Effect of Gas Dispersion Properties and Bias in Scheelite Column Flotation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment and Procedures
2.3. Gas Dispersion Properties and Bias
3. Results
3.1. Gas Dispersion Properties and Bias
3.1.1. Gas Holdup
3.1.2. Bubble Size
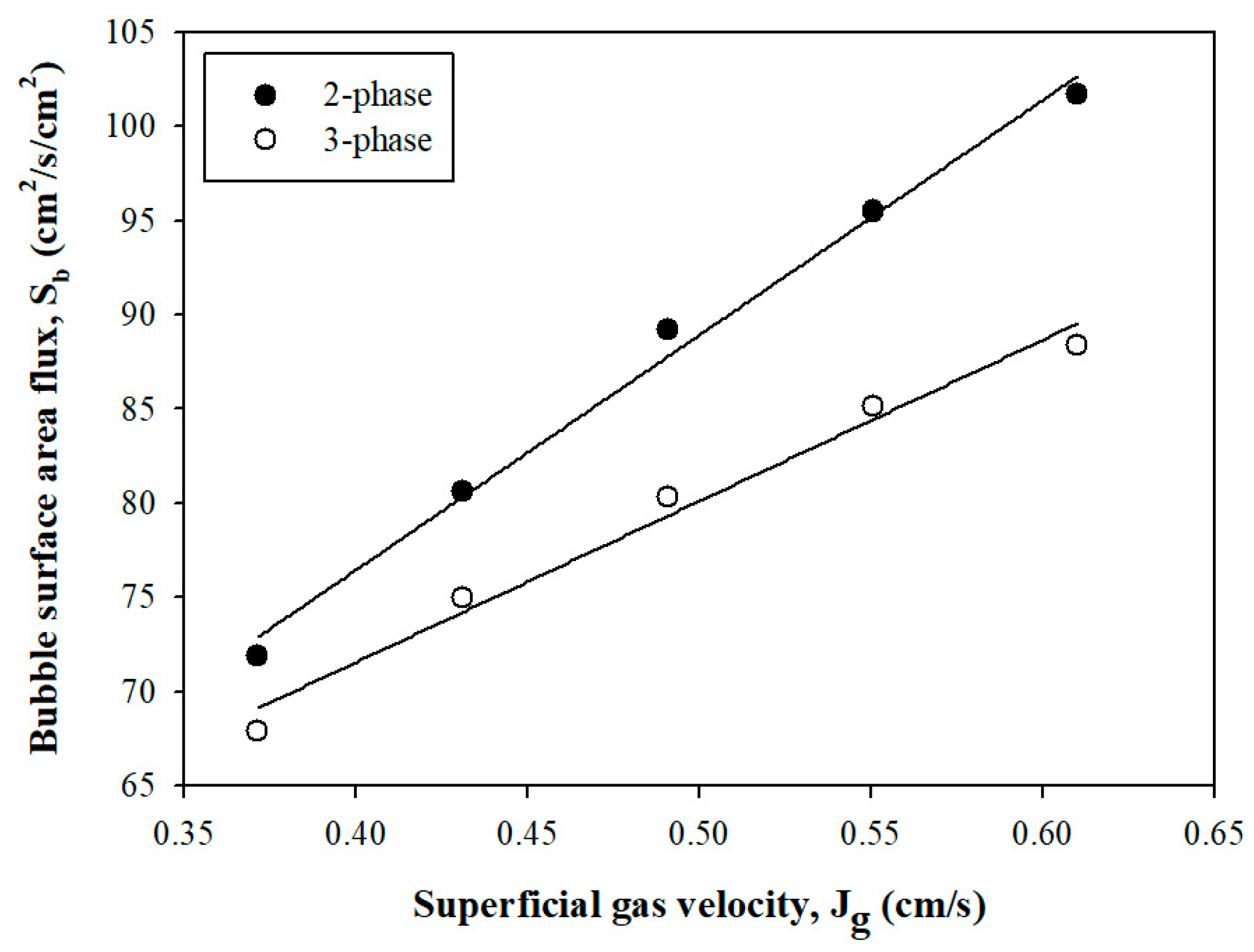
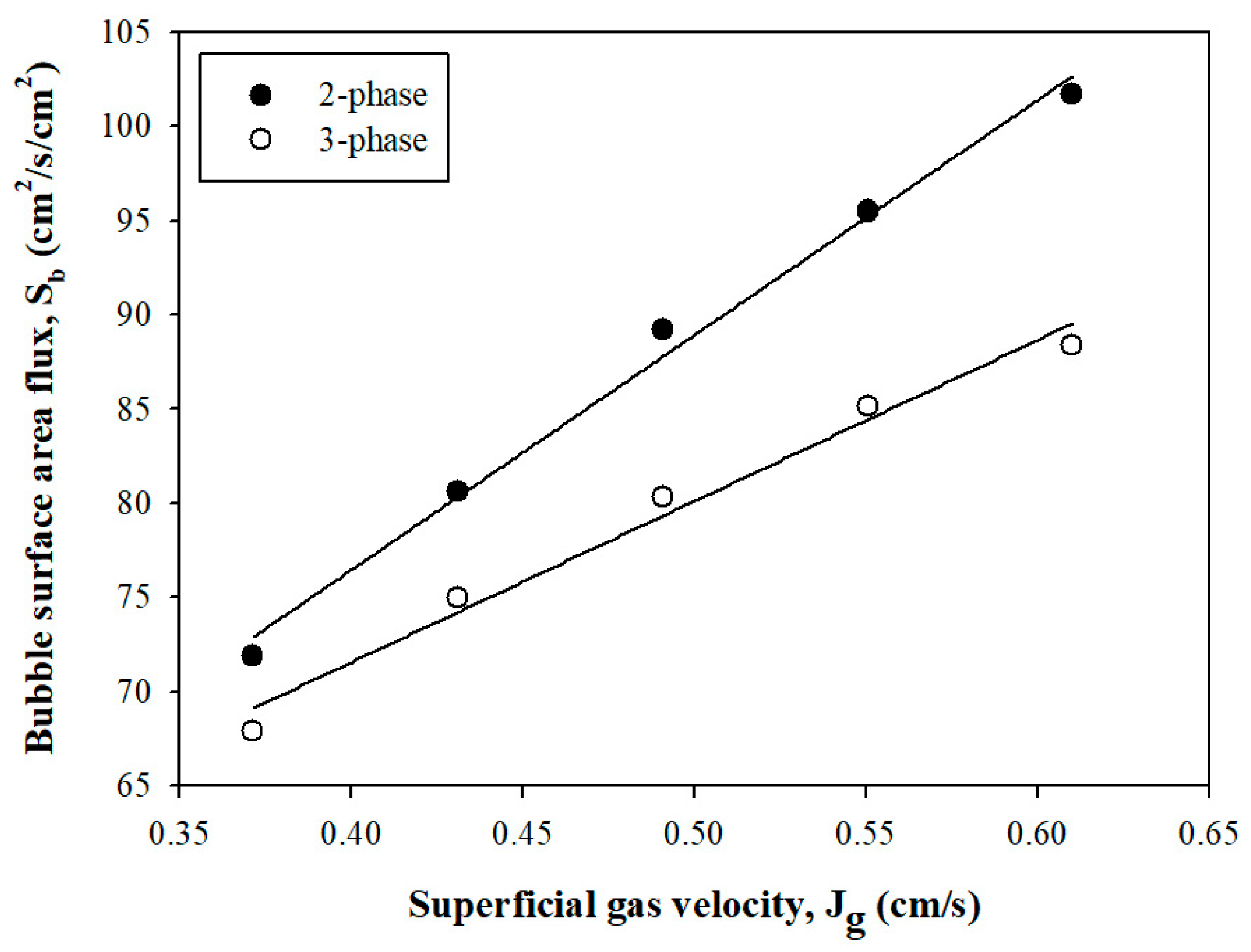
3.1.3. Bubble Surface Area Flux
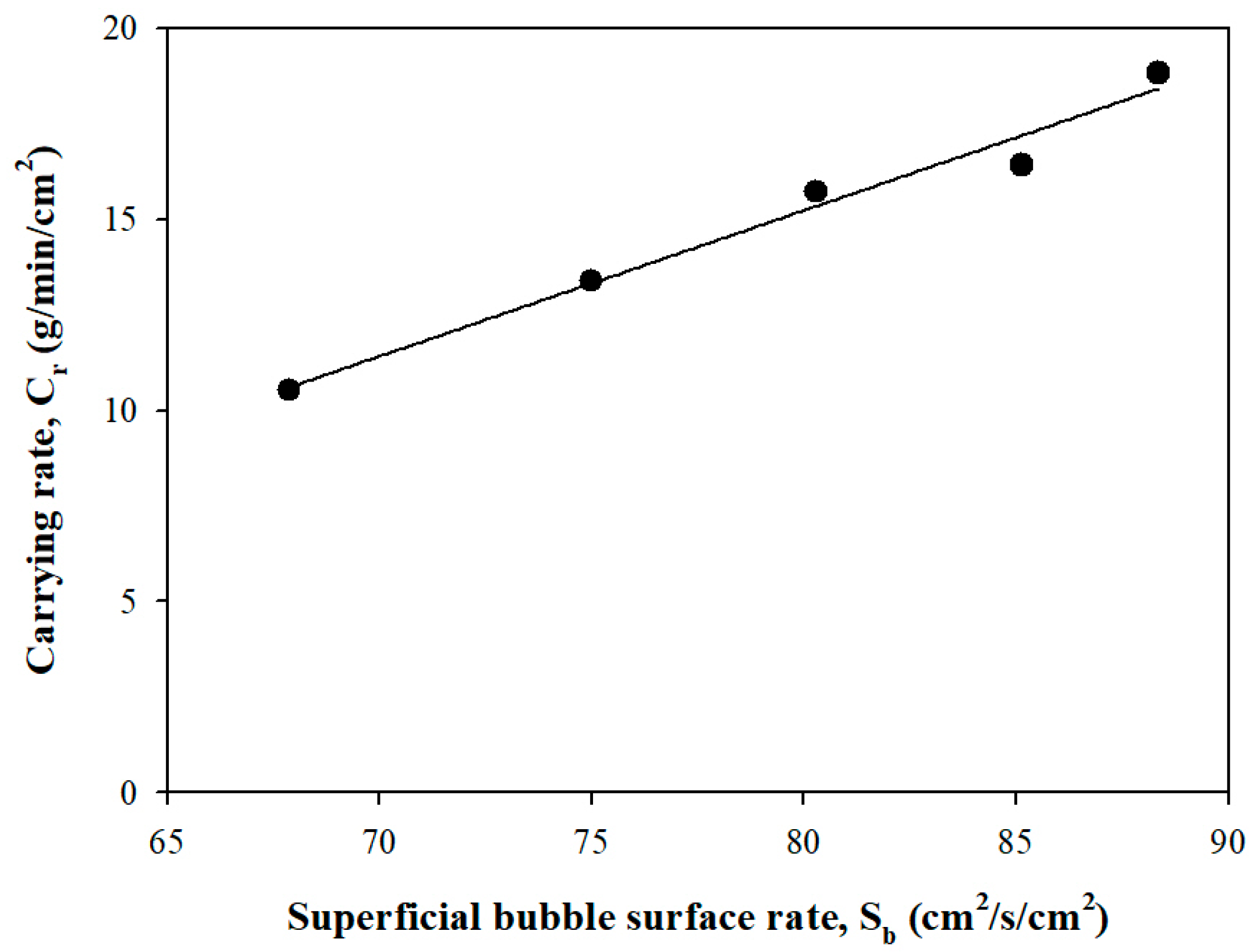
3.1.4. Carrying Rate
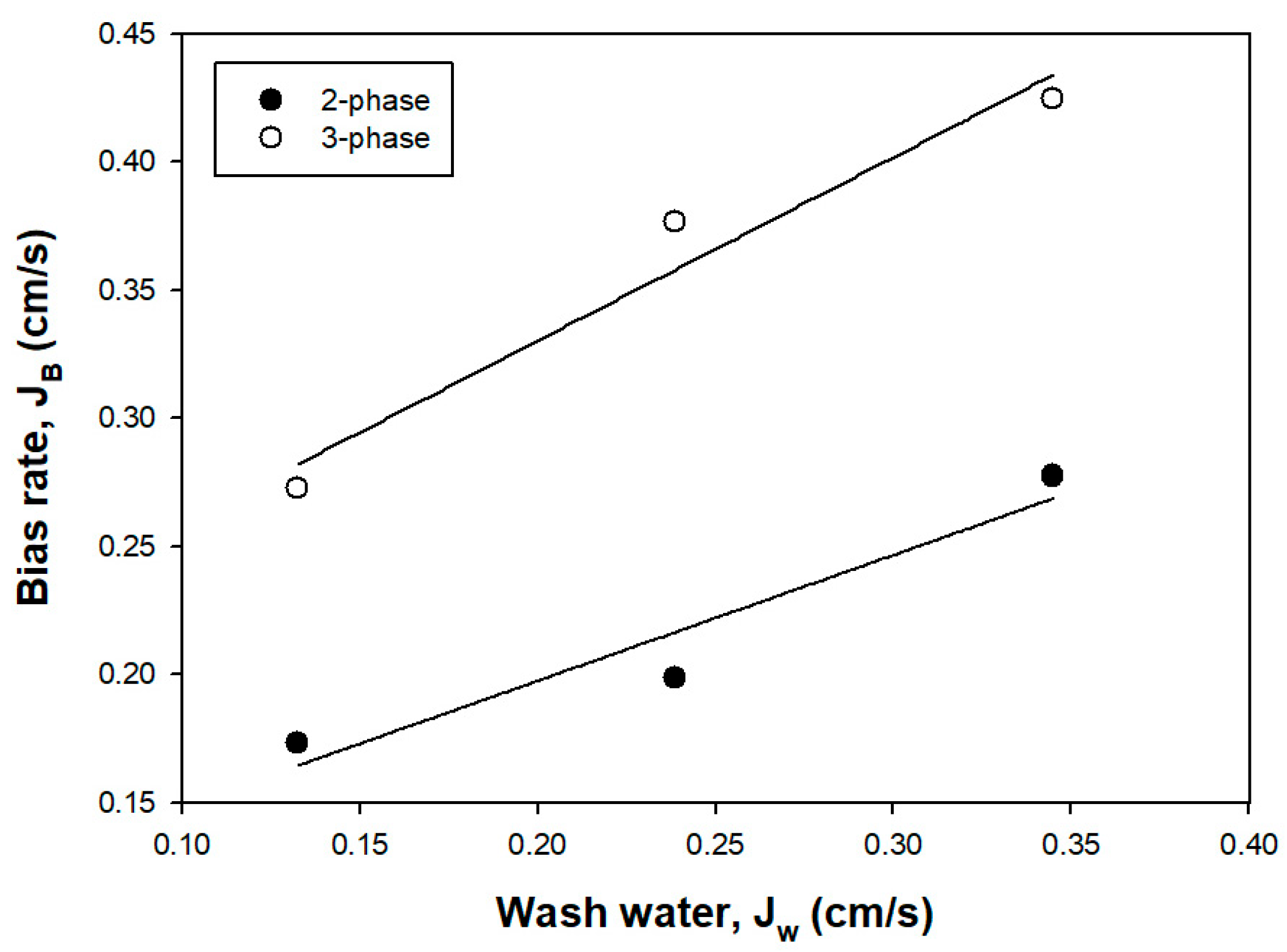
3.1.5. Bias Rate
3.2. Performance of Column Flotation
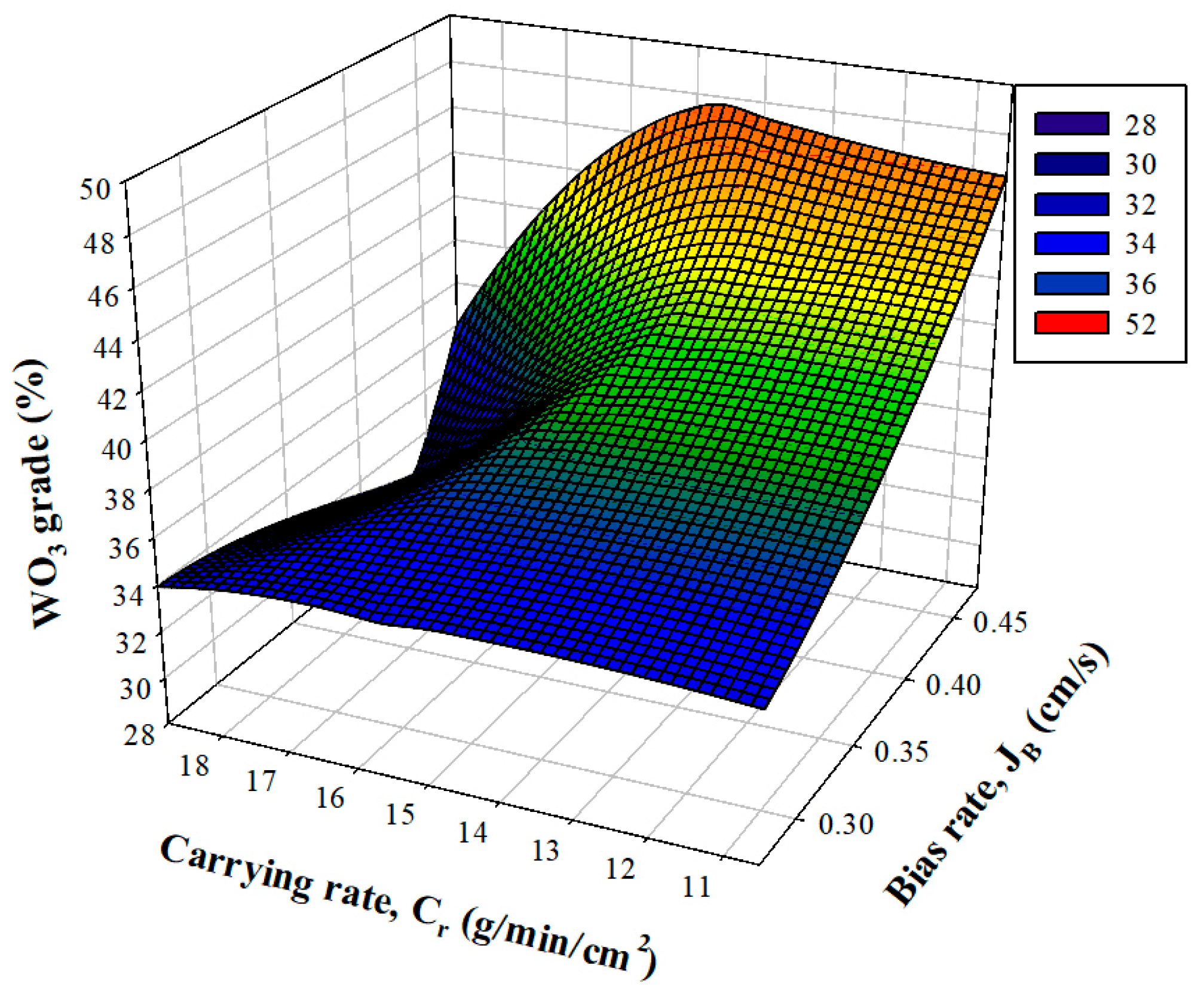
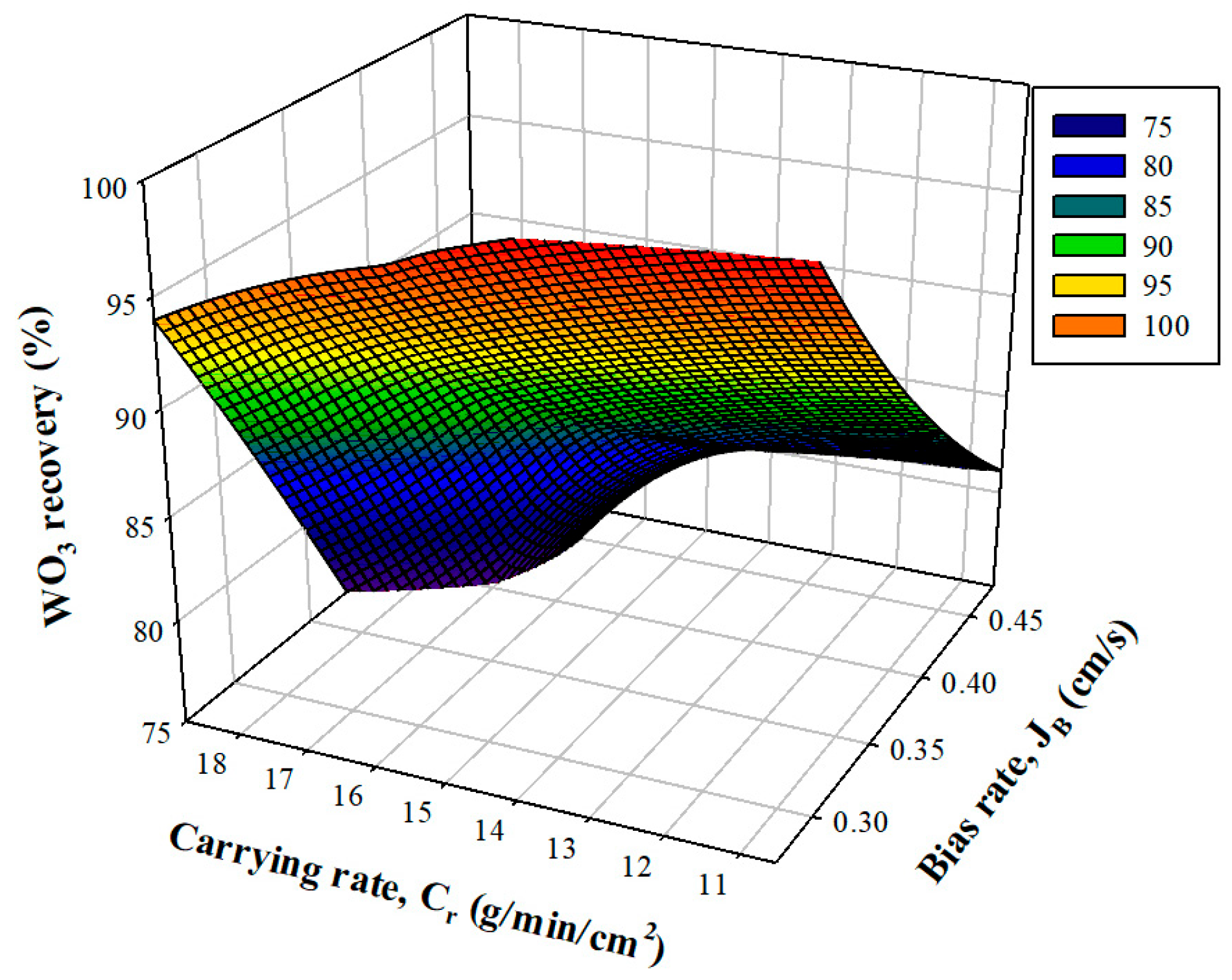
3.2.1. Effect of Bias and Carrying Rate on Grade and Recovery
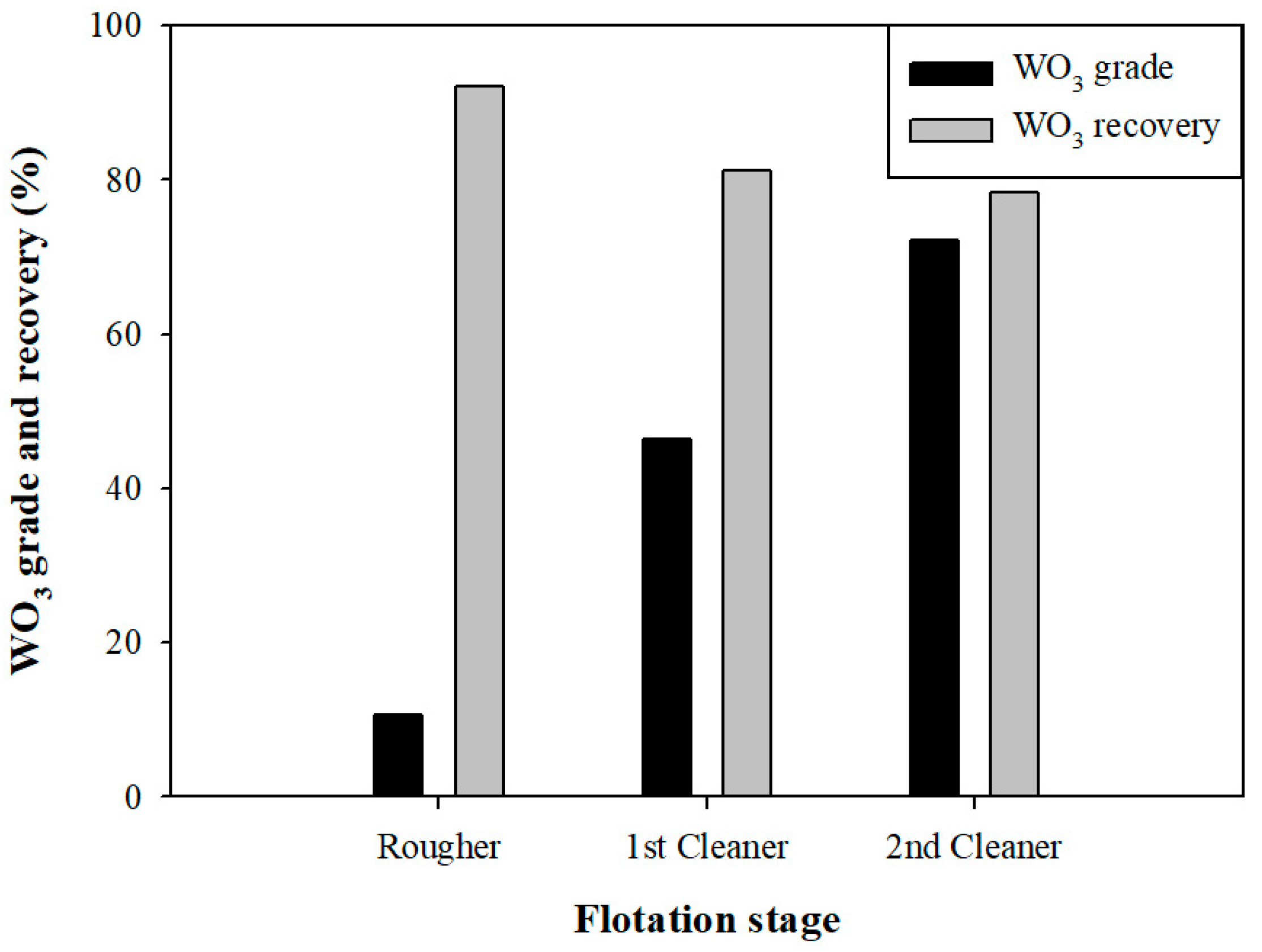
3.2.2. Grade and Recovery of WO3
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shemi, A.; Magumise, A.; Ndlovu, S.; Sacks, N. Recycling of tungsten carbide scrap metal: A review of recycling methods and future prospects. Miner. Eng. 2018, 122, 195–205. [Google Scholar] [CrossRef]
- Wu, Y.C.; Hou, Q.Q.; Luo, L.M.; Zan, X.; Zhu, X.Y.; Li, P.; Xu, Q.; Cheng, J.G.; Luo, G.N.; Chen, J.L. Preparation of ultrafine-grained/nanostructured tungsten materials: An overview. J. Alloys Compd. 2019, 779, 926–941. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, H.; Feng, B.; Zhang, L.; Chen, Y.; Gao, Z. Flotation separation of scheelite and apatite by polysaccharide depressant xanthan gum. Miner. Eng. 2021, 170, 107045. [Google Scholar] [CrossRef]
- Foucaud, Y.; Filippov, L.; Filippova, I.; Badawi, M. The challenge of tungsten skarn processing by froth flotation: A review. Front. Chem. 2020, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Li, M.; Zhang, M.; Cui, R.; Shi, J.; Ning, J. Decoupling the effects of solid and liquid phases in a Pb-water glass mixture on the selective flotation separation of scheelite from calcite. Miner. Eng. 2020, 154, 106423. [Google Scholar] [CrossRef]
- Hu, Y.-h.; Yang, F.; Sun, W. The flotation separation of scheelite from calcite using a quaternary ammonium salt as collector. Miner. Eng. 2011, 24, 82–84. [Google Scholar] [CrossRef]
- Feng, B.; Luo, X.; Wang, J.; Wang, P. The flotation separation of scheelite from calcite using acidified sodium silicate as depressant. Miner. Eng. 2015, 80, 45–49. [Google Scholar]
- Yin, W.-z.; Wang, J.-z. Effects of particle size and particle interactions on scheelite flotation. Trans. Nonferrous Met. Soc. China 2014, 24, 3682–3687. [Google Scholar] [CrossRef]
- Foucaud, Y.; Filippova, I.; Dehaine, Q.; Hubert, P.; Filippov, L. Integrated approach for the processing of a complex tungsten Skarn ore (Tabuaço, Portugal). Miner. Eng. 2019, 143, 105896. [Google Scholar] [CrossRef]
- Tao, X.; Liu, Y.; Jiang, H.; Chen, R. Microbubble generation with shear flow on large-area membrane for fine particle flotation. Chem. Eng. Process.-Process Intensif. 2019, 145, 107671. [Google Scholar] [CrossRef]
- Huang, G.; Feng, Q.; Ou, L.; Wu, X.; Lu, Y.; Zhang, G. A comparative study of recovering fine scheelite in tailings by flotation cell and flotation column. J. Solid Waste Technol. Manag. 2010, 36, 61–68. [Google Scholar] [CrossRef]
- Sobhy, A.; Tao, D. Nanobubble column flotation of fine coal particles and associated fundamentals. Int. J. Miner. Process. 2013, 124, 109–116. [Google Scholar] [CrossRef]
- Peng, F.F.; Yu, X. Pico–nano bubble column flotation using static mixer-venturi tube for Pittsburgh No. 8 coal seam. Int. J. Min. Sci. Technol. 2015, 25, 347–354. [Google Scholar] [CrossRef]
- Finch, J.A.; Dobby, G. Column flotation. In Flotation Science and Engineering; CRC Press: Boca Raton, FL, USA, 1990; pp. 291–329. [Google Scholar]
- Finch, J.; Xiao, J.; Hardie, C.; Gomez, C. Gas dispersion properties: Bubble surface area flux and gas holdup. Miner. Eng. 2000, 13, 365–372. [Google Scholar] [CrossRef]
- Bhunia, K.; Kundu, G.; Mukherjee, D. Application of statistical analysis on the bubble surface area flux in a column flotation cell. Sep. Sci. Technol. 2015, 50, 1230–1238. [Google Scholar] [CrossRef]
- Li, H.; Villar, R.D.; Gomez, C. Reviewing the experimental procedure to determine the carrying capacity in flotation columns. Can. Metall. Q. 2004, 43, 513–520. [Google Scholar] [CrossRef]
- Şahbaz, O.; Uçar, A.; Öteyaka, B.; Taş, O.Ö.; Özdemir, O. Separation of colemanite from tailings using the pilot scale flotation column. Powder Technol. 2017, 309, 31–36. [Google Scholar] [CrossRef]
- Banisi, S.; Finch, J.; Laplante, A.; Weber, M. Effect of solid particles on gas holdup in flotation columns—I. Measurement. Chem. Eng. Sci. 1995, 50, 2329–2334. [Google Scholar] [CrossRef]
- Shukla, S.C.; Kundu, G.; Mukherjee, D. Study of gas holdup and pressure characteristics in a column flotation cell using coal. Miner. Eng. 2010, 23, 636–642. [Google Scholar] [CrossRef]
- Qiu, G.; Hu, Y.; Wang, D. Interaction of Particles and Flotation Techniques of Fine Particles; Central South University of Technology Press: Changsha, China, 1993. (In Chinese) [Google Scholar]
- Krishna, R.; De Swart, J.W.; Ellenberger, J.; Martina, G.B.; Maretto, C. Gas holdup in slurry bubble columns: Effect of column diameter and slurry concentrations. AIChE J. 1997, 43, 311–316. [Google Scholar] [CrossRef]
- Pandit, A.; Joshi, J. Three phase sparged reactors—Some design aspects. Rev. Chem. Eng. 1984, 2, 1–84. [Google Scholar] [CrossRef]
- Vazirizadeh, A.; Bouchard, J.; Chen, Y. Effect of particles on bubble size distribution and gas hold-up in column flotation. Int. J. Miner. Process. 2016, 157, 163–173. [Google Scholar] [CrossRef]
- Ostadrahimi, M.; Gharibi, K.; Dehghani, A.; Farrokhpay, S. Estimating bubble loading in industrial flotation cells. Minerals 2019, 9, 222. [Google Scholar] [CrossRef]
- Panjipour, R.; Karamoozian, M.; Albijanic, B. Bubble size distributions in gas–liquid–solid systems and their influence on flotation separation in a bubble column. Chem. Eng. Res. Des. 2021, 167, 96–106. [Google Scholar] [CrossRef]
- Sarhan, A.; Naser, J.; Brooks, G. CFD model simulation of bubble surface area flux in flotation column reactor in presence of minerals. Int. J. Min. Sci. Technol. 2018, 28, 999–1007. [Google Scholar] [CrossRef]
- Panjipour, R.; Karamoozian, M.; Albijanic, B. Investigations of gas holdup, interfacial area of bubbles and bubble size distributions in a pilot plant flotation column. Miner. Eng. 2021, 164, 106819. [Google Scholar] [CrossRef]
- Leiva, J.; Vinnett, L.; Contreras, F.; Yianatos, J. Estimation of the actual bubble surface area flux in flotation. Miner. Eng. 2010, 23, 888–894. [Google Scholar] [CrossRef]
- Yianatos, J.; Contreras, F. On the carrying capacity limitation in large flotation cells. Can. Metall. Q. 2010, 49, 345–352. [Google Scholar] [CrossRef]
- Martínez-Gómez, V.; Pérez-Garibay, R.; Rubio, J. Factors involving the solids-carrying flotation capacity of microbubbles. Miner. Eng. 2013, 53, 160–166. [Google Scholar] [CrossRef]
- Garibay, R.P. Effect of collection zone height and operating variables on recovery of overload flotation columns. Miner. Eng. 2002, 15, 325–331. [Google Scholar] [CrossRef]
- Davis, V., Jr.; Stanley, F.; Bethell, P.; Luttrell, G.; Mankosa, M. Column flotation at the Middle Fork preparation facility. Coal Perparation 1994, 14, 133–145. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L. Improving column flotation of oxidized or ultrafine coal particles by changing the flow pattern of air supply. Miner. Eng. 2018, 124, 98–102. [Google Scholar] [CrossRef]
- Mohanty, M.; Honakeri, R. Performance optimization of Jameson flotation technology for fine coal cleaning. Miner. Eng. 1999, 12, 367–381. [Google Scholar] [CrossRef]
- Patwardhan, A.; Honaker, R. Development of a carrying-capacity model for column froth flotation. Int. J. Miner. Process. 2000, 59, 275–293. [Google Scholar] [CrossRef]
- Perez, R.; Del Villar, R. Estimation of bias and entrainment in flotation columns using conductivity measurements. Can. Metall. Q. 1997, 36, 299–307. [Google Scholar] [CrossRef]
- Ucar, A.; Yargan, M. Selective separation of boron values from the tailing of a colemanite processing plant. Sep. Purif. Technol. 2009, 68, 1–8. [Google Scholar] [CrossRef]
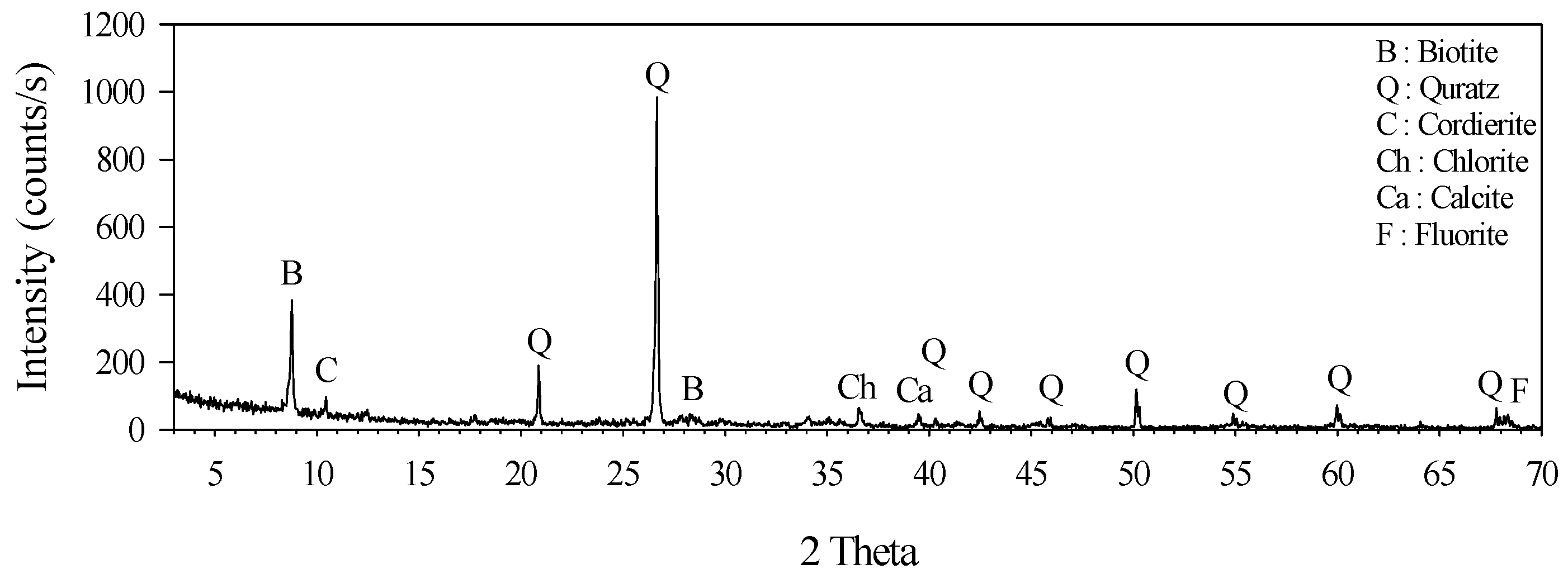
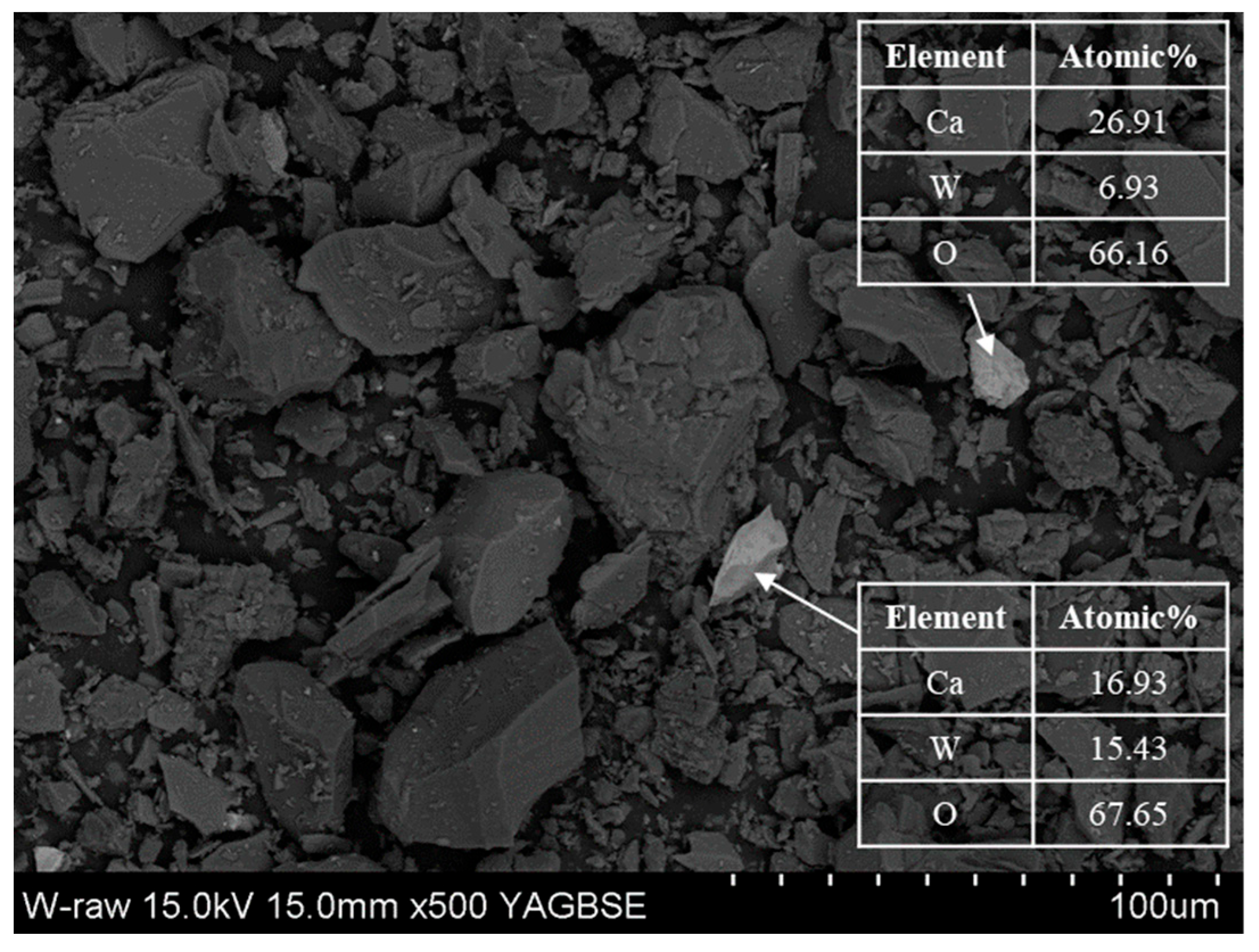
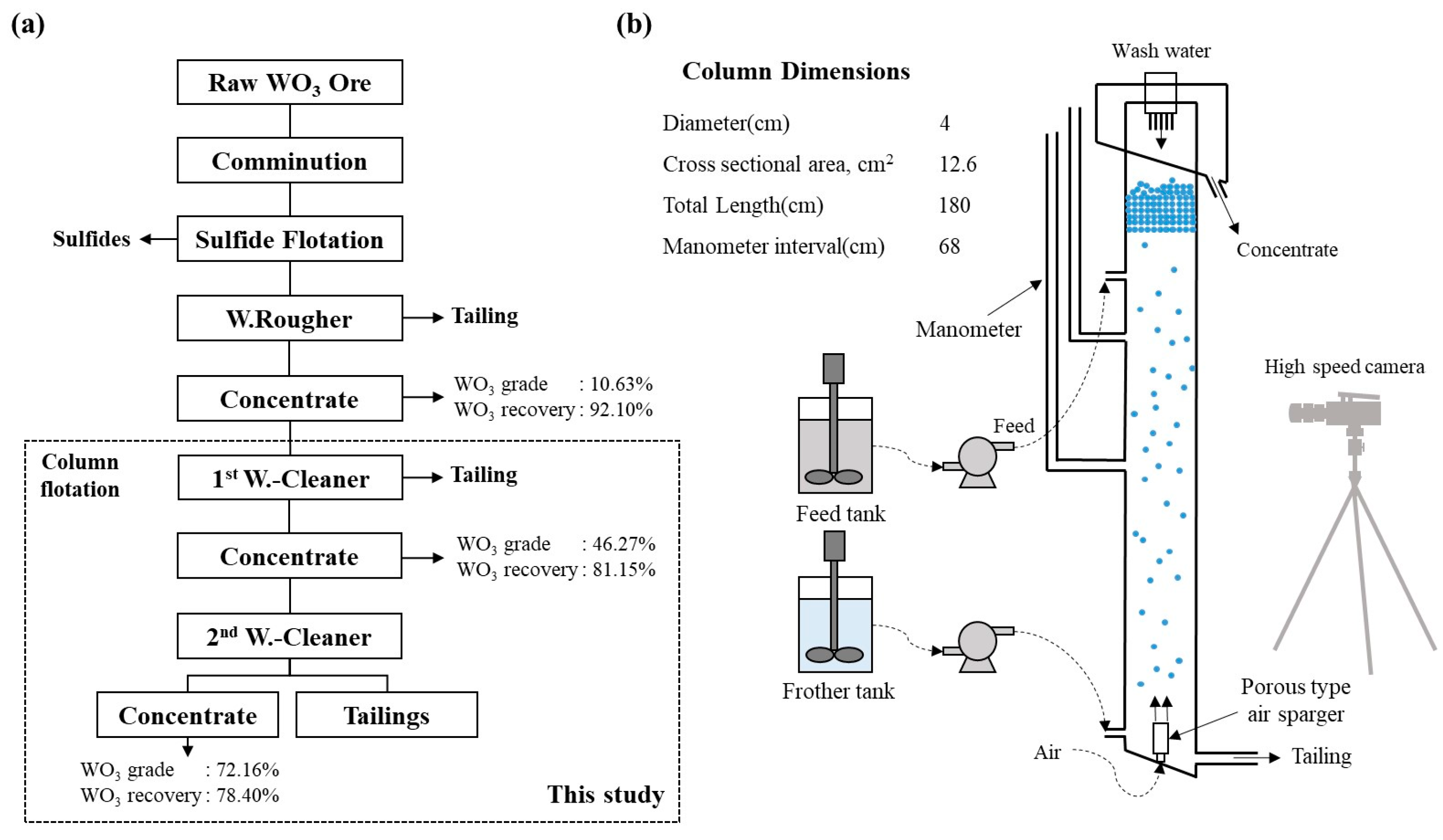
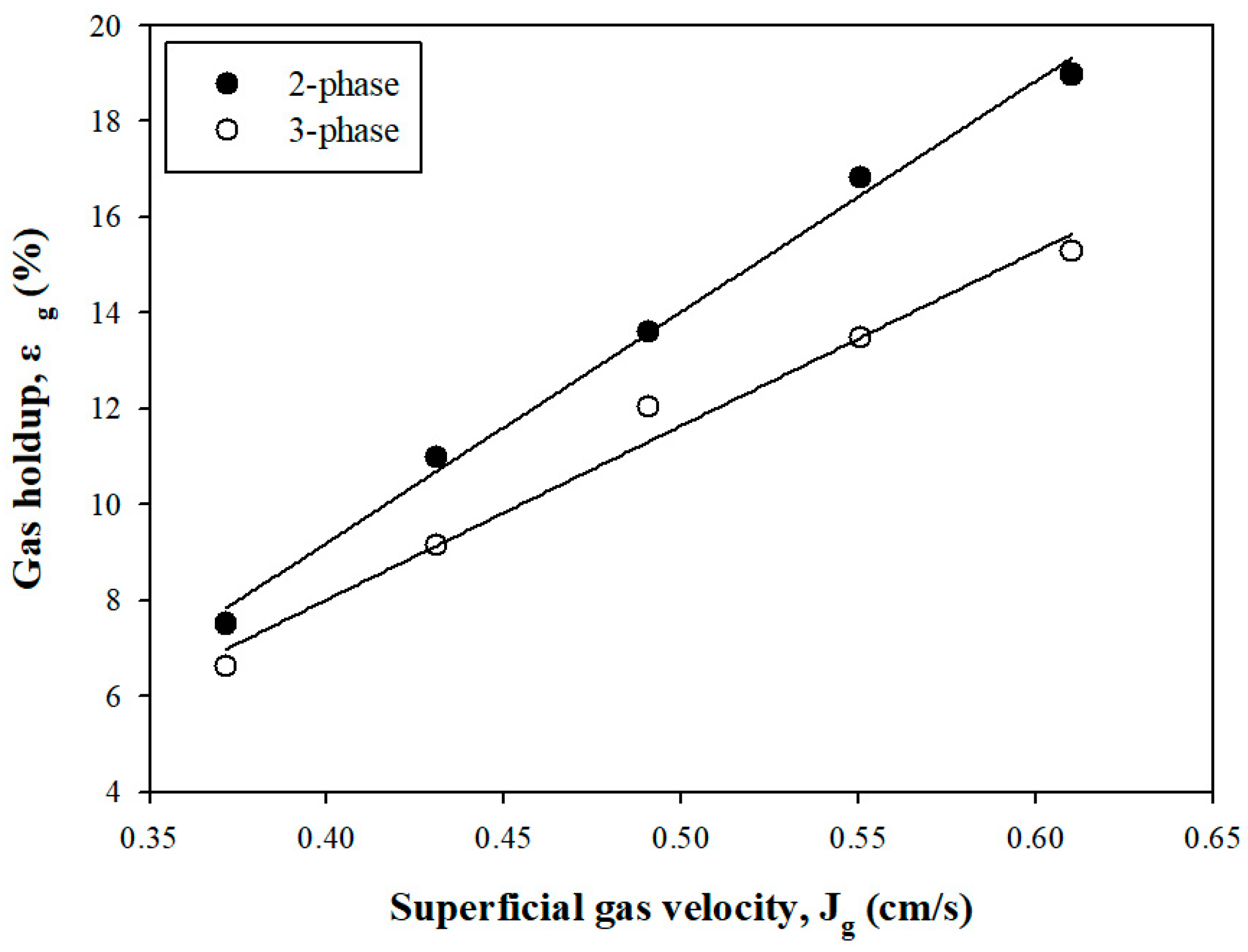
| Element | SiO2 | Fe2O3 | Al2O3 | CaO | MgO | K2O | SO3 | TiO2 | WO3 | Etc. |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 53.44 | 13.97 | 12.35 | 9.42 | 3.42 | 2.99 | 0.91 | 0.88 | 0.37 | 2.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Park, C.-H. Effect of Gas Dispersion Properties and Bias in Scheelite Column Flotation. Minerals 2024, 14, 881. https://doi.org/10.3390/min14090881
Kim H, Park C-H. Effect of Gas Dispersion Properties and Bias in Scheelite Column Flotation. Minerals. 2024; 14(9):881. https://doi.org/10.3390/min14090881
Chicago/Turabian StyleKim, HyunSoo, and Chul-Hyun Park. 2024. "Effect of Gas Dispersion Properties and Bias in Scheelite Column Flotation" Minerals 14, no. 9: 881. https://doi.org/10.3390/min14090881






