Curing Agent for High-Concentration Unclassified Tailings Stockpiling: A Case Study of Tailings from a Gold Mine
Abstract
1. Introduction
2. Curing Reaction Mechanisms
2.1. Reaction Mechanism of Cement
- (1)
- Hydration of C3S
- (2)
- Hydration of C2S
- (3)
- Hydration of C3A
- (4)
- Hydration of C4AF
2.2. Reaction Mechanism of Quicklime
2.3. Reaction Mechanism of Slag
2.4. Mechanism of Gypsum Hydration
2.5. Reaction Mechanism of Bentonite
3. Experimental Materials and Equipment
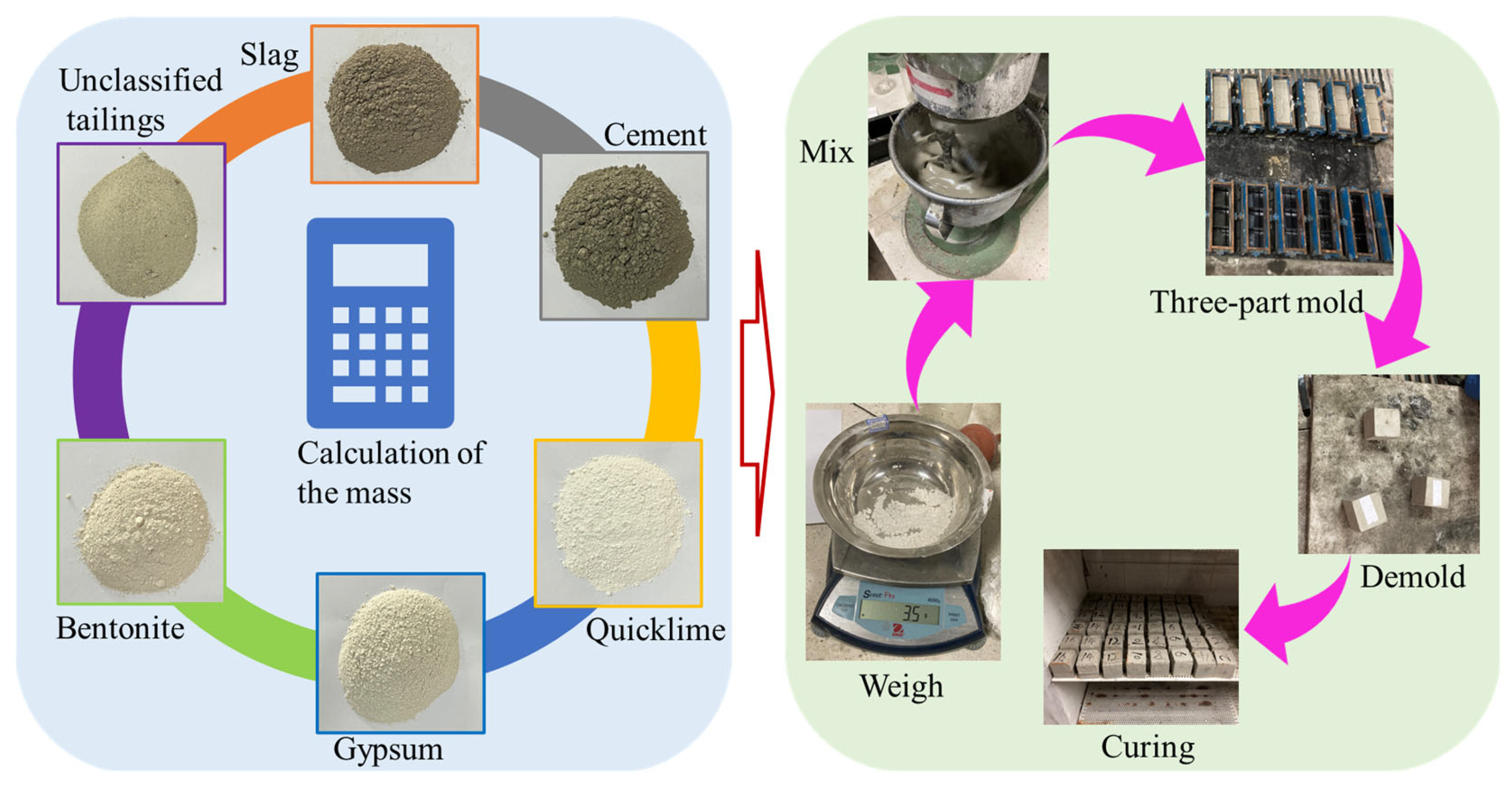
3.1. Experimental Materials
- (1)
- Unclassified tailings
- (2)
- Slag
- (3)
- Quicklime
- (4)
- Cement
- (5)
- Gypsum
- (6)
- Bentonite
3.2. Experimental Equipment
- (1)
- Load Testing Machine
- (2)
- Rheometer
- (3)
- SEM
- (4)
- XRD
4. Compressive Strength Testing Experiment Scheme
4.1. Orthogonal Experimental Design
4.2. Sample Preparation
4.3. Compression Strength Testing
4.4. Rheological Characteristics Testing Experiment Plan
5. Results
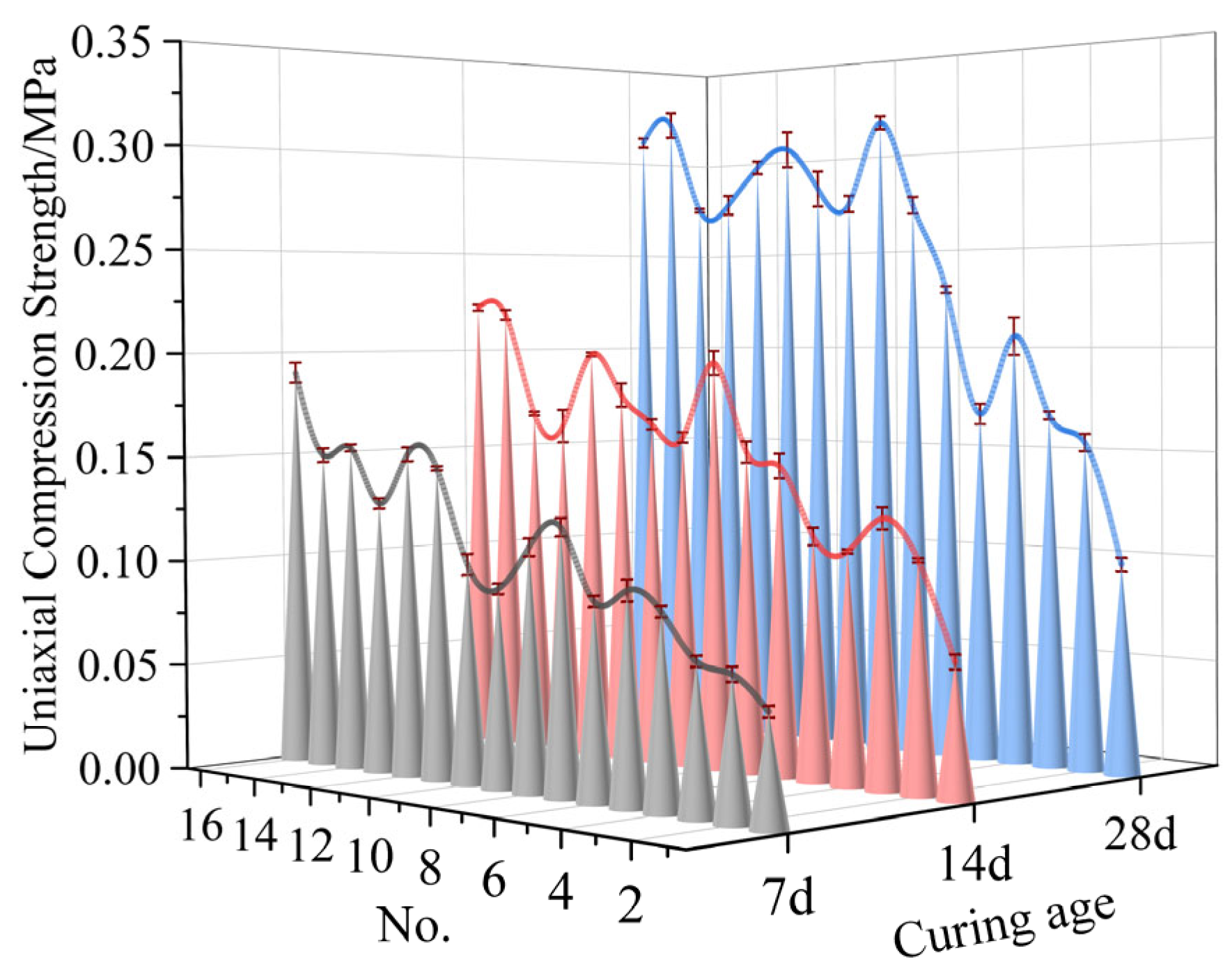
5.1. Compression Strength Test Results
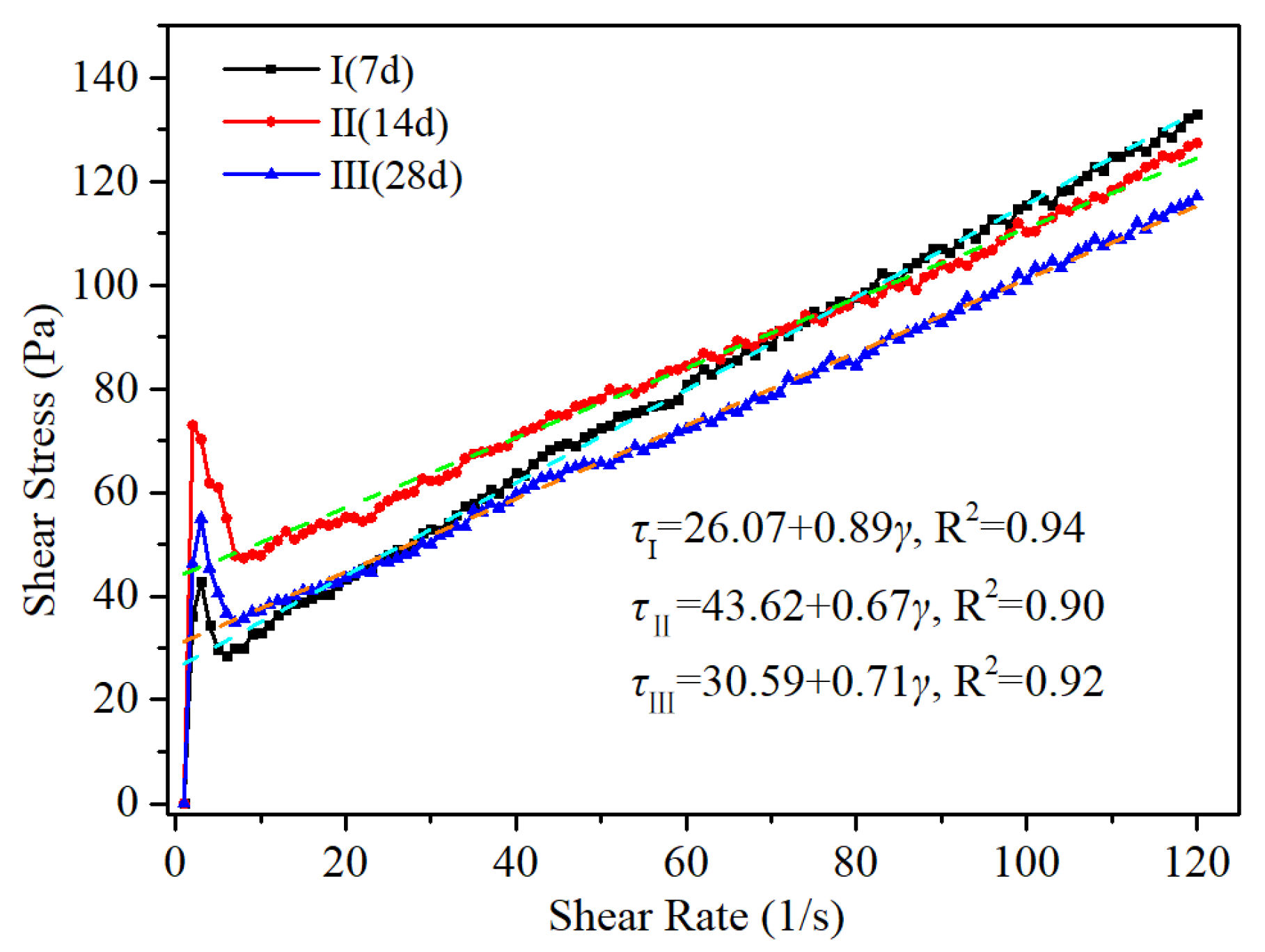
5.2. Rheological Characteristics Test Results
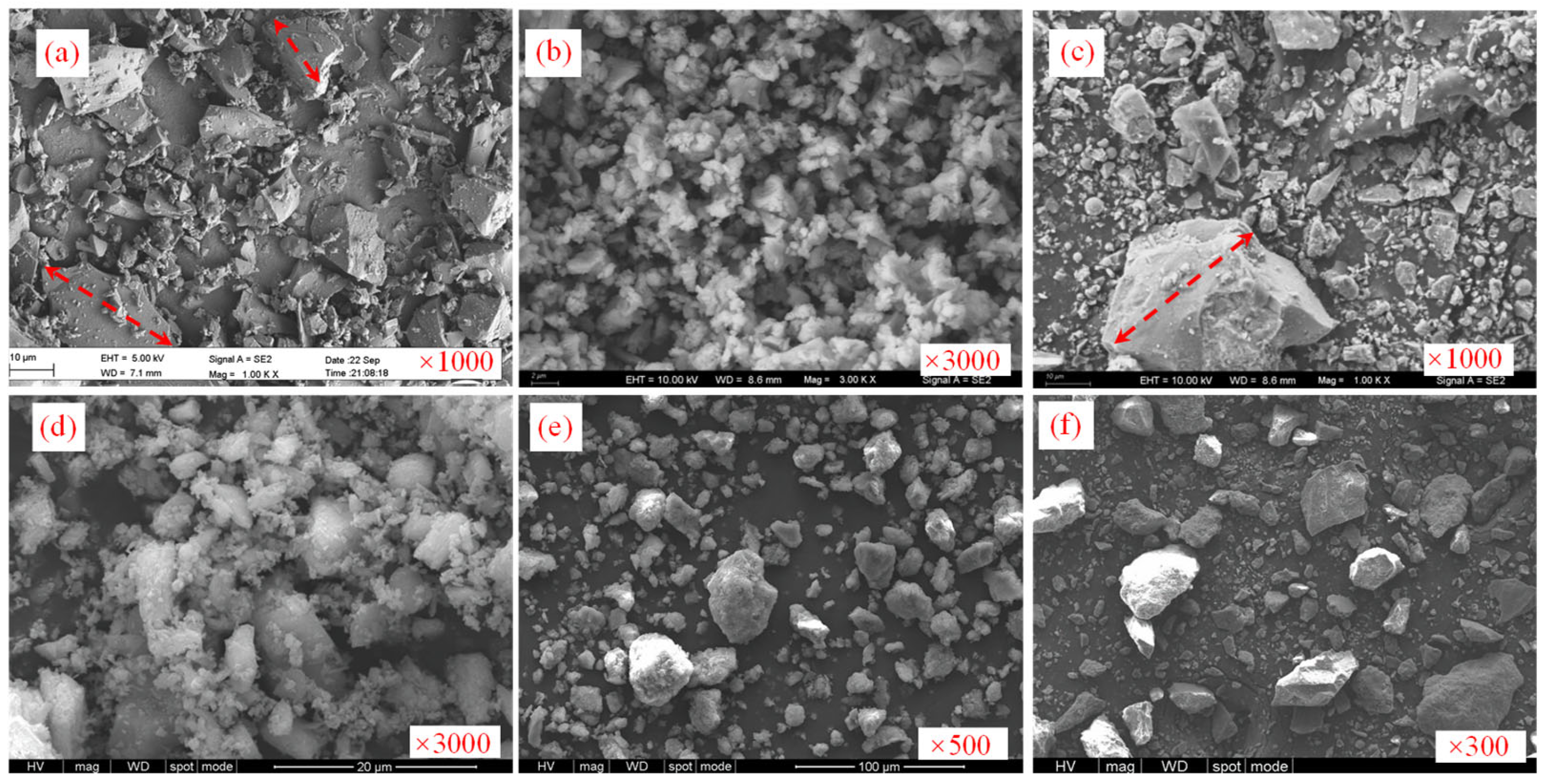
5.3. SEM Experimental Results
6. Discussion
7. Conclusions
- (1)
- The curing agent system for tailings consolidation and stockpiling composed of slag, quicklime, cement, gypsum, and bentonite is feasible. The reaction mechanism involves synergistic interactions among these compositions, promoting the dissolution of active compositions in slag and tailings. In addition to their inherent cementitious activity, the excitation agents such as cement, quicklime, and gypsum in the curing agent also serve as alkaline excitation agents for the volcanic ash reactions of slag and tailings. The formation of calcium hydroxide and various C-S-H/C-A-H enhances the disintegration of the slag glass network. The presence of gypsum aids in the formation of AFt or AFm through reactions with active compositions. Bentonite contributes to ion exchange and volumetric expansion, reinforcing the solidifying system;
- (2)
- The optimal proportions of each material in the curing agent are as follows: slag, 58%; quicklime, 15%; cement, 8%; gypsum, 9%; and bentonite, 10%. The proportion of industrial waste steel slag reached 58%, which reflects the low-cost nature of the curing agent. Under this condition, the modified unclassified tailings slurry exhibited a yield stress of 43.62 Pa and a viscosity coefficient of 0.67 Pa·s at a slurry concentration of 75%, and the average uniaxial compression strength values of the MUTSSs were 0.20 MPa, 0.25 MPa, and 0.34 MPa at 7 d, 14 d, and 28 d, respectively;
- (3)
- The hydration products of the curing agent are mainly AFt, C-S-H and Ca (OH)2. With the extension of the hydration time, the contents of AFt and Ca (OH)2 decreased, while the content of C-S-H increased, forming a gel system and spatial structures. The tailings particles that did not fully react acted as a skeleton and supported the strength of the solidified samples. These two aspects worked together to improve the overall strength of the solidified sample and achieved effective solidification of the unclassified tailings.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tabelin, C.B.; Park, I.; Phengsaart, T.; Jeon, S.; Villacorte-Tabelin, M.; Alonzo, D.; Yoo, K.; Ito, M.; Hiroyoshi, N. Copper and critical metals production from porphyry ores and E-wastes: A review of resource availability, processing/recycling challenges, socio-environmental aspects, and sustainability issues. Resour. Conserv. Recycl. 2021, 170, 105610. [Google Scholar] [CrossRef]
- Liu, L.; Xin, J.; Huan, C.; Zhao, Y.J.; Fan, X.; Guo, L.J.; Song, K.-I. Effect of curing time on the mesoscopic parameters of cemented paste backfill simulated using the particle flow code technique. Int. J. Miner. Metall. Mater. 2021, 28, 590–602. [Google Scholar] [CrossRef]
- Edraki, M.; Baumgartl, T.; Manlapig, E.; Bradshaw, D.; Franks, D.M.; Moran, C.J. Designing mine tailings for better environmental, social and economic outcomes: A review of alternative approaches. J. Clean. Prod. 2014, 84, 411–420. [Google Scholar] [CrossRef]
- Boger, D.V. Personal perspective on paste and thickened tailings: A decade on. Min. Technol. 2012, 121, 29–36. [Google Scholar] [CrossRef]
- Psarropoulos, P.N.; Tsompanakis, Y. Stability of tailings dams under static and seismic loading. Can. Geotech. J. 2008, 45, 663–675. [Google Scholar] [CrossRef]
- Birch, W.J.; Dixon-Hardy, D.W.; Engels, J.M. Tailings management facility topography modelling to identify flow accumulations and pond geometry. Min. Technol. 2006, 115, 65–74. [Google Scholar] [CrossRef]
- Cross, A.T.; Lambers, H. Young calcareous soil chronosequences as a model for ecological restoration on alkaline mine tailings. Sci. Total Environ. 2017, 607, 168–175. [Google Scholar] [CrossRef]
- Schoenbrunn, F.; Bach, M. The Development of paste thickening and its application to the minerals industry; an industry review. BHM Berg-Und Hüttenmännische Monatshefte 2015, 160, 257–263. [Google Scholar] [CrossRef]
- Newman, L.; Arnold, K.; Wittwer, D. Dry stack tailings design for the Rosemont Copper project. In Tailings and Mine Waste; CRC Press: Boca Raton, FL, USA, 2010; pp. 315–326. [Google Scholar] [CrossRef]
- Alshawmar, F.; Fall, M. Dynamic response of thickened tailings in shaking table testing. Int. J. Geo-Eng. 2021, 12, 28. [Google Scholar] [CrossRef]
- Wang, Z.W.; Li, F.; Mei, G.D. OpenMP Parallel Finite-Discrete Element Method for Modeling Excavation Support with Rockbolt and Grouting. Rock Mech. Rock Eng. 2024, 57, 3635–3657. [Google Scholar] [CrossRef]
- Fourie, A. Paste and thickened tailings: Has the promise been fulfilled. In GeoCongress 2012: State of the Art and Practice in Geotechnical Engineering; American Society of Civil Engineers: Reston, VA, USA, 2012; pp. 4126–4135. [Google Scholar]
- Eng, F.P.P. Paste Thickening: Considerations for Backfill vs. Tailings Management. Eng. Min. J. 2011, 212, 34–40. [Google Scholar]
- Yang, P.Y.; Li, L. Investigation of the short-term stress distribution in stopes and drifts backfilled with cemented paste backfill. Int. J. Min. Sci. Technol. 2015, 25, 721–728. [Google Scholar]
- Kouame, K.J.A.; Feng, Y.; Jiang, F.; Zhu, S. A Study of Technical Measures for Increasing the Roof-Contacted Ratio in Stope and Cavity Filling. J. Mater. Sci. Res. 2016, 5, 54. [Google Scholar] [CrossRef]
- Chen, O.S.; Zhang, O.L.; Fourie, A.; Chen, X.; Qi, C.C. Experimental investigation on the strength characteristics of cement paste backfill in a similar stope model and its mechanism. Constr. Build. Mater. 2017, 154, 34–43. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Y.; Bao, S.; Chen, T. Utilization of Iron Ore Tailings as Raw Material for Portland Cement Clinker Production. Adv. Mater. Sci. Eng. 2016, 2016, 1596047. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Wang, X. Dual waste utilization in cemented paste backfill using steel slag and mine tailings and the heavy metals immobilization effects. Powder Technol. 2022, 403, 117413. [Google Scholar] [CrossRef]
- Wang, J.; Fu, J.; Song, W.; Zhang, Y. Effect of rice husk ash (RHA) dosage on pore structural and mechanical properties of cemented paste backfill. J. Mater. Res. Technol. 2022, 17, 840–851. [Google Scholar] [CrossRef]
- Chen, S.J.; Du, Z.W.; Zhang, Z.; Yin, D.W.; Feng, F.; Ma, J.B. Effects of red mud additions on gangue-cemented paste backfill properties. Powder Technol. 2020, 367, 833–840. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Ma, Z.Y.; Qiu, J.P.; Sun, X.G.; Gu, X.W. Experimental study on the utilization of steel slag for cemented ultra-fine tailings backfill. Powder Technol. 2020, 375, 284–291. [Google Scholar]
- Zhao, Y.L.; Wu, P.Q.; Qiu, J.P.; Guo, Z.B.; Tian, Y.S.; Sun, X.G.; Gu, X.W. Recycling hazardous steel slag after thermal treatment to produce a binder for cemented paste backfill. Powder Technol. 2022, 395, 652–662. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Qiu, J.P.; Zhang, S.Y.; Guo, Z.B.; Wu, P.Q.; Sun, X.G.; Gu, X.W. Low carbon binder modified by calcined quarry dust for cemented paste backfill and the associated environmental assessments. J. Environ. Manag. 2021, 300, 113760. [Google Scholar] [CrossRef] [PubMed]
- Telesca, A.; Marroccoli, M.; Calabrese, D.; Valenti, G.L.; Montagnaro, F. Flue gas desulfurization gypsum and coal fly ash as basic components of prefabricated building materials. Waste Manag. 2013, 33, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Yum, W.S.; Jeong, Y.; Song, H.; Oh, J.E. Recycling of limestone fines using Ca(OH)2 and Ba(OH)2 activated slag systems for eco-friendly concrete brick production. Constr. Build. Mater. 2018, 185, 275–284. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.H.; Bai, J.; Li, L.T. Innovative methodology for comprehensive utilization of iron ore tailings: Part I. The recovery of iron from iron ore tailings using magnetic separation after magnetizing roasting. J. Hazard. Mater. 2010, 174, 71–77. [Google Scholar] [CrossRef]
- Yao, G.; Wang, Q.; Su, Y.W.; Wang, J.X.; Qiu, J.; Lyu, X.J. Mechanical activation as an innovative approach for the preparation of pozzolan from iron ore tailings. Miner. Eng. 2020, 145, 106068. [Google Scholar] [CrossRef]
- Barati, S.; Tabatabaie Shourijeh, P.; Samani, N.; Asadi, S. Stabilization of iron ore tailings with cement and bentonite: A case study on Golgohar mine. Bull. Eng. Geol. Environ. 2020, 79, 4151–4166. [Google Scholar] [CrossRef]
- Fall, M.; Célestin, J.C.; Han, F.S. Suitability of bentonite-paste tailings mixtures as engineering barrier material for mine waste containment facilities. Miner. Eng. 2009, 22, 840–848. [Google Scholar] [CrossRef]
- Opiso, E.M.; Tabelin, C.B.; Maestre, C.V.; Aseniero, J.P.J.; Arima, T.; Villacorte-Tabelin, M. Utilization of Palm Oil Fuel Ash (POFA) as an Admixture for the Synthesis of a Gold Mine Tailings-Based Geopolymer Composite. Minerals 2023, 13, 232. [Google Scholar] [CrossRef]
- Opiso, E.M.; Tabelin, C.B.; Maestre, C.V.; Aseniero, J.P.J.; Park, I.; Villacorte-Tabelin, M. Synthesis and characterization of coal fly ash and palm oil fuel ash modified artisanal and small-scale gold mine (ASGM) tailings based geopolymer using sugar mill lime sludge as Ca-based activator. Heliyon 2021, 7, e06654. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, A.X.; Zhang, L.F.; Wang, H.J.; Jin, F. Investigation of the Sedimentation Property of Backfill Material on the Basis of Rheological Test: A Case Study of Iron Tailings. J. Chem. 2018, 2018, 9530767. [Google Scholar] [CrossRef]
- Boger, D.V. Rheology of slurries and environmental impacts in the mining industry. Annu. Rev. Chem. Biomol. 2013, 4, 239–257. [Google Scholar] [CrossRef]
- Yang, L.; Jia, H.; Wu, A.; Jiao, H.; Chen, X.; Kou, Y.; Dong, M. Particle Aggregation and Breakage Kinetics in Cemented Paste Backfill. Int. J. Miner. Metall. Mater. 2023, 220, 172–186. [Google Scholar] [CrossRef]
- Mizani, S.; He, X.; Simms, P. Application of lubrication theory to modeling stack geometry of high density mine tailings. J. Non-Newton. Fluid Mech. 2013, 198, 59–70. [Google Scholar] [CrossRef]
- Yang, L.; Gao, Y.; Chen, H.; Jiao, H.; Dong, M.; Bier, T.A.; Kim, M. Three-dimensional concrete printing technology from a rheology perspective: A review. Adv. Cem. Res. 2024, 126, 72–86. [Google Scholar] [CrossRef]
- Li, G.Y.; Zhao, X.H. Properties of concrete incorporating fly ash and ground granulated blast-furnace slag. Cem. Concr. Comp. 2003, 25, 293–299. [Google Scholar] [CrossRef]
- Double, D.D. New developments in understanding the chemistry of cement hydration. Phil. Trans. R. Soc. Lond. A 1983, 310, 53–66. [Google Scholar]
- Wang, B.W.; Li, Q.L.; Dong, P.B.; Gan, S.; Yang, L.; Wang, R.Z. Performance investigation of blast furnace slag based cemented paste backfill under low temperature and low atmospheric pressure. Constr. Build. Mater. 2023, 363, 129744. [Google Scholar] [CrossRef]
- Zhang, M.G.; Li, K.Q.; Ni, W.; Zhang, S.Q.; Liu, Z.Y.; Wang, K.; Wei, X.L.; Yu, Y. Preparation of mine backfilling from steel slag-based non-clinker combined with ultra-fine tailing. Constr. Build. Mater. 2022, 320, 126248. [Google Scholar] [CrossRef]
- Hou, Y.Q.; Yang, K.; Yin, S.H.; Yu, X.; Wang, L.M.; Yang, X.B. Enhancing the physical properties of cemented ultrafine tailings backfill (CUTB) with fiber and rice husk ash: Performance, mechanisms, and optimization. J. Mater. Res. Technol. 2024, 29, 4418–4432. [Google Scholar] [CrossRef]
- Salami, B.A.; Ibrahim, M.; Algaifi, H.A.; Alimi, W.; Oladapo, E.A. A review on the durability performance of alkali-activated binders subjected to chloride-bearing environment. Constr. Build. Mater. 2022, 317, 125947. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, S.Y. Effects of titanium gypsum on high calcium fly ash system: Physical and microscopic properties, hydration, and Fe transformation. J. Build. Eng. 2023, 78, 107653. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, M.H.; Zhao, Y.D.; Chi, X.F.; Guo, J.Z.; Du, D.H.; Du, J.X. Hydration mechanism and phase assemblage of blended cement with iron-rich sewage sludge ash. J. Build. Eng. 2023, 63, 105579. [Google Scholar] [CrossRef]
- Liu, X.H.; Ma, B.G.; Tan, H.B.; Gu, B.Q.; Zhang, T.; Chen, P.; Li, H.N.; Mei, J.P. Effect of aluminum sulfate on the hydration of Portland cement, tricalcium silicate and tricalcium aluminate. Constr. Build. Mater. 2020, 232, 117179. [Google Scholar] [CrossRef]
- Li, C.B.; Ma, B.G.; Tan, H.B.; Zhang, T.; Liu, X.H.; Chen, P. Effect of triisopropanolamine on chloride binding of cement paste with ground-granulated blast furnace slag. Constr. Build. Mater. 2020, 256, 119494. [Google Scholar] [CrossRef]
- Soliman, M.A.; Rashad, G.M.; Mahmoud, M.R. Organo-modification of montmorillonite for enhancing the adsorption efficiency of cobalt radionuclides from aqueous solutions. Environ. Sci. Pollut. Res. 2019, 26, 10398–10413. [Google Scholar] [CrossRef]
- Kazutoshi, H.; Li, H.J.; MatSuda, K.; Takehisa, T.; Elliott, E. Mechanism of forming organic/inorganic network structures during in-situ free-radical polymerization in PNIPA-Clay nanocomposite hydrogels. Macromolecules 2005, 38, 3482. [Google Scholar]
- Segad, M.; Jonsson, B.; Akesson, T.; Cabane, B. Ca/Na Montmorillonite: Structure, Forces and Swelling Properties. Langmuir 2010, 26, 5782–5790. [Google Scholar] [CrossRef]
- Liu, L.; Xin, J.; Huan, C.; Qi, C.C.; Zhou, W.W.; Song, K.-I. Pore and strength characteristics of cemented paste backfill using sulphide tailings: Effect of sulphur content. Constr. Build. Mater. 2020, 237, 117452. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Yang, K.; He, X.; Wei, Z.; Zhang, J.Q. Study on proportioning experiment and performance of solid waste for underground backfilling. Mater. Today Commun. 2022, 32, 103863. [Google Scholar] [CrossRef]
- Grabinsky, M.W.; Theriault, J.; Welch, D. An overview of paste and thickened tailings disposal on surface. In Symposium 2002 on Environment and Mine; Canadian Institute of Mining, Metallurgy and Petroleum: Montreal, QC, Canada, 2002; p. 533. [Google Scholar]
- Leong, Y.K. Controlling the rheology of iron ore slurries and tailings with surface chemistry for enhanced beneficiation performance and output, reduced pumping cost and safer tailings storage in dam. Miner. Eng. 2021, 166, 106874. [Google Scholar] [CrossRef]
- Gao, R.G.; Zhou, K.P.; Zhou, Y.L.; Yang, C. Research on the fluid characteristics of cemented backfill pipeline transportation of mineral processing tailings. Alex Eng. J. 2020, 59, 4409–4426. [Google Scholar] [CrossRef]
- Gu, X.W.; Yang, B.H.; Li, Z.J.; Liu, B.N.; Liu, J.P.; Wang, Q.; Nehdif, M.L. Elucidating the reaction of seashell powder within fly ash cement: A focus on hydration products. Constr. Build. Mater. 2024, 428, 136331. [Google Scholar] [CrossRef]
- Jha, A.K.; Sivapullaiah, P.V. Volume change behavior of lime treated gypseous soil—Influence of mineralogy and microstructure. Appl. Clay Sci. 2016, 119, 202–212. [Google Scholar] [CrossRef]
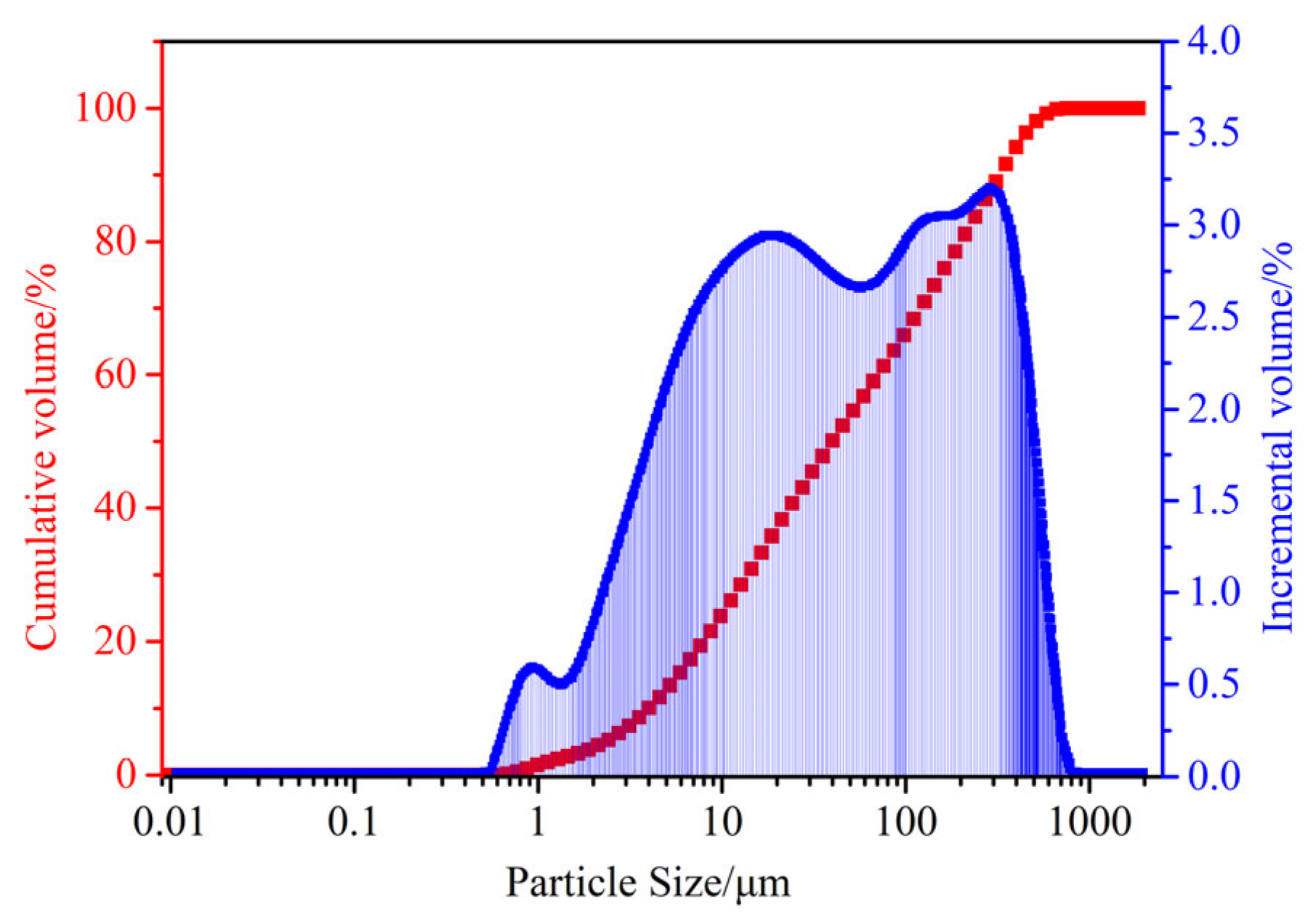





| Specific Gravity | Bulk Density (t/m) | Porosity (%) | Angle of Repose (°) |
|---|---|---|---|
| 2.863 | 1.694 | 36.134 | 40.538 |
| Upper Limit/% | Lower Limit/% | Equal Division/% | |
|---|---|---|---|
| B (Quicklime) | 30 | 15 | 5 |
| C (Cement) | 16 | 4 | 4 |
| D (Gypsum) | 12 | 3 | 3 |
| E (Bentonite) * | 10 | 1 | 3 |
| No. | O (Unclassified Tailings Slurry Concentration) * | B (Quicklime) | C (Cement) | D (Gypsum) | E (Bentonite) | A (Slag) |
|---|---|---|---|---|---|---|
| 1 | 69% | 30% | 16% | 12% | 10% | 32% |
| 2 | 69% | 25% | 12% | 9% | 7% | 47% |
| 3 | 69% | 20% | 8% | 6% | 4% | 62% |
| 4 | 69% | 15% | 4% | 3% | 1% | 77% |
| 5 | 71% | 30% | 12% | 6% | 1% | 51% |
| 6 | 71% | 25% | 16% | 3% | 4% | 52% |
| 7 | 71% | 20% | 4% | 12% | 7% | 57% |
| 8 | 71% | 15% | 8% | 9% | 10% | 58% |
| 9 | 73% | 30% | 8% | 3% | 7% | 52% |
| 10 | 73% | 25% | 4% | 6% | 10% | 55% |
| 11 | 73% | 20% | 16% | 9% | 1% | 54% |
| 12 | 73% | 15% | 12% | 12% | 4% | 57% |
| 13 | 75% | 30% | 4% | 9% | 4% | 53% |
| 14 | 75% | 25% | 8% | 12% | 1% | 54% |
| 15 | 75% | 20% | 12% | 3% | 10% | 55% |
| 16 | 75% | 15% | 16% | 6% | 7% | 56% |
| Curing Age | O | B | C | D | E | |
|---|---|---|---|---|---|---|
| Range R | 7 d | 0.117 | 0.059 | 0.017 | 0.019 | 0.023 |
| 14 d | 0.123 | 0.075 | 0.019 | 0.020 | 0.023 | |
| 28 d | 0.183 | 0.103 | 0.032 | 0.039 | 0.021 | |
| Main-Sub Sequence | 7 d | O > B > E > D > C | ||||
| 14 d | O > B > E > D > C | |||||
| 28 d | O > B > D > C > E | |||||
| Optimal Level | 7 d | O4 | B4 | C1 | D1 | E4 |
| 14 d | O4 | B4 | C3 | D2 | E1 | |
| 28 d | O4 | B4 | C4 | D2 | E1 | |
| Curing Age | Slurry Mass Concentration | Mass Proportion | ||||
|---|---|---|---|---|---|---|
| Slag | Quicklime | Cement | Gypsum | Bentonite | ||
| 7d | 75% | 56% | 15% | 16% | 12% | 1% |
| 14d | 75% | 58% | 15% | 8% | 9% | 10% |
| 28d | 75% | 62% | 15% | 4% | 9% | 10% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Li, K.; Guo, L.; Wang, S.; Chu, Y.; Lu, Y. Curing Agent for High-Concentration Unclassified Tailings Stockpiling: A Case Study of Tailings from a Gold Mine. Minerals 2024, 14, 884. https://doi.org/10.3390/min14090884
Wang W, Li K, Guo L, Wang S, Chu Y, Lu Y. Curing Agent for High-Concentration Unclassified Tailings Stockpiling: A Case Study of Tailings from a Gold Mine. Minerals. 2024; 14(9):884. https://doi.org/10.3390/min14090884
Chicago/Turabian StyleWang, Weixiang, Kun Li, Lijie Guo, Sha Wang, Yifan Chu, and Yao Lu. 2024. "Curing Agent for High-Concentration Unclassified Tailings Stockpiling: A Case Study of Tailings from a Gold Mine" Minerals 14, no. 9: 884. https://doi.org/10.3390/min14090884
APA StyleWang, W., Li, K., Guo, L., Wang, S., Chu, Y., & Lu, Y. (2024). Curing Agent for High-Concentration Unclassified Tailings Stockpiling: A Case Study of Tailings from a Gold Mine. Minerals, 14(9), 884. https://doi.org/10.3390/min14090884









