Abstract
The concealed Molai Zn-Pb±(Ag,Ge) stratiform deposit in southeastern Peloponnese is hosted in Triassic intermediate tuffs, ignimbrites and subaerial andesitic flows. The host rocks display trace element signatures of a Supra-Subduction Zone (SSZ) setting. Three ore-forming stages are recognized, with stages I and II related to formation of the epigenetic, stratiform, massive-to-semi-massive ore and a late stage III associated with vein-type mineralization. The O and D isotope geochemistry of gangue chlorite and epidote reveal mixing with fresh meteoric water during the weaning stages of the hydrothermal activity of the late stage II due to uplifting of the hydrothermal system. Sphalerite is the major ore phase, with three different varieties formed during stages I (Sp-I) and II (Sp-II and Sp-III). All sphalerite varieties coexist, depicting gradual change in the chemistry of the ore-forming fluids. Molai ores are characterized by elevated Ag and Ge contents. Tetrahedrite is the major Ag carrier, while among the three sphalerite varieties, early Sp-I comprises the highest Ge contents. The Molai Zn-Pb±(Ag,Ge) deposit is characterized by intermediate features between bimodal felsic massive sulfides and subaerial epithermal systems based on the shallow formation depth, the presence of hydraulic breccias associated with phase separation, the ore formation along high-angle faults, the relatively low ore-forming temperatures below 250 °C obtained from geothermometry, and the absence of the typical structure of bimodal felsic type ores.
1. Introduction
Volcanogenic Massive Sulfides (VMSs), also known as Volcanic-Associated [1], or Volcanic-Hosted Massive Sulfides [2], are among the most common, exploited and well-studied ore deposit types by economic geologists—e.g., [3,4,5,6]. They were formed throughout Earth’s history, spanning from the Paleoarchean [7,8], approximately 3.5 Ga ago, to the present, i.e., modern seafloor massive sulfides (SMS) [9,10,11].
Volcanogenic massive sulfide deposits are typically formed in marine environments related to a diversity of geotectonic settings, e.g., both convergent and divergent margins and in both fore-arc and back-arc basins [12,13,14]. They are genetically associated with volcanic and volcanosedimentary sequences and are major sources of base (e.g., Cu, Zn and Pb) and precious metals. The VMS contemporary classification schemes take under consideration the ore mineralogy and grade-tonnage, as well as the composition, petrochemistry and geotectonic setting of their host volcanic and volcanosedimentary strata [15,16]. The contemporary VMS categorization includes the “Felsic”, “Bimodal Mafic” and “Mafic” types, with further subdivision of the “Felsic” type into “Felsic-to-Intermediate” and “Bimodal Felsic” (e.g., Kuroko type), and of the “Mafic” type into “Mafic-to-Pelitic Siliciclastic-Mafic” (e.g., Besshi-type) and “Mafic” (e.g., Cyprus-type) [4,6].
Recent studies have also proven the correlation between VMS and epithermal type mineralizations, particularly in shallow marine environments and in settings where hydrothermal convection is active for prolonged periods [17,18] and references therein]. Current exploration strategies have targeted such settings for Critical Raw Materials (CRMs), as the advances in analytical techniques during the late 20th century have also proven their potential for various critical metals, especially in polymetallic sulfide ores associated with intermediate-felsic volcanic sequences—see [19,20,21]. At the same time, several VMS deposits have been re-examined for their precious and strategic metal content, proving that VMS deposits still hold a very large potential for non-typical critical and/or strategic metals needed for the contemporary and future needs of mankind [4,6,22,23,24,25].
In this context, the Molai massive sulfide ores were a major base metal exploration target in Greece until the 1980s. Early studies proposed a syngenetic character of the sulfide ore to the host volcanic rocks (based on Pb/Pb galena geochronology [26,27] and the stratiform sulfide ore development), and, based on the classification schemes of the early 80s, it has been described as a Kuroko-type VMS deposit [27,28,29]. Interestingly, no other studies have been performed on the Molai massive sulfide ore since, especially regarding the petrography, mineral chemistry, and trace element characteristics of the Molai sulfide ore and its strategic/critical metal potential. In 2022, the Greek government awarded a 30-year exploration and mining license to the Hellenic Minerals mining company (subsidiary of Rockfire Resources Ltd.), which has successfully verified the drilling campaign of the 1980s through resampling of the pre-existing drill cores (see [30,31]).
The objective of this paper is to provide a multidisciplinary approach to dissect the Molai deposit, combining geological, mineralogical, petrographical, geochemical and stable isotope data. The aim is to provide detailed information on the geologic environment of ore formation, its spatial development and the trace element signatures, with a special interest in CRMs—see regulation OJ 202401252 EU, [32]. The results of this study could be employed in future exploration strategies regarding concealed VMS-style deposits in which prior exploration has focused solely on their base metal contents. This study could prove beneficial for the metallogenic potential of Greece, as well as the globe.
2. Regional Geology
In southeastern Peloponnese, four nappes of the External Hellenides are exposed, including the “Phyllite-Quartzite” unit (P-Q), the “Gavrovo-Tripolitza” unit (G-T) and the “Pindos” unit (P) stacked over the basement “Plattenkalk” unit (PLK) (Figure 1a) [33,34,35,36,37,38]. The PLK series comprises an Upper Triassic neritic sequence [39] overlain by pelagic carbonate rocks of Jurassic to Upper Eocene age [40,41] while above them, a lower Oligocene flysch sequence is resting conformably [42]. Beneath the carbonate rocks, the Kastania phyllites [43,44] occur, only in the Taygetos area, which are probably of Permo-Triassic age. The PLK unit underwent greenschist facies conditions metamorphism (P = 7–8 kbar, T = 310–360 °C) during its obduction on the Eurasian Continental margin [38].
The P-Q unit—also referred to as the “Arna unit”—in central Peloponnese [45] is wedged between the basement PLK unit and the unmetamorphosed neritic platform of the G-T unit [46]. The P-Q unit comprises mainly phyllites and quartzites, as well as marbles; schists; alkaline meta-tholeiites, -sandstones, -shales, -carbonates, -pelites and -conglomerates; and remnants of Hercynian orthogneisses overlain by an Oligocene meta-flysch [35,38]. It is considered as a Carboniferous-Permian-to-Triassic rift sequence [47,48]. Chatzaras et al. [48] also proposed a depositional environment, in the southern part of Paleotethys and along the northern Gondwana margin. The P-Q unit has undergone two metamorphic events: the first occurred ca. 36 to 29 Ma in blueschist-facies conditions (P ~ 17 Kbar, T = 450 ± 30 °C; garnet-glaucophane), while the second occurred in greenschist-facies conditions (P = 4.5–6.0 kbar, T = 250–310 °C; chloritoid-albite) between 17 and 15 Ma [36,37].
The overlying G-T unit comprises a lower Carboniferous-to-late Triassic volcanosedimentary sequence, namely the “Tyros Beds”, and an upper Triassic-to-Cretaceous sequence composed of neritic platform carbonates followed by an upper Eocene flysch [49,50]. The G-T (meta)-limestones indicate metamorphism of very low grade to lower epizone (T = 240–320° C) [51,52]. An inundation conglomerate, including conglomerate breccias and breccias, separates the lower Tyros Beds from the overlain carbonates [53].
The “Tyros Beds” (TBs) which host the Molai ore deposit, comprise the lowermost segment of the G-T unit (Figure 1a) [33,54,55]. The TBs are considered to be a Carboniferous-to-late Triassic volcanosedimentary succession overprinted by very low-grade metamorphism (prehnite-pumpellyite facies) during the Eocene-Oligocene [28,56]. Two different successions have been distinguished in the TBs, i.e., the lower “meta-clastic” and the upper “volcanosedimentary” series [28,57,58,59,60,61,62]. The lower “meta-clastic” series comprises ca. 500 m thick massive, coarse-grained marbles overlain by a ca. 150 to 200 m thick meta-clastic sequence involving mica and chlorite schists, pelites, sandstones, conglomerates, Fe-formations and evaporitic deposits indicative of a Sabkha-lacustrine-like environment [63,64,65]. The upper volcanosedimentary series consists of volcanic rocks, namely the “Tyros volcanic rocks” (TVR), including transitional subaerial-to-submarine andesitic lava flows, andesite sills and pyroclastic rocks such as felsic tuffs and rhyolites, ignimbrites and lahar deposits (Figure 2a). Dolomites, limestones, marbles and metapelites are intercalated with volcanic rocks [28,35,38,66].
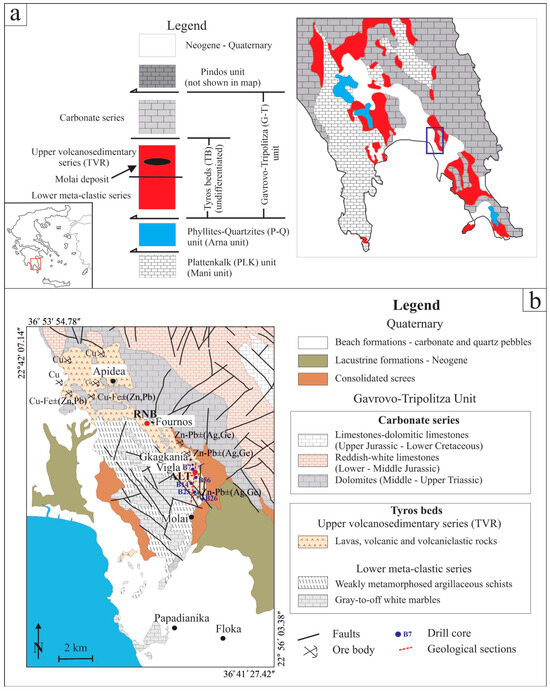
Figure 1.
(a) Simplified geological map of the geotectonic regime of external Hellenides in SE Peloponnese—with modifications after Xypolias et al. [67] and Chatzaras et al. [48]. (b) Geological map of the Molai deposit including the major sulfide ore locations.
Figure 1.
(a) Simplified geological map of the geotectonic regime of external Hellenides in SE Peloponnese—with modifications after Xypolias et al. [67] and Chatzaras et al. [48]. (b) Geological map of the Molai deposit including the major sulfide ore locations.
3. Deposit Geology and Exploration History
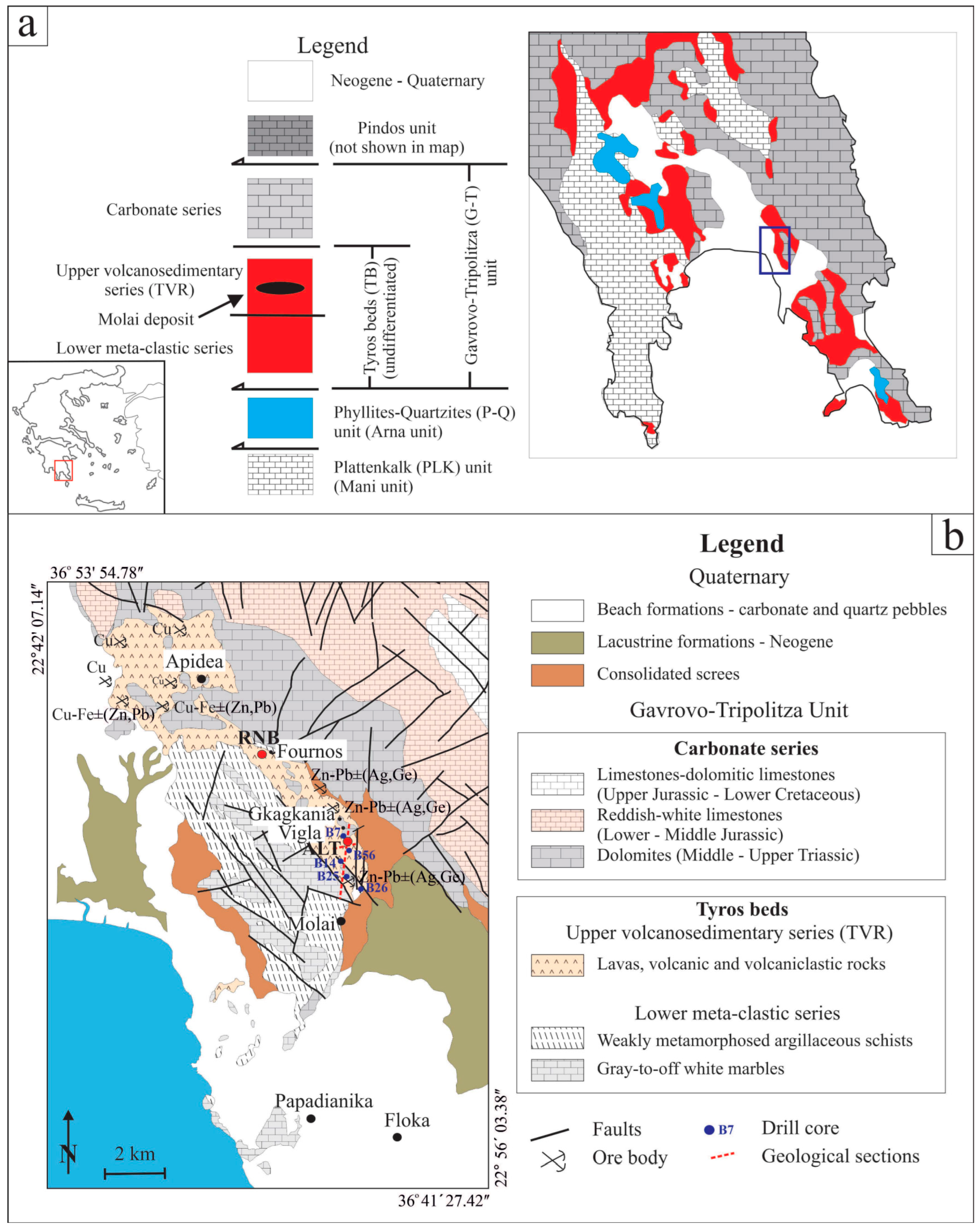
The Molai deposit was originally discovered in 1938 through regional assessment by the Greek Geological Survey of the Ministry of Finance (currently named the Institute of Geology and Mineral Exploration-IGME). The deposit is situated ~5 km north of the town of Molai and comprises nine multiple, stratabound massive orebodies over a total length along the strike of ~7 km, grouped in three different mining zones (e.g., west with one orebody, central with six and east with two) and four mining sectors; a. Molai-Vigla-Mesovouni-Gkagkania-Fournos (also the focus of this study), b. Apidea, c. Floka, and d. Papadianika, (Figure 1b). The stratiform Molai ores were considered by early studies, i.e., [26,27,28,29], as syngenetic to the host volcanic rocks. The researchers describe them as layered orebodies and they were dated at ca. 240 Ma (Pb/Pb geochronology on galena). They have also deduced many similarities with Kuroko-type VMSs, and so have classified the Molai ore deposit as a Kuroko-type VMS.
Limited work was conducted on the deposit until the early 1970s, when IGME performed semi-regional lead isotope studies and geophysical surveys. By 1979, exploration had focused on the Molai deposit, and diamond drilling commenced the same year [28,53]. The drilling exploration included 173 drill cores (of ca. 1500 m along strike N-to-S, 200 m across dip and ~300 m in depth). The orebodies follow a general N-S direction and are placed along two major fault systems, forming “flower structures” trending between N20° and N40° W, and N20° E, whereas their sub-vertical trends (E-W and ESE-WNW trending) are barren [68]. The orebodies dip from intermediate (40°) to steep (70–80°) to the east (Figure 2b). The thickness of the orebodies ranges between <1 and 15 m located at absolute levels between +165 m and −50 m [27] (Figure 2c). An oxidation zone (with width up to 20 cm) is developed around the exposed and near-surface orebodies. An initial non-compliant mineral estimate was calculated in 1981, whereas a second, non-compliant mineral estimate was calculated in 1988, which was significant enough for IGME to commence feasibility studies into mining at Molai. Yet, operations ceased in the late 1980s due to a drastic drop in the international price of zinc.
The economic value of the Molai ore deposit is based on geological, geochemical, geophysical and core-logging data that prove that it contains ~10.6 to ~26.5% Zn, ~1.5 to ~2.9% Pb and ~60 to ~90 g/t Ag [28]. The reported maiden JORC 2012-compliant resources of the Molai deposit are 3.2 ± 0.2 Mt with 11% Zn eq. (e.g., 9.4% Zn, 1.8% Pb, 47 g/t Ag and a cut-off grade of 4% Zn) for lodes of ~210 Kt Zn, ~39 Kt Pb and ~3.5 Moz Ag and a recovery rate of 90% using the cut-and-fill method and bulk floatation for recovery of 20% [69]. Rockfire Resources Ltd. also announced in 2022 that ore concentrates for the Molai deposit yield ~57% Zn, ~64% Pb, ~2.6% Cu, ~860 g/t Ag, ~0.5 g/t Au and a weighted average grade of Ge at ~50 g/t, with maximum values of 197 g/t (based on 51 assayed samples re-analyzed from the pre-existing drill cores). Hellenic Minerals IKE (Nicosia, Cyprus), a subsidiary of Rockfire Resources Ltd. (London, UK), is currently employing a drilling project that aims at extending the zinc resources both at depth and to the north.
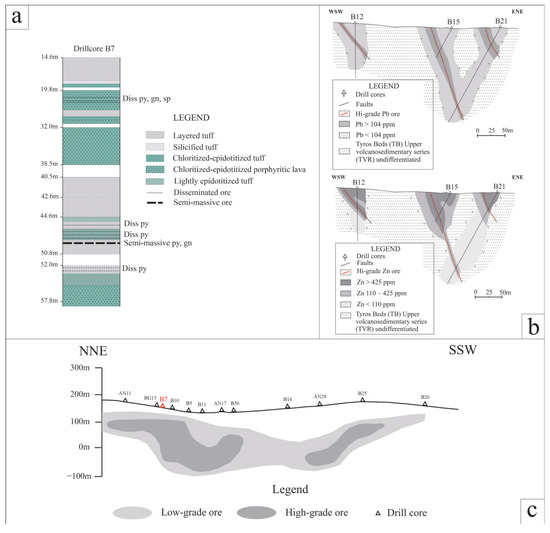
Figure 2.
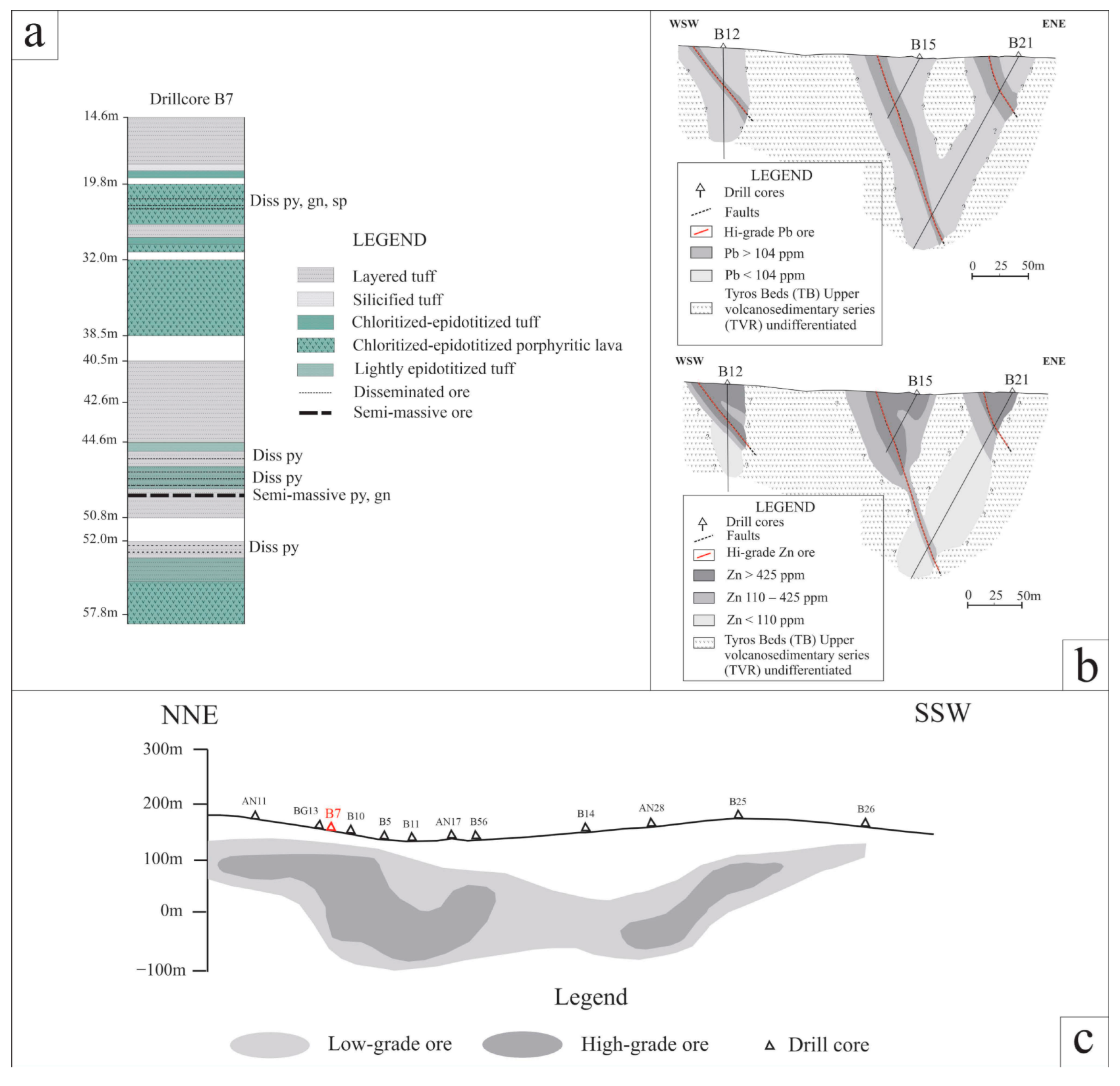
(a) Generalized stratigraphy of the upper volcanosedimentary series of Tyros Beds (TVR) hosting the Zn-Pb±(Ag,Ge) sulfide ore at Gkagkania and Vigla sites. (b) Simplified WSW-ENE geological sections with Pb and Zn distributions from the Molai sulfide ore (Vigla site) based on drill-core data from HSGME—with modifications after Ilias [70]. See Figure 1b for section reference. (c) Simplified NNE-SSW section of the Molai (Vigla and Gkagkania) sulfide ore (see Figure 1b for section reference).
Figure 2.
(a) Generalized stratigraphy of the upper volcanosedimentary series of Tyros Beds (TVR) hosting the Zn-Pb±(Ag,Ge) sulfide ore at Gkagkania and Vigla sites. (b) Simplified WSW-ENE geological sections with Pb and Zn distributions from the Molai sulfide ore (Vigla site) based on drill-core data from HSGME—with modifications after Ilias [70]. See Figure 1b for section reference. (c) Simplified NNE-SSW section of the Molai (Vigla and Gkagkania) sulfide ore (see Figure 1b for section reference).
4. Sampling and Analytical Techniques
Sampling included a total number of 230 surface and sub-surface samples for lithological, mineralogical and geochemical analyses (refer to Table S1a for sample locations). Additionally, samples were collected from 15 historical drill cores, along a N-S axis, covering a distance of approximately 1 km (Table S1b). Samples were taken from various depths in order to cover all possible lithotypes and ore textures. Prior analyses of the samples were processed in the Minerals and Ore Deposits Lab, Department of Geology, University of Patras.
An Axioscop 40 optical microscope was employed for the study of ore mineralogy and texture. Photographs were taken by a Jenoptik Progres Gryphax camera attached to the Gryphax software for registration and processing of the obtained material. Mineralogical microanalyses were performed using a JEOL JSM-6300 SEM equipped with energy dispersive and wavelength spectrometers (EDS and WDS) and INCA software at the Laboratory of Electron Microscopy and Microanalysis, University of Patras, Greece. Operating conditions consisted of an accelerating voltage of 25 kV and beam current of 3.3 nA, with a 4-μm beam diameter. The total counting time was 60 s, and dead time, 40%. Standards used for gangue and ore minerals were natural marialite (Cl), tourmaline (B, F), orthoclase (K), diopside (Ca, Si), ilmenite (Ti), rhodonite (Mn), fayalite (Fe), jadeite (Na), forsterite (Mg), corundum (Al), chlorite, epidote, plagioclase and muscovite, natural chalcopyrite, tetrahedrite, tennantite, stibnite, pyrite, sphalerite, galena, hausmannite (Mn2+), manganite (Mn3+), pyrolusite (Mn4+) and synthetic CoNiAs, SnO2 and CdTe, as well as the native metals Ag, Au, Te and Se. Detection limits were ~0.01% and accuracy better that 5% was obtained.
Ore mineral compositions (651 analyses in total) were determined using a JEOL 8900 Superprobe equipped with wavelength, energy dispersive and back-scatter detectors and a xClent system, at the Microprobe Center of the Department of Earth and Planetary Sciences Department, at McGill University, Montreal, QC, Canada. Operating conditions were an acceleration voltage of 15 kV, a beam current of 10 nA, and a counting time of 20 s for all elements, except 100 s for Ag and 50 s for As and Te. Scans in WDS mode were also used to detect trace element contents. The measured X-ray lines were Ag La, Sb La, Cu Ka, Fe Ka, Ge Ka, Ga Ka, In Ka, Pb Ka, Sn Ka, Au Ma, Mn Ka, Se La, Bi La, Cd La, Te La and S Ka. ZAF corrections were made with proprietary JEOL software. The detection limits (in wt %) of the trace elements were Ag: 0.0419, Zn: 0.054, Fe: 0.0476, Cu: 0.0718, Pb: 0.2072, Au: 0.0371, Sn: 0.0572, Ni: 0.0487, Mn: 0.0306, S: 0.022, As: 0.0662, Sb: 0.0611, Te: 0.0429, Sr: 0.0671, Bi: 0.0535, Se: 0.0271, Ga: 0.0344, In: 0.0271 and Ge: 0.0439. A minimum of ten analyses were obtained from each sample and three from each grain.
Whole-rock lithogeochemical analyses were performed on 115 samples from the Molai host volcanic rocks to determine their bulk compositions. The samples were commercially prepared (using the ES6 code), and then analyzed for their major (10 in total) and trace element (45 in total) contents using the lithium metaborate fusion methods ICP-AES (FUS-ICP) and ICP-MS (FUS-MS), respectively, by ActLabs, Ancaster, ON, Canada. The loss on ignition (LOI) was also measured at ActLabs by implementation of the wet chemical method. Detection limits were 0.001 wt % for MnO and TiO2 and 0.01 wt % for the rest of the major elements. For the trace elements, the detection limits were as follows: 0.01 ppm for Lu; 0.05 ppm for Pr, Eu and Tm; 0.1 ppm for La, Ce, Nd, Sm, Gd, Tb, Dy, Ho, Er, Yb, Ta, Tl, Th and U; 0.2 ppm for In and Hf; 0.4 ppm for Bi; 0.5 ppm for Ag, Sb and Cs; 1 ppm for Sc, Be, Y, Co, Ga, Ge, Nb, Sn and W; 2 ppm for Ba, Zr, Sr, Rb and Mo; 5 ppm for V, As and Pb; 10 ppm for Cu; 20 ppm for Cr and Ni; and 30 ppm for Zn. The “NIST 694”, “DNC-1”, “W-2a”, “SY-4”, “B25-88” and “BIR-1a” standards were employed by ActLabs for major elements, and the “NIST-694”, “DNC-1”, “LKSD-3”, “TDB-1”, “W-2a”, “SY-4”, “CTA-AC-1”, “BIR-1a”, “NCS DC70009”, “OREAS 100a”, “OREAS 101a”, “OREAS 101b” and “B25-88” standards for trace element analyses. Duplicates were analyzed for precision (standard deviation—SD) calculations. For major elements, the SD ranges between 0% (for K and Cr) and 1.8% (for Si), whereas for trace elements, the SD ranges between 0% (for Cr, Sn and W) and 1.58% (for Rb).
A total of 21 oxygen (δ18O), and 16 hydrogen (δD) isotope compositions were obtained from bulk rock samples of the TVR basalts at Krokees, host andesites of the Molai deposit, mineral separates of gangue chlorite, and epidote (e.g., Vigla, Papadianika and Krokees sites) from clear quartz veinlets mineralized with galena (three chlorite and eight epidote separates). Moreover, three clear quartz separates from the same veins were analyzed for their δ30Si composition. The oxygen and hydrogen isotopic composition chlorite and epidote were measured by a Triple-Quad Mass Spectrometer (MAT-253) at the State Key Laboratory of Geological Processes and Mineral Resources, Beijing, China. Oxygen was released following the method of De Groot [71]. The δ18O and δD values are reported based on V-SMOW, and the analytical errors are ±0.1‰ and ±1‰, respectively. The δ18OH2O and δDH2O values in chlorite and epidote were calculated using the equations of Zheng [72,73]. The deuterium isotopic compositions were measured from fluids released from the host andesites, and chlorite and epidote.
5. Results
5.1. The Hosts of the Molai Deposit
5.1.1. Lithopetrography and Mineralogy
The TVR, at the Apidea and Molai (Gkagkania and Vigla) mining area, comprise lava flows intercalated with pyroclastic and volcaniclastic rocks (Figure 3, Table S2). The lavas are mainly aphanitic, of up to 95 vol.% fine-grained groundmass, and rarely porphyritic basaltic andesites-to-andesites (Figure 3a–c and Figure 4a), overlying their apophyseal “feeders”, i.e., sills and plugs. The lavas are stratified on the mesoscopic scale, i.e., they develop mauve-colored flow tops, indicating subaerial oxidation of magnetite, and dark-green-colored flow bases. The bases display flow foliation defined by oligoclase and rare biotite, hornblende, and pyroxene phenocrysts.
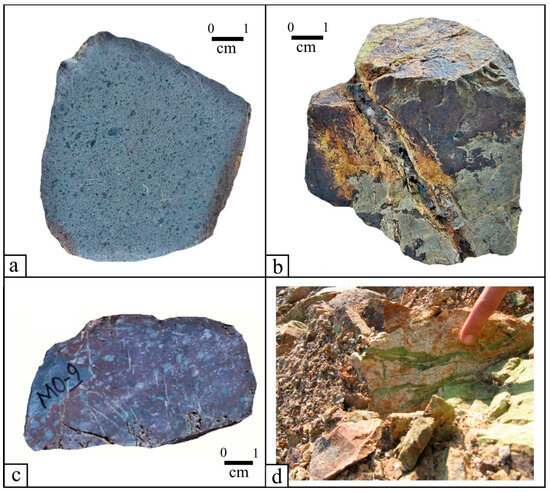
Figure 3.
Macroscopic photos of the Molai Zn-Pb±(Ag,Ge) deposit host rocks. (a) Aphanitic lavas. (b) lava sample cut by mineralized veinlets comprising pyrite affected by supergene alteration. (c) Porphyritic lavas. (d) Quartz-chlorite-epidote veinlets cutting the host andesites.
Figure 3.
Macroscopic photos of the Molai Zn-Pb±(Ag,Ge) deposit host rocks. (a) Aphanitic lavas. (b) lava sample cut by mineralized veinlets comprising pyrite affected by supergene alteration. (c) Porphyritic lavas. (d) Quartz-chlorite-epidote veinlets cutting the host andesites.
The base porphyritic andesites contain up to ~45 vol % coarse-grained phenocrysts (with sizes reaching up to ~1 cm) of prismatically lath-shaped subhedral plagioclases, i.e., oligoclase (An~20) altered to albite (Ab~95), and rare pyroxenes (Aeg~95 and rare Di-Hd), biotite and hornblende. Pyroxenes are altered to fine-grained sericite, albite, chlorite, actinolite, calcite and epidote (Ps% ~20) prisms (Figure S1). The phenocrystals are dispersed in a groundmass with common glomero-porphyritic textures which comprises chlorite, epidote, quartz, feldspars, hematite, titanite, calcite, and rare sericite, and devitrified glass (composed of ~80 wt % SiO2 and ~10 wt % Al2O3 and ~6 wt % Na2O, Table 1). The sill and plug “feeders” are mainly porphyritic to glomero-porphyritic and, rarely, amygdaloid lavas (Figure 3c) which, at Krokees, form a small lava dome (with length of ~2 km and width of ~500 m) that has been exploited during antiquity as a source of decorative stone, i.e., the famous “Lapis Lacedaemonius” [74,75]. This basaltic porphyry is characterized by a variable ratio of plagioclase phenocrysts to groundmass and rare vesicular textures filled with chlorite and quartz ± epidote (Ps% ~78) (Figure S1). The base andesites are crosscut by veins and veinlets filled with sericite, chlorite, vermiculite, dolomite, calcite, quartz and albite. The veins are antitaxial with epidote growing at the margins and sericite in the median planes.
The top aphanitic andesites comprise only up to 15 vol % phenocrysts, i.e., albite-to-andesine with smaller sizes relative to the porphyries, suggesting higher cooling rates (Figure 3a). The groundmass is fine-to-micro-grained and even glassy, microlithic and flow-foliated and comprises hornblende and pyroxene replaced by Fe- (hypogene hematite) and Ti-oxides and -hydroxides and sometimes devitrified glass, montmorillonite, orthoclase, albite, sericite, chlorite, epidote, quartz and calcite. They are also crosscut by syntaxial veins of quartz and calcite, comprising disseminated pyrite and sphalerite, where pyrite is placed at the margins and sphalerite in the median plane (Figure 3b). The lava tops appear less altered in respect to the bottom andesites.
The Tyros volcaniclastic and pyroclastic rocks, i.e., tuffs and ashes, are the main hosts to the Zn-Pb±(Ag,Ge) Molai massive-to-semi-massive ores. They are represented mainly by intermediate composition (predominantly andesites, Figure 4a, Table 2). At the Gkagkania and Vigla mining sites (Figure 1b), the volcanoclastic and subaerial pyroclastic rocks comprise predominately agglomerates and subordinate air-fall tuffs, ash tuffs and ashes, tuffaceous lapilli, ignimbrites and lahar deposits and flows, pumicites and volcanic breccias. These are intercalated with tuffaceous and volcaniclastic sandstones, cobble conglomerates, shales, conglomerates and, rarely, limestones.

Table 1.
Representative whole rock analyses of unaltered host TVR lavas and volcanic glass of the Molai Zn-Pb±(Ag,Ge) ore (major elements in wt%, trace elements in ppm). Please refer to Figure 1b and Table S1a for sample locations.
| Sample | RNB 01 | RNB 03 | RNB 04 | RNB 05 | MO19B-C6-Glass/Mixture | TYR-12A | TYR-13B | TYR-33A | TYR-41B | TYR-5C |
|---|---|---|---|---|---|---|---|---|---|---|
| Major elements | ||||||||||
| SiO2 | 57.29 | 58.45 | 55.99 | 55.02 | 82.42 | 54.3 | 59.12 | 55.74 | 49.76 | 57.55 |
| Al2O3 | 17.71 | 16.27 | 15.16 | 19.19 | 10.38 | 19.83 | 17.43 | 14,00 | 20.36 | 18.07 |
| Fe2O3(T) | 7.78 | 6.74 | 6.62 | 6.68 | 0.89 | 6.06 | 7.46 | 8.71 | 8.49 | 7.47 |
| MnO | 0.11 | 0.07 | 0.1 | 0.07 | n.a. | 0.08 | 0.07 | 0.08 | 0.09 | 0.10 |
| MgO | 2.09 | 2.6 | 3.27 | 1.69 | 0.32 | 1.4 | 1.81 | 7.51 | 4.76 | 3.43 |
| CaO | 2.36 | 2.74 | 4.99 | 3.46 | 0.29 | 7.83 | 4.1 | 2.34 | 4.72 | 3.41 |
| Na2O | 4.34 | 3.01 | 2.22 | 4.79 | 6.00 | 3.19 | 1.19 | 1.95 | 2.32 | 2.44 |
| K2O | 1.98 | 2.84 | 3.61 | 2.37 | 0.04 | 2.79 | 5.73 | 3.61 | 3.99 | 4.07 |
| TiO2 | 0.76 | 0.86 | 0.79 | 0.78 | 0.08 | 0.91 | 0.74 | 0.7 | 0.7 | 0.68 |
| P2O5 | 0.17 | 0.16 | 0.14 | 0.16 | n.a. | 0.16 | 0.21 | 0.11 | 0.13 | 0.09 |
| LOI | 3.97 | 4.65 | 6.26 | 4.21 | n.a. | 3.17 | 2.95 | 5.89 | 5.18 | 3.2 |
| Total | 98.56 | 98.39 | 99.14 | 98.43 | 100.43 | 99.72 | 100.8 | 100.6 | 100.5 | 100.5 |
| Trace elements | ||||||||||
| Sc | 21 | 27 | 26 | 26 | n.a. | 23 | 18 | 33 | 22 | 21 |
| Be | <1 | 1 | 1 | 1 | n.a. | 1 | 1 | <1 | 1 | <1 |
| V | 201 | 155 | 132 | 212 | n.a. | 237 | 82 | 215 | 175 | 158 |
| Ba | 254 | 236 | 318 | 341 | n.a. | 289 | 343 | 114 | 284 | 229 |
| Sr | 75 | 69 | 124 | 88 | n.a. | 271 | 255 | 22 | 319 | 158 |
| Y | 18 | 21 | 22 | 16 | n.a. | 16 | 45 | 17 | 16 | 19 |
| Zr | 62 | 124 | 132 | 74 | n.a. | 89 | 164 | 96 | 80 | 73 |
| Cr | 40 | 50 | 50 | 40 | n.a. | <20 | <20 | 150 | <20 | <20 |
| Co | 21 | 15 | 23 | 30 | n.a. | 10 | 10 | 24 | 25 | 16 |
| Ni | <20 | <20 | <20 | 20 | n.a. | <20 | <20 | 30 | <20 | <20 |
| Cu | 140 | 100 | <10 | 120 | n.a. | 170 | 30 | 10 | 80 | 20 |
| Zn | <30 | 50 | 60 | 720 | n.a. | 110 | 60 | 120 | 80 | 90 |
| Ga | 16 | 18 | 16 | 20 | n.a. | 19 | 19 | 14 | 19 | 17 |
| Ge | <1 | 1 | 1 | 1 | n.a. | 1 | 2 | 2 | <1 | 2 |
| As | <5 | <5 | <5 | 38 | n.a. | <5 | <5 | <5 | <5 | <5 |
| Rb | 40 | 60 | 76 | 41 | n.a. | 45 | 146 | 51 | 40 | 49 |
| Nb | 2 | 5 | 5 | 3 | n.a. | 3 | 5 | 4 | 2 | 2 |
| Mo | <2 | <2 | <2 | <2 | n.a. | <2 | <2 | <2 | <2 | <2 |
| Ag | <0.5 | 0.6 | <0.5 | <0.5 | n.a. | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| In | <0.2 | <0.2 | <0.2 | <0.2 | n.a. | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 |
| Sn | 1 | 1 | 1 | 2 | n.a. | <1 | 1 | <1 | <1 | <1 |
| Sb | <0.5 | <0.5 | <0.5 | 0.5 | n.a. | 0.6 | <0.5 | <0.5 | <0.5 | <0.5 |
| Cs | 2.2 | 2.3 | 3.2 | 2.2 | n.a. | 1.4 | 5.0 | 1.5 | 1.8 | 2.5 |
| La | 13.4 | 21.3 | 37.8 | 13.7 | n.a. | 11,0 | 19.3 | 17.2 | 9.7 | 7.0 |
| Ce | 31.3 | 48.3 | 68.4 | 28.3 | n.a. | 23.5 | 43.8 | 44.2 | 19.8 | 16.5 |
| Pr | 3.75 | 5.87 | 7.46 | 3.84 | n.a. | 2.85 | 5.63 | 4.91 | 2.6 | 2.1 |
| Nd | 15.5 | 23.1 | 28.0 | 15.5 | n.a. | 11.9 | 24.0 | 18.7 | 11.5 | 9.3 |
| Sm | 3.7 | 4.9 | 5.4 | 3.7 | n.a. | 2.7 | 6.5 | 3.8 | 2.9 | 2.7 |
| Eu | 0.98 | 1.21 | 1.33 | 1.01 | n.a. | 1.00 | 1.77 | 1.00 | 1.07 | 0.88 |
| Gd | 3.3 | 4.6 | 5,0 | 3.4 | n.a. | 3,0 | 7.2 | 3.5 | 3,0 | 3.1 |
| Tb | 0.6 | 0.7 | 0.8 | 0.6 | n.a. | 0.4 | 1.2 | 0.6 | 0.5 | 0.5 |
| Dy | 3.5 | 4.2 | 4.5 | 3.2 | n.a. | 2.7 | 7.3 | 3.2 | 3.1 | 3.3 |
| Ho | 0.7 | 0.8 | 0.9 | 0.6 | n.a. | 0.5 | 1.5 | 0.6 | 0.6 | 0.7 |
| Er | 2.0 | 2.3 | 2.5 | 1.7 | n.a. | 1.5 | 4.6 | 1.7 | 1.9 | 2.0 |
| Tm | 0.29 | 0.33 | 0.37 | 0.23 | n.a. | 0.22 | 0.66 | 0.26 | 0.29 | 0.31 |
| Yb | 1.9 | 2.2 | 2.4 | 1.6 | n.a. | 1.6 | 4.6 | 1.7 | 2.0 | 2.0 |
| Lu | 0.28 | 0.35 | 0.36 | 0.23 | n.a. | 0.25 | 0.69 | 0.24 | 0.3 | 0.31 |
| Hf | 1.8 | 3.1 | 3.3 | 2.0 | n.a. | 2.5 | 4.3 | 2.4 | 2.3 | 2.1 |
| Ta | 0.1 | 0.6 | 0.6 | 0.4 | n.a. | 0.2 | 0.4 | 0.3 | 0.2 | 0.2 |
| W | 47 | 33 | 17 | 66 | n.a. | 1 | <1 | <1 | <1 | <1 |
| Tl | <0.1 | <0.1 | <0.1 | <0.1 | n.a. | 0.1 | 0.3 | 0.1 | 0.1 | 0.2 |
| Pb | <5 | <5 | <5 | 95 | n.a. | 33 | 11 | 27 | 22 | 22 |
| Bi | <0.4 | <0.4 | <0.4 | <0.4 | n.a. | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 |
| Th | 4.5 | 7.2 | 6.8 | 4.7 | n.a. | 3.4 | 5.5 | 4.1 | 3.3 | 2.2 |
| U | 1.1 | 1.6 | 1.3 | 1.4 | n.a. | 0.9 | 1.5 | 0.8 | 1.1 | 0.5 |
| AI | 37.79 | 48.61 | 48.83 | 32.98 | 27.55 | 58.77 | 72.16 | 55.41 | 56.18 | |
| CCPI | 58.99 | 59.70 | 61.28 | 51.82 | 53.41 | 55.19 | 73.41 | 66.28 | 60.93 | |
| CIA | 67.11 | 65.45 | 58.35 | 64.37 | 58.95 | 61.27 | 63.93 | 64.86 | 64.56 | |
| Zr/Y | 3.44 | 5.90 | 6.00 | 4.63 | 5.56 | 3.64 | 5.65 | 5.00 | 3.84 | |
| Rb/Sr | 0.53 | 0.87 | 0.61 | 0.47 | 0.17 | 0.57 | 2.32 | 0.13 | 0.31 | |
| Rb/Ba | 0.16 | 0.25 | 0.24 | 0.12 | 0.16 | 0.43 | 0.45 | 0.14 | 0.21 | |
| ΣLREEs | 68.63 | 104.68 | 148.39 | 66.05 | 52.95 | 101 | 89.81 | 47.57 | 38.48 | |
| ΣHREEs | 12.57 | 15.48 | 16.83 | 11.56 | 10.17 | 27.75 | 11.8 | 11.69 | 12.22 | |
| ΣREEs | 81.2 | 120.16 | 165.22 | 77.61 | 63.12 | 128.75 | 101.61 | 59.26 | 50.7 | |
| ΣLREEs/ΣHREEs | 5.46 | 6.76 | 8.82 | 5.71 | 5.21 | 3.64 | 7.61 | 4.07 | 3.15 | |
| 1 (LaN/SmN) | 0.53 | 0.63 | 1.02 | 0.54 | 0.59 | 0.43 | 0.66 | 0.49 | 0.38 | |
| 2 (SmN/YbN) | 0.99 | 1.13 | 1.14 | 1.18 | 0.86 | 0.72 | 1.14 | 0.74 | 0.69 | |
| 3 Eu/Eu * | 1.30 | 1.27 | 1.25 | 1.34 | 1.88 | 1.28 | 1.31 | 1.78 | 1.53 | |
| 4 Eu/Eu * | 1.32 | 1.20 | 1.20 | 1.34 | 1.64 | 1.21 | 1.29 | 1.70 | 1.41 | |
| 5 Ce/Ce * | 1.01 | 0.99 | 0.94 | 0.90 | 0.97 | 0.96 | 1.10 | 0.91 | 0.98 | |
1 (La/LaN)/(Sm/SmN), 2 (Sm/SmN)/(Yb/YbN), 3 EuN/(0.66 SmN + 0.33 TbN), 4 EuN/(0.5 SmN + 0.5 GdN), 5 CeN/(0.5 LaN + 0.5 PrN), n.a. not analyzed. * REE normalizing values: Eu: 1.08; Sm: 5.55; Tb: 0.0774; Gd: 4.66; La: 38.2; Ce: 79.6; Pr: 8.83; Yb: 2.82; Lu: 0.433; Nb: 33.9.
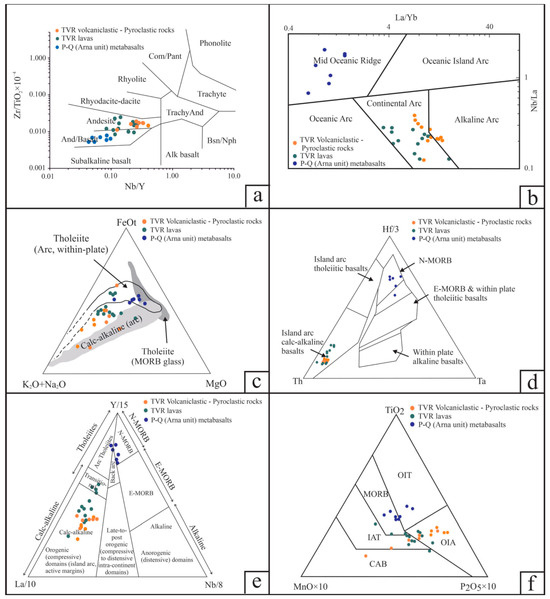
Figure 4.
Discrimination and geotectonic setting diagrams for the TVR volcanic (lavas) and volcaniclastic-pyroclastic rocks and P-Q (Arna unit) metabasalts. (a) Nb/Y vs. Zr/Ti binary diagram—after Wincehster and Floyd (1977) [76]. (b) La/Yb vs. Nb/La—after Hollocher et al. [77]. (c) FeO-−MgO−Na2O + K2O ternary diagram—after Irvine and Baragar [78]. (d) Hf-Ta-Th ternary diagram—after Wood [79]. (e) Y-Nb-La ternary diagram—after Cabanis and Lecolle [80]. (f) Ti-P-Mn ternary diagram—after Mullen [81].
Figure 4.
Discrimination and geotectonic setting diagrams for the TVR volcanic (lavas) and volcaniclastic-pyroclastic rocks and P-Q (Arna unit) metabasalts. (a) Nb/Y vs. Zr/Ti binary diagram—after Wincehster and Floyd (1977) [76]. (b) La/Yb vs. Nb/La—after Hollocher et al. [77]. (c) FeO-−MgO−Na2O + K2O ternary diagram—after Irvine and Baragar [78]. (d) Hf-Ta-Th ternary diagram—after Wood [79]. (e) Y-Nb-La ternary diagram—after Cabanis and Lecolle [80]. (f) Ti-P-Mn ternary diagram—after Mullen [81].
The tuffs are light grey-colored and are either crystalline or lithic tuffs or a mixture of both, containing vesicles filled with sericite and epidote. Their crystal fragments are mainly quartz and sericite, followed by calcite, dolomite, ankerite, albite, orthoclase and chlorite. The groundmass comprises sericite, illite, orthoclase remnants, chlorite, epidote, hematite, calcite and dolomite or mixtures of them. Scarce fine-grained basaltic tuffs contain abundant quartz, in addition to pyroxenes, chlorite, epidote and hematite. Close to the orebodies, the tuffs are intensively silicified and sericitized, and mineralized along their S0/S1 planes. The ashes and ash-flows contain fragments of quartz crystals, hyaloclasts, fine-grained lavas and sedimentary rocks clasts (sizes up to ~0.2 mm), dispersed in a fine-grained groundmass comprising quartz, sericite, illite, montmorillonite, albite, orthoclase, chlorite, epidote and Fe- and sometimes Ti-oxides, and/or hydroxides (Table S3). The lapilli tuffs are massive, polymictic and lithoclastic. They mainly consist of subangular lava fragments or blocks (sizes up to ~80 mm) of the base porphyritic andesites with various degrees of silicification, sericitization, chloritization and carbonatization, and, to a lesser extent, of tuffs, ashes, pumices and hyaloclasts (sizes up to ~30 mm). Their groundmass comprises sericite, chalcedony, calcite ± chlorite and Fe-oxides.
5.1.2. Petrochemistry
The silica and alkali (Na2O + K2O) contents of the Tyros top aphanitic and base porphyritic lavas range between ~51 and ~65 wt % and ~3.2 and ~7.2 wt %, respectively for the least altered samples (Table 1 and Table S4). Accordingly, for the volcaniclastic and pyroclastic rocks, the silica and alkali (Na2O + K2O) contents range between ~51 and ~63 wt % and ~4.2 and ~8.6 wt %, respectively (Table 2 and Table S4). These samples display an Ishikawa Alteration Index—AI [82] between 22 and 79 (e.g., 22 and 58 for the lavas and 39 and 78 for the volcanoclastic and pyroclastic rocks, Table 1 and Table 2) and a Chlorite-Carbonate-Pyrite Index—CCPI [83] ranging between 51 and 69 for the lavas and 28 and 73 for the volcanoclastic and pyroclastic rocks (Table 1 and Table 2). The TVR volcaniclastic and pyroclastic rocks show slightly higher silica content than the TVR lavas and range in composition from basaltic andesites-to-andesites (Figure 4a).
The La/Yb and Nb/La systematics (Figure 4b) for all TVR samples are typical of “Continental Arc” volcanism [77]. Based on the major and trace element geochemistry, the TVR display a predominant calc-alkaline affinity (Figure 4c–e) supporting a typical subduction setting with a small population of aphanitic and porphyritic lavas displaying a transitional character towards more tholeiitic compositions (Figure 4e). On the immobile Ti-P-Mn ternary diagram of Mullen [81], the Tyros volcanic rocks range from calc-alkaline basalts to island arc, and ocean island tholeiites (Figure 4f).

Table 2.
Representative whole rock analyses of unaltered host TVR volcaniclastic and pyroclastic rocks of the Molai Zn-Pb±(Ag,Ge) ore (major elements in wt%, trace elements in ppm). Please refer to Figure 1b and Table S1a for sample locations; Samples B2, B7, AN22 and B25 are from drill cores (see Table S1b).
Table 2.
Representative whole rock analyses of unaltered host TVR volcaniclastic and pyroclastic rocks of the Molai Zn-Pb±(Ag,Ge) ore (major elements in wt%, trace elements in ppm). Please refer to Figure 1b and Table S1a for sample locations; Samples B2, B7, AN22 and B25 are from drill cores (see Table S1b).
| Sample | ALT 1K | ALT-1J | ALT 2C | ALT-2B | B2 31-25 | B7 55 | AN22 140-142 | B25-88 * | B25 90-60 * | B25 91-40 * |
|---|---|---|---|---|---|---|---|---|---|---|
| Major elements | ||||||||||
| SiO2 | 62.67 | 62.94 | 63.11 | 56.77 | 60.35 | 58.14 | 58.26 | 40.14 | 61.85 | 43.85 |
| Al2O3 | 19.51 | 17.87 | 15.22 | 14.8 | 18.34 | 18.2 | 16.84 | 6.55 | 12.74 | 9.19 |
| Fe2O3(T) | 2.39 | 4.23 | 5.3 | 7.48 | 2.25 | 6.62 | 8.1 | 7.17 | 2.71 | 4.96 |
| MnO | 0.04 | 0.04 | 0.09 | 0.06 | 0.1 | 0.05 | 0.1 | 0.01 | 0.06 | 0.03 |
| MgO | 1.22 | 0.91 | 1.93 | 2.15 | 2.19 | 3.3 | 3.53 | 0.35 | 0.99 | 0.53 |
| CaO | 0.5 | 0.41 | 0.83 | 3.38 | 2.7 | 1.14 | 1.34 | 0.85 | 6.8 | 2.82 |
| Na2O | 2.75 | 3.75 | 1.47 | 1.24 | 1.98 | 3.16 | 3.07 | 0.05 | 0.16 | 0.48 |
| K2O | 5.84 | 4.41 | 6.29 | 5.42 | 5.65 | 4.64 | 4.72 | 2.26 | 4.39 | 2.99 |
| TiO2 | 1.07 | 1.02 | 1.24 | 1.12 | 0.93 | 1.05 | 0.7 | 0.55 | 1.08 | 0.74 |
| P2O5 | 0.25 | 0.19 | 0.27 | 0.22 | 0.2 | 0.22 | 0.16 | 0.12 | 0.24 | 0.19 |
| LOI | 3.98 | 3.63 | 4.61 | 6.32 | 5.94 | 3.32 | 3.94 | 9.56 | 6.53 | 7.66 |
| Total | 100.2 | 99.39 | 100.4 | 98.96 | 100.6 | 99.84 | 100.7 | 67.6 | 97.54 | 73.44 |
| Trace elements | ||||||||||
| Sc | 20 | 19 | 19 | 18 | 17 | 19 | 22 | 9 | 18 | 13 |
| Be | 2 | 1 | 1 | 2 | 2 | 2 | 2 | <1 | 2 | <1 |
| V | 202 | 171 | 200 | 198 | 179 | 187 | 172 | 95 | 173 | 128 |
| Ba | 217 | 187 | 432 | 489 | 69 | 361 | 422 | 95 | 164 | 163 |
| Sr | 17 | 20 | 36 | 55 | 32 | 59 | 74 | 7 | 36 | 29 |
| Y | 18 | 23 | 25 | 32 | 19 | 26 | 17.4 | 8 | 22 | 15 |
| Zr | 152 | 140 | 201 | 192 | 149 | 142 | 109 | 76 | 147 | 107 |
| Cr | 50 | 20 | <20 | <20 | <20 | 30 | <20 | 20 | <20 | <20 |
| Co | 4 | 5 | 39 | 12 | 7 | 14 | 43 | 9 | 12 | 19 |
| Ni | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| Cu | 50 | 50 | 80 | 20 | 40 | 20 | <10 | 140 | 70 | 210 |
| Zn | 140 | 170 | 670 | 700 | 450 | 300 | 70 | >10,000 | 8210 | >10,000 |
| Ga | 20 | 17 | 15 | 17 | 18 | 18 | 19 | 25 | 23 | 21 |
| Ge | 2 | 2 | 3 | 2 | 2 | 2 | 1.8 | 95 | 6 | 38 |
| As | 10 | 13 | 14 | 24 | 35 | 14 | <5 | 212 | 44 | 227 |
| Rb | 84 | 60 | 113 | 73 | 80 | 92 | 89 | 57 | 91 | 79 |
| Nb | 7 | 6 | 8 | 8 | 6 | 7 | 3.6 | 3 | 4 | 5 |
| Mo | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 |
| Ag | 0.6 | <0.5 | <0.5 | 0.6 | 0.7 | <0.5 | 0.5 | 23.6 | 3.0 | 15.9 |
| In | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.01 | 0.4 | <0.2 | <0.2 |
| Sn | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| Sb | 2.2 | 2.1 | 1.5 | 1.6 | 4.7 | 3.8 | 0.5 | 71.3 | 16.9 | 77.7 |
| Cs | 3.6 | 3.1 | 3.4 | 3.5 | 4.6 | 2.6 | 5.6 | 3.7 | 9.9 | 2.5 |
| La | 31.0 | 26.6 | 29.3 | 36.7 | 15.5 | 21.8 | 17.3 | 11.0 | 9.8 | 13.2 |
| Ce | 56.7 | 49.1 | 57.8 | 70.1 | 37.1 | 49.4 | 37.7 | 23.9 | 27.2 | 31.7 |
| Pr | 6.81 | 6.42 | 7.05 | 8.19 | 4.31 | 5.79 | 4.31 | 2.85 | 3.34 | 4.02 |
| Nd | 25.7 | 24.9 | 26.7 | 31.3 | 17.5 | 23.2 | 18.0 | 11.4 | 13.8 | 15.8 |
| Sm | 5.0 | 5.1 | 5.6 | 6.8 | 3.8 | 5.3 | 4.0 | 2.3 | 3.7 | 3.6 |
| Eu | 1.00 | 1.29 | 1.22 | 1.29 | 0.72 | 1.1 | 0.95 | 0.26 | 0.84 | 0.78 |
| Gd | 4.0 | 4.5 | 5.2 | 6.2 | 3.2 | 4.9 | 3.32 | 2.0 | 4.0 | 3.4 |
| Tb | 0.6 | 0.7 | 0.8 | 1.0 | 0.5 | 0.8 | 0.56 | 0.3 | 0.6 | 0.5 |
| Dy | 3.6 | 4.1 | 4.8 | 5.7 | 3.1 | 4.8 | 3.28 | 2.0 | 3.9 | 3.3 |
| Ho | 0.8 | 0.9 | 1.0 | 1.1 | 0.7 | 1.0 | 0.65 | 0.4 | 0.8 | 0.7 |
| Er | 2.4 | 2.5 | 3.0 | 3.2 | 2.1 | 2.8 | 1.87 | 1.3 | 2.4 | 2.1 |
| Tm | 0.37 | 0.38 | 0.44 | 0.47 | 0.32 | 0.41 | 0.29 | 0.19 | 0.38 | 0.33 |
| Yb | 2.4 | 2.6 | 2.9 | 3.1 | 2.2 | 2.8 | 1.81 | 1.3 | 2.6 | 2.2 |
| Lu | 0.36 | 0.37 | 0.42 | 0.48 | 0.35 | 0.43 | 0.30 | 0.19 | 0.43 | 0.36 |
| Hf | 3.8 | 3.7 | 4.8 | 4.5 | 3.8 | 3.9 | 2.7 | 2.4 | 3.7 | 3.3 |
| Ta | 0.7 | 0.6 | 0.9 | 0.7 | 0.6 | 0.6 | 0.51 | 0.2 | 0.3 | 0.3 |
| W | 3 | <1 | 225 | 1 | 1 | <1 | 166 | 5 | 2 | 4 |
| Tl | 0.3 | 0.4 | 0.7 | 0.2 | 0.4 | 0.4 | 0.31 | 0.8 | 0.7 | 0.7 |
| Pb | 480 | 28 | 12 | 7 | 630 | 9 | 7 | >10,000 | 2000 | >10,000 |
| Bi | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | 0.8 | <0.4 | <0.4 | <0.4 |
| Th | 9.4 | 8.9 | 10.1 | 9.4 | 8.8 | 8.9 | 6,0 | 4.3 | 6.9 | 5.8 |
| U | 2.3 | 2.1 | 2.6 | 2.3 | 2.3 | 2.4 | 1.49 | 1.3 | 2.2 | 1.9 |
| AI | 68.48 | 56.12 | 78.14 | 62.10 | 62.62 | 64.87 | 65.17 | |||
| CCPI | 28.18 | 36.63 | 46.33 | 57.15 | 35.58 | 54.27 | 58.14 | |||
| CIA | 68.22 | 67.59 | 63.92 | 59.58 | 63.97 | 67.06 | 64.84 | |||
| Zr/Y | 8.44 | 6.09 | 8.04 | 6.00 | 7.84 | 5.46 | 6.26 | 9.50 | 6.68 | 7.13 |
| Rb/Sr | 4.94 | 3.00 | 3.14 | 1.33 | 2.50 | 1.56 | 1.20 | |||
| Rb/Ba | 0.39 | 0.32 | 0.26 | 0.15 | 1.16 | 0.25 | 0.21 | |||
| ΣLREEs | 126.21 | 113.41 | 127.67 | 154.38 | 78.93 | 106.59 | 82.26 | |||
| ΣHREEs | 14.53 | 16.05 | 18.56 | 21.25 | 12.47 | 17.94 | 12.08 | |||
| ΣREEs | 140.74 | 129.46 | 146.23 | 175.63 | 91.4 | 124.53 | 94.35 | |||
| ΣLREEs/ΣHREEs | 8.69 | 7.07 | 6.88 | 7.26 | 6.33 | 5.94 | 6.81 | |||
| 1 (LaN/SmN) | 0.90 | 0.76 | 0.76 | 0.78 | 0.59 | 0.60 | 0.63 | |||
| 2 (SmN/YbN) | 1.06 | 1.00 | 0.98 | 1.11 | 0.88 | 0.96 | 1.12 | |||
| 3 Eu/Eu ** | 1.09 | 1.32 | 1.12 | 0.97 | 1.00 | 1.05 | 1.24 | |||
| 4 Eu/Eu ** | 1.05 | 1.27 | 1.06 | 0.93 | 0.97 | 1.02 | 1.23 | |||
| 5 Ce/Ce ** | 0.90 | 0.87 | 0.93 | 0.93 | 1.04 | 1.01 | 1.01 | |||
1 (La/LaN)/(Sm/SmN), 2 (Sm/SmN)/(Yb/YbN), 3 EuN/(0.66 SmN + 0.33 TbN), 4 EuN/(0.5 SmN + 0.5 GdN), 5 CeN/(0.5 LaN + 0.5 PrN), * Sulfide ore. ** REE normalizing values: Eu: 1.08; Sm: 5.55; Tb: 0.0774; Gd: 4.66; La: 38.2; Ce: 79.6; Pr: 8.83; Yb: 2.82; Lu: 0.433; Nb: 33.9.
The total REE abundance of the TVR aphanitic and porphyritic lavas ranges between ~50 and ~240 ppm (average of ~100 ppm ± 12 ppm), whereas for the volcaniclastic and pyroclastic rocks, it ranges between ~90 and ~170 ppm (average of ~125 ppm ± 8 ppm). The chondrite-normalized REE patterns of all samples are essentially sub-parallel, with moderate negative slopes for LREE and almost horizontal HREE segments (Figure 5a, Table 1 and Table 2; ΣLREE/ΣHREE = 5.2 to 8.8 for the lavas, and 4.8 to 8.6 for the volcaniclastic and pyroclastic rocks). The volcaniclastic and pyroclastic rocks display a minor negative Eu anomaly relative to the lavas, indicating fractionation of Eu during plagioclase crystallization, whereas no Ce anomaly is observed for all the analyzed host rocks (Figure 5, Table 1 and Table 2). The MORB-normalized spider diagrams display sub-parallel trends for all lithotypes, with enrichment in LILE and depletion in HFSE (Figure 5b). The Y/Ho ratio of the pyroclastic and volcaniclastic rocks range from ~22 to ~36, whereas the aphanitic and porphyritic lavas show a similar yet narrower range (~24 to ~32). All the features support the differentiation of a single enriched mantle source in a supra-subduction zone (SSZ) setting, during all stages of Tyros volcanism. Our geochemical data suggest that the partial melts relate to a clay-poor and plagioclase-rich source (e.g., CaO/Na2O = 3.11 ± 5.37) derived from ~70 to ~90% anatexis of CAB for Apidea (+Krokees) and Tyros lavas, and ~40 to ~70% CAB and shales for Molai host volcaniclastic and pyroclastic rocks (Figure 6a).
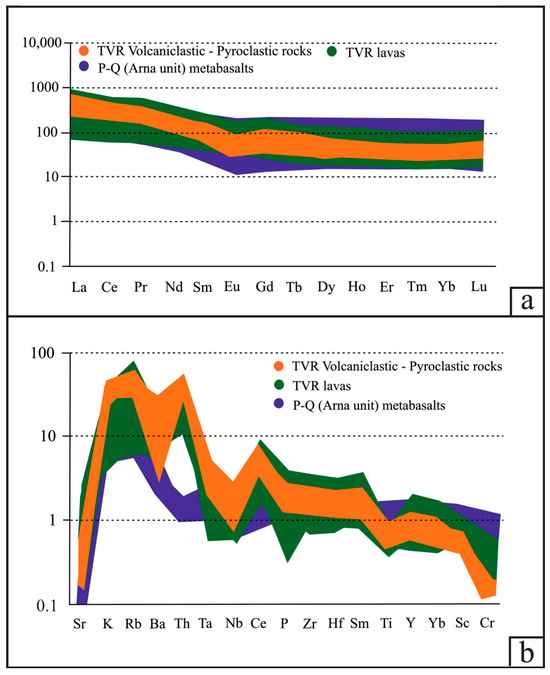
Figure 5.
Spider diagrams for the Tyros volcanic and volcaniclastic host rocks. (a) MORB normalized—normalizing values after Pearce [84]. (b) Chondrite normalized—normalizing values after Nakamura [85].
Figure 5.
Spider diagrams for the Tyros volcanic and volcaniclastic host rocks. (a) MORB normalized—normalizing values after Pearce [84]. (b) Chondrite normalized—normalizing values after Nakamura [85].
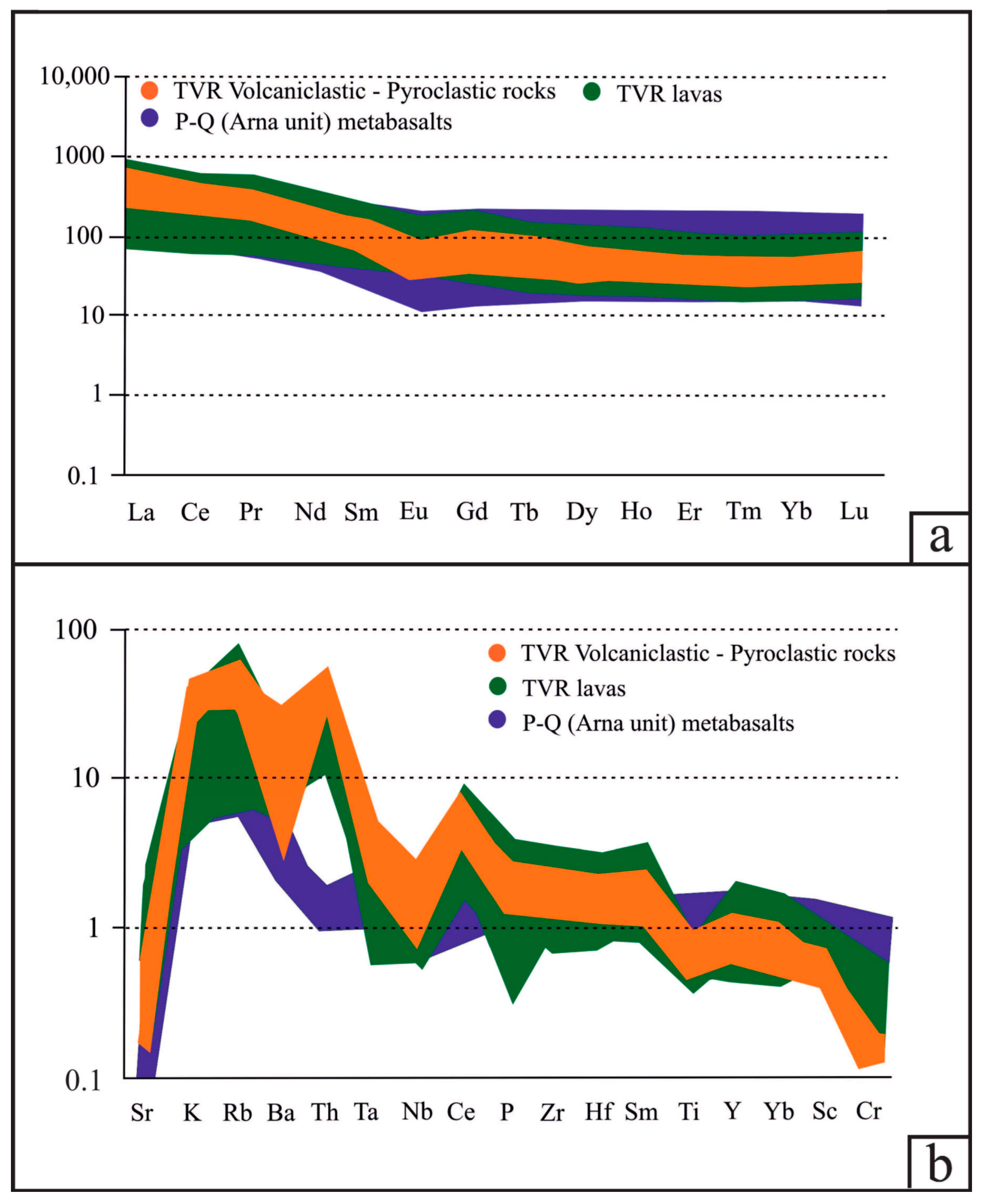
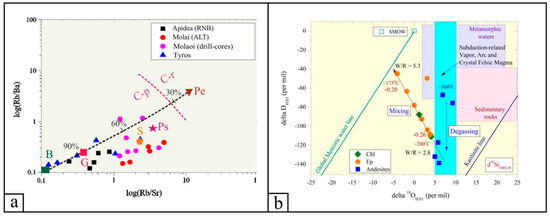
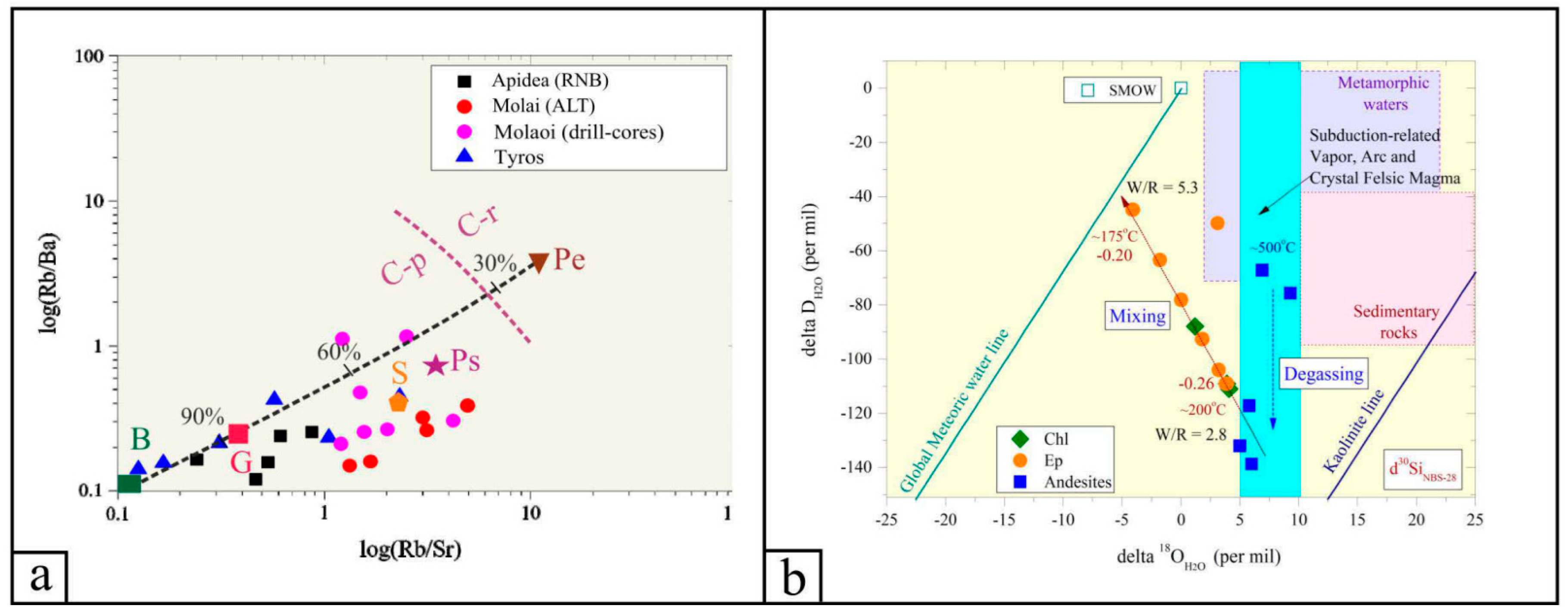
Figure 6.
(a) log(Rb/Sr) versus log(Rb/Ba) ratios for the volcanic, volcaniclastic and pyroclastic rocks of the TVR sequence. The dashed purple line separates magmatic rocks formed by anatexis of clay-poor with higher plagioclase and CaO/Na2O content source (C-p) in respect to those produced from clay-rich with lower plagioclase and CaO/Na2O content source (C-r). Modeling of the calculated compositions of pelite (Pe)- and psammite (Ps)-derived magmas, the average compositions of basalt (B), shale (S) and greywacke (G), and the calculated melt mixing curve (black dashed line) are also shown—after Sylvester [86] and Yiang et al. [87]. (b) Hydrogen versus oxygen isotope binary diagram of gangue phases from the Molai ore. The “Meteoric Water and Kaolinite Lines, Subduction-Related Vapor, Arc and Crystal Felsic Magma, I- and S-type magmas, Sedimentary rocks, Metamorphic water box, Formation waters and Primary magmatic water” are after Bowman [88] and Kuşcu et al. [89].
Figure 6.
(a) log(Rb/Sr) versus log(Rb/Ba) ratios for the volcanic, volcaniclastic and pyroclastic rocks of the TVR sequence. The dashed purple line separates magmatic rocks formed by anatexis of clay-poor with higher plagioclase and CaO/Na2O content source (C-p) in respect to those produced from clay-rich with lower plagioclase and CaO/Na2O content source (C-r). Modeling of the calculated compositions of pelite (Pe)- and psammite (Ps)-derived magmas, the average compositions of basalt (B), shale (S) and greywacke (G), and the calculated melt mixing curve (black dashed line) are also shown—after Sylvester [86] and Yiang et al. [87]. (b) Hydrogen versus oxygen isotope binary diagram of gangue phases from the Molai ore. The “Meteoric Water and Kaolinite Lines, Subduction-Related Vapor, Arc and Crystal Felsic Magma, I- and S-type magmas, Sedimentary rocks, Metamorphic water box, Formation waters and Primary magmatic water” are after Bowman [88] and Kuşcu et al. [89].
5.2. Alteration Petrography
Our study has focused on two different cross-sections, namely the RNB (between Gkagkania orebody and Apidea mineralization) and ALT (between Vigla and Gkagkania ores, Figure 1b). The hydrothermally altered pyroclastic rocks are primarily silicified and enveloped by extensive sericitization, as substantiated by the increased alkali content of the host tuffs adjacent to the ore deposits (Table 2). They are crosscut by veins filled by quartz, calcite-dolomite and marginal sericite. The tuffs display the same alteration patterns as the ashes, with the exception of tourmaline formation. They are crosscut by early barren quartz and late calcite ± dolomite veins, as well as mineralized quartz veins filled by pyrite, sphalerite and galena. The tuffs also appear intensively altered adjacent to the orebodies, with pervasive sericitization, followed by late carbonatization. They are also crosscut by stockworks with predominant quartz and/or minor calcite, dolomite, sericite, albite, disseminated pyrite and hematite (both hypogene and supergene).
In the ALT cross-section, the plagioclases of the base andesites are pseudomorphosed and replaced by sericite, and then albite, pyroxenes and hornblende are replaced by clinochlore. The groundmass comprises clinochlore, or clinochlore and chamosite, or hematite and chamosite, albite ± clay minerals, sericite and calcite. Clinochlore was formed during the metamorphism of the host rocks in the prehnite-pumpellyite facies [57], as chamosite replaces it. Sericite and chlorite, which belong to the celadonite, clinochlore and chamosite varieties (Figure S1, Table S3), and minor albite (Ab~90 to 95) replace the plagioclases and clinopyroxenes. They also fill open spaces, i.e., amygdaloids and stockworks comprising the assemblage subhedral-to-anhedral quartz, sericite, chlorite, epidote, calcite and albite ± actinolite that are crosscut by veinlets of clear quartz and galena. These veins and veinlets comprise quartz, barite, calcite, epidote, albite and sericite. Comb-textured clinochlore, albite and colloidal chalcedony are placed at the margins, whereas calcite, sericite and quartz occupy the median planes of the veins and amygdaloids. Groundmass plagioclase is replaced by sericite, prisms of epidote, and calcite (Figure 3d).
Two generations of hydrothermal quartz are recognized: (i) coarse-grained milky quartz (estimated at ~25 vol.%), mainly developed in the amygdaloids (with sizes of up to 2 mm), in hydraylic breccias and median parts of the veins intergrowing with subhedral medium-grained chlorite (ridipolite and pycnochlorite/brunsvigite), sericite (siderophyllite-to-celadonite) (both ~25 vol.%), magnetite, ilmenite (XIlm~90), and pyrite; and (ii) fine-grained clear quartz associated with galena (~20 vol.%) that intergrows with epidote and medium-to-coarse-grained anhedral calcite–dolomite–ankerite–siderite mixtures (~15 vol.%, that fill open spaces and veins with widths up to 10 cm, either with the assemblage chlorite-quartz-sulfides or barren). Epidote in these veins (Ps% between ~7 to ~54 and decreasing Ca/(Al + Fe3+) ratio from cores ~40 to rims ~35%) develops a negative trend in the Al3+ versus Ps% plot, indicating that trivalent Fe3+ substitutes bivalent Ca in the A-site (Figure S1).
5.3. Oxygen, Hydrogen and Silicon Isotopes of the Hosts
The bulk-rock δ18OV-SMOW values from the Krokees basalts and Molai host andesites range from +7.2 to +16.5 (+13.6 ± 3.7) per mil, whereas the ones from quartz, chlorite and epidote mineral separates range from +9.7 to +18.1 (+12.1 ± 7.3) per mil. The highest δ18OV-SMOW values were measured on clear quartz from the quartz–galena veinlets, in which the δ30SiNBS values range from −0.26 to −0.20 per mil. The δDV-SMOW values for bulk andesites and basalts range from ~−146 to −74, whilst those for epidote and chlorite range from ~−154 to −87 per mil (Table 3). The calculated isotope temperatures using the epidote–chlorite pair (e.g., sample MO17; Vigla site) range between ~155° and ~190 °C (Figure 6b). The calculated δ18OH2O values of the ore fluid in equilibrium with the host andesites and clear quartz are between +6.9 and +16.2, and +4.1 ± 0.2 per mil. The calculated δ18OH2O and δDH2O values of the ore fluid in equilibrium with epidote and chlorite are between −4.1 and +3.9, and +1.2 and +4.1 per mil, and −109 and −41, and −111 and −88 per mil, respectively (T = 175 °C, Table 3). The water-to-rock ratios for the host andesites rose from 2.8 to 5.3 (between T = 200° and 175 °C, Figure 6b).

Table 3.
Oxygen, hydrogen and silicon isotopes obtained from the TVR basalts and andesites, and chlorite, epidote and clear quartz from the Molai Zn-Pb±(Ag,Ge) deposit. Samples MO11, MO17 and MO19 are surface samples from the Vigla site (Figure 1b). Please refer to Table S1a for sample locations.
| Sample | Mineral | δ18OV-SMOW (per Mil) | δ18OH2O (per Mil) | δDV-SMOW (per Mil) | δDH2O (per Mil) | δ30SiNBS-28 (per Mil) |
|---|---|---|---|---|---|---|
| MO19D0H3 | Chlorite | 17.9 | 4.1 | −154 | −111 | - |
| MO11D0H | Chlorite 1 | 15 | 1.2 | −130 | −87.9 | - |
| MO17B0H | Chlorite 1 | 17.7 | 3.9 | −152 | −109 | - |
| MO17D0H2 | Epidote | 17.6 | 3.8 | −151 | −109 | - |
| MO11D0H4 | Epidote 1 | 17.7 | 3.9 | −152 | −109 | - |
| MO11D0H2 | Epidote | 15.6 | 1.8 | −135 | −92.6 | - |
| MO11D0H6 | Epidote | 16.9 | 3.1 | −92.2 | −49.8 | - |
| MO17B0H | Epidote 1 | 17 | 3.2 | −146 | −104 | - |
| MO17D0H | Epidote | 13.8 | 0 | −121 | −78.1 | - |
| MO19D0H5 | Epidote | 9.7 | −4.1 | −87.2 | −44.8 | - |
| MO11D0H3 | Epidote | 12 | −1.8 | −106 | −63.4 | - |
| KR1201 | Clear Quartz | 18 | 4.2 | - | - | −0.26 |
| KR1202 | Clear Quartz | 17.7 | 3.9 | - | - | −0.23 |
| KR1203 | Clear Quartz | 18.1 | 4.3 | - | - | −0.20 |
| MO19 | Andesite 2 | 14.4 | 14.1 | - | - | - |
| MO19 | Andesite | 16.5 | 16.2 | - | - | - |
| MO19 | Andesite | 16.3 | 6.0 | −145.7 | −138.7 | - |
| KR1 | Basalt | 15.3 | 5.0 | −139.0 | −132 | - |
| KR1 | Basalt | 16.1 | 5.8 | −124.2 | −117.2 | - |
| KR1 | Basalt | 7.2 | 6.9 | −74.2 | −67.2 | - |
| KR1 | Basalt | 9.6 | 9.3 | −82.7 | −75.7 | - |
1 Temperatures obtained from the epidote–chlorite pair range between T ~155° and T ~190 °C (e.g., sample MO17; Vigla site), utilizing the chlorite- and epidote–water equations of Zhao and Zheng (2003) [90] and the hydrogen fractionation factors of De Hoog et al. (2009) [91]. 2 εHf = −7.9 to −6.2.
5.4. Ore Texture, Mineralogy and Chemistry
5.4.1. Ore Types and Textures
In the Molai area, two major ore types are distinguished based on ore mineralogy, texture and host rock lithology:
- A Cu-Fe±(Pb,Zn) mineralization exposed in Apidea and Krokees, with predominant pyrite ((Cu + Ni + Co)/Fe% = 1.74 ± 0.59) and chalcopyrite ((Ni + Co + Ta)/(Fe + Cu)% = 1.05 ± 0.1), minor hematite and magnetite and traces of bornite and sphalerite, hosted in the basaltic andesite lavas. The mineralization is syngenetic to the host lavas and is predominantly disseminated as veinlets and lesser within the hydrothermally altered lavas and has no economic importance. According to Melidonis and Constantinides [53], the veins crosscut the primary Cu-Fe disseminated ore and, besides pyrite and chalcopyrite, they also contain minor galena and sphalerite.
- A Zn-Pb±(Ag,Ge) bedded and massive-to-semi-massive epigenetic sulfide ore with predominant sphalerite, galena and pyrite, hosted in tuffs. This ore type continues to be the main focus of the exploration target in the area [29,53]. The stratiform sulfide ore is both meso- and micro-layered, particularly between the major ore phases, e.g., galena, sphalerite and pyrite.
Three major ore-texture styles are identified in the stratabound epigenetic Molai Zn-Pb±(Ag,Ge) ore, including:
- Bedded, massive-to-semi-massive epigenetic sulfide ore developed along the layering of the host tuffs and ashes with predominant sphalerite, galena and chalcopyrite, and subordinate pyrite, tetrahedrite and traces of arsenopyrite (Figure 7a,b). In mega-scale, the thickness of the orebodies varies greatly and ranges between a few cm to ~15 m;
- Clastic, massive-to-semi-massive epigenetic ore where massive sphalerite and galena are hosted in fine-grained volcaniclastic rocks after erosion of the formerly deposited tuffs and ashes, and the fine-grained ore phases cement the tuff and ash fragments;
- Hydaylic breccias of tuffs and ashes cemented with chalcopyrite, sphalerite, galena and minor pyrite. The sulfide phases are fine-to-coarse-grained with milky quartz and calcite as gangue (Figure 7c). These breccias appear chaotic, massive, matrix-supported and poorly sorted. Breccia fragments are angular-to-subrounded and of different sizes (up to several μm) with jigsaw and open space filling textures. The ore minerals are commonly concentrated at clast rims.
Moreover, a vein-type ore is developed, forming mainly veinlets and, to a lesser extent, stockworks comprising clear quartz, calcite and minor chlorite and epidote with predominant galena. This ore type is associated with a later hydrothermal and ore-forming event following the earlier massive-to-semi-massive ore, and crosscuts the host tuffs and ashes, as well as the overlying andesitic flows (Figure 7d).
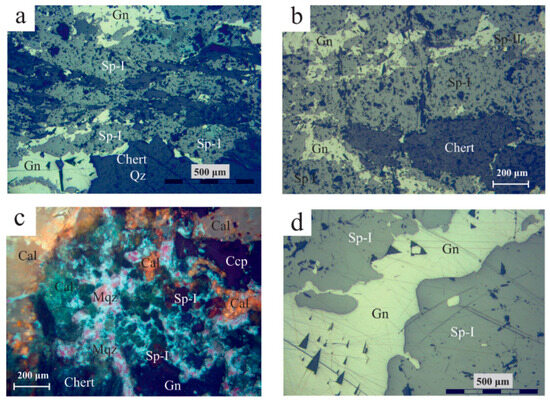
Figure 7.
Reflected light optical microscopy photos of Molai ore textures. (a,b) Bedded ore with bands of sphalerite (Sp-I variety) and galena (Gn) following the host tuff bedding (plain polars). (c). Sulfide breccia with sphalerite (Sp I), chalcopyrite (Ccp) and galena (Gn), cemented by milky quartz (Mqz) and fine-to-coarse-grained calcite (Cal) (crossed polars). (d) Vein-type ore (stage III) with late-stage galena (Gn) cutting and replacing early sphalerite (Stage I–Sp-I) (plain polars). Abbreviations after Whitney and Evans [92].
Figure 7.
Reflected light optical microscopy photos of Molai ore textures. (a,b) Bedded ore with bands of sphalerite (Sp-I variety) and galena (Gn) following the host tuff bedding (plain polars). (c). Sulfide breccia with sphalerite (Sp I), chalcopyrite (Ccp) and galena (Gn), cemented by milky quartz (Mqz) and fine-to-coarse-grained calcite (Cal) (crossed polars). (d) Vein-type ore (stage III) with late-stage galena (Gn) cutting and replacing early sphalerite (Stage I–Sp-I) (plain polars). Abbreviations after Whitney and Evans [92].
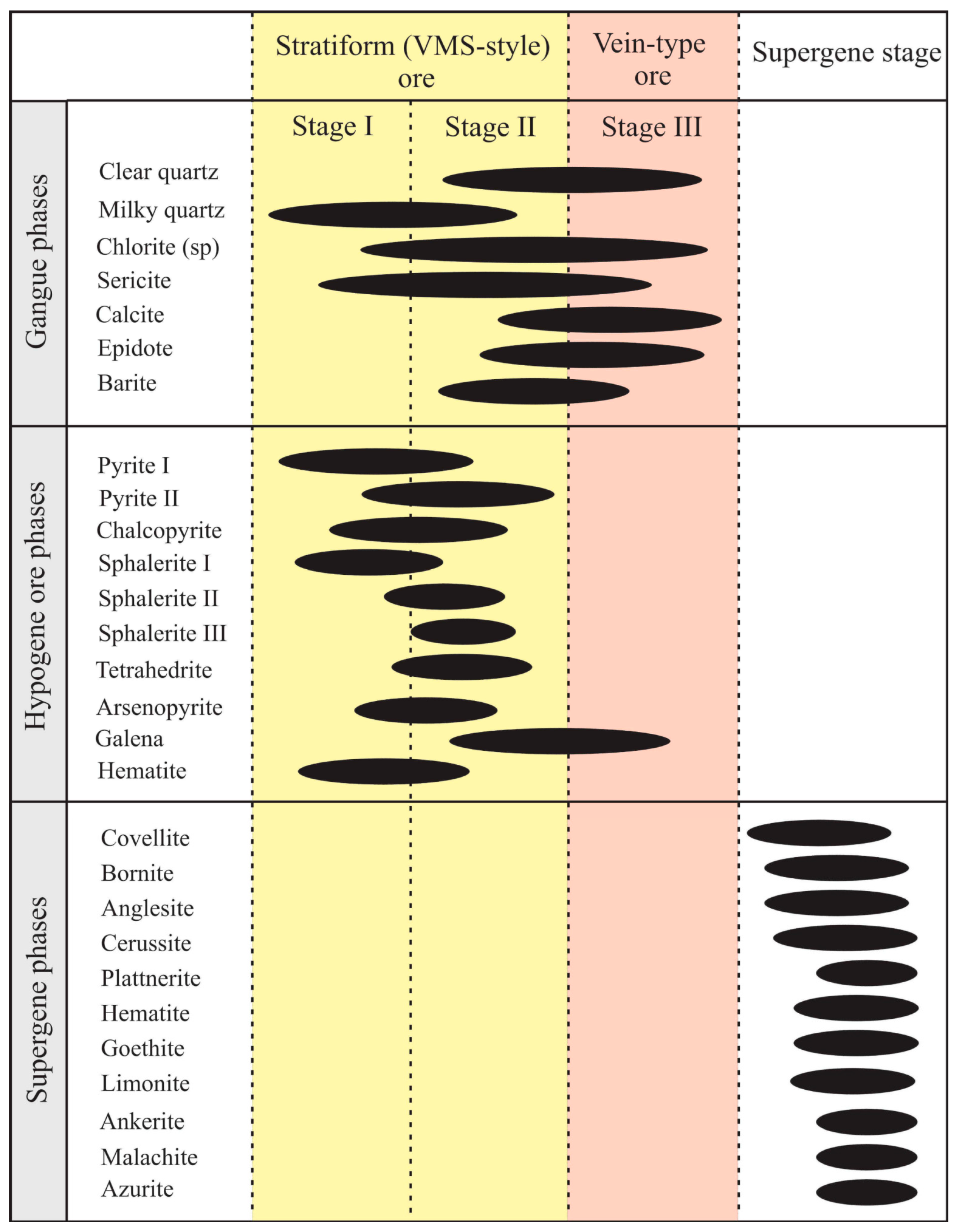
Based on the aforementioned ore textures identified, three main ore-forming stages are distinguished in the Molai Zn-Pb±(Ag,Ge) ore (Figure 8). Stages I and II are associated with the formation of the massive-to-semi-massive, stratiform VMS-style mineralization, while stage III is associated with the vein-type ore after fracturing of the hydrothermal system, which is expressed as quartz–galena veins and veinlets that crosscut the host tuffs, ashes and overlying lavas. The three hypogene stages are followed by a later supergene stage. The paragenesis of each mineralization stage are as follows (refer to Section 6.1 for details):
- Stage I: Pyrite (Py-I) + sphalerite (Sp-I) + chalcopyrite + arsenopyrite ± pyrrhotite-magnetite-hematite;
- Stage II: Sphalerite (Sp-II) + sphalerite (Sp-III) + tetrahedrite + pyrite (Py-II) + galena;
- Stage III: Galena.
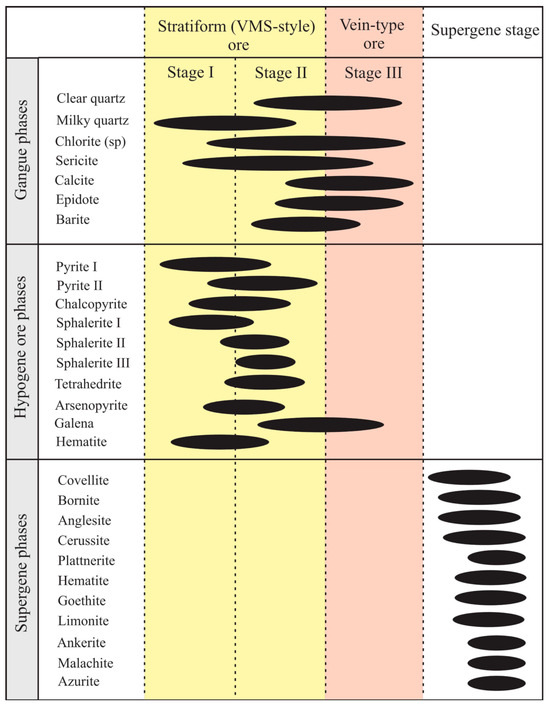
Figure 8.
Paragenetic sequence of the Molai Zn-Pb±(Ag,Ge) ore and the corresponding stages.
Figure 8.
Paragenetic sequence of the Molai Zn-Pb±(Ag,Ge) ore and the corresponding stages.
5.4.2. Ore Mineralogy and Geochemistry
The main ore minerals of the Molai Zn-Pb±(Ag,Ge) deposit are sphalerite, galena, pyrite and chalcopyrite, followed by minor tetrahedrite and traces of arsenopyrite. Sphalerite predominates in the Molai Zn-Pb±(Ag,Ge) ores, with three different varieties (Sp-I, Sp-II and Sp-III) distinguished based on reflected-light optical petrography [93] and ore chemistry (Table 4 and Table S5, Figure 9):
- Sp-I, a Fe-rich variety of orange-red internal reflections (Figure 9) with XFeS molar, Fe/Zn and Zn/S atomic ratios of 20.5 ± 2.7, 0.229 ± 0.04 and 0.79 ± 0.06, respectively. Sp-I is the most abundant among the three varieties (≥30 vol.% in the ore) and forms masses of euhedral-to-subhedral, coarse grained crystals (sizes up to 500 μm) that follow the layering of the host tuffs and ashes. In many cases, coarse-grained Sp-I appears fragmented and develops a typical cataclastic texture (Figure 9e,f). The Sp-I fragments are cemented by stage II Sp-II, fine-grained pyrite (Py-II) and tetrahedrite, and is replaced by stage III galena,
- Sp-II, a low-Fe variety of yellow internal reflections (Figure 9a–d) with XFeS molar, Fe/Zn and Zn/S atomic ratios at 6.3 ± 1.7, 0.089 ± 0.03 and 0.91 ± 0.02, respectively. Sp-II is the second most abundant variety (<5 vol.% in the ore) and follows, or occasionally replaces, Sp-I. Sp-II occurs as disseminated euhedral-to-subhedral medium-grained crystals (sizes up to 200 μm), or in the interstices and boundaries of Sp-I crystals and fragments. Similarly to Sp-I, Sp-II forms masses that follow the layering of the host tuffs and ashes. Sp-II also appears cataclastic and is replaced by stage III galena,
- Sp-III, a very low-Fe variety of white internal reflections (Figure 9a–d) with XFeS molar, Fe/Zn and Zn/S atomic ratios at 0.5 ± 0.1, 0.005 ± 0.001 and 0.97 ± 0.01, respectively. Sp-III is a trace constituent of the Molai ore and follows the formation of Sp-I and Sp-II. Sp-III forms fine-grained, euhedral crystals (sizes between 10 μm to 30 μm) along the planar edges of Sp-I and Sp-II.

Table 4.
Representative EPMA analyses of sphalerite varieties from the Molai Zn-Pb±(Ag,Ge) sulfide ore (results in wt%). Samples are from historical drill cores B7, B14, B25, B26 and B56 (please refer to Table S1b for details).
Table 4.
Representative EPMA analyses of sphalerite varieties from the Molai Zn-Pb±(Ag,Ge) sulfide ore (results in wt%). Samples are from historical drill cores B7, B14, B25, B26 and B56 (please refer to Table S1b for details).
| Sp-I | Sp-I | Sp-I | Sp-II | Sp-II | Sp-II | Sp-III | Sp-III | Sp-III | |
|---|---|---|---|---|---|---|---|---|---|
| Fe | 11.04 | 11.11 | 11.68 | 4.68 | 4.80 | 4.81 | 0.25 | 0.31 | 0.3 |
| As | 0.04 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ag | n.d. | n.d. | n.d. | 0.01 | 0.01 | 0.01 | n.d. | n.d. | n.d. |
| Te | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Sb | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Cu | 0.01 | n.d. | n.d. | n.d. | 0.01 | n.d. | n.d. | n.d. | n.d. |
| Zn | 55.10 | 55.25 | 52.12 | 61.68 | 61.37 | 61.52 | 66.55 | 66.82 | 66.21 |
| Au | 0.01 | n.d. | 0.01 | n.d. | n.d. | n.d. | n.d. | 0,01 | n.d. |
| Se | n.d. | n.d. | 0.02 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Co | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ni | n.d. | n.d. | 0.020 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Cd | 0.23 | 0.25 | 0.26 | 0.11 | 0.12 | 0.2 | 0.26 | 0.26 | 0.25 |
| Bi | 0.02 | 0.02 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Sn | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Mn | 0.27 | 0.18 | 0.16 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ga | n.d. | n.d. | 0.02 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| In | n.d. | 0.02 | 0.02 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ge | 0.01 | 0.01 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| S | 33.46 | 33.44 | 33.09 | 33.38 | 33.63 | 33.54 | 33.46 | 33.61 | 33.82 |
| Total | 100.19 | 100.28 | 97.39 | 99.85 | 99.94 | 100.07 | 100.53 | 100.99 | 100.57 |
| Atoms per formula unit (apfu) | |||||||||
| S | 0.998 | 0.997 | 1.01 | 1.006 | 1.011 | 1.008 | 1.009 | 1.009 | 1.017 |
| Cu | − | − | − | − | − | − | − | − | − |
| Fe | 0.189 | 0.190 | 0.205 | 0.081 | 0.083 | 0.083 | 0.004 | 0.005 | 0.005 |
| As | 0.001 | − | − | − | − | − | − | − | − |
| Ag | − | − | − | − | − | − | − | − | − |
| Te | − | − | − | − | − | − | − | − | − |
| Sb | − | − | − | − | − | − | − | − | − |
| Zn | 0.806 | 0.808 | 0.78 | 0.912 | 0.905 | 0.907 | 0.984 | 0.984 | 0.976 |
| Au | − | − | − | − | − | − | − | − | − |
| Se | − | − | − | − | − | − | − | − | − |
| Co | − | − | − | − | − | − | − | − | − |
| Ni | − | − | 0.001 | − | − | − | − | − | − |
| Cd | 0.002 | 0.002 | 0.002 | 0.001 | 0.001 | 0.002 | 0.002 | 0.002 | 0.002 |
| Bi | − | − | − | − | − | − | − | − | − |
| Sn | − | − | − | − | − | − | − | − | − |
| Mn | 0.005 | 0.003 | 0.003 | − | − | − | − | − | − |
| Ga | − | − | − | − | − | − | − | − | − |
| In | − | − | − | − | − | − | − | − | − |
| Ge | 0.001 | 0.001 | 0.001 | − | − | − | − | − | − |
| TOTAL | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Zn/S | 0.808 | 0.810 | 0.772 | 0.906 | 0.895 | 0.900 | 0.975 | 0.975 | 0.960 |
| Fe/Zn | 0.235 | 0.235 | 0.262 | 0.089 | 0.092 | 0.092 | 0.004 | 0.005 | 0.005 |
| XFeS | 0.190 | 0.191 | 0.208 | 0.082 | 0.083 | 0.084 | 0.004 | 0.005 | 0.005 |
n.d. not detected
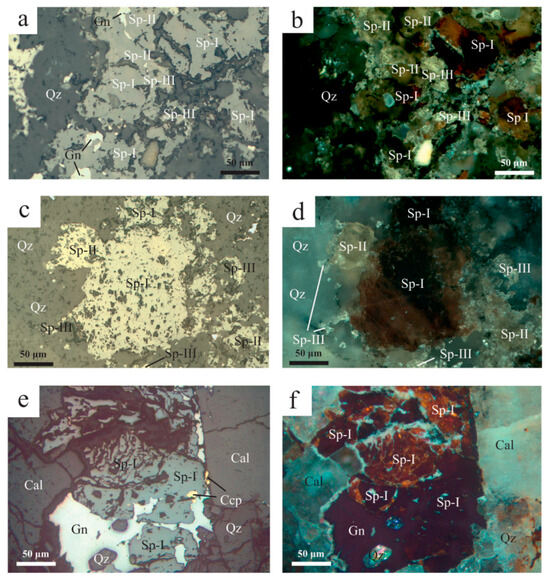
Figure 9.
Sphalerite varieties of Molai sulfide ore. (a–d) Sp-I (high Fe) is an early sulfide phase and predates Sp-II (low Fe) and Sp-III (very low Fe), forming coarse-grained, euhedral-to-subhedral crystals. Sp-II and Sp-III varieties follow the deposition of Sp-I, forming finer-grained euhedral-to-subhedral crystals at the planar edges of Sp-I. (e,f) Cataclastic texture of early Sp-I with galena (Gn) and chalcopyrite (Ccp) in veinlet associated with fine-grained quartz (Qz) and coarse-grained calcite (Cal). Abbreviations after Whitney and Evans [92].
Figure 9.
Sphalerite varieties of Molai sulfide ore. (a–d) Sp-I (high Fe) is an early sulfide phase and predates Sp-II (low Fe) and Sp-III (very low Fe), forming coarse-grained, euhedral-to-subhedral crystals. Sp-II and Sp-III varieties follow the deposition of Sp-I, forming finer-grained euhedral-to-subhedral crystals at the planar edges of Sp-I. (e,f) Cataclastic texture of early Sp-I with galena (Gn) and chalcopyrite (Ccp) in veinlet associated with fine-grained quartz (Qz) and coarse-grained calcite (Cal). Abbreviations after Whitney and Evans [92].
Sphalerite comprises low Cd content (max. 0.003 apfu for all varieties). Sp-I incorporates traces of Ge, whereas Sp-II and Sp-III are barren. The Cu content is very low in all sphalerite varieties, with the Fe-rich sphalerite presenting the highest content (max. 0.05 apfu, average 0.001 apfu). Traces of Au and Ag are found in all sphalerite varieties, whereas Bi and Te are detected exclusively in Sp-I (Table 4 and Table S5).
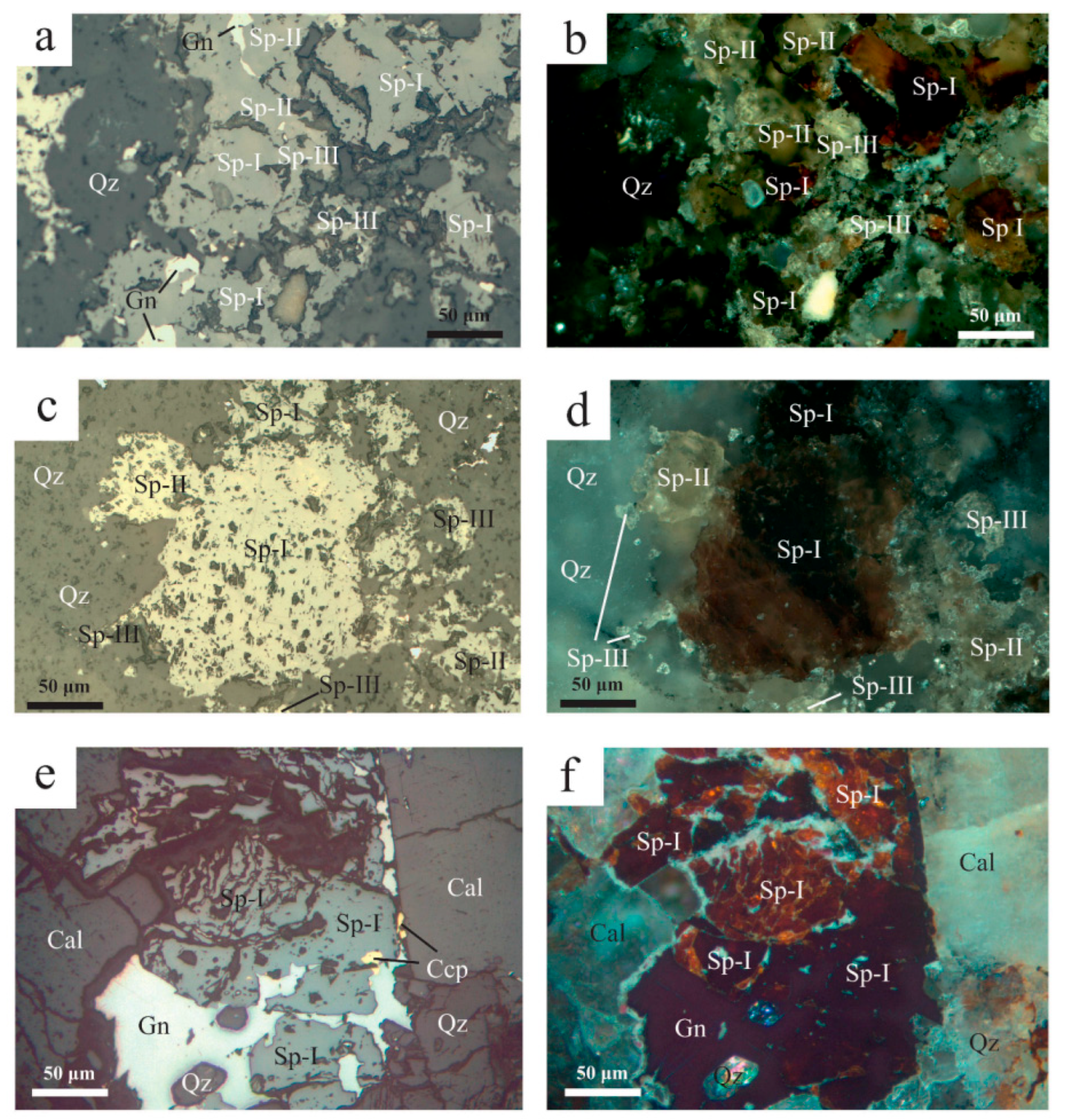
Galena is the second most abundant ore phase in the Molai Zn-Pb±(Ag,Ge) ore. Galena is coarse-grained and anhedral, and associated with the later stages of the Molai paragenesis, replacing early chalcopyrite, sphalerite (Sp-I and Sp-II) and tetrahedrite (Figure 10a,b,d,e). Galena incorporates Zn (up to 0.12 apfu), Cu (up to 0.01 apfu) and Fe (up to 0.008). It shows very limited substitution of sulfur by As (up to 0.002 apfu) and less by Te and Se (up to 0.001 apfu) (Table S5). At the uppermost and exposed to surface weathering part of the sulfide ore, galena was subjected to supergene weathering to anglesite, cerussite and plattnerite.
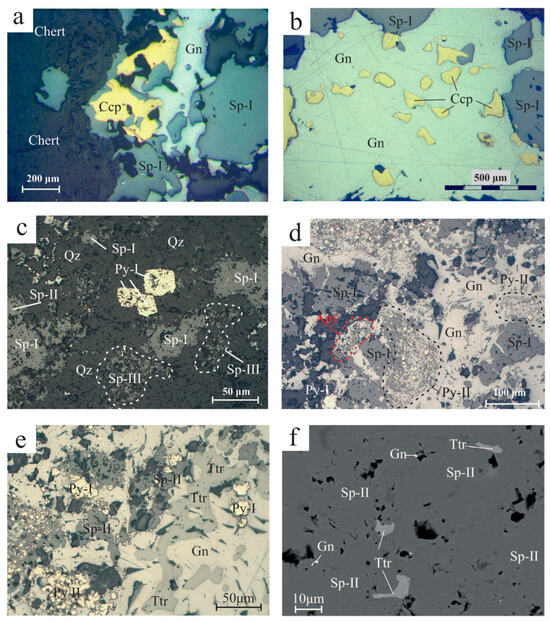
Figure 10.
Reflected light optical microscopy and back-scattered electron microscopy photos from Molai ore. (a) Sphalerite (Sp-I) replaced by chalcopyrite (Ccp) and late-stage galena (Gn) replacing both aforementioned phases. (b) Advanced replacement of sphalerite (Sp-I) and chalcopyrite (Ccp) by galena (Gn). (c) Stage I coarse-grained, euhedral pyrite (Py-I) in quartz groundmass and intergrown with Sp-I, Sp-II and Sp-III. (d) Massive sulfide ore with predominant galena (Gn) and swarms of fine-grained pyrite (Py-II). Sphalerite (Sp-I) appears brecciated and replaced by late-stage galena. Masses of fine-grained euhedral arsenopyrite (Apy) are also developed. (e) Anhedral tetrahedrite (Ttr) after replacement by late-stage galena (Gn). Minor sphalerite (Sp-II) and pyrite (Py-I and Py-II) are also present. Sphalerite shows signs of replacement by late-stage galena (rounded crystals). Pyrite forms euhedral grains either as isolated crystals (Py-I), or aggregates (Py-II). (f) Tetrahedrite (Ttr) and galena (Gn) inclusions in sphalerite (Sp-II). Abbreviations after Whitney and Evans [92].
Figure 10.
Reflected light optical microscopy and back-scattered electron microscopy photos from Molai ore. (a) Sphalerite (Sp-I) replaced by chalcopyrite (Ccp) and late-stage galena (Gn) replacing both aforementioned phases. (b) Advanced replacement of sphalerite (Sp-I) and chalcopyrite (Ccp) by galena (Gn). (c) Stage I coarse-grained, euhedral pyrite (Py-I) in quartz groundmass and intergrown with Sp-I, Sp-II and Sp-III. (d) Massive sulfide ore with predominant galena (Gn) and swarms of fine-grained pyrite (Py-II). Sphalerite (Sp-I) appears brecciated and replaced by late-stage galena. Masses of fine-grained euhedral arsenopyrite (Apy) are also developed. (e) Anhedral tetrahedrite (Ttr) after replacement by late-stage galena (Gn). Minor sphalerite (Sp-II) and pyrite (Py-I and Py-II) are also present. Sphalerite shows signs of replacement by late-stage galena (rounded crystals). Pyrite forms euhedral grains either as isolated crystals (Py-I), or aggregates (Py-II). (f) Tetrahedrite (Ttr) and galena (Gn) inclusions in sphalerite (Sp-II). Abbreviations after Whitney and Evans [92].
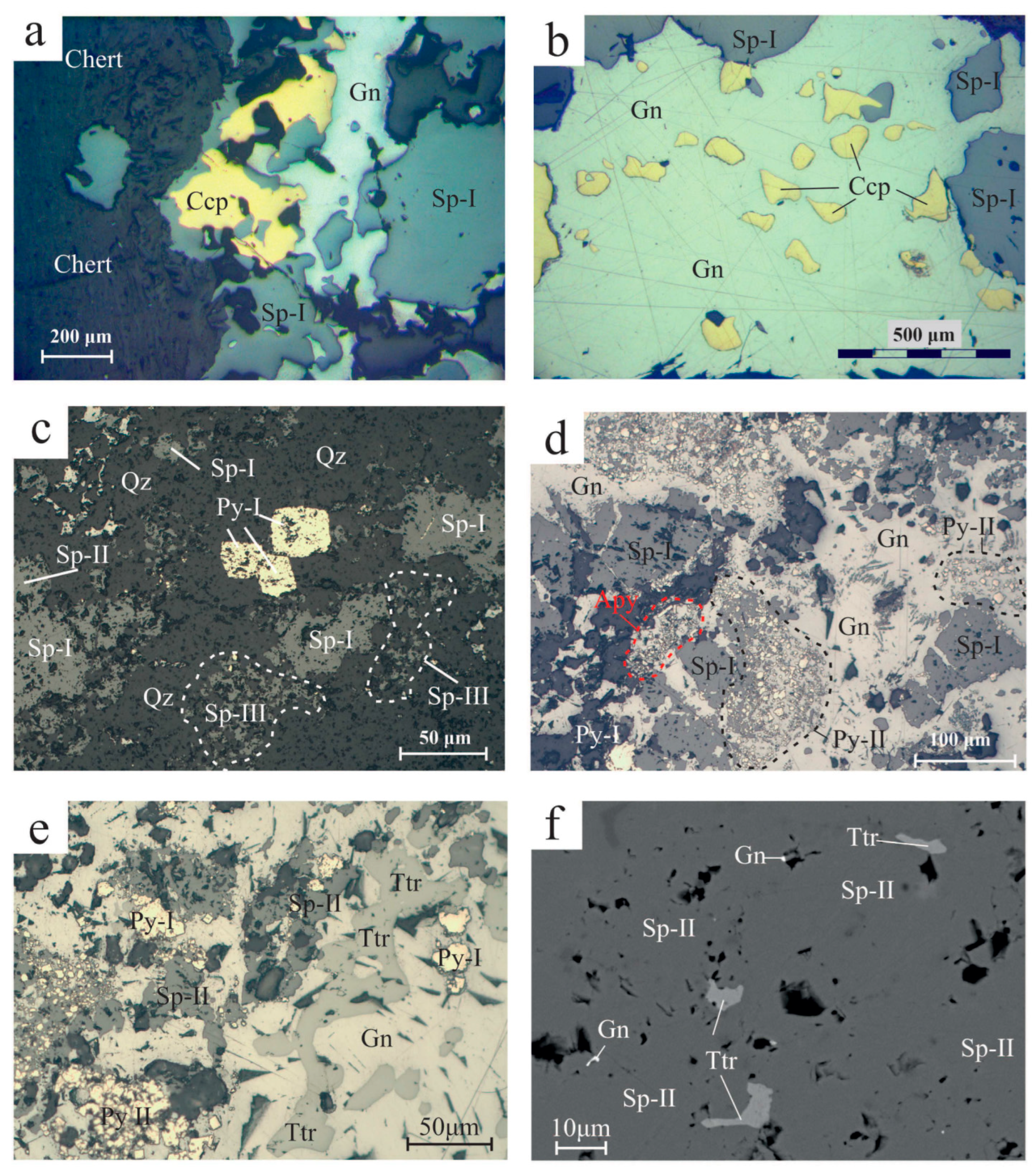
Pyrite is the third most abundant sulfide phase after sphalerite and galena in the MolaiZn-Pb±(Ag,Ge) ore. Two generations of pyrite are distinguished: an early euhedral-to-subhedral, coarse-grained type (Py-I with sizes of ~ 300 μm, Figure 10c,e) forming disseminations and aggregates, and a later euhedral-to-subhedral, fine-grained variety (Py-II, sizes of ~10 to 30 μm) forming swarms, clusters and aggregates associated with late galena. Py-II replaces both Sp-I and Sp-II (Figure 10d,e). Py-II disseminations are frequent in the host tuffs and ashes in distance from the orebodies and are genetically associated to their sericitization. Moreover, substitution of Fe by Cu, Zn and Ni is common, although traces of Mn, Ag and Au were also detected (Table 5 and Table S5). Py-I comprises Cu (up to 0.02 apfu) and traces of Pb, Co, Au, Te, Ag, As and Ni (≤~430 ppm). It displays Fe/S, (Ag + Cu + Zn + Cd + Mn)/Fe%, (Zn + Cd)/Fe% and (As + Sb + Te + Se)/S% ratios of 0.51 ± 0.1, 3.8 ± 5.6 and 5.8 ± 10.2, respectively.

Table 5.
Representative EPMA analyses of sulfides and sulfosalts from the Molai Zn-Pb±(Ag,Ge) ore (results in wt %). Samples are from historical drill cores B7, B14, B25, B26 and B56 (please refer to Table S1b for details).
Table 5.
Representative EPMA analyses of sulfides and sulfosalts from the Molai Zn-Pb±(Ag,Ge) ore (results in wt %). Samples are from historical drill cores B7, B14, B25, B26 and B56 (please refer to Table S1b for details).
| Py-I | Py-I | Py-II | Ttr | Ttr | Ttr | Ccp-0 | Ccp | Gn | Gn | Gn | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | 47.06 | 46.88 | 47.01 | 4.01 | 4.01 | 3.99 | 31.89 | 30.67 | 0.03 | 0.05 | 0.03 |
| As | 0.01 | 0.01 | 0.05 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ag | n.d. | n.d. | 0.01 | 3.37 | 3.28 | 3.48 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Pb | n.d. | n.d. | n.d. | 0.03 | n.d. | n.d. | n.d. | n.d. | 86.57 | 86.24 | 86.93 |
| Te | n.d. | n.d. | 0.01. | 0.04 | 0.05 | 0.02 | n.d. | 0.15 | n.d. | n.d. | n.d. |
| Sb | n.d. | n.d. | n.d. | 28.57 | 28.95 | 28.81 | n.d. | n.d. | n.d. | 0,01 | n.d. |
| Cu | 0.02 | 0.02 | 0.01 | 34.32 | 34.34 | 34.03 | 32.97 | 33.43 | 0.02 | n.d. | 0.06 |
| Zn | n.d. | n.d. | 0.01 | 4.91 | 5.02 | 5.43 | n.d. | 0.02 | 0.05 | 0.01 | 0.01 |
| Au | n.d. | n.d. | 0.01 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.02 | 0.02 |
| Co | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.21 | 0.02 | n.d. | n.d. | n.d. |
| Ni | 0.01 | n.d. | 0.01 | n.d. | n.d. | n.d. | 0.35 | n.d. | n.d. | n.d. | n.d. |
| Cd | 0.01 | 0.02 | 0.04 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Bi | n.d. | n.d. | 0.01 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.014 | n.d. | n.d. |
| Mn | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ga | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| In | 0.01 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ge | 0.03 | 0.05 | 0.01 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.04 | 0.04 | 0.04 |
| Ta | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.38 | n.d. | n.d. | n.d. | n.d. |
| S | 53.25 | 53.25 | 52.80 | 25.1 | 25.18 | 25.04 | 33.97 | 35.43 | 13.22 | 13.37 | 13.39 |
| Total | 100.39 | 100.22 | 100.33 | 100.36 | 100.83 | 100.79 | 99.77 | 99.72 | 99.95 | 99.74 | 100.47 |
| Atoms per formula unit (apfu) | |||||||||||
| S | 1.989 | 1.992 | 1.994 | 13.074 | 13.066 | 13.014 | 2.00 | 2.025 | 0.990 | 0.999 | 0.995 |
| Cu | − | − | − | 9.023 | 8.992 | 8.927 | 1.00 | 0.964 | 0.001 | − | 0.002 |
| Fe | 1.009 | 1.007 | 1.003 | 1.200 | 1.195 | 1.192 | 0.99 | 1.007 | 0.001 | 0.002 | 0.001 |
| As | − | − | 0.001 | − | − | − | − | − | − | − | − |
| Ag | − | − | − | 0.522 | 0.506 | 0.538 | − | − | − | − | − |
| Pb | − | − | − | − | − | − | − | − | 1.004 | 0.997 | 0.999 |
| Te | − | − | − | 0.006 | 0.006 | 0.002 | − | 0.002 | − | − | − |
| Sb | − | − | − | 3.919 | 3.956 | 3.943 | − | − | − | − | − |
| Zn | − | − | − | 1.254 | 1.278 | 1.383 | − | − | 0.002 | − | − |
| Au | − | − | − | − | − | − | − | − | − | − | − |
| Co | − | − | − | 0.001 | 0.001 | − | − | 0.001 | − | − | − |
| Ni | − | − | − | 0.001 | − | − | 0.01 | − | − | − | − |
| Cd | − | − | 0.001 | − | − | − | − | − | − | − | − |
| Bi | − | − | − | − | − | − | − | − | − | − | − |
| Mn | − | − | − | − | − | − | − | − | − | − | − |
| Ga | − | − | − | − | − | − | − | − | − | − | − |
| In | − | − | − | − | − | − | − | − | − | − | |
| Ge | − | 0.001 | 0.001 | − | − | − | − | 0.001 | 0.001 | 0.001 | |
| TOTAL | 3 | 3 | 3 | 29 | 29 | 29 | 4 | 4 | 2 | 2 | 2 |
| (Cu + Ag + Au) TR: | 6.00 | 6.00 | 6.00 | ||||||||
| Cu TET: | 3.54 | 3.50 | 3.46 | ||||||||
| (Fe + Zn) TET: | 2.45 | 2.47 | 2.57 | ||||||||
| As + Sb + Se: | 3.92 | 3.96 | 3.94 | ||||||||
| S + Te: | 13.08 | 13.07 | 13.02 | ||||||||
Py—pyrite; Ttr—tetrahedrite; Ccp—chalcopyrite; Gn—galena (abbreviations after Whitney and Evans [92]. Ccp-0: Apidea mineralization. Py-I, Py-II, Ccp, Ttr and gn: Molai (Vigla) mineralization. n.d. not detected.
Chalcopyrite is a subordinate ore phase in the Molai Zn-Pb±(Ag,Ge) ore, and occurs in stage I. Chalcopyrite either forms masses or disseminations of medium-grained anhedral crystals (sizes up to 150 μm), replacing sphalerite (Sp-I), and then is replaced by galena (Figure 10a,b). Chalcopyrite incorporates Zn (up to 0.002 apfu) and Cd (up to 0.007 apfu) and traces of Co, Ag, Au and Te (Table 5 and Table S5).
Tetrahedrite is subordinate in the Molai deposit and forms two populations. The predominant population involves subhedral-to-anhedral and medium-grained tetrahedrite (sizes up to 150 μm) forming aggregates and clusters in close association with galena and sphalerite (Sp-I and Sp-II varieties). Tetrahedrite succeeds Sp-I and Sp-II and shows signs of dissolution from later galena (e.g., rounded planes, Figure 10e). The second population is subordinate and includes fine-grained tetrahedrite filling cracks in fragmented coarse-grained Sp-I. Moreover, tetrahedrite is found as fine inclusions (less than 10 μm in diameter) in Sp-II (Figure 10f). It is a pure tetrahedrite end-member with (Sb/(Sb + As)% = 100, although Grossou-Valta et al. [27] have reported an intermediate end-member with Sb/(Sb + As)% = 0.4. Tetrahedrite is the main Ag carrier in the Molai ore, with Ag being significantly incorporated in the tetrahedrite structure, reaching up to 0.5 apfu (~3 wt %). Tetrahedrite also contains Zn (up to 1.4 apfu), Fe (up to 1.2 apfu) and traces of Te (up to 0.008 apfu) (Table 5 and Table S5). Application on the Fe/Zn sphalerite–tetrahedrite exchange geothermometer (Sp-II, XFeS = 0.084 and tetrahedrite Zn between 1.25 and 1.38 apfu) of Raabe and Sack [94], Sack and Loucks [95] and Lusk and Calder [96] suggests that these coexisting phases were formed at temperatures of 232° ± 10° and 177 ± 9 °C, respectively.
Arsenopyrite is a trace constituent of the Molai Zn-Pb±(Ag,Ge) ore. Arsenopyrite forms fine-grained euhedral crystals (size less than 10 μm) either as disseminations or fine layers in contact with Sp-I and, rarely, tetrahedrite that follow the layering of the host ashes and tuffs (Figure 10d). In places, arsenopyrite fills the cracks of Sp-I along with tetrahedrite.
6. Discussion
6.1. Deposit Type of the Molai Sulfide Ore
The Molai Zn-Pb±(Ag,Ge) ore deposit is a stratiform VMS-style ore, which was falsely described as bimodal felsic VMS type (former Kuroko-type) [28,29]. We propose that the epigenetic Molai Zn-Pb±(Ag,Ge) ore deposit presents a transitional character between a marine bimodal felsic VMS and a subaerial epithermal system [97]. It bears many similarities to the massive sulfide deposit at the Palinuro Seamount in the Thyrrenian sea [98], and the submarine-to-subaerial mineralization in western Milos [99].
Firstly, the stratiform massive-to-semi-massive sulfide ore is developed along high-angle faults, supporting the epigenetic character of the mineralization (Figure 2b). Secondly, the ore is hosted in a shallow submarine-to-subaerial environment, possibly less than 500 m, much shallower relatively to typical VMS formation settings (~2 km) [100], as evident by the texture of the host lithologies, e.g., the absence of pillow basalts that would testify of a deeper setting, and the presence of typical subaerial ignimbrites, tuffs, ashes and lahar deposits [101]. Thirdly, there is petrographic evidence of phase separation (boiling), based on the presence of hydrothermal but no tectonic breccias (Figure 7c), another atypical feature for VMS deposits, where overpressure conditions due to large seawater depths prevent phase separation [10]. Moreover, the Molai mineralization is concealed and is hosted within the volcaniclastic and pyroclastic rocks (sub-seafloor type). This is also supported by the development of “flower structures” [102] within the host rocks, indicating both the conduits for the ore-bearing hydrothermal fluids and the unconsolidated nature of the host lithologies. The Molai ore mineralogy is rather simple, with sphalerite, galena and pyrite predominating (>90% modal), followed by minor chalcopyrite and tetrahedrite (<10% modal), while other sulfides and sulfosalts (e.g., arsenopyrite, pyrrhotite and jamesonite are found as traces (<0.5% modal). Moreover, the epigenetic Molai ores were formed at temperatures between 200 and 250 °C based on the chlorite and sphalerite–tetrahedrite geothermometers, much lower temperatures relative to the majority of VMS deposits [4]. Such temperatures could support both the shallow formation setting and the epithermal character of the ore deposit. Most importantly, the typical bimodal felsic metal zoning, with Cu-rich “Oko” (yellow) ore in the center and Pb-Zn-rich “Kuroko” (black) ore at the flanks [103,104] is absent, whereas the texture of the Molai ores only bears evidence of successive pulses of hydrothermal activity and gradual change in the geochemistry of the ore-forming fluids.
The first two ore-forming stages (I and II, Figure 8) are associated with the formation of a stratiform VMS-style ore along the layering of the host tuffs and ashes. Early stage I involved circulation of hydrothermal fluids within the unconsolidated tuffs and ashes, which is also supported by the increased silica content of the host tuffs relative to the TVR lavas, depicting silicification during ore formation. At this stage, major ore phases include coarse-grained pyrite (Py-I) and sphalerite (Sp-I), minor chalcopyrite and traces of arsenopyrite. The ore-bearing fluids diffused and dispersed in the tuff S0/S1 planes (Figure 7a,b) and volcanic breccia discontinuities [105], depositing their metal load in a stratiform but epigenetic manner. During this stage, gradual uplifting caused the development of faults and joints that further enabled hydrothermal convection within the tuffs and ashes. Progressive mixing with percolating water resulted in temperature drop that induced, together with pressure drop, the development of hydrothermal breccias with deposition of Sp-I, minor chalcopyrite and milky quartz (Figure 7c). As tectonism was still active at this stage, micro-breccia textures of massive Sp-I are formed and “flower structures” are developed, the latter acting as “funnels” of alteration and upward migration of hydrothermal fluids.
During stage II, the physicochemical conditions and geochemistry of the ore-forming fluids changes and minor low-Fe (Sp-II) and traces of very low-Fe (Sp-III) sphalerite are deposited, whilst Fe-rich sphalerite (Sp-I) deposition is ceased. Very limited replacement between the three sphalerite varieties is observed (Figure 9), indicating that the hydrothermal fluids were gradually depleted in iron due to cooling. The iron content of Sp-II and Sp-III is compatible to sphalerite s in intermediate-to-high sulfidation epithermal environments in equilibrium with pyrite [106,107], and co-crystallization of Sp-II with tetrahedrite and enargite (reported by [27]) further supports our hypothesis [108]. Stage II sulfides are deposited along the same pathways and conduits used by the ore minerals of stage I, either by replacing stage I sulfides (e.g., galena replacing chalcopyrite and Sp-I) or by depositing finer-grained stage II sulfides along the boundaries and interstices of stage I phases. As the system evolved and reached even shallower levels, stage III vein-type ore was formed along the already mineralized pathways of stages I and II, with predominant galena replacing both stage I (e.g., Sp-I and chalcopyrite) and stage II phases (e.g., Sp-II and tetrahedrite).
The stable isotope geochemistry of bulk rock (host andesite) and gangue phases (chlorite and epidote) clearly supports a mixing mechanism between ascending magmatic fluids and fresh meteoric water during late stage III (vein type ore with galena-quartz-chlorite-epidote veinlets, Figure 3d). Our calculations show that the host andesite melt contained a low initial water content (between 0.14 and 0.27 wt % H2O, at T = 500 °C, using the approach of De Hoog et al. [91], and was subjected to extensive degassing. The δ18OH2O and δDH2O values suggest a primarily volcanogenic origin of the ore-forming fluids for the Molai deposit. Then, at T ≤ 200 °C, at the late stages of the ore formation (stage III, vein-type ore), the magmatic fluids were gradually mixed and diluted with meteoric waters (Figure 6b), although metamorphism in prehnite–pumpellyite facies may have modified the isopotic compositions of chlorite. Mixing was combined with cooling of the ore fluids and an increase in the permeability of the host volcanic and volcaniclastic rocks that correlate with the thermal collapse of the hydrothermal system, and the development of flower structures. Clear quartz from stage III galena–quartz–chlorite–epidote veinlets shows δ30SiNBS values ranging between −0.20 and −0.26 per mill (Table 3), which fall in the typical range of intermediate-to-acidic volcanic rocks [109], pin-pointing the Si source in the volcanic rocks hosting the Molai ore [110,111].
The proposed model of formation includes an early high-temperature stage with predominant Fe-rich sphalerite (Sp-I), pyrite and chalcopyrite. As stated by Chon and Shimazaki [112] and Frenzel et al. [113], the Fe content in sphalerite is indicative of both the formation temperatures and the sulfidation state of the hydrothermal system. Based on ore mineralogy and mineral chemistry, the hydrothermal system evolved from an early low sulfidation state as Sp-I was precipitated, to a gradually higher sulfidation state where Sp-II and Sp-III were deposited with decreasing temperature. Preliminary studies deduced formation temperatures higher than 200 °C (232 ± 10 °C) for Sp-II and lower than 200 °C (177 ± 9 °C) for tetrahedrite, based on the sphalerite (Sp-II)-tetrahedrite geothermometer.
6.2. Metal Zoning and Ore Geochemistry
As stated earlier, no typical bimodal felsic zoning is observed in the Molai ore, yet the Pb/Zn and log[(Cu·Pb·Zn)/(103·Co·Ni·Cr)] ratios increase from depth to surface, depicting the predominance of vein-type galena on the upper part (stage III) and sphalerite (all varieties) at lower levels (stages I and II). At the same time, the log(Cu·Pb·Zn)/105 ratio decreases towards the surface, suggesting downwards fluid circulation of meteoric-dominated fluids, as suggested by the isotopic compositions of the ore-forming fluids.
Ore geochemistry indicates the presence of precious metals (Ag ± Au) in the ore-forming fluids. Tetrahedrite is the major Ag carrier. The Molai ore is also characterized by increased Ge content (Table 4 and Table 5), with the predominant Ge-bearing phases being Sp-I, Py-I and galena (Table 5). Following the general consensus that sphalerite is the major Ge carrier in sulfide ores [113,114], Sp-I sphalerite incorporates traces of Ge, whereas Sp-III incorporates In. The Ge geochemistry in the Molai ore clearly shows that Ge is largely associated with early-stage (Stage I) phases (Py-I and Sp-I) and less with late-stage phases (e.g., stage III galena). The Ge content in Py-I is rather high (up to 0.001 apfu; Table 4) and approximately up to two orders of magnitude higher than in typical pyrite crystals from other hydrothermal-type mineralizations (e.g., Beishan Pb-Zn ore district, China [115]; Kipushi Cu-Zn deposit, Tongo [116]; the submarine Niuatahi hydrothermal system, Lau basin, Tonga [117]). Possibly, the increased content of Ge in Py-I is related to the presence of Ge-bearing sub-microscopic inclusions. On the other hand, Ga and In seem to mostly associate with the host volcanic and volcaniclastic lithologies, as their content is similar in both the host rocks and the massive-to-semi-massive sulfide ore (Table 1, Table 2, Table 3, Table 4 and Table 5; Tables S4 and S5).
The presence of Ag and Au in the sulfide ore may indicate a possible development of an upper precious metal-rich section formed at even shallower levels which was later subjected to surface erosion and weathering and is now absent.
Interestingly, the Cu-Fe±(Pb,Zn) Apidea mineralization displays similar geochemical signatures to the ones observed for the Molai Zn-Pb±(Ag,Ge) ores. Despite the limited data on the Apidea mineralization, based on host rock lithology and the disseminated character of the sulfide mineralization, we propose that the Apidea Cu-Fe±(Pb,Zn) mineralization represents a syngenetic sulfide mineralization resembling the intrusion-hosted magmatic Cu-Ni-Co type genetically related to the basaltic andesite lava suite.
This study proved the importance of EPMA and trace element geochemistry in defining both the ore-type and the major critical and strategic metal carriers in stratiform and massive sulfide deposits. The results may be employed in future exploration strategies regarding fossilized hydrothermal systems and the re-examination and re-evaluation of formerly explored VMS systems, with beneficial outcome for the metallogenic potential of Greece and the Mediterranean region in general.
7. Conclusions
The Molai Zn-Pb±(Ag,Ge) ore deposit is concealed and hosted within unconsolidated tuffs and ashes (sub-seafloor), replacing the host lithologies and leading to the formation of the bedded/stratiform sulfide ore. Despite the predominant layered character (VMS-style) of the Molai ore, it is characterized by intermediate features between bimodal felsic massive sulfides and epithermal systems based on the following:
- The epigenetic character of the stratiform massive-to-semi-massive ore, replacing the host tuffs and ashes;
- The development of the stratiform massive-to-semi-massive sulfide ore along high angle faults;
- The shallow formation depth, possibly less than 500 m, as supported by the subaerial pyroclastic products hosting the sulfide ores (ashes, tuffs, ignimbrites and lahar deposits), and the development of flower structures and petrographic evidence of phase separation (boiling) during hydrothermal convection and ore formation;
- The relatively low ore-forming temperatures, below 250 °C, based on the sphalerite-tetrahedrite and chlorite geothermometers;
- The concealed character of the massive-to-semi-massive ore within the tuffs and ashes and not on the seabed;
- The absence of the typical structure of a Kuroko-type mineralization.
The simple ore mineralogy with predominant sphalerite, galena and pyrite (>90% modal) is another peculiar characteristic of the Molai Zn-Pb±(Ag,Ge) ore, that distinguishes it from the typical bimodal felsic VMS deposits.
Three different ore-forming stages are recognized based on ore mineralogy, geochemistry and texture, with stages I and II related to the formation of the epigenetic stratiform massive-to-semi-massive ore and the late vein-type stage III with predominant galena.
Sphalerite is the major ore phase, with three different varieties formed during stages I (Sp-I) and II (Sp-II and Sp-III), with progressively smaller grain size and lower Fe content with decreasing temperature. Very limited replacement is observed between the three sphalerite varieties, depicting gradual change in the chemistry of the ore-forming fluids. The stable isotope geochemistry of gangue chlorite and epidote reveal mixing with fresh meteoric waters during the weaning stages of the hydrothermal activity.
The Molai ore is characterized by increased CRM contents, in particular, Ag and Ge. Tetrahedrite is the major Ag carrier, while Sp-I and Py-I are the major Ge carriers. The high Ge contents in Py-I are attributed to the presence of Ge-bearing sub-microscopic inclusions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/min14090885/s1, Figures S1 and S2: Alteration mineralogy discrimination diagrams and Sphalerite (Sp-II_—tetrahedrite pairs for geothermometry; Table S1a: Sample coordinates; Table S1b: Drill cores; Table S2: Host-rock lithology mineralogy; Table S3: Alteration minerals analyses; Table S4: Geochemical analyses TVR samples; Table S5: Molai sulfides analyses. The following references were cited in the Supplementary Materials: [94,95,96,118,119].
Author Contributions
Conceptualization, E.K., K.St.S., S.S.T. and S.F.T.; methodology, E.K., S.S.T. and S.F.T.; software, E.K., S.F.T. and S.K.; validation, S.S.T., S.F.T., S.K., D.Z. (Deghao Zhai), M.F. and P.V.; formal analysis, E.K., S.S.T. and S.F.T.; investigation, E.K., S.S.T., S.F.T., S.K., K.K., K.P., J.P. and I.K.; resources, E.K. and K.St.S.; data curation, S.S.T., S.F.T., K.St.S., P.V., D.Z. (Dimitrios Zouzias) and E.K.; writing—original draft preparation, E.K., S.S.T., S.F.T. and K.St.S.; writing—review and editing, S.S.T.; visualization, S.S.T., S.F.T. and E.K.; supervision, S.S.T., S.F.T. and K.St.S.; project administration, E.K., K.St.S. and S.S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Acknowledgments
The authors would like to thank Lang Shi for performing detailed microanalyses of ore phases from Molai mineralization at McGill University, Canada; Kimon Christanis and Stavros Kalaitzidis for their valuable assistance during field work, sample preparation and geochemical and mineralogical analyses; and the IGME branch in Peloponnese for providing access to the cores and samples for logging and sampling. This paper is dedicated to the memory of our colleague, Karen St. Seymour, who recently passed away.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Franklin, J.M. Volcanic-associated massive sulfide base metals. In Geology of Canadian Mineral Deposit Types; Eckstrand, O.R., Sinclair, W.D., Thorpe, R.I., Eds.; Geological Survey of Canada, Geology of Canada: Ottawa, ON, Canada, 1996; Volume 8, pp. 158–183. [Google Scholar]
- Large, R.R.; McPhie, J.; Gemmell, J.B.; Herrmann, W.; Davidson, G.J. The spectrum of ore deposit types, volcanic environments, alteration halos, and related exploration vectors in submarine volcanic successions: Some examples from Australia. Econ. Geol. 2001, 96, 1037–1054. [Google Scholar] [CrossRef]
- Franklin, J.M.; Sangster, D.M.; Lydon, J.W. Volcanic associated massive sulfide deposits. In Economic Geology 75th Anniversary Volume; Skinner, B.J., Ed.; Society Economic Geologists: Littleton, CO, USA, 1981; pp. 485–627. [Google Scholar]
- Franklin, J.M.; Gibson, H.L.; Jonasson, I.R.; Galley, A.G. Volcanogenic massive sulfide deposits. In One Hundredth Anniversary Volume; Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2005; pp. 523–560. [Google Scholar]
- Lydon, J.W. Volcanogenic massive sulfide deposits: I. A descriptive model. In Ore Deposit Models; Roberts, R.G., Sheanan, P.A., Eds.; Reprint Series; Geoscience Canada: St. John’s, NL, Canada, 1988; Volume 3, pp. 14–56. [Google Scholar]
- Tornos, F.; Peter, J.M.; Allen, R.; Conde, C. Controls on the sitting and style of volcanogenic massive sulfide deposits. Ore Geol. Rev. 2015, 68, 142–163. [Google Scholar] [CrossRef]
- Huston, D.L.; Morant, P.; Pirajno, F.; Cummins, B.; Baker, D.; Mernagh, T.P. Paleoarchean mineral deposits of the Pilbara craton: Genesis, tectonic environment and comparisons with younger deposits. Dev. Precambrian Geol. 2007, 15, 411–450. [Google Scholar]
- Huston, D.L.; Pehrsson, S.; Eglington, B.M.; Zaw, K. The geology and metallogeny of volcanic-hosted massive sulfide deposits: Variations through geologic time and with tectonic setting. Econ. Geol. 2010, 105, 571–591. [Google Scholar] [CrossRef]
- Rona, P.A. Hydrothermal mineralization at oceanic ridges. Canad. Mineral. 1988, 26, 431–465. [Google Scholar]
- Hannington, M.D.; de Ronde, C.E.J.; Peterson, S. Sea-Floor Tectonics and Submarine Hydrothermal Systems; Society of Economic Geologists: Littleton, CO, USA, 2005; pp. 111–141. [Google Scholar]
- Hannington, M.D.; Jamieson, J.; Monecke, T.; Petersen, S. Modern sea-floor massive sulfides and base metal resources: Toward an estimate of global sea-floor massive sulfide potential. Soc. Econ. Geol. 2010, 15, 317–338. [Google Scholar]
- Sawkins, F.J. Integrated tectonic–genetic model for volcanic—Hosted massive sulfide deposits. Geology 1990, 18, 1061–1064. [Google Scholar] [CrossRef]
- Ludden, J.N.; Peloquin, S.A. A geodynamic model for the evolution of the Abitibi Belt—Implications for the origins of volcanic massive sulfide deposits. Geol. Assoc. Can. Short Course Notes 1996, 12, 205–238. [Google Scholar]
- Piercey, S.J.; Paradis, S.; Murphy, D.C.; Mortensen, J.K. Geochemistry and paleotectonic setting of felsic volcanic rocks in the Finlayson Lake volcanic hosted massive sulfide district, Yukon, Canada. Econ. Geol. 2001, 96, 1877–1905. [Google Scholar] [CrossRef]
- Piercey, S. The setting, style and role of magmatism in the formation of volcanogenic massive sulfide deposits. Miner. Depos. 2001, 46, 449–471. [Google Scholar] [CrossRef]
- Shanks, W.C.P., III; Koski, R.A. Introduction. In Volcanogenic Massive Sulfide Occurrence Model; Shanks, W.C.P., III, Thurston, R., Eds.; U.S. Geological Survey Scientific Investigations Report 2010–5070–C; U.S. Geological Survey: Preston, VA, USA, 2012; Chapter 1; pp. 5–8. [Google Scholar]
- Sillitoe, R.H.; Hannington, M.D.; Thompson, J.F.H. High sulfidation deposits in the volcanogenic massive sulfide environment. Econ. Geol. 1996, 91, 204–212. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Daneshfar, B. Strategic Prospectivity Review of Mineral Potential: Middle East Region; Report to Metal Mining Agency of Japan; Hedenquist Consulting Inc.: Ottawa, ON, Canada, 2001; p. 68. [Google Scholar]
- Barrie, C.T.; Nielsen, F.W.; Aussant, C.H. The Bisha Volcanic-Associated Massive Sulfide Deposit, Western Nakfa Terrane, Eritrea. Econ. Geol. 2007, 102, 717–738. [Google Scholar] [CrossRef]
- Glasby, G.P.; Iizasa, K.; Hannington, M.; Kubota, H.; Notsu, K. Mineralogy and composition of Kuroko deposits from northeastern Honshu and their possible modern analogues from the Izu-Ogasawara (Bonin) Arc south of Japan: Implications for mode of formation. Ore Geol. Rev. 2008, 34, 547–560. [Google Scholar] [CrossRef]
- Ridley, W.I. Geochemical characteristics. In Volcanogenic Massive Sulfide Occurrence Model; Shanks, W.C.P., III, Thurston, R., Eds.; U.S. Geological Survey Scientific Investigations Report 2010–5070–C; U.S. Geological Survey: Preston, VA, USA, 2012; Chapter 14; pp. 205–225. [Google Scholar]
- Galley, A.G.; Hannington, M.; Jonasson, I. Volcanogenic massive sulfide deposits. In Mineral Deposits of Canada: A Synthesis of Major Deposit-Types, District Metallogeny, the Evolution of Geological Provinces, and Exploration Methods; Goodfellow, W.D., Ed.; Geological Association of Canada, Mineral Deposits Division: St. John’s, NL, Canada, 2007; pp. 141–161. [Google Scholar]
- Koski, R.A.; Mosier, D.L. Deposit type and associated commodities. In Volcanogenic Massive Sulfide Occurrence Model; Shanks, W.C.P., III, Thurston, R., Eds.; U.S. Geological Survey Scientific Investigations Report 2010–5070–C; U.S. Geological Survey: Preston, VA, USA, 2012; Chapter 2; pp. 13–21. [Google Scholar]
- Lobanov, K.V.; Gaskov, I.V. The Karchiga copper massive sulfide deposit in the high-grade metamorphosed rocks of the Kurchum block: Geologic structure, formation, and metamorphism (Rudny Altai). Russ. Geol. Geophys. 2012, 53, 77–91. [Google Scholar] [CrossRef]
- Hannington, M.D. Volcanogenic Massive Sulfide Deposits. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 463–488. [Google Scholar] [CrossRef]
- Brauer, R. Das Praneogen im Raum Molaoi-Talanta/SE Lakonien (Peloponnes Griechenland). In Frankfurter Geowiss Abh; Geologisch-Paläontologisches Institute: Münster, Germany, 1982; pp. 1–300. [Google Scholar]
- Grossou-Valta, M.; Adam, K.; Constantinides, D.C.; Prevosteau, J.M.; Dimou, E. Mineralogy of and potential beneficiation process for the Molai complex sulfide orebody, Greece. In Sulfide Deposits-Their Origin and Processing; Gray, P.M.J., Bowyer, G.L., Castle, J.F., Vaughan, D.J., Warner, N.A., Eds.; Institution of Mining and Metallurgy: London, UK, 1990; pp. 119–133. [Google Scholar]
- Skarpelis, N. Metallogeny of Massive Sulfides and Petrology of the External Metamorphic Belt of the Hellenides (SE Peloponnesus). Ph.D. Thesis, National Kapositrian University of Athens, Athens, Greece, 1982. (In Greek). [Google Scholar]
- Angelopoulos, K.; Constantinides, D. The Zinc–Silver–Lead deposit, Molai, Laconia. Bull. Geol. Soc. Greece 1986, 20, 305–320. [Google Scholar]
- High Grade Germanium Intersected at Molaoi. Available online: https://polaris.brighterir.com/public/rockfire_resources/news/rns/story/w3pjq3x (accessed on 6 June 2024).
- Drilling Finds Zinc Beneath the Main Resource at Molaoi. Available online: https://polaris.brighterir.com/public/rockfire_resources/news/rns/story/x2v71mw (accessed on 6 June 2024).
- Regulation (EU) 2024/1252 of the European Parliament and of the Council of 11 April 2024 Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) No 168/2013, (EU) 2018/858, (EU) 2018/1724 and (EU) 2019/1020, OJ (L) 2024/1252, 3.5.2024, 2024, 67p. Available online: https://www.eur-lex.europa.eu/eli/reg/2024/1252/oj (accessed on 17 July 2024).
- Thiebault, F.; Triboulet, T. Alpine metamorphism and deformation in Phyllite nappes (external Hellenides southern Peloponnesus Greece): Geodynamic implication. J. Geol. 1984, 92, 185–199. [Google Scholar] [CrossRef]
- Doutsos, T.; Koukouvelas, I.; Poulimenos, G.; Kokkalas, S.; Xypolias, P.; Skourlis, K. An exhumation model of the south Peloponnesus, Greece. Int. J. Earth Sci. 2000, 89, 350–365. [Google Scholar] [CrossRef]
- Pe-Piper, G.; Piper, D.J.W. The Igneous Rocks of Greece: The Anatomy of an Orogen; Gebrüder Borntraeger: Stuttgart, Germany, 2002; p. 573. [Google Scholar]
- Romer, T.; Völs, S.; Schulz, B.; Xypolias, P.; Zulauf, G.; Krenn, E. Metamorphism and P–T paths of the pre-Alpine basement and the Phyllite-Quartzite unit s.str. of Kythira (External Hellenides, Greece). Z. Dtsch. Ges. Geowiss. 2008, 159, 469–484. [Google Scholar]
- Lode, S.; Romer, T.; Völs, S.; Xypolias, P.; Zulauf, G. The pre-Alpine basement of Kythira (Greece). Z. Dtsch. Ges. Geowiss. 2008, 159, 457–468. [Google Scholar] [CrossRef]
- Micheuz, P.; Krenn, K.; Fritz, H.; Kurz, W. Tectonometamorphic evolution of blue-schist-facies rocks in the Phyllite-Quartzite Unit of the External Hellenides (Mani, Greece). Austrian J. Earth Sci. 2015, 108, 109–122. [Google Scholar] [CrossRef]
- Tataris, A.; Maragoudakis, N. On the stratigraphy of Triassic and Jurassic of the Tripolis zone at Kynouria (Peloponnes). Bull. Geol. Soc. Greece 1967, 6, 353–364. [Google Scholar]
- Thiebault, F. Evolution geodynamique des Hellenides externes en Peloponnèse meridional (Grece). Soc. Geol. Nord. 1982, 6, 574. [Google Scholar]
- Blumor, T. Die Phyllit-Quartzit-Serie SE-Lakoniens (Peloponnes, Griechenland): Hochdruckmetamorphite in einem orogen Keil. In Frankfurter Geowiss Abh; Geologisch-Paläontologisches Institute: Münster, Germany, 1998; Volume 17, pp. 1–190. [Google Scholar]
- Kowalczyk, G.; Zugel, P. Die Vathia-Schichten–Flysch der Plattenkalk-Serie des Peloponnes. Kontribution Forschungen Inst. Senckenberg 1997, in press. [Google Scholar]
- Psonis, K. Presence of Permo(?) Lower Triassic beds at the base of Plattenkalk series in Taygetos, description of a continuous section. Ann. Geol. Pays Hellen. 1981, 30, 578–587. [Google Scholar]
- Dittmar, U.; Kowalczyk, G. Das Liegende der Plattenkalk-KarbonateimTaygetos (Sud Peloponnes). Nachrichten Dtsch. Geol Ges. 1989, 41, 88–89. [Google Scholar]
- Papanikolaou, D.; Skarpelis, N. The blueschists in the external metamorphic belt of the Hellenides: Composition, structure and geotectonic significance of the Arna unit. Ann. Geol. Pays Hellen. 1986, 32, 47–68. [Google Scholar]
- Ring, U.; Fassoulas, C.; Uysal, I.T.; Bolhar, R.; Tong, K.; Todd, A. Nappe imbrication within the Phyllite-Quartzite Unit of West Crete: Implications for sustained high-pressure metamorphism in the Hellenide subduction orogen, Greece. Tectonics 2022, 41, e2022TC007430. [Google Scholar] [CrossRef]
- Robertson, A.H.F. Sedimentary evidence from the south Mediterranean region (Sicily, Crete, Peloponnese, Evia) used to test alternative models for the regional tectonic setting of Tethys during Late Palaeozoic–Early Mesozoic time. In Tectonic Development of the Eastern Mediterranean Region; Robertson, A.H.F., Mountrakis, D., Eds.; Geol Soc London: London, UK, 2006; pp. 91–154. [Google Scholar]
- Chatzaras, V.; Dörr, W.; Gerdes, A.; Krahl, J.; Xypolias, P.; Zulauf, G. Tracking the late Paleozoic to early Mesozoic margin of northern Gondwana in the Hellenides: Paleotectonic constraints from U–Pb detrital zircon ages. Int. J. Earth Sci. (Geol. Rundsch) 2016, 105, 1881–1899. [Google Scholar] [CrossRef]
- Mariolakos, I.; Zagorchev, I.; Fountoulis, I.; Ivanov, M. Neotectonic Transect Moesia Apulia. In Proceedings of the 32nd International Geological Congress, Florence, Italy, 20–28 August 2004. Field Trip B26, 7p, and Greek route B26 (appendix) 25p. [Google Scholar]
- Zulauf, G.; Dörr, W.; Xypolias, P.; Gerdes, A.; Kowakczyk, G.; Linckens, J. Triassic evolution of the western Neotethys: Constraints from microfabrics and U–Pb detrital zircon ages of the Plattenkalk Unit (External Hellenides, Greece). Int. J. Earth Sci. 2019, 108, 2493–2529. [Google Scholar] [CrossRef]
- Klein, T.; Craddock, J.P.; Zulauf, G. Constraints on the geodynamical evolution of Crete: Insights from illite crystallinity, Raman spectroscopy and calcite twinning above and below the “Cretan detachment”. Int. J. Earth Sci. (Geol. Rundsch) 2012, 102, 139–182. [Google Scholar] [CrossRef]
- Rahl, J.M.; Anderson, K.M.; Brandon, M.T.; Fassoulas, C. Raman spectroscopic carbonaceous material thermometry of low-grade metamorphic rocks: Calibration and application to tectonic exhumation in Crete, Greece. Earth Planet. Sci. Lett. 2005, 240, 339–354. [Google Scholar] [CrossRef]
- Melidonis, N.; Constantinides, D. Volcanic-Hosted Sulfide Mineralization, Apidea Region, Laconia, Greece; Ore Deposit Research No 10; Institute of Geology and Mineral Exploration Greece: Athens, Greece, 1979. [Google Scholar]
- Bassias, Y.; Triboulet, C. Tectono-Metamorphic Evolution of Blueschist Formations in the Peloponnesus (Parnon and Taygetos Massifs, Greece): A Model of Nappe Stacking during Tertiary Orogenesis. J. Geol. 1994, 102, 697–708. [Google Scholar] [CrossRef]
- Zulauf, G.; Dörr, W.; Fisher-Spurlock, S.C.; Gerdes, A.; Chatzaras, V.; Xypolias, P. Closure of the Paleotethys in the External Hellenides: Constraints from U–Pb ages of magmatic and detrital zircons (Crete). Gondwana Res. 2015, 28, 642–667. [Google Scholar] [CrossRef]
- Pe-Piper, G.; Kotopouli, C.N. Very low-grade metamorphism of (?) Triassic volcanics. West Hellenic Nappes, Southern Peloponnese, Greece. Geol. Soc. Am. Bull. 1981, 92, 1762–1806. [Google Scholar] [CrossRef]
- Fytrolakis, N. The previously unknown Palaeozoic of Kalamai. Bull. Geol. Soc. Greece 1971, 8, 70–81. (In Greek) [Google Scholar]
- Lys, M.; Thiebault, F. Donnees nouvelles sur l’ age des schistesen Peloponnese meridional. Compte Rendus L’academie Des Sci. Paris (Ser. D) 1971, 272, 196–197. [Google Scholar]
- Panagos, A.; Pe, G.G.; Piper, D.J.W.; Kotopouli, C.N. Age and stratigraphic subdivision of the phyllite series, Krokee region, Peloponnese, Greece. Neues Jahrb. Für Geol. Und Paläontologie Monatshefte 1979, 3, 181–190. [Google Scholar]
- Thiébault, F.; Kozur, H. Précisions sur l’age de la formation de Tyros (Palaéozoique supérieur-Carnien) et de la base de la series de Gavrovo-Tripolitza (Carnian), Peloponnèse méridional, Grèce. Compte Rendus L’academie Des Sci. Paris 1979, 288, 23–26. [Google Scholar]
- Brauer, R.; Ittner, R.; Kowalczyk, G. Ergebnisseaus der ‘Phyllit-Serie’ SE-Lakoniens (Peloponnes, Griechenland). Neues Jahrb. Für Geol. Und Paläontologie Monatshefte 1980, 3, 129–132. [Google Scholar] [CrossRef]
- Doert, U.; Kowalczyk, G.; Kaufmann, G.; Krahl, J. Zur stratigraphischen Einstufung der “Phyllit-Serie” von Krokee und der Halbinsel Xyli (Lakonien, Peloponnes). Erlanger Geol. Abh. 1985, 112, 1–10. [Google Scholar]
- Varti-Mataragka, M. Lithofacies and depositional environment of the sedimentary sequence of Tyros Beds, Asfakorachi, Molai, SE Peloponnese. Bull. Geol. Soc. Greece 1993, 28, 567–577. [Google Scholar]
- Gerolymatos, I. Metamorphose und Tektonik der Phyllit-Quartzit-Serie und Der Tyros Formation auf dem Peloponnes und Kythira. Berl. Geowiss. Abh. 1994, 164, 161. [Google Scholar]
- Zulauf, G.; Blau, J.; Dörr, W.; Klein, T.; Krahl, J.; Kustatscher, E.; Petschick, R.; van de Schootbrugge, B. New U–Pb zircon and biostratigraphic data of the Tyros Unit, eastern Crete: Constraints on Triassic palaeogeography and depositional environment of the eastern Mediterranean. Z. Dtsch. Ges. Geowiss. 2013, 164, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Pe-Piper, G. The triassic volcanic rocks of Tyros, Zarouhla, Kalamae, and Epidavros, Peloponnese, Greece. Schweiz. Mineral. Petrogr. Mitteilungen 1983, 63, 249–266. [Google Scholar]
- Xypolias, P.; Koukouvelas, I.; Zulauf, G. Cenozoic tectonic evolution of northeastern Apulia: Insights from a key study area in the Hellenides (Kythira, Greece). Z. Dtsch. Ges. Geowiss. 2008, 159, 439–455. [Google Scholar] [CrossRef]
- Ripis, K. Geological–Mineralogical Report of Exploration Gallery 165, Molai Polymetallic Sulfide Deposit; Institute of Geology and Mineral Exploration Greece: Athens, Greece, 1987; p. 22. [Google Scholar]
- Rockfire Resources. Available online: www.rockfireresources.com/projects/molaoi/ (accessed on 29 June 2024).
- Ilias, P. Lithogeochemical Investigation of Drill-Cores from Molai Deposit, Lakonia, Greece; Institute of Geology and Mineral Exploration: Athens, Greece, 1985; Report; p. 26. [Google Scholar]
- De Groot, P.A. Handbook of Stable Isotope Analytical Techniques: Stable Isotope Geochemistry, 6th ed.; Elsevier Science: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Zheng, Y.F. Calculation of oxygen isotope fractionation in anhydrous silicate minerals. Geochim. Cosmochim. Acta 1993, 57, 1079–1091. [Google Scholar] [CrossRef]
- Zheng, Y.F. Calculation of oxygen-isotope fractionation in hydroxyl-bearing silicates. Earth Planet. Sci. Lett. 1993, 120, 247–263. [Google Scholar] [CrossRef]
- Zezza, U.; Lazzarini, L. Krokeatis Lithos (Lapis Lacedaemonius): Source, history of use, scientific characterization. In Interdisciplinary Studies on Ancient Stone (2002), Proceedings of the ASMOSIA VI: Padua, Italy, 15–18 June 2000; Lazzarini, L., Ed.; Bottega d’Erasmo: Turin, Italy, 2002. [Google Scholar]
- Koutsovitis, P.; Kanellopoulos, C.; Passa, S.; Foni, K.; Tsapara, E.; Oikonomou, G.; Xirokostas, N.; Vallianatou, K.; Mouxiou, E. Mineralogical, petrological and geochemical features of the unique Lapis Lacedaemonius (Krokeatis Lithos) from Laconia, Greece: Approach on petrogenetic processes within the Triassic volcanic context. Bull. Geol. Soc. Greece 2016, 50, 1903–1912. [Google Scholar] [CrossRef][Green Version]
- Winchester, J.A.; Floyd, P.A. Geochemical discrimination of different magma series and their differentiation products using immobile elements. Chem. Geol. 1977, 20, 325–343. [Google Scholar] [CrossRef]
- Hollocher, K.T.; Robinson, P.C.; Walsh, O.E.; Roberts, D.F. Geochemistry of amphibolite-facies volcanics and gabbros of the Støren Nappe in extensions west and southwest of Trondheim, western gneiss region, Norway: A key to correlations and paleotectonic settings. Am. J. Sci. 2012, 312, 357–416. [Google Scholar] [CrossRef]
- Irvine, T.N.; Baragar, W.R.A. A guide to the chemical classification of the common volcanic rocks. Can. J. Sci. 1971, 8, 523–548. [Google Scholar] [CrossRef]
- Wood, D.A. The application of a Th-Hf-Ta diagram to problems of tectonomagmatic classification and to establishing the nature of crustal contamination of basaltic lavas of the British Tertiary volcanic province. Earth Planet. Sci. Lett. 1980, 50, 11–30. [Google Scholar] [CrossRef]
- Cabanis, B.; Lecolle, M. Le diagramme La/10-Y/15-Nb/8: Un outil pour la discrimination des series volcaniques et la mise en evidence des processus de mèlange et/ou de contamination crustale. Compte Rendus L’academie Sci. Paris 1989, 309, 2023–2029. [Google Scholar]
- Mullen, E.D. MnO/TiO2/P2O5: A minor element discriminant for basaltic rocks of oceanic environments and its implications for petrogenesis. Earth Planet. Sci. Lett. 1983, 62, 53–62. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Sawaguchi, T.; Iwaya, S.; Horiuchi, M. Delineation of prospecting targets for Kuroko deposits based on modes of volcanism of underlying dacite and alteration halos. Min. Geol. 1976, 26, 105–117. [Google Scholar]
- Large, R.R.; Gemmell, J.B.; Paulick, H. The alteration box plot: A simple approach to understanding the relationship between alteration mineralogy and lithogeochemistry associated with VHMS deposits. Econ. Geol. 2001, 96, 957–971. [Google Scholar] [CrossRef]
- Pearce, J.A. Role of the sub-continental lithosphere in magma genesis at active continental margins. In Continental Basalts and Mantle Xenoliths; Hawkesworth, C.J., Norry, M.J., Eds.; Shiva Publications: Nantwich Cheshire, UK, 1983; pp. 230–249. [Google Scholar]
- Nakamura, N. Determination of REE, Ba, Mg, Na and K in carbonaceous and ordinary chondrites. Geochim. Cosmochim. Acta 1974, 38, 757–775. [Google Scholar] [CrossRef]
- Sylvester, P.J. Post-collisional strongly peraluminous granites. Lithos 1998, 45, 29–44. [Google Scholar] [CrossRef]
- Jiang, Y.D.; Schulmann, K.; Sun, M.; Štípská, P.; Guy, A.; Janoušek, V.; Lexa, O.; Yuan, C. Anatexis of accretionary wedge, Pacific-type magmatism, and formation of vertically stratified continental crust in the Altai Orogenic Belt. Tectonics 2016, 35, 3095–3118. [Google Scholar] [CrossRef]
- Bowman, J.R. Stable-isotope systematics of skarns. In Mineralized Intrusion-Related Skarn Systems; Lentz, D.R., Ed.; Mineralogical Association of Canada Short Course Series 26; Mineralogical Association of Canada: Ottawa, ON, Canada, 1998; pp. 99–145. [Google Scholar]
- Kuşcu, I.; Yılmazer, E.; Güleç, N.; Bayır, S.; Demirela, G.; Gençalioğlu-Kuşcu, G.; Kuru, S.G.; Kaymakçı, N. U-Pb and 40Ar-39Ar geochronology and isotopic constraints on the genesis of copper-gold-bearing iron oxide deposits in the Hasançelebi district, Eastern Turkey. Econ. Geol. 2011, 106, 261–288. [Google Scholar]
- Zhao, Z.F.; Zheng, Y.F. Calculation of oxygen isotope fractionation in magmatic rocks. Chem. Geol. 2003, 193, 59–80. [Google Scholar] [CrossRef]
- De Hoog, J.C.; Taylor, B.E.; Van Bergen, M.J. Hydrogen-isotope systematics in degassing basaltic magma and application to Indonesian arc basalts. Chem. Geol. 2009, 266, 256–266. [Google Scholar] [CrossRef][Green Version]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Awadh, S.M. Iron content variations in sphalerite and their effects on reflectance and internal reflections under reflected light. Arab. J. Geosci. 2008, 2, 139–142. [Google Scholar] [CrossRef]
- Raabe, K.C.; Sack, R.O. Growth zoning in tetrahedrite-tennantite from the Hock Hocking Mine, Alma, Colorado. Can. Miner. 1984, 22, 577–582. [Google Scholar]
- Sack, R.O.; Loucks, R.R. Thermodynamic properties of tetrahedrite-tennantites: Constraints on the interdependence of the Ag↔Cu, Fe↔Zn, Cu↔Fe, and As↔Sb exchange reactions. Am. Mineral. 1985, 70, 1270–1289. [Google Scholar]
- Lusk, J.; Calder, B.O.E. The composition of sphalerite and associated sulfides in reactions of the Cu–Fe–Zn–S, Fe–Zn–S and Cu–Fe–S systems at 1 bar and temperatures between 250 and 535 °C. Chem. Geol. 2004, 203, 319–345. [Google Scholar] [CrossRef]
- Hannington, M.D.; Poulsen, K.H.; Thompson, J.F.H.; Sillitoe, R.H. Volcanogenic gold in the massive sulfide environment. In Volcanic-Associated Massive Sulfide Deposits: Processes and Examples in Modern and Ancient Settings; Barrie, C.T., Hannington, M.D., Eds.; Society of Economic Geologists, Inc.: Littleton, CO, USA, 1999; Volume 8, pp. 325–356. [Google Scholar]
- Gemmell, J.C.; Petersen, S.; Monecke, T.; Hannington, M.D.; Lackschewitz, K.; Augustin, N.; Gibson, H.; Perrin, K.; Sharpe, R.; Simpson, K. High to Intermediate Sulfidation, Shallow Marine, Sulfide Mineralization in the South-Eastern Tyrrhenian Sea, Italy. In Proceedings of the Smart Science for Exploration and Mining, Tenth Biennial SGA Meeting, Townsville, Australia, 17–20 August 2009. [Google Scholar]
- Alfieris, D.; Voudouris, P.; Spry, P.G. Shallow submarine epithermal Pb-Zn-Cu-Au-Ag-Te mineralization on western Milos Island, Aegean Volcanic Arc, Greece: Mineralogical, geological, and geochemical constraints. Ore Geol. Rev. 2013, 53, 159–180. [Google Scholar] [CrossRef]
- Stix, J.; Kennedy, B.; Hannington, M.; Gibson, H.; Fiske, R.; Mueller, W.; Franklin, J. Caldera-forming processes, and the origin of submarine volcanogenic massive sulfide deposits. Geology 2003, 31, 375–378. [Google Scholar] [CrossRef]
- Morgan, L.A.; Schulz, K.J. Physical volcanology of volcanogenic massive sulfide deposits. In Volcanogenic Massive Sulfide Occurrence Model; Shanks, W.C.P., III, Thurston, R., Eds.; U.S. Geological Survey Scientific Investigations Report 2010–5070–C; U.S. Geological Survey: Preston, VA, USA, 2012; Chapter 5; pp. 64–100. [Google Scholar]
- Huang, L.; Liu, C.-Y. Three types of flower structures in a divergent-wrench fault zone. J. Geophys. Res. Solid Earth 2017, 122, 10478–10497. [Google Scholar] [CrossRef]
- Horikoshi, E.; Shikazono, N. Sub-types and their characteristics of Kuroko-type deposits. Min. Geol. 1978, 28, 267–276. [Google Scholar]
- Ohmoto, H.; Takahashi, T. Geological setting of the Kuroko deposits, Japan—Part III. Submarine calderas and Kuroko genesis. In The Kuroko and Related Volcanogenic Massive Sulfide Deposits; Ohmoto, H., Skinner, B.J., Eds.; Monograph 5; Economic Geology, Yale Station: New Heaven, CT, USA, 1983; pp. 39–54. [Google Scholar]
- Kevrekidis, E.; St. Seymour, K.; Tombros, S.; Koukouvelas, I.; Oyman, T.; Zhai, D.; Liu, J.; Kalaitzidis, S. The Molaoi Pb-Zn(-Ag) deposit in Southeastern Peloponnese, Hellas. In Proceedings of the GAC-MAC 2017, Kingston, ON, Canada, 14–18 May 2017. [Google Scholar]
- Barton, P.B.; Toulmin, P. Phase relations involving sphalerite in the Zn-Fe-S system. Econ. Geol. 1966, 61, 815–849. [Google Scholar] [CrossRef]
- Hannington, M.D.; Scott, S.D. Sulfidation equilibria as guides to gold mineralization in volcanogenic sulfides: Evidence from sulfide mineralogy and the composition of sphalerite. Econ. Geol. 1989, 84, 1978–1995. [Google Scholar] [CrossRef]
- Torró, L.; Benites, D.; Vallance, J.; Laurent, O.; Ortiz-Benavente, B.A.; Chelle-Michou, C.; Proenza, J.A.; Fontboté, L. Trace element geochemistry of sphalerite and chalcopyrite in arc-hosted VMS deposits. J. Geochem. Explor. 2020, 232, 106882. [Google Scholar] [CrossRef]
- Chen, A.-X.; Li, Y.-H.; Chen, Y.; Yu, H.-M.; Huang, F. Silicon isotope composition of subduction zone fluids as recorded by jadeites from Myanmar. Contrib. Mineral. Petrol. 2020, 175, 6. [Google Scholar] [CrossRef]
- Savage, P.S.; Georg, R.B.; Williams, H.M.; Burton, K.W.; Halliday, A.N. Silicon isotope fractionation during magmatic differentiation. Geochim. Cosmochim. Acta 2011, 75, 6124–6139. [Google Scholar] [CrossRef]
- Kleine, B.I.; Stefánsson, A.; Halldórsson, S.A.; Whitehouse, M.J.; Jónasson, K. Silicon and oxygen isotopes unravel quartz formation processes in the Icelandic crust. Geochem. Perspect. Lett. 2018, 7, 5–11. [Google Scholar] [CrossRef]
- Chon, H.T.; Shimazaki, H. Iron, manganese, and cadmium contents of sphalerites and their genetical implications to hydrothermal metallic ore deposits in Korea. J. Korean Inst. Min. Geol. 1986, 19, 139–149. [Google Scholar]
- Frenzel, M.; Hirsch, T.; Gutzmer, J. Gallium, Germanium, Indium, and other trace and minor elements in sphalerite as a function of deposit type—A meta-analysis. Ore Geol. Rev. 2016, 76, 52–78. [Google Scholar] [CrossRef]
- Meng, Y.-M.; Zhang, X.; Huang, X.-W.; Hu, R.; Bi, X.; Meng, S.; Zhou, L.; Zheng, Y. A review of the Zn-Pb deposits in Sichuan-Yunnan-Guizhou metallogenic region with emphasis on the enrichment mechanism of Ge, Ga, and In. Ore Geol. Rev. 2024, 164, 105853. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, Z.; Sun, H.; Koua, K.A.D.; Lyu, C. LA-ICP-MS trace element analysis of sphalerite and pyrite from the Beishan Pb-Zn ore district, south China: Implications for ore genesis. Ore Geol. Rev. 2022, 150, 105128. [Google Scholar] [CrossRef]
- Kelvin, M.; Whiteman, E.; Petrus, J.; Leybourne, M.; Nkuma, V. Application of LA-ICP-MS to process metallurgy: Gallium and Germanium recovery at Kipushi copper-zinc deposit. Miner. Eng. 2022, 176, 107332. [Google Scholar] [CrossRef]
- Falkenberg, J.J.; Keith, M.; Haase, K.M.; Klemd, R.; Bach, W.; Strauss, H.; Storch, B.; Peters, C.; Rubin, K.H.; Anderson, M.O. In-situ mineral, bulk sulfide-sulfate, S- and Pb isotope, and whole rock major and chalcophile element data from the submarine Niuatahi hydrothermal system, Lau basin, Tonga rear-arc [dataset bundled publication]. PANGAEA 2022. [Google Scholar] [CrossRef]
- Armbruster, T.; Bonazzi, P.; Akasaka, M.; Bermanec, V.; Chopin, C.; Gieré, R.; Heuss-Assbichler, S.; Liebscher, A.; Menchetti, S.; Pan, Y.; et al. Recommended nomenclature of epidote-group minerals. Eur. J. Mineral. 2006, 18, 551–567. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R. Crystal chemistry and paragenesis of compositionally unique (Al-, Fe-, Nb-, and Zr-rich) titanite from Afrikanda, Russia. Am. Mineral. 2004, 89, 1752–1762. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
