Study on Column Leaching Behavior of Low-Grade High Calcium and Magnesium Copper Ore
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Analysis
2.2. Leaching Process
3. Process Mineralogy
3.1. Elemental Analysis
3.2. Particle Size Analysis
3.3. Chemical Phase Analysis
3.4. Mineral Composition and Relative Abundance
3.5. Mineral Distribution Characteristics
3.6. Occurrence States of Copper, Cobalt, Calcium, and Magnesium
4. Analysis of Leaching Mechanisms
4.1. Leaching Mechanism of Copper-Bearing Minerals
- (1)
- Leaching of copper oxide minerals: Malachite is a copper carbonate mineral (Cu2CO3(OH)2) belonging to the monoclinic crystal system. Its crystals are often prismatic or needle-like. Malachite is readily leached with acids. In a sulfuric acid leaching system, the chemical reaction proceeds as shown in Equation (2) [28]:
- (2)
- Leaching of copper sulfide minerals: Copper sulfide minerals include chalcocite and bornite, although they are present in very low quantities and are difficult to leach under conventional conditions. Leaching typically requires bacterial action or high-temperature oxidative conditions. In a sulfuric acid leaching system, chalcocite and bornite react directly with sulfuric acid, potentially undergoing the chemical reactions shown in Equations (3) and (4) [28]:
4.2. Leaching Mechanism of Cobalt-Bearing Minerals
4.3. Leaching Mechanism of Other Metal Minerals
4.4. Leaching Mechanism of Gangue Minerals
- (1)
- Quartz: a gangue mineral with stable physical and chemical properties, belonging to the trigonal crystal system. As an oxide mineral, quartz reacts with hydrofluoric acid but does not react with sulfuric acid, hydrochloric acid, or nitric acid.
- (2)
- Dolomite: a calcium magnesium carbonate CaMg(CO3)2, belonging to the trigonal crystal system. In a sulfuric acid leaching system, dolomite reacts with sulfuric acid, as given in Equation (13) [26]:
- (3)
- Muscovite: a silicate mineral KAl2[AlSi3O10](OH)2, belonging to the monoclinic crystal system. The ideal composition involves Al3+ in the octahedral sites, which can be partially substituted by Fe3+, Mg2+, Fe2+, Mn2+, V, and other metal ions. In a sulfuric acid leaching system, muscovite can react with sulfuric acid, as given in Equation (14) [26]:
- (4)
- Talc: a tri-octahedral mineral Mg3(Si4O10)(OH)2. It typically occurs in massive, platy, fibrous, or radial forms, known for its softness and greasy feel. In a sulfuric acid leaching system, talc may react with sulfuric acid, as given in Equation (15) [26]:
- (5)
- Chlorite: a special 2:1-type hydrated layered aluminosilicate mineral with a general formula Y3[Z4O10](OH)2·Y3(OH)6, in the formula, Y represents Mg2+, Fe2+, Al3+, and Fe3+, while Z represents Si and Al.
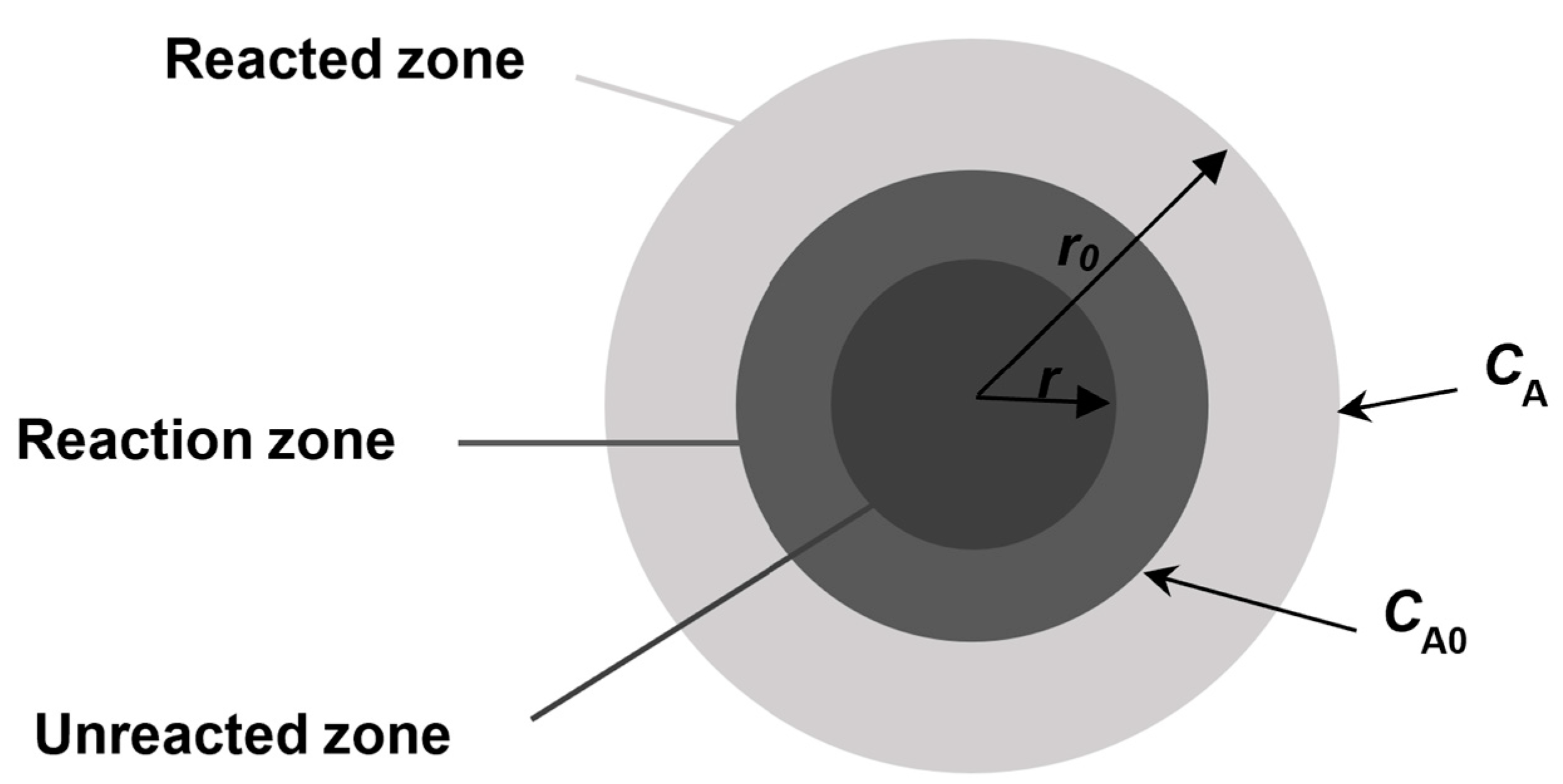
5. Leaching Kinetics Analysis
- (1)
- When the chemical reaction rate is very fast, Equation (32) can be simplified to , indicating that the leaching process is controlled by solid material layer diffusion.
- (2)
- When the solid material layer diffusion rate is very high, the leaching process is controlled by chemical reactions.
- (3)
- When the rates are comparable, the leaching process is controlled by a combination of chemical reaction and solid material layer diffusion, and the dynamic equation is Equation (32).
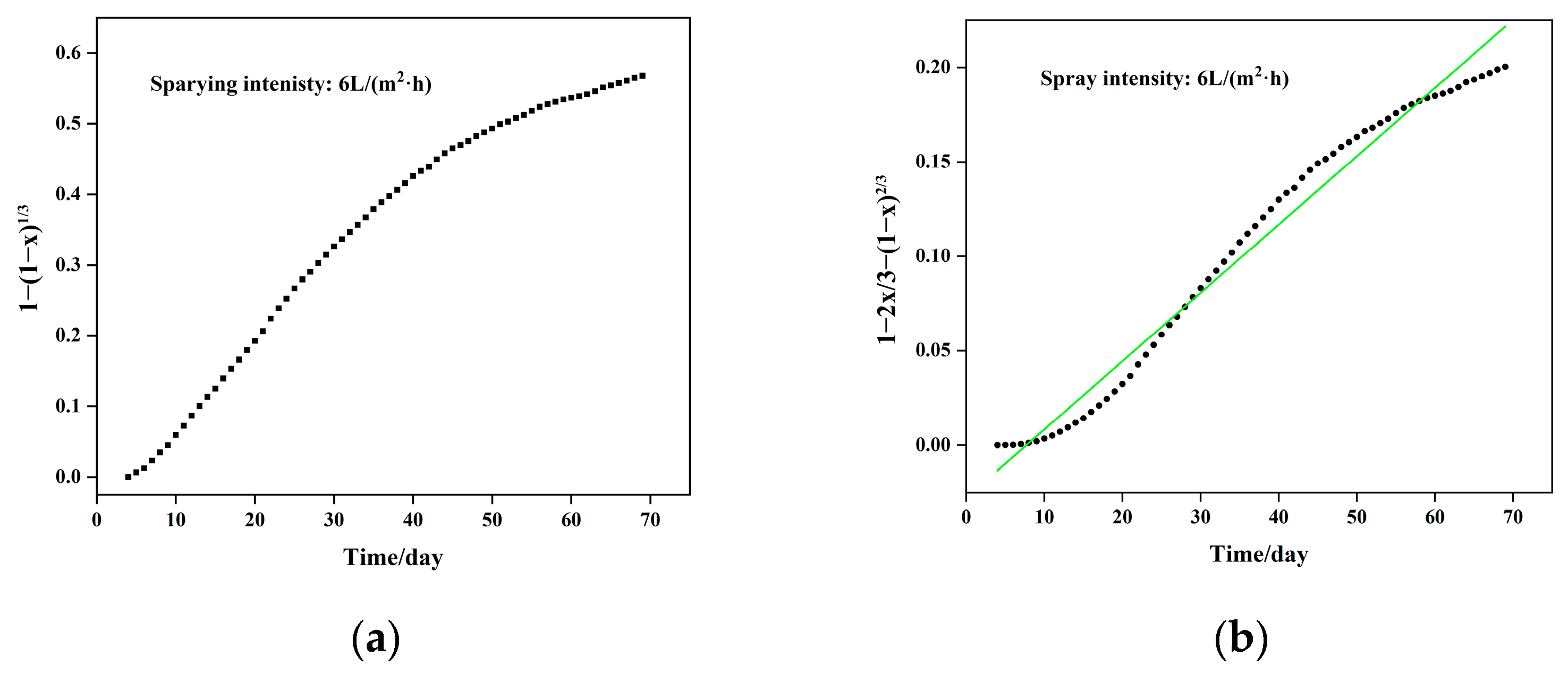
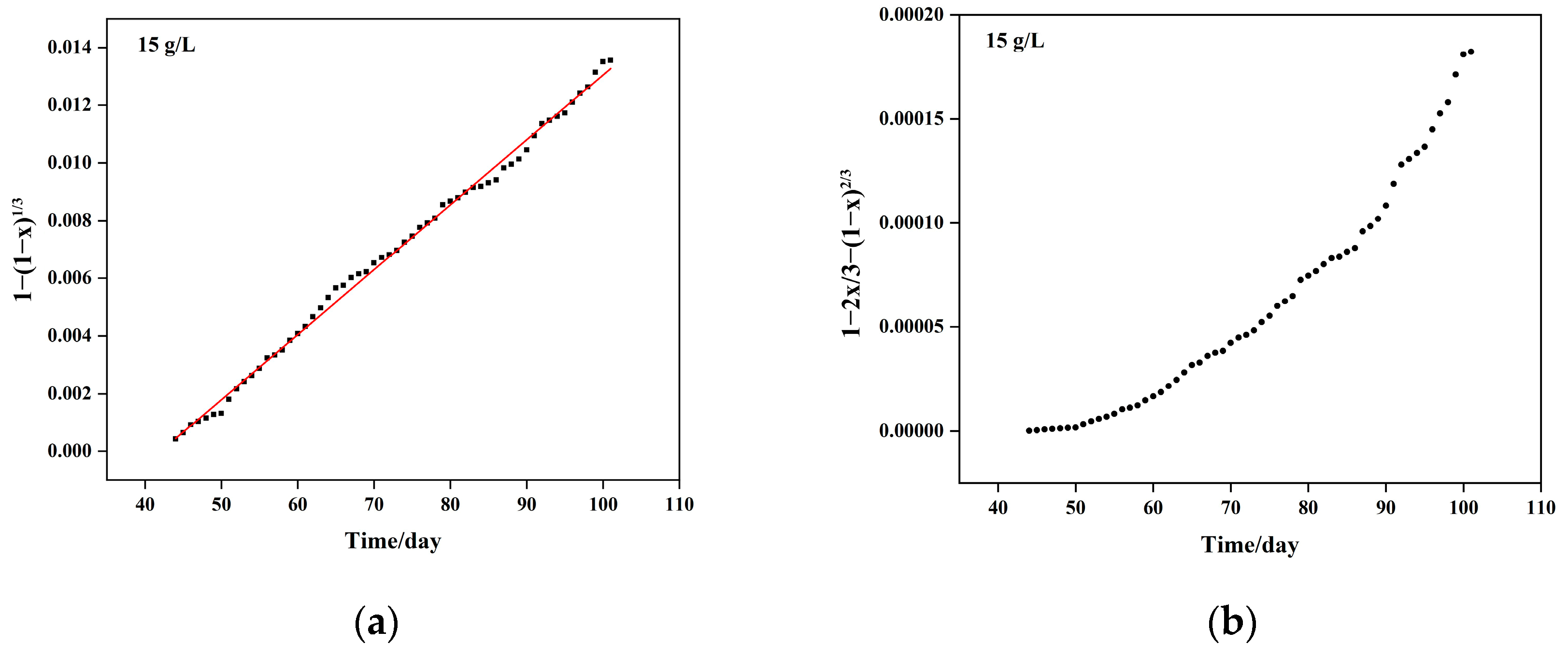
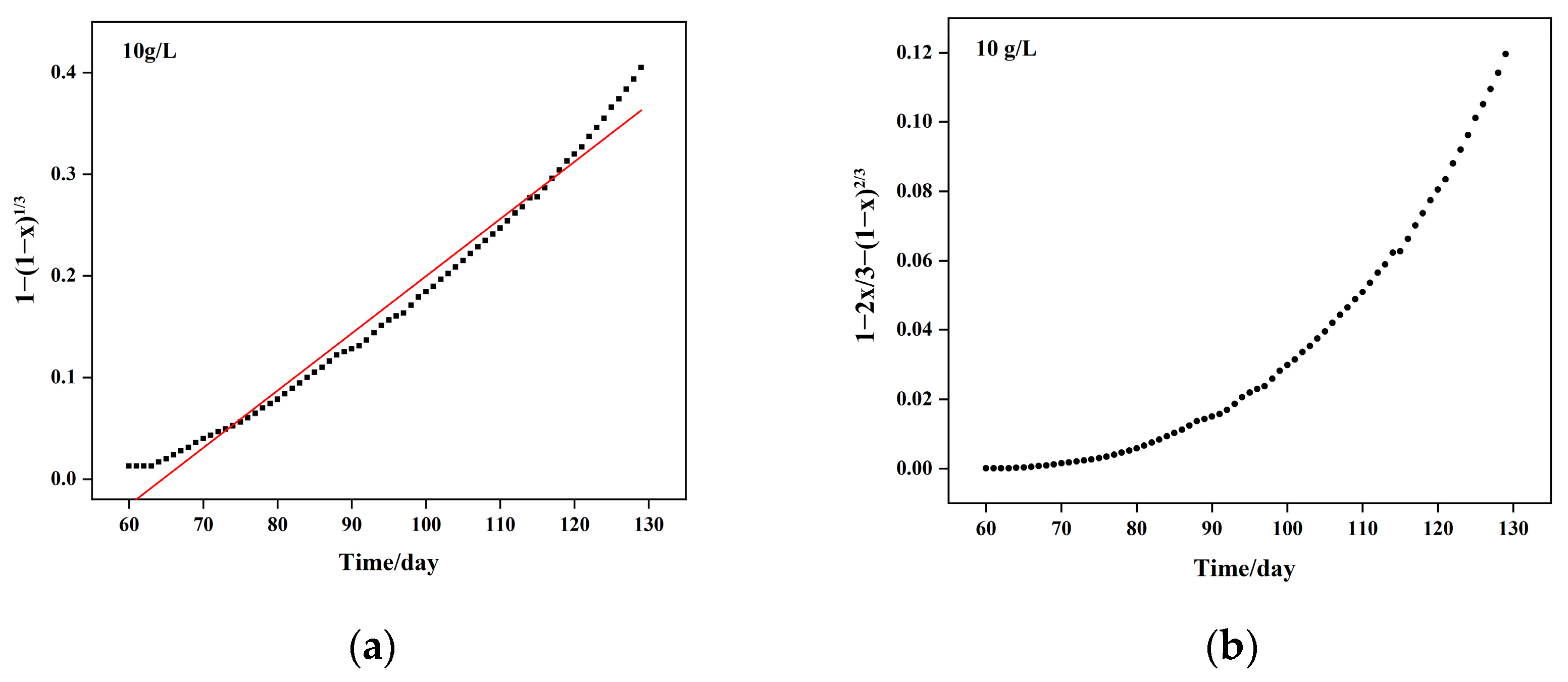
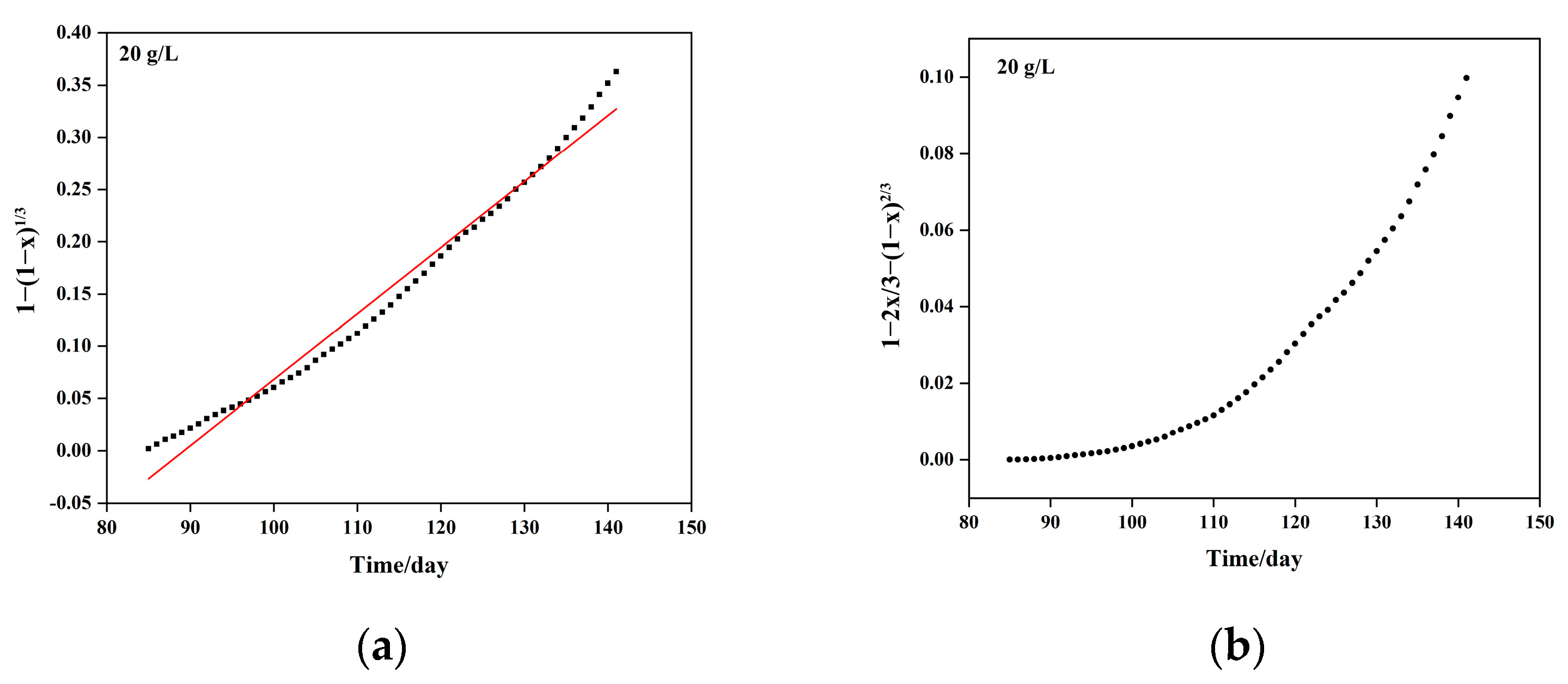
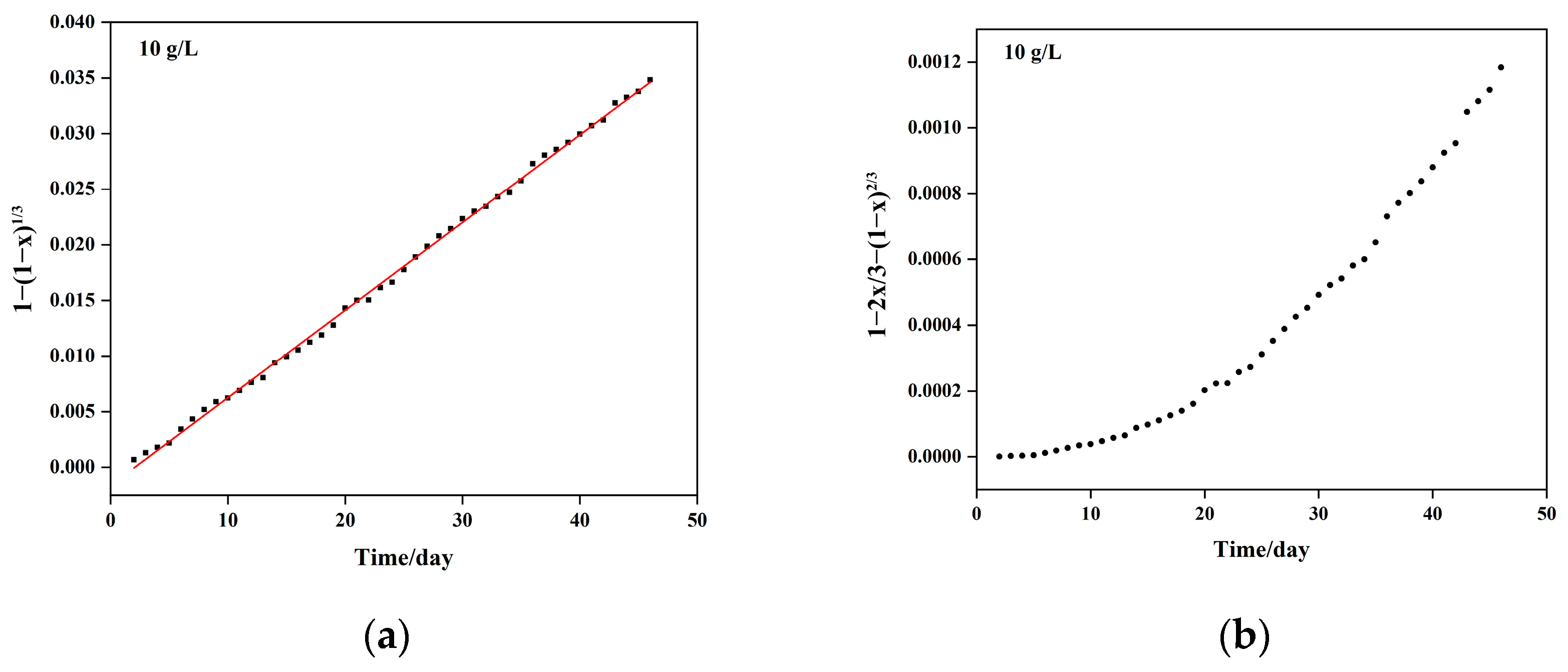
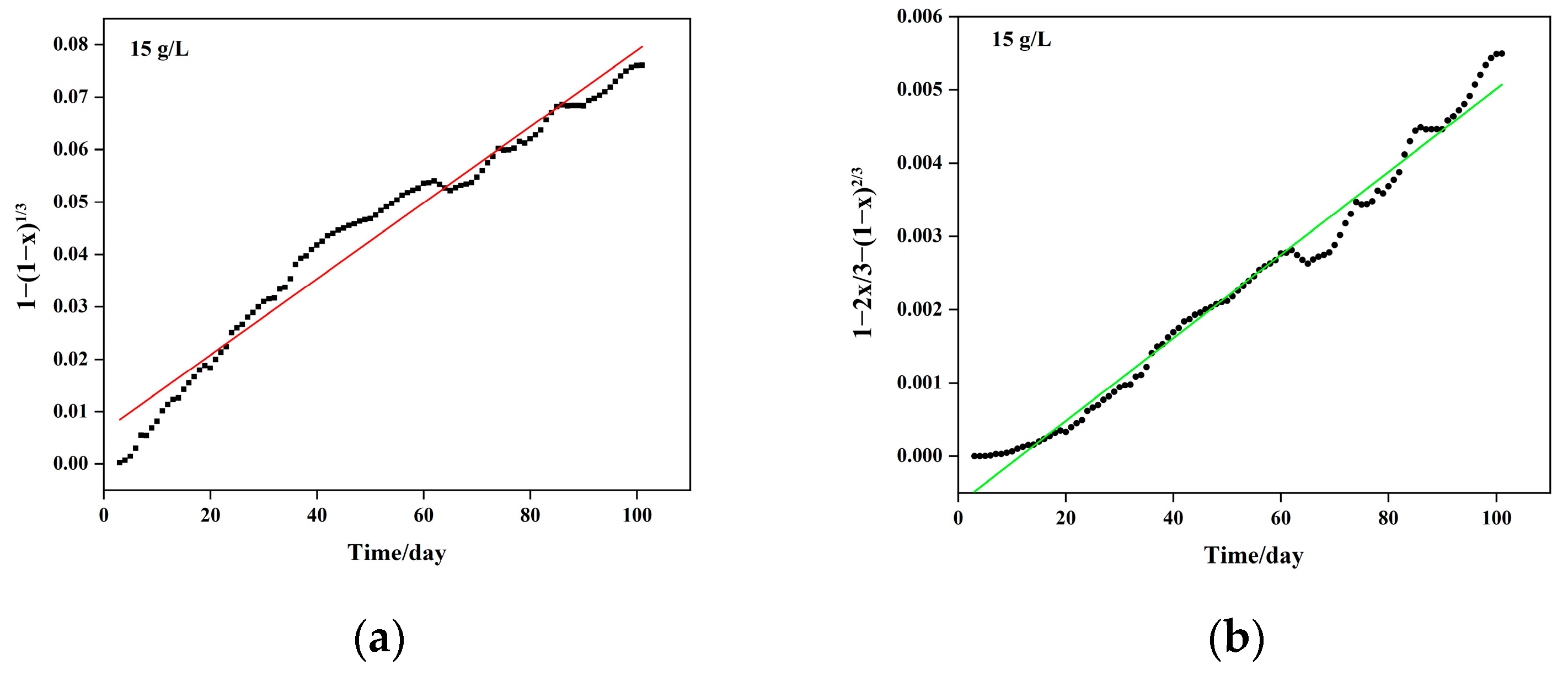
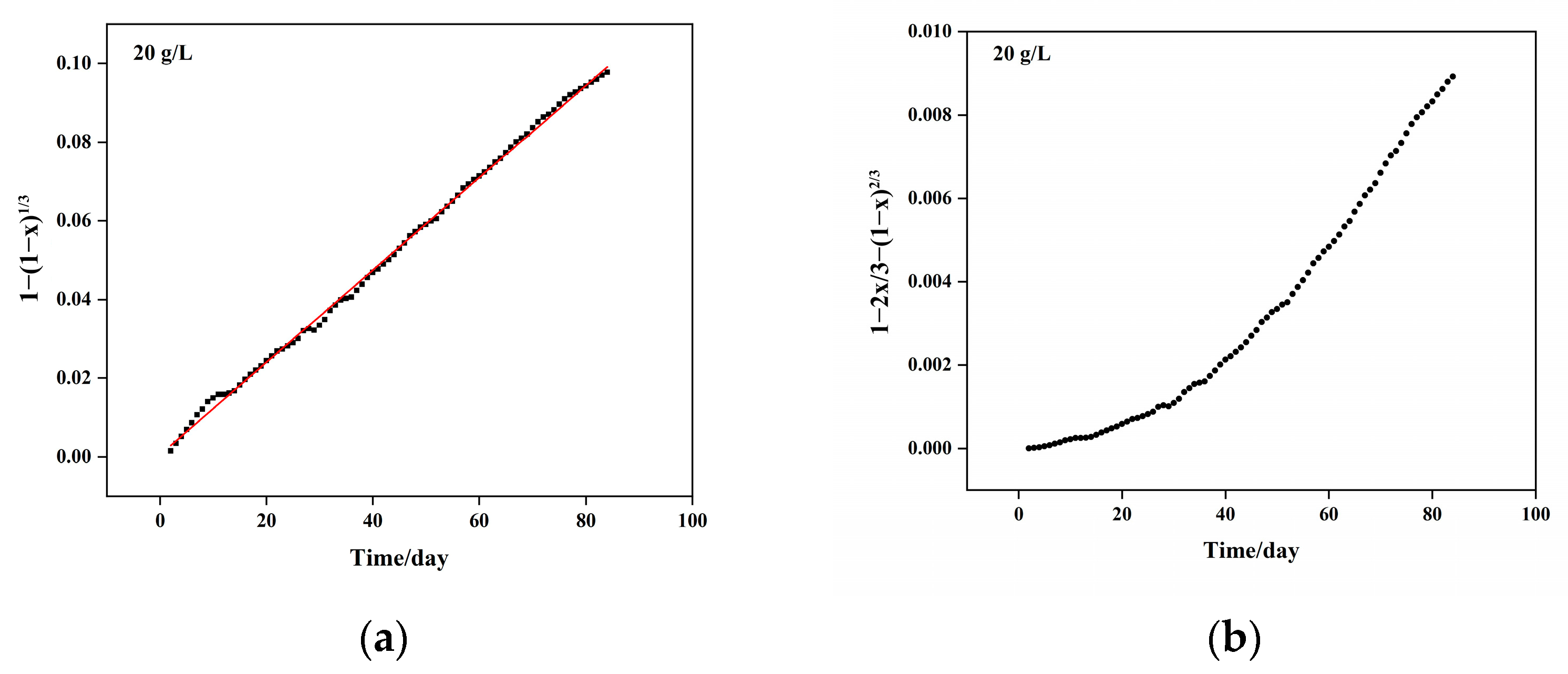
5.1. Column Leaching Kinetics of Copper
- (1)
- Chemical reaction control kinetics equation: , with a correlation coefficient of R2 = 0.9918;
- (2)
- Solid material diffusion control kinetics equation: , with a correlation coefficient of R2 = 0.9828. Combining the chemical reaction and diffusion control kinetics equations, the resulting mixed control kinetics equation is represented as: , with an apparent reaction rate constant of 4.090 × 10−3 day−1.
- (1)
- Chemical reaction control kinetic equation: , with a correlation coefficient of R2 = 0.9746;
- (2)
- Solid material layer diffusion control kinetic equation: , with a correlation coefficient of R2 = 0.9912. Combining the kinetic equations for chemical reaction control and diffusion control, the mixed control kinetic equation is derived as , with an apparent reaction rate constant of 2.729 × 10−2 per day.
- (1)
- Chemical reaction control kinetic equation: , with a correlation coefficient of R2 = 0.9918;
- (2)
- Solid material diffusion control kinetic equation: , with a correlation coefficient R2 = 0.9828. By merging the chemical reaction control and diffusion control kinetic equations, the combined control kinetic equation is obtained as follows: , with an apparent reaction rate constant of 4.092 × 10−3 per day.
5.2. Leaching Kinetics of Iron
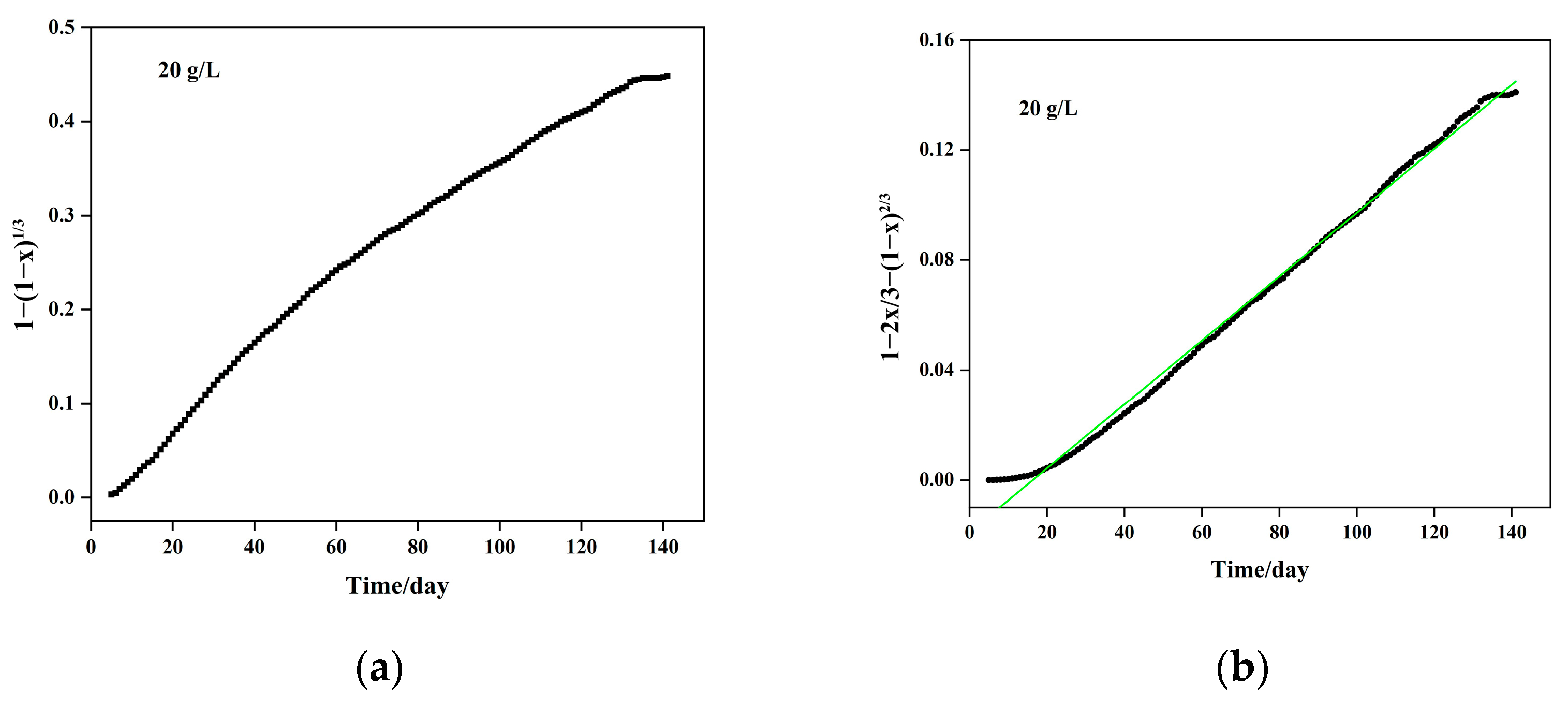
5.3. Column Leaching Kinetics of Calcium
5.4. Column Leaching Kinetics of Magnesium
- (1)
- Chemical reaction control kinetic equation: , with a coefficient of determination of R2 = 0.9663;
- (2)
- Solid material layer diffusion control kinetic equation: , with a coefficient of determination of R2 = 0.9834. By combining the chemical reaction control and diffusion control kinetic equations, the mixed control kinetic equation is derived as follows: , with an apparent reaction rate constant of per day.
- (1)
- Chemical reaction control kinetic equation: , with a coefficient of determination of R2 = 0.9663;
- (2)
- Solid material layer diffusion control kinetic equation: , with a coefficient of determination of R2 = 0.9834. By combining the chemical reaction control and diffusion control kinetic equations, the mixed control kinetic equation is derived as follows: , with an apparent reaction rate constant of per day.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borodianskiy, K. Effect of carbon nanotubes’ addition on mechanical properties and thermal conductivity of copper. J. Mater. Sci. 2019, 54, 13767–13774. [Google Scholar] [CrossRef]
- Jona, F.; Marcus, P.M. Structural properties of copper. Phys. Rev. B 2001, 63, 094113. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, P.; Yu, Z.; Shi, H.; Wu, D.; Yan, H.; Ye, X.; Lu, Q.; Tian, Y. A review on additive manufacturing of pure copper. Coatings 2021, 11, 740. [Google Scholar] [CrossRef]
- Murphy, M.C. The engineering fatigue properties of wrought copper. Fatigue Fract. Eng. Mater. Struct. 1981, 4, 199–234. [Google Scholar] [CrossRef]
- Miya, N.; Machogo-Phao, L.F.E.; Ntsendwana, B. Exploring copper oxide and copper sulfide for non-enzymatic glucose sensors: Current progress and future directions. Micromachines 2023, 14, 1849. [Google Scholar] [CrossRef] [PubMed]
- Habashi, F. The future of copper metallurgy. Miner. Process. Extr. Metall. Rev. 1995, 15, 5–12. [Google Scholar] [CrossRef]
- Schlesinger, M.E.; Sole, K.C.; Davenport, W.G.; Flores, G.R.A. Extractive Metallurgy of Copper; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Biswas, A.K.; Davenport, W.G. Extractive Metallurgy of Copper: International Series on Materials Science and Technology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 20. [Google Scholar]
- Bai, X.; Wen, S.M.; Feng, Q.C.; Liu, J.; Lin, Y.L.; Yao, Z.H. A new technique for recovering copper from complex copper oxide ore by flotation and metallurgical processing. JOM 2019, 71, 784–790. [Google Scholar] [CrossRef]
- Cao, Z.F.; Zhong, H.; Liu, G.Y.; Zhao, S.J. Techniques of copper recovery from Mexican copper oxide ore. Min. Sci. Technol. 2009, 19, 45–48. [Google Scholar] [CrossRef]
- Petersen, J. Heap leaching as a key technology for recovery of values from low-grade ores–A brief overview. Hydrometallurgy 2016, 165, 206–212. [Google Scholar] [CrossRef]
- Dixon, D.G. Heap leach modeling–the current state of the art. Hydrometallurgy 2003, 1, 289–314. [Google Scholar]
- Trujillo, J.Y.; Cisternas, L.A.; Gálvez, E.D.; Mellado, M.E. Optimal design and planning of heap leaching process. Application to copper oxide leaching. Chem. Eng. Res. Des. 2014, 92, 308–317. [Google Scholar] [CrossRef]
- Mellado, M.E.; Gálvez, E.D.; Cisternas, L.A. On the optimization of flow rates on copper heap leaching operations. Int. J. Miner. Process. 2011, 101, 75–80. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Franzidis, J.P.; Petersen, J. Heap leaching technology—Current state, innovations, and future directions: A review. Miner. Process. Extr. Metall. Rev. 2016, 37, 73–119. [Google Scholar] [CrossRef]
- Shayestehfar, M.R.; Nasab, S.K.; Mohammadalizadeh, H. Mineralogy, petrology, and chemistry studies to evaluate oxide copper ores for heap leaching in Sarcheshmeh copper mine, Kerman, Iran. J. Hazard. Mater. 2008, 154, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Norris, P.R.; Calvo-Bado, L.A.; Brown, C.F.; Davis-Belmar, C.S. Ore column leaching with thermophiles: I, copper sulfide ore. Hydrometallurgy 2012, 127, 62–69. [Google Scholar] [CrossRef]
- Hoseinian, F.S.; Abdollahzade, A.; Mohamadi, S.S.; Hashemzadeh, M. Recovery prediction of copper oxide ore column leaching by hybrid neural genetic algorithm. Trans. Nonferrous Met. Soc. China 2017, 27, 686–693. [Google Scholar] [CrossRef]
- de Andrade Lima, L.R.P. A mathematical model for isothermal heap and column leaching. Braz. J. Chem. Eng. 2004, 21, 435–447. [Google Scholar] [CrossRef]
- Liu, M.; Wen, J.; Tan, G.; Liu, G.; Wu, B. Experimental studies and pilot plant tests for acid leaching of low-grade copper oxide ores at the Tuwu Copper Mine. Hydrometallurgy 2016, 165, 227–232. [Google Scholar] [CrossRef]
- Panda, S.; Mishra, G.; Sarangi, C.K.; Sanjay, K.; Subbaiah, T.; Das, S.K.; Sarangi, K.; Ghosh, M.K.; Pradhan, N.; Mishra, B.K. Reactor and column leaching studies for extraction of copper from two low grade resources: A comparative study. Hydrometallurgy 2016, 165, 111–117. [Google Scholar] [CrossRef]
- Renman, R.; Jiankang, W.; Jinghe, C. Bacterial heap-leaching: Practice in Zijinshan copper mine. Hydrometallurgy 2006, 83, 77–82. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Li, X.; Ma, B.; Wang, H.; Wang, C. Selective extraction of cobalt and copper From cobalt-rich copper sulfide ores. Metall. Mater. Trans. B 2023, 54, 2332–2346. [Google Scholar] [CrossRef]
- Liu, W.; Granata, G. Temperature control in copper heap bioleaching. Hydrometallurgy 2018, 176, 26–32. [Google Scholar] [CrossRef]
- Yin, S.; Wang, L.; Kabwe, E.; Chen, X.; Yan, R.; An, K.; Zhang, L.; Wu, A. Copper bioleaching in China: Review and prospect. Minerals 2018, 8, 32. [Google Scholar] [CrossRef]
- Zheng, C.; Jiang, K.; Cao, Z.; Northwood, D.O.; Waters, K.E.; Wang, H.; Liu, S.; Zhu, K.E.; Ma, H. Agitation leaching behavior of copper–cobalt oxide ores from the Democratic Republic of the Congo. Minerals 2023, 13, 743. [Google Scholar] [CrossRef]
- Xu, J.; Qin, S.; Zheng, C.; Wang, J.; Yang, B.; Qiu, G.; Cui, S.; Ma, H. Characterization and optimization of copper-cobalt oxide ores during acid leaching. JOM 2023, 75, 5785–5795. [Google Scholar] [CrossRef]
- Zheng, C.; Jiang, K.; Cao, Z.; Wang, H.; Liu, S.; Waters, K.E.; Ma, H. Pressure leaching behaviors of copper-cobalt sulfide concentrate from Congo. Sep. Purif. Technol. 2023, 309, 123010. [Google Scholar] [CrossRef]
- Park, I.; Yoo, K.; Alorro, R.D.; Kim, M.S.; Kim, S.K. Leaching of copper from cuprous oxide in aerated sulfuric acid. Mater. Trans. 2017, 58, 1500–1504. [Google Scholar] [CrossRef]
- Yang, J.; Chen, L.; Wu, D.; Cao, J.; Guo, J. Leaching of cuprite with ozone as an oxidant in sulfuric acid solution and its oxidation leaching mechanism. Arab. J. Chem. 2023, 16, 105159. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, K.; Ma, H.; Qin, S.; Zheng, C. The behavior of zinc and iron in neutralized residue during pressure leaching. Min. Metall. Explor. 2022, 39, 847–854. [Google Scholar] [CrossRef]
- Braun, R.L.; Lewis, A.E.; Wadsworth, M.E. In-place leaching of primary sulfide ores: Laboratory leaching data and kinetics model. Metall. Trans. 1974, 5, 1717–1726. [Google Scholar] [CrossRef]


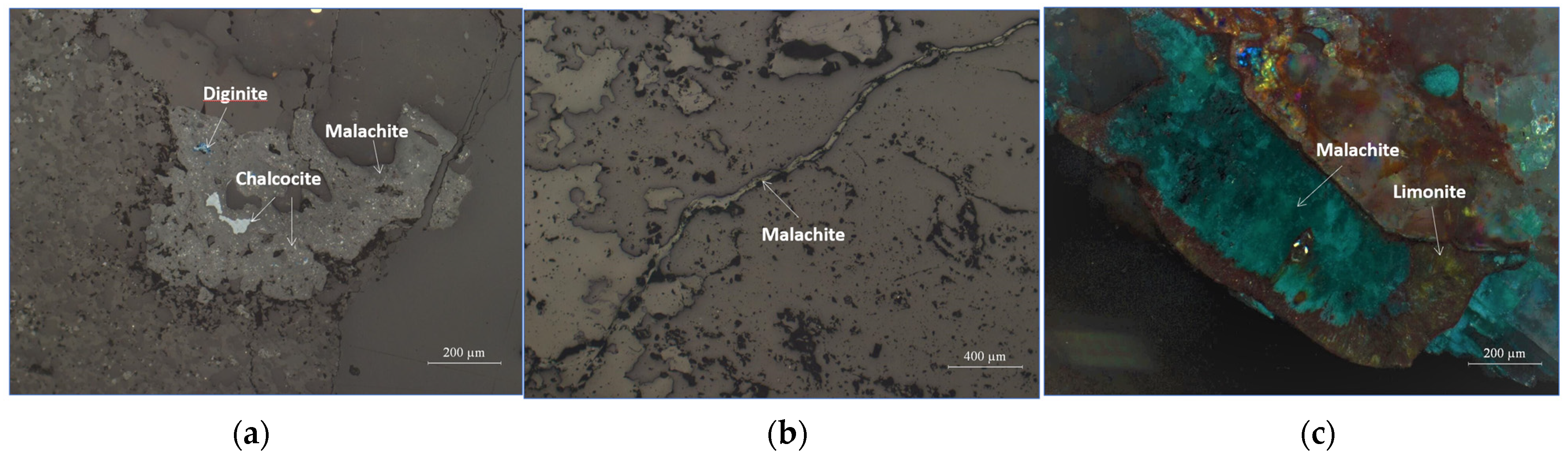









| No. | Cu | Co | Fe | Mn | Ca | Mg |
|---|---|---|---|---|---|---|
| Sample-1 | 0.70 | 0.67 | 0.078 | 0.36 | 0.59 | 4.45 |
| Sample-2 | 0.67 | 0.68 | 0.078 | 0.36 | 0.58 | 4.33 |
| Sample-3 | 0.70 | 0.68 | 0.078 | 0.37 | 0.58 | 4.23 |
| Average | 0.69 | 0.68 | 0.080 | 0.36 | 0.58 | 4.34 |
| Element | Cu | Co | Fe | S | Zn | Pb | Ni | As | TiO2 |
| Content (%) | 0.75 | 0.13 | 1.18 | 0.01 | 0.03 | 0.05 | 0.01 | 0.01 | 0.03 |
| Element | Ca | Mg | Al2O3 | SiO2 | K2O | Na2O | P2O5 | MnO | C |
| Content (%) | 4.11 | 4.60 | 4.31 | 61.07 | 0.93 | 0.04 | 0.07 | 0.05 | 3.55 |
| Particle Size (mm) | Weight (g) | Percentage (%) | Cumulative Percentage (%) |
|---|---|---|---|
| 13.2–26.5 | 346.50 | 31.29 | 31.29 |
| 4.75–13.2 | 259.10 | 23.40 | 54.69 |
| 1–4.75 | 149.40 | 13.49 | 68.18 |
| 0.45–1 | 69.60 | 6.29 | 74.47 |
| 0.154–0.45 | 71.10 | 6.42 | 80.89 |
| 0.075–0.154 | 53.80 | 4.86 | 85.75 |
| <0.075 | 157.80 | 14.25 | 100.00 |
| Total | 1107.30 |
| Particle Size (mm) | Cu | Co | Fe | Mn | Ca | Mg |
|---|---|---|---|---|---|---|
| 13.2–26.5 | 29.21 | 30.99 | 21.72 | 30.34 | 41.03 | 39.80 |
| 4.75–13.2 | 20.60 | 9.89 | 16.24 | 14.73 | 25.13 | 22.97 |
| 1–4.75 | 16.20 | 15.14 | 12.37 | 10.33 | 12.68 | 12.07 |
| 0.45–1 | 7.54 | 10.79 | 7.82 | 7.80 | 5.44 | 6.30 |
| 0.154–0.45 | 5.65 | 10.17 | 7.49 | 9.50 | 4.75 | 5.15 |
| 0.075–0.154 | 3.50 | 6.09 | 5.79 | 7.19 | 2.98 | 3.56 |
| <0.075 | 17.30 | 16.93 | 28.56 | 20.12 | 7.99 | 10.15 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Copper | Phase | Oxidized Copper | Secondary Sulfide Copper | Goethite | Other | Total |
| Copper content (wt%) | 0.61 | 0.011 | 0.009 | 0.004 | 0.634 | |
| Distribution (%) | 96.21 | 1.74 | 1.42 | 0.63 | 100.00 | |
| Cobalt | Phase | Oxidized cobalt | Carbonate | Goethite | Other | Total |
| Cobalt content (wt%) | 0.105 | 0.003 | 0.004 | 0.003 | 0.115 | |
| Distribution (%) | 91.30 | 2.61 | 3.48 | 2.61 | 100.00 | |
| Calcium | Phase | Calcium carbonate | Calcium fluoride | Calcium silicate | Calcium sulfate | Total |
| Calcium content (wt%) | 1.80 | 0.10 | 6.12 | 0.05 | 8.07 | |
| Distribution (%) | 22.30 | 1.24 | 75.84 | 0.62 | 100.00 |
| Mineral | Content (%) | Mineral Name | Content (%) |
|---|---|---|---|
| Malachite | 0.89 | Chlorite | 2.75 |
| Chrysocolla | 0.29 | Talc | 2.59 |
| Covellite | 0.01 | Biotite | 0.32 |
| Heterogenite | 0.17 | Amphibole | 0.71 |
| Goethite | 0.62 | Apatite | 0.12 |
| Siderite | 0.13 | Kaolinite | 0.24 |
| Quartz | 52.14 | Celadonite | 0.11 |
| Dolomite | 27.68 | Potassium Feldspar | 0.40 |
| Muscovite | 10.52 | Other | 0.31 |
| Mineral | Mineral Weight (wt%) | Copper Content in Mineral (%) | Copper Metal Weight (wt%) | Copper Distribution (%) |
| Malachite | 0.89 | 54.95 | 0.489 | 77.97 |
| Chrysocolla | 0.29 | 34.99 | 0.101 | 16.18 |
| Heterogenite | 0.17 | 9.21 | 0.016 | 2.50 |
| Covellite | 0.01 | 79.86 | 0.008 | 1.27 |
| Goethite | 0.62 | 1.52 | 0.009 | 1.50 |
| Talc | 2.59 | 0.10 | 0.003 | 0.41 |
| Biotite | 0.32 | 0.34 | 0.001 | 0.17 |
| Total | 0.627 | 100.00 |
| Mineral | Mineral Weight (wt%) | Cobalt Content in Mineral (%) | Cobalt Metal Weight (wt%) | Cobalt Distribution (%) |
|---|---|---|---|---|
| Heterogenite | 0.17 | 57.35 | 0.0975 | 85.13 |
| Malachite | 0.89 | 1.05 | 0.0093 | 8.16 |
| Chrysocolla | 0.29 | 0.13 | 0.0004 | 0.32 |
| Goethite | 0.62 | 0.65 | 0.0040 | 3.52 |
| Calcite | 27.68 | 0.01 | 0.0028 | 2.42 |
| Talc | 2.59 | 0.02 | 0.0005 | 0.45 |
| Total | 0.1145 | 100.00 |
| Mineral | Mineral Weight (wt%) | Calcium Content in Mineral (%) | Calcium Metal Weight (wt%) | Calcium Distribution (%) |
|---|---|---|---|---|
| Dolomite | 27.68 | 20.70 | 5.73 | 96.21 |
| Amphibole | 0.71 | 17.71 | 0.13 | 2.11 |
| Apatite | 0.12 | 39.65 | 0.05 | 0.80 |
| Wollastonite | 0.24 | 14.46 | 0.03 | 0.57 |
| Celadonite | 0.11 | 17.36 | 0.02 | 0.31 |
| Total | 5.96 | 100.00 |
| Mineral | Mineral Weight (wt%) | Magnesium Content in Mineral (%) | Magnesium Metal Weight (wt%) | Magnesium Distribution (%) |
|---|---|---|---|---|
| Dolomite | 27.68 | 13.60 | 3.7645 | 74.62 |
| Celadonite | 2.75 | 18.50 | 0.5088 | 10.08 |
| Talc | 2.59 | 17.59 | 0.4556 | 9.03 |
| Biotite | 10.52 | 1.82 | 0.1915 | 3.79 |
| Wollastonite | 0.71 | 10.21 | 0.0725 | 1.44 |
| Chrysocolla | 0.24 | 11.77 | 0.0282 | 0.56 |
| Biotite | 0.32 | 5.58 | 0.0179 | 0.35 |
| Malachite | 0.89 | 0.35 | 0.0031 | 0.06 |
| Chrysocolla | 0.29 | 0.99 | 0.0029 | 0.06 |
| Goethite | 0.62 | 0.09 | 0.0006 | 0.01 |
| Total | 5.0454 | 100.00 |
| Sulfuric Acid Concentration (g/L) | Kinetic Equation | Apparent Reaction Rate Constant (Days−1) | Controlling Step |
|---|---|---|---|
| 10 | 2.23 × 10−3 | Chemical reaction | |
| 15 | 4.09 × 10−2 | Mixed control: Chemical and diffusion control | |
| 20 | 1.16 × 10−3 | Diffusion control |
| Spraying Intensity (L/(m2·h)) | Kinetic Equation | Apparent Reaction Rate Constant (Day−1) | Controlling Step |
|---|---|---|---|
| 6 | 3.62 × 10−3 | Diffusion control | |
| 10 | 1.16 × 10−3 | Diffusion control | |
| 15 | 2.512 × 10−2 | Diffusion control |
| Particle Size | Kinetic Equation | Apparent Reaction Rate Constant (per Day) | Controlling Step |
|---|---|---|---|
| <10 mm | 2.729 × 10−2 | Mixed control | |
| <40 mm | 4.092 × 10−3 | Mixed control |
| Sulfuric Acid Concentration (g/L) | Kinetic Equation | Apparent Reaction Rate Constant (Day−1) | Controlling Step |
|---|---|---|---|
| 15 | 1.05 × 10−3 | Mixed control | |
| 20 | 1.38 × 10−3 | Chemical reaction control |
| Sulfuric Acid Concentration (g/L) | Kinetic Equation | Apparent Reaction Rate Constant (Day−1) | Controlling Step |
|---|---|---|---|
| 10 | 5.63 × 10−3 | Chemical reaction control | |
| 15 | 1.30 × 10−6 | Diffusion control | |
| 20 | 6.32 × 10−3 | Chemical reaction control |
| Sulfuric Acid Concentration (g/L) | Kinetic Equation | Apparent Reaction Rate Constant (Day−1) | Controlling Step |
|---|---|---|---|
| 10 | 7.88 × 10−3 | Chemical reaction control | |
| 15 | 7.83 × 10−4 | Mixed control | |
| 20 | 1.17 × 10−3 | Chemical reaction control |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Qin, S.; Zheng, C.; Sun, H.; Yang, B.; Liu, S.; Qiu, G.; Northwood, D.O.; Waters, K.E.; Ma, H. Study on Column Leaching Behavior of Low-Grade High Calcium and Magnesium Copper Ore. Minerals 2024, 14, 822. https://doi.org/10.3390/min14080822
Xu J, Qin S, Zheng C, Sun H, Yang B, Liu S, Qiu G, Northwood DO, Waters KE, Ma H. Study on Column Leaching Behavior of Low-Grade High Calcium and Magnesium Copper Ore. Minerals. 2024; 14(8):822. https://doi.org/10.3390/min14080822
Chicago/Turabian StyleXu, Jingyuan, Shuchen Qin, Chaozhen Zheng, Honghong Sun, Baojun Yang, Sanping Liu, Guanzhou Qiu, Derek O. Northwood, Kristian E. Waters, and Hao Ma. 2024. "Study on Column Leaching Behavior of Low-Grade High Calcium and Magnesium Copper Ore" Minerals 14, no. 8: 822. https://doi.org/10.3390/min14080822
APA StyleXu, J., Qin, S., Zheng, C., Sun, H., Yang, B., Liu, S., Qiu, G., Northwood, D. O., Waters, K. E., & Ma, H. (2024). Study on Column Leaching Behavior of Low-Grade High Calcium and Magnesium Copper Ore. Minerals, 14(8), 822. https://doi.org/10.3390/min14080822









