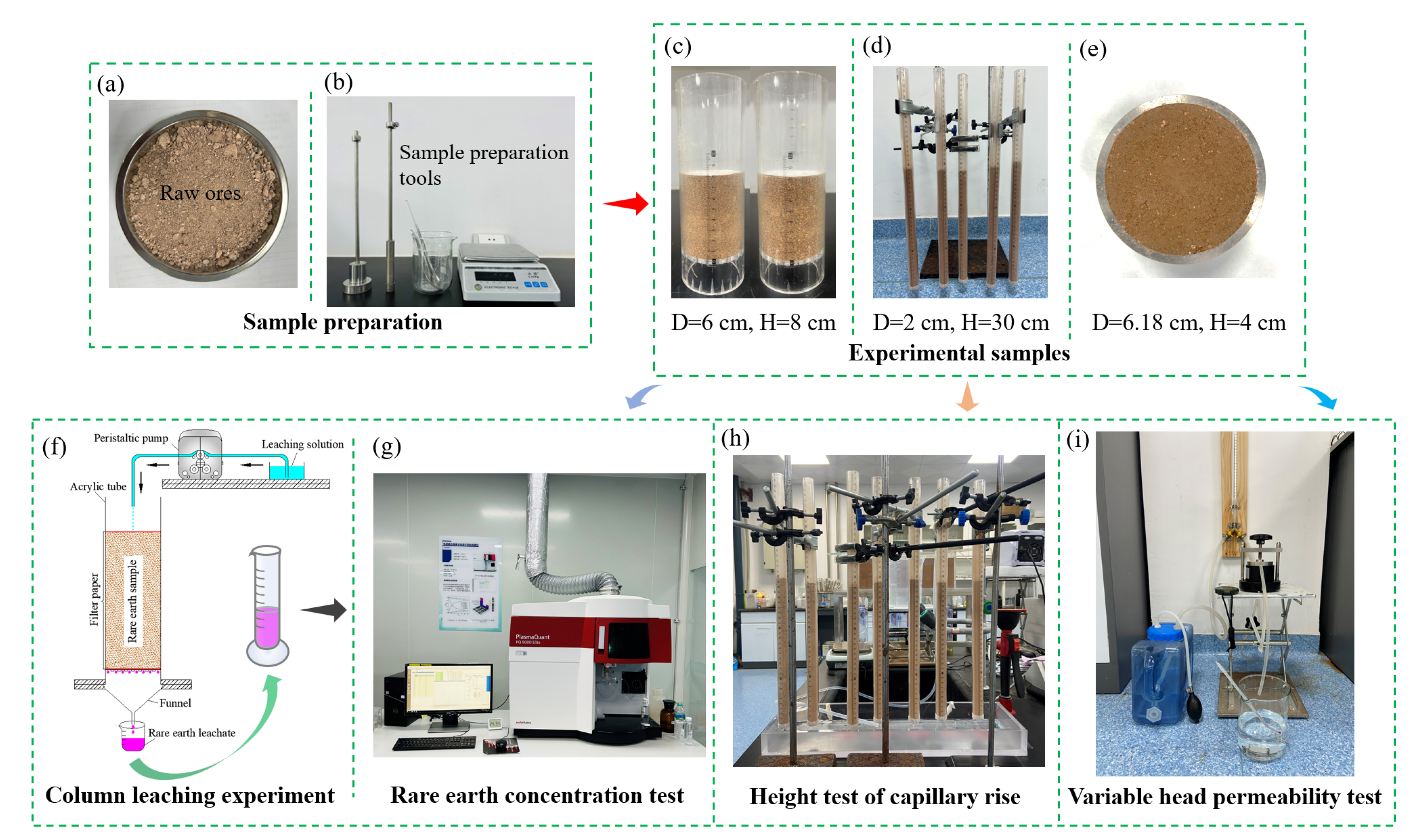
2.2.1. Sample Preparation
2.2.2. Column Leaching Experiment
2.2.3. Height Test of Capillary Rise
2.2.4. Variable Head Permeability Test
3. Results and Discussion
3.1. Experimental Results of Column Leaching
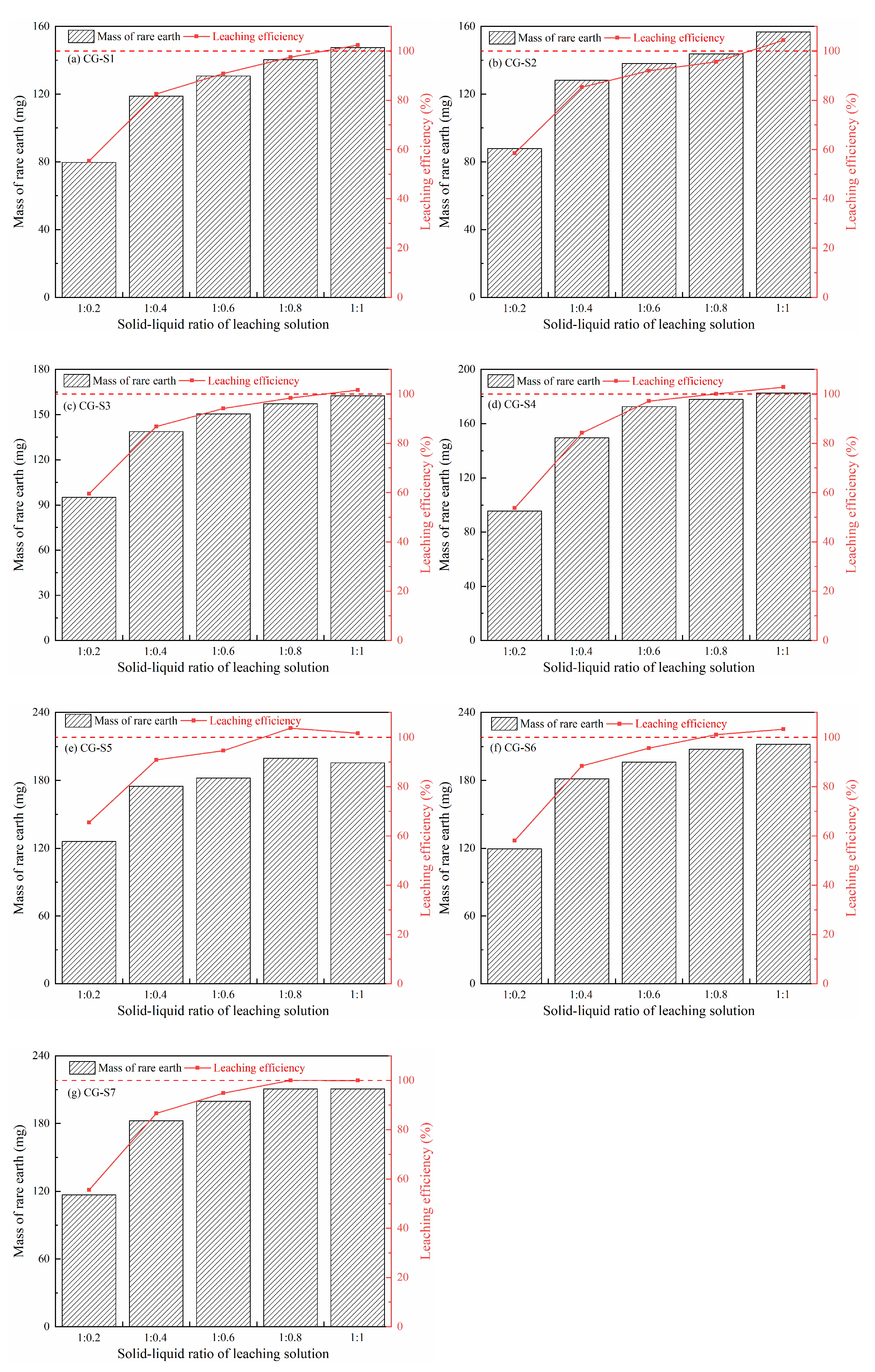
3.1.1. Effect of Different Solid–Liquid Ratios of the Leaching Solution on Rare Earth Leaching
3.1.2. Mass Transfer Characteristics of Rare Earth Ore
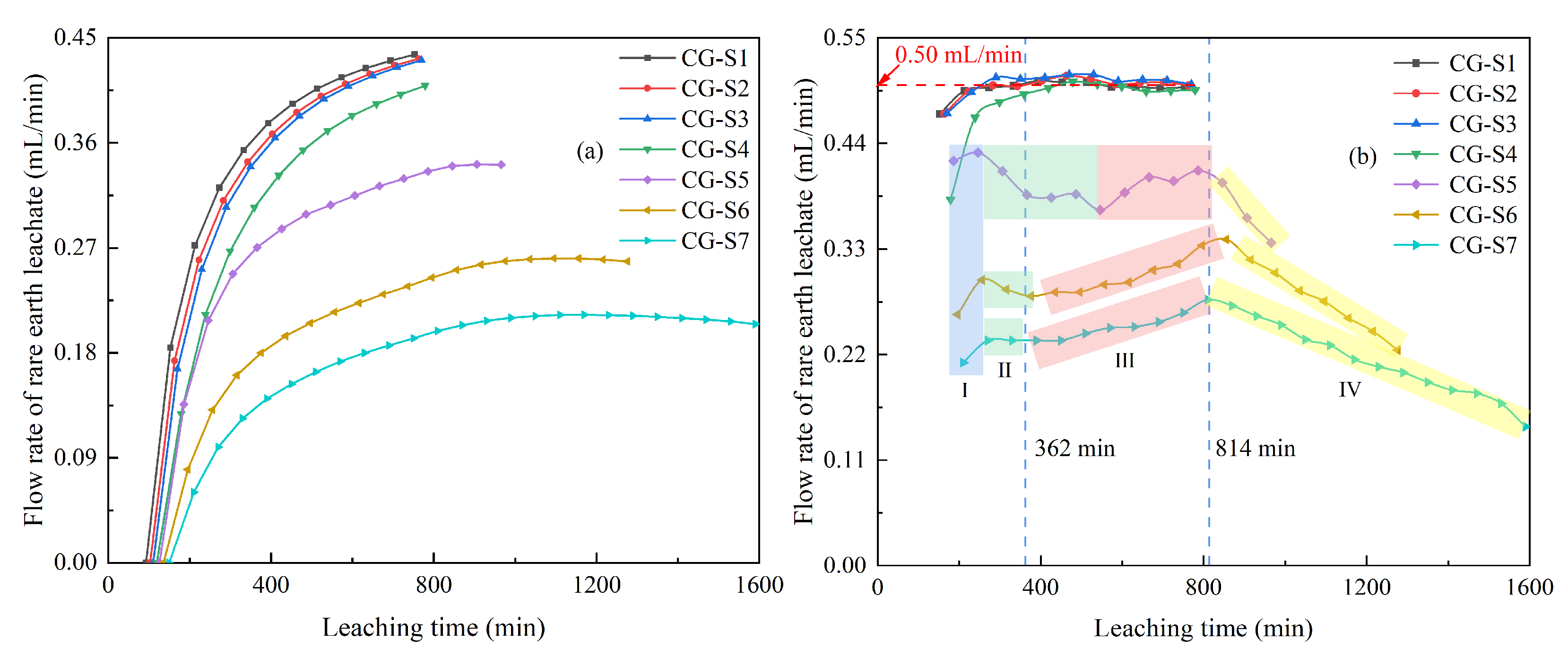
3.1.3. Flow Rate of the Rare Earth Leachate
3.2. Effect of Fine Particle Content on the Rising Process of Capillary Water
3.3. Effect of Fine Particle Content on Permeability Characteristics of Rare Earth Ore
4. Conclusions
- (1)
- With the increasing content of fine particles, the time for peak rare earth concentration of the leachate was prolonged, and the peak was increased. When the fine particle content exceeded 20%, all ion-exchangeable-phase rare earth ions could be replaced at a low dosage of the leaching solution. And the flow rate of the leachate showed a multi-stage change, which was affected by the combined effects solution permeation, ion exchange, and fluctuations in the height of accumulated liquid.
- (2)
- According to the plate theory, a higher fine particle content corresponded to a smaller H and a larger n, indicating that the increase in the fine particle content could improve the mass transfer of rare earth and promote the leaching process. When the fine particle content reached 25%, the H and n changed little with the increasing content of fine particles, which had little improvement in the leaching process.
- (3)
- In the height tests of capillary rise, the whole rising process could be divided into three stages: the rapid rise stage (0–60 min), the slow rise stage (60–300 min), and the steady rise stage (300–900 min). The capillary water eventually rose to a lower height as the fine particle content increased, and the rising rate varied across different stages for samples with different fine particle content.
- (4)
- The effect of fine particle content on the flow rate of leachate and the permeability coefficient of the sample was negative. When the proportion of fine particles was elevated from 5% to 20%, the permeability coefficient of the sample decreased from 0.01623 cm/s to 0.00096 cm/s, with a decrease of 94%. But when the fine particle content of the sample increased beyond 20%, the decrease in the permeability coefficient was not obvious.
- (5)
- The increase in fine particle content could improve leaching efficiency, which could be attributed to the decrease in porosity and permeability of the sample, making the infiltration of the leaching solution more uniform and reducing the blind leaching area. According to the results of this study and the actual conditions of ion-adsorption-type rare earth ores, the optimal fine particle content of the ore is 20%. Under this condition, rare earth leaching has the greatest effectiveness.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ji, L.Q.; Chen, M.X.; Gu, H.; Zhao, J.H.; Yang, X. Actuality of light rare earth resources and application in field of new energy vehicles. J. Chin. Soc. Rare Earths 2020, 38, 129–138. (In Chinese) [Google Scholar]
- Liu, T.C.; Chen, J. Extraction and separation of heavy rare earth elements: A review. Sep. Purif. Technol. 2021, 276, 119263. [Google Scholar] [CrossRef]
- Zhang, S.; Saji, S.E.; Yin, Z.Y.; Zhang, H.B.; Du, Y.P.; Yan, C.H. Rare-earth incorporated alloy catalysts: Synthesis, properties, and applications. Adv. Mater. 2021, 33, 2005988. [Google Scholar] [CrossRef]
- Zhou, B.L.; Li, Z.X.; Chen, C.C. Global potential of rare earth resources and rare earth demand from clean technologies. Minerals 2017, 7, 203. [Google Scholar] [CrossRef]
- Zheng, G.D.; Wang, K.; Chen, Q.S.; Zhang, Y.F.; Xing, J.Y.; Long, T.; Dong, Y.T.; Ni, J.P. The change of world rare earth industrial structure and the problems faced by China’s rare earth industry. Acta. Geol. Sin. 2021, 42, 265–272. (In Chinese) [Google Scholar]
- Yan, D.R.; Ro, S.; Sunam, O.; Kim, S. On the global rare earth elements utilization and its supply-demand in the future. IOP Conf. Ser. Earth Environ. Sci. 2020, 508, 012084. [Google Scholar] [CrossRef]
- Feng, J.; Zhou, F.; Chi, R.A.; Liu, X.; Xu, Y.L.; Liu, Q. Effect of a novel compound on leaching process of weathered crust elution deposited rare earth ore. Miner. Eng. 2018, 129, 63–70. [Google Scholar] [CrossRef]
- Li, Y.H.M.; Zhao, W.W.; Zhou, M.F. Nature of parent rocks, mineralization styles and ore genesis of regolith-hosted REE deposits in South China: An integrated genetic model. J. Asian Earth Sci. 2017, 148, 65–95. [Google Scholar] [CrossRef]
- Chen, X.; He, Q.; Chen, J.F.; Huang, L.; Tan, C.M.; Yin, Y.Q.; Jiao, Y.F.; Xiao, Y.F. Development of leaching technology and theory of ion-adsorption type rare earth ore. J. Chin. Soc. Rare Earths 2022, 40, 936–947. (In Chinese) [Google Scholar]
- Deng, Z.X.; Qin, L.; Wang, G.S.; Luo, S.H.; Peng, C.L.; Li, Q. Metallogenic process of ion adsorption REE ore based on the occurrence regularity of La in kaolin. Ore Geol. Rev. 2019, 112, 103022. [Google Scholar] [CrossRef]
- Li, F.J.; Xiao, Z.; Zeng, J.Y.; Chen, J.Q.; Sun, X.Q. Recovery of REEs from leaching liquor of ion-adsorbed-type rare earths ores using ionic liquid based on cooking oil. Hydrometallurgy 2020, 196, 105449. [Google Scholar] [CrossRef]
- Zhou, L.B.; Yang, J.; Kang, S.J.; Wang, X.J.; Yu, H.D.; Wan, Y.H. Enhancing leaching efficiency of ion adsorption rare earths by ameliorating mass transfer effect of rare earth ions by applying an electric field. J. Rare Earths 2024, 42, 172–180. [Google Scholar] [CrossRef]
- Yang, L.F.; Wang, D.S.; Li, C.C.; Sun, Y.Y.; Zhou, X.Z.; Li, Y.X. Searching for a high efficiency and environmental benign reagent to leach ion-adsorption rare earths based on the zeta potential of clay particles. Green Chem. 2018, 20, 4528–4536. [Google Scholar] [CrossRef]
- Li, C. The generalization and application of new technology on lixiviating mineral at the original Place for ionic rare earths nonferrous metals science and engineering. Non-Ferr. Met. Sci. Eng. 2011, 2, 63–67. (In Chinese) [Google Scholar]
- Huang, X.W.; Long, Z.Q.; Wang, L.S.; Feng, Z.Y. Technology development for rare earth cleaner hydrometallurgy in China. Rare Mets. 2015, 34, 215–222. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, Q.; Feng, J.; Su, J.X.; Liu, X.; Chi, R.A. Role of initial moisture content on the leaching process of weathered crust elution-deposited rare earth ores. Sep. Purif. Technol. 2019, 217, 24–30. [Google Scholar] [CrossRef]
- Li, M.Y.H.; Zhou, M.F. The role of clay minerals in formation of the regolith-hosted heavy rare earth element deposits. Am. Miner. 2020, 105, 92–108. [Google Scholar] [CrossRef]
- Tian, J.; Yin, J.Q.; Chi, R.A.; Rao, G.H.; Jiang, M.T.; Ouyang, K.X. Kinetics on leaching rare earth from the weathered crust elution-deposited rare earth ores with ammonium sulfate solution. Hydrometallurgy 2010, 101, 166–170. [Google Scholar]
- Lee, T.K.; Ro, H.M. Estimating soil water retention function from its particle-size distribution. Geosci. J. 2014, 18, 219–230. [Google Scholar] [CrossRef]
- Xie, F.F.; Wang, G.S.; Wen, T. Discussion on reasons for difference of ionic phase rare earth grade distribution in different grain size fractions. Chin. J. Nonferr. Met. 2023, 33, 1672–1681. (In Chinese) [Google Scholar]
- Luo, S.H.; Luo, T.; Wang, G.S.; Liu, J.; Hu, S.L.; Zhu, D.M. Effect of heterogeneity of leaching solution on leaching rate in ionic rare earth ore body. Soils 2018, 50, 421–427. (In Chinese) [Google Scholar]
- Tian, J.; Yin, J.Q.; Tang, X.K.; Chen, J.; Luo, X.P.; Rao, G.H. Enhanced leaching process of a low-grade weathered crust elution-deposited rare earth ore with carboxymethyl sesbania gum. Hydrometallurgy 2013, 139, 124–131. [Google Scholar] [CrossRef]
- Luo, X.P.; Zhang, Y.B.; Zhou, H.P.; He, K.Z.; Zhang, B.Y.; Zhang, D.M.; Xiao, W.J. Pore structure characterization and seepage analysis of ionic rare earth orebodies based on computed tomography images. Int. J. Min. Sci. Technol. 2022, 32, 411–421. [Google Scholar] [CrossRef]
- Wang, M.Z.; Long, P.; Hu, S.L.; Wang, G.S.; Luo, S.H.; Hong, B.G. An estimation method for spatial permeability distribution of the ion-adsorption type rare earth ore body. Non-Ferr. Met. Sci. Eng. 2023, 14, 280–287. (In Chinese) [Google Scholar]
- Yin, S.H.; Qi, Y.; Xie, F.F.; Chen, X.; Wang, L.M. Permeability characteristic of weathered crust elution-deposited rare earth ores under different pore structures. Chin. J. Nonferrous Met. 2018, 28, 1043–1049. (In Chinese) [Google Scholar]
- Guo, Z.Q.; Lai, Y.M.; Jin, J.F.; Zhou, J.R.; Zhao, K.; Sun, Z. Effect of particle size and grain composition on two-dimensional infiltration process of weathered crust elution-deposited rare earth ores. Trans. Nonferrous Met. Soc. China 2020, 30, 1647–1661. [Google Scholar] [CrossRef]
- Zou, H.L.; Zhang, Z.Y.; Chen, Z.; Liu, D.F.; Chai, X.W.; Zhang, H.; Chi, R.A. Seepage process on weathered crust elution-deposited rare earth ores with ammonium carboxylate solution. Physicochem. Probl. Miner. Process. 2020, 56, 90–102. [Google Scholar]
- Liu, D.F.; Zhang, Z.Y.; Chi, R.A. Seepage mechanism during in-situ leaching process of weathered crust elution-deposited rare earth ores with magnesium salt. Physicochem. Probl. Miner. Process. 2020, 56, 349–361. [Google Scholar] [CrossRef]
- Wu, K.X.; Zhang, L.; Zhu, P.; Chen, L.K.; Tian, Z.F.; Xing, X.Y. Research on particle size distribution and its variation of ion-adsorption type rare earth ore. Chin. Rare Earths 2016, 37, 67–74. (In Chinese) [Google Scholar]
- Wei, D.D.; Wang, H.N.; Yang, Z.Z. Experimental research on the leaching of ionic rare earth ore with weak permeability. Min. Res. Dev. 2016, 36, 55–58. (In Chinese) [Google Scholar]
- Zhang, Q.; Zhang, Z.Y.; Liu, D.F.; Guo, W.D.; Yan, W.X. Analysis of influencing factors and model construction of electrical resistivity of weathered crust elution-deposited rare earth ore. Nonferr. Met. Eng. 2023, 13, 55–65. (In Chinese) [Google Scholar]
- Zhou, L.B.; Kang, S.J.; Yang, J.; Wang, X.J.; Yu, H.D.; Wan, Y.H. Effect of particle size on the leaching of a weathered crust elution-deposited rare earth ore. Hydrometallurgy 2023, 222, 106200. [Google Scholar] [CrossRef]
- GB/T 50123-2019; Standard for Geotechnical Testing Method. China Planning Press: Beijing, China, 2019. Available online: https://www.mohurd.gov.cn/gongkai/zhengce/zhengcefilelib/201908/20190801_241309.html (accessed on 21 July 2024).



| Parameter Type | Ore Grade (%) | Bulk Density (g/cm3) | Dry Density (g/cm3) | Moisture Content (%) | Specific Density |
|---|---|---|---|---|---|
| Value | 0.04 | 1.54 | 1.36 | 13.00 | 2.66 |
| REO Type | La2O3 | CeO2 | Pr6O11 | Nd2O3 | Sm2O3 | Eu2O3 | Gd2O3 | Tb4O7 |
|---|---|---|---|---|---|---|---|---|
| Value (%) | 21.52 | 30.85 | 5.14 | 19.58 | 3.66 | 0.50 | 3.30 | 0.42 |
| REO type | Dy2O3 | Ho2O3 | Er2O3 | Tm2O3 | Yb2O3 | Lu2O3 | Y2O3 | REO |
| Value (%) | 2.27 | 0.37 | 0.98 | <0.20 | 0.75 | <0.20 | 9.95 | 0.042 |
| Sample | CG-S1 | CG-S2 | CG-S3 | CG-S4 | CG-S5 | CG-S6 | CG-S7 |
|---|---|---|---|---|---|---|---|
| Fine particle content | 5.00 | 10.00 | 15.00 | 20.00 | 25.00 | 30.00 | 35.00 |
| Ore grade (%) | 0.048 | 0.050 | 0.053 | 0.059 | 0.064 | 0.069 | 0.070 |
| d50 (mm) | 1.55 | 1.34 | 1.14 | 0.97 | 0.83 | 0.69 | 0.56 |
| Cu | 13.43 | 28.45 | 48.84 | 75.52 | 97.11 | 109.75 | 111.91 |
| Cc | 1.04 | 1.83 | 2.37 | 2.63 | 1.97 | 0.40 | 0.42 |
| Sample | Fine Particle Content (%) | Column Height (mm) | Retention Volume (mL) | Half Peak Height Width (mL) | Theoretical Plate Number | Plate Height (mm) |
|---|---|---|---|---|---|---|
| CG-S1 | 5 | 80 | 63.08 | 94.57 | 2.47 | 32.43 |
| CG-S2 | 10 | 80 | 62.50 | 92.85 | 2.51 | 31.84 |
| CG-S3 | 15 | 80 | 63.53 | 80.11 | 3.49 | 22.94 |
| CG-S4 | 20 | 80 | 77.56 | 85.15 | 4.60 | 17.38 |
| CG-S5 | 25 | 80 | 75.86 | 71.54 | 6.24 | 12.83 |
| CG-S6 | 30 | 80 | 72.38 | 67.89 | 6.30 | 12.69 |
| CG-S7 | 35 | 80 | 69.98 | 63.98 | 6.63 | 12.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Yu, H.; Kang, S.; Sun, G.; Deng, Y.; Wang, X.; Zhao, H.; Xu, J. Effect of Fine Particle Content on Solution Flow and Mass Transfer of Ion-Adsorption-Type Rare Earth Ores. Minerals 2024, 14, 879. https://doi.org/10.3390/min14090879
Zhou L, Yu H, Kang S, Sun G, Deng Y, Wang X, Zhao H, Xu J. Effect of Fine Particle Content on Solution Flow and Mass Transfer of Ion-Adsorption-Type Rare Earth Ores. Minerals. 2024; 14(9):879. https://doi.org/10.3390/min14090879
Chicago/Turabian StyleZhou, Lingbo, Hongdong Yu, Shijie Kang, Guidong Sun, Yang Deng, Xiaojun Wang, Hanlin Zhao, and Jingtao Xu. 2024. "Effect of Fine Particle Content on Solution Flow and Mass Transfer of Ion-Adsorption-Type Rare Earth Ores" Minerals 14, no. 9: 879. https://doi.org/10.3390/min14090879
APA StyleZhou, L., Yu, H., Kang, S., Sun, G., Deng, Y., Wang, X., Zhao, H., & Xu, J. (2024). Effect of Fine Particle Content on Solution Flow and Mass Transfer of Ion-Adsorption-Type Rare Earth Ores. Minerals, 14(9), 879. https://doi.org/10.3390/min14090879








