Open-Circuit Technology of Zinc Oxide Ore Flotation with Ternary Collector and Its Adsorption Characteristics on Smithsonite Surface
Abstract
:1. Introduction
2. Experiment
2.1. Materials and Reagents
2.2. Flotation Tests
2.3. Zeta Potential
2.4. Adsorption Tests
2.5. XPS Analysis
2.6. Contact-Angle Test
3. Results and Discussion
3.1. Flotation of Zinc Oxide Ores
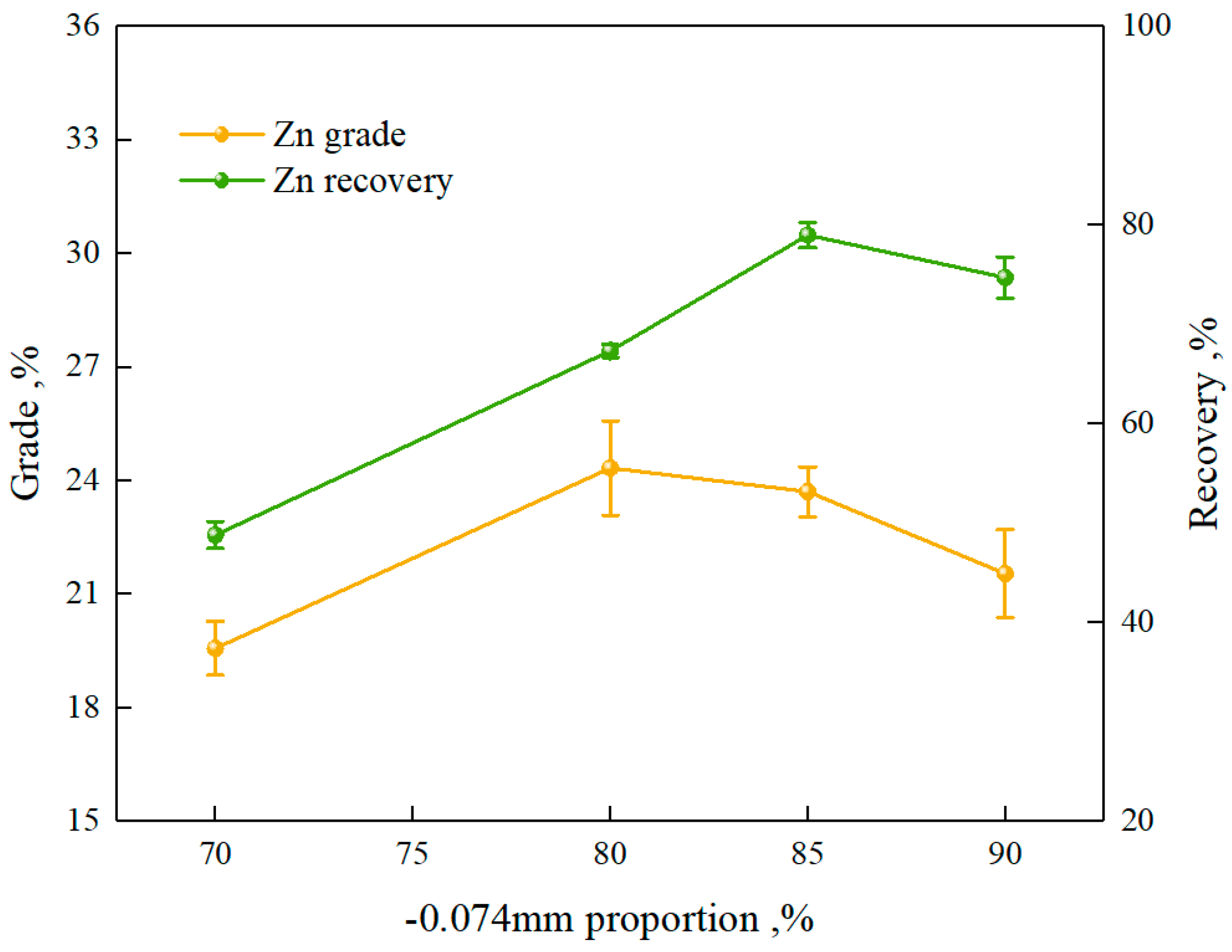
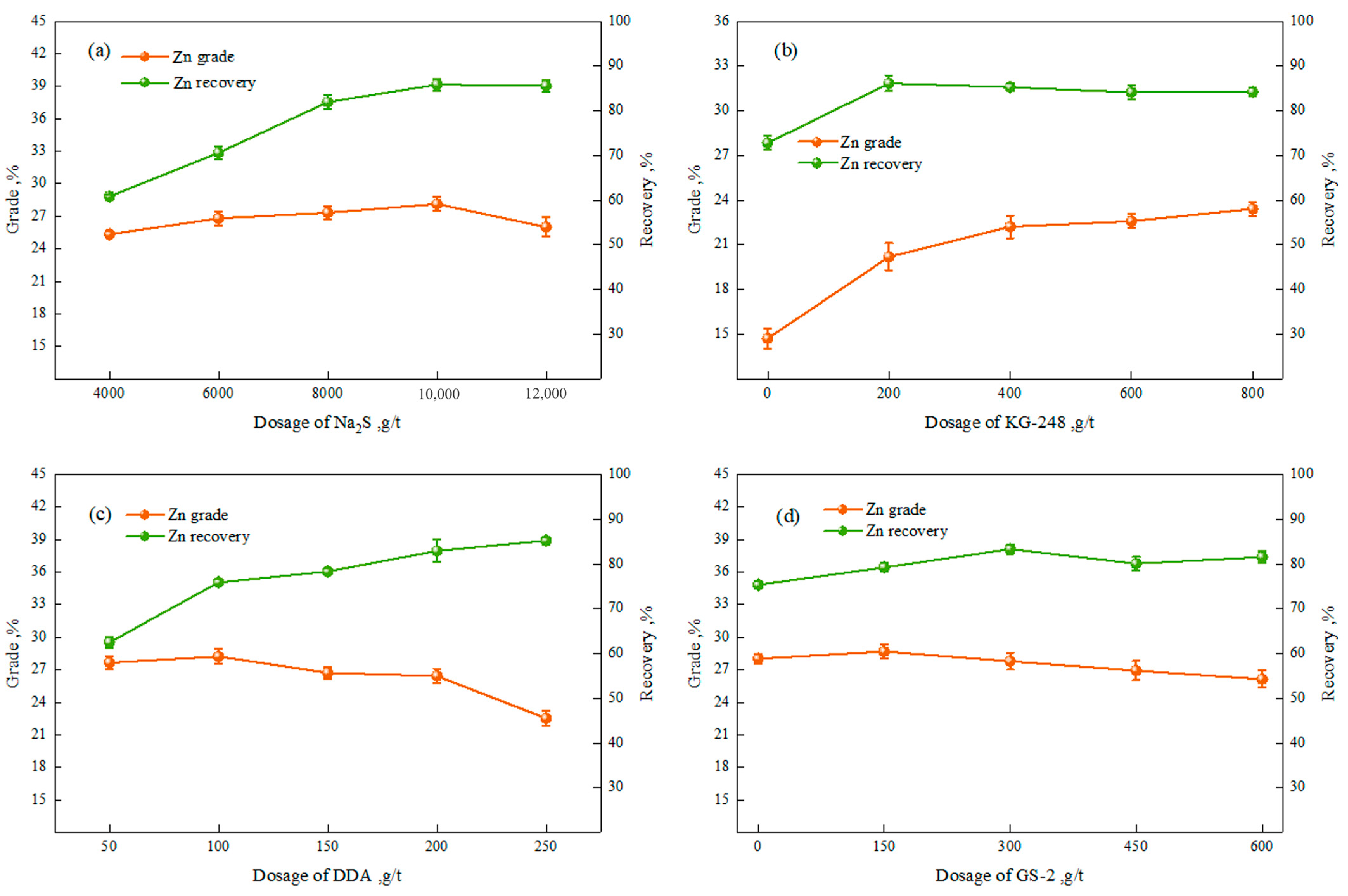
3.1.1. Flotation Test of Zinc
3.1.2. Flotation Open-Circuit Test
3.2. Flotation Behavior and Surface-Adsorption Analysis of Smithsonite
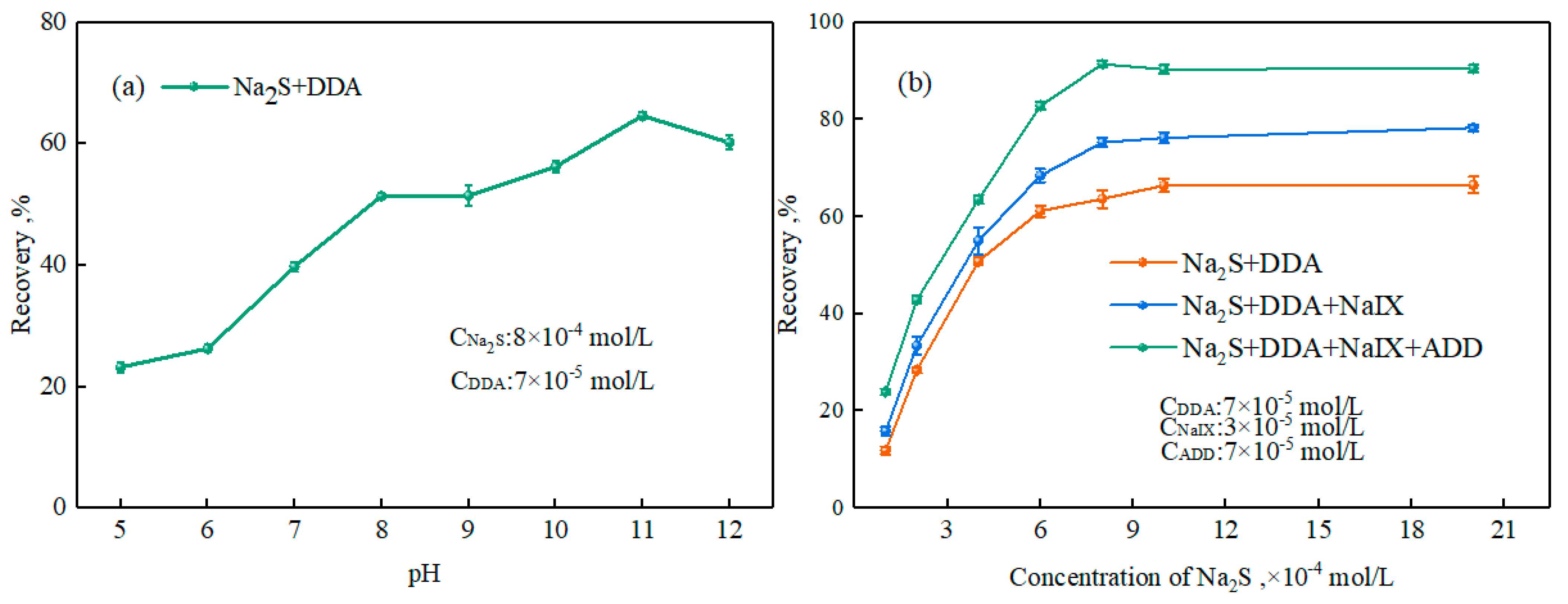
3.2.1. Effect of Ternary Collector on Smithsonite Flotation Behavior
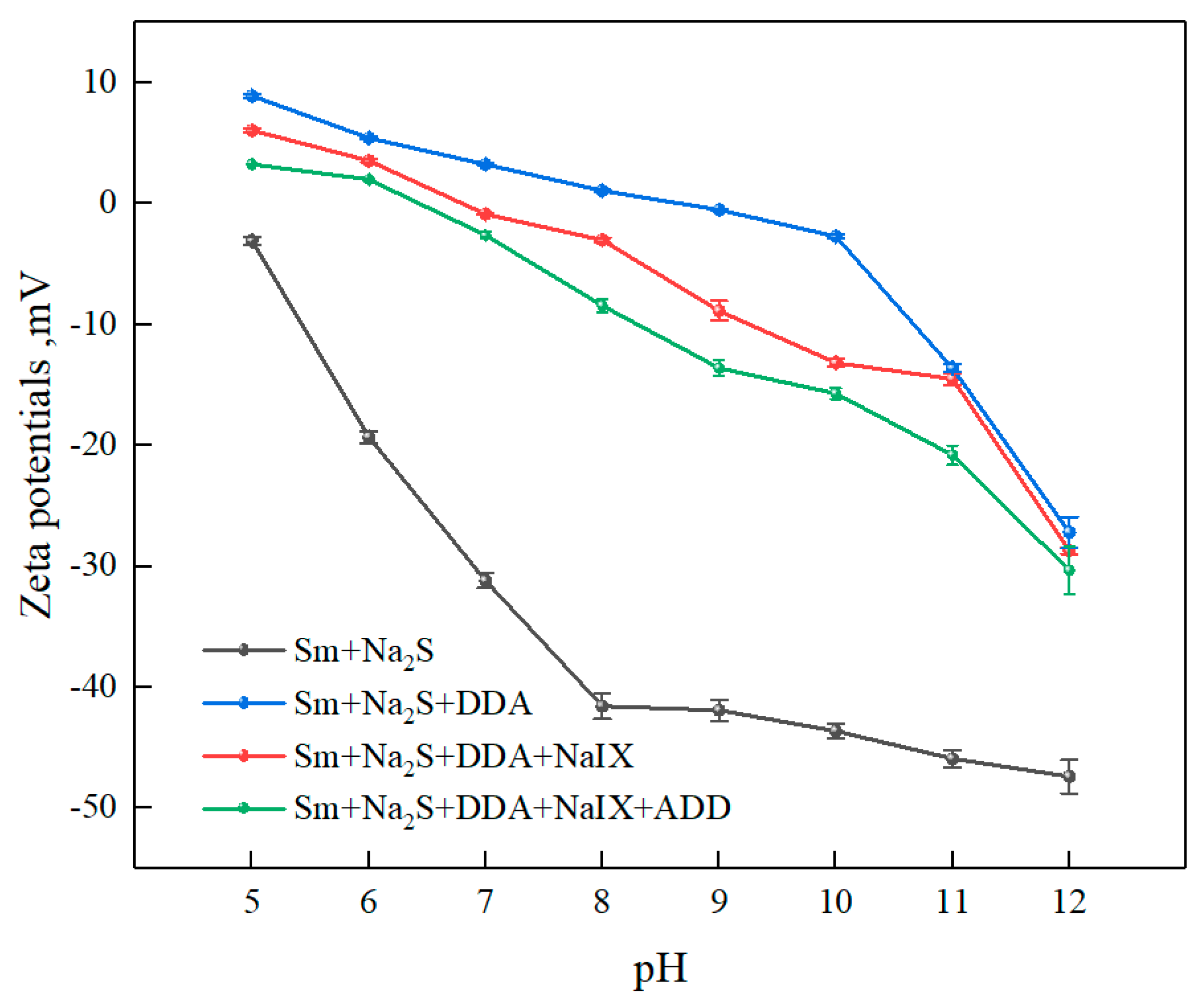
3.2.2. Zeta-Potential Measurements
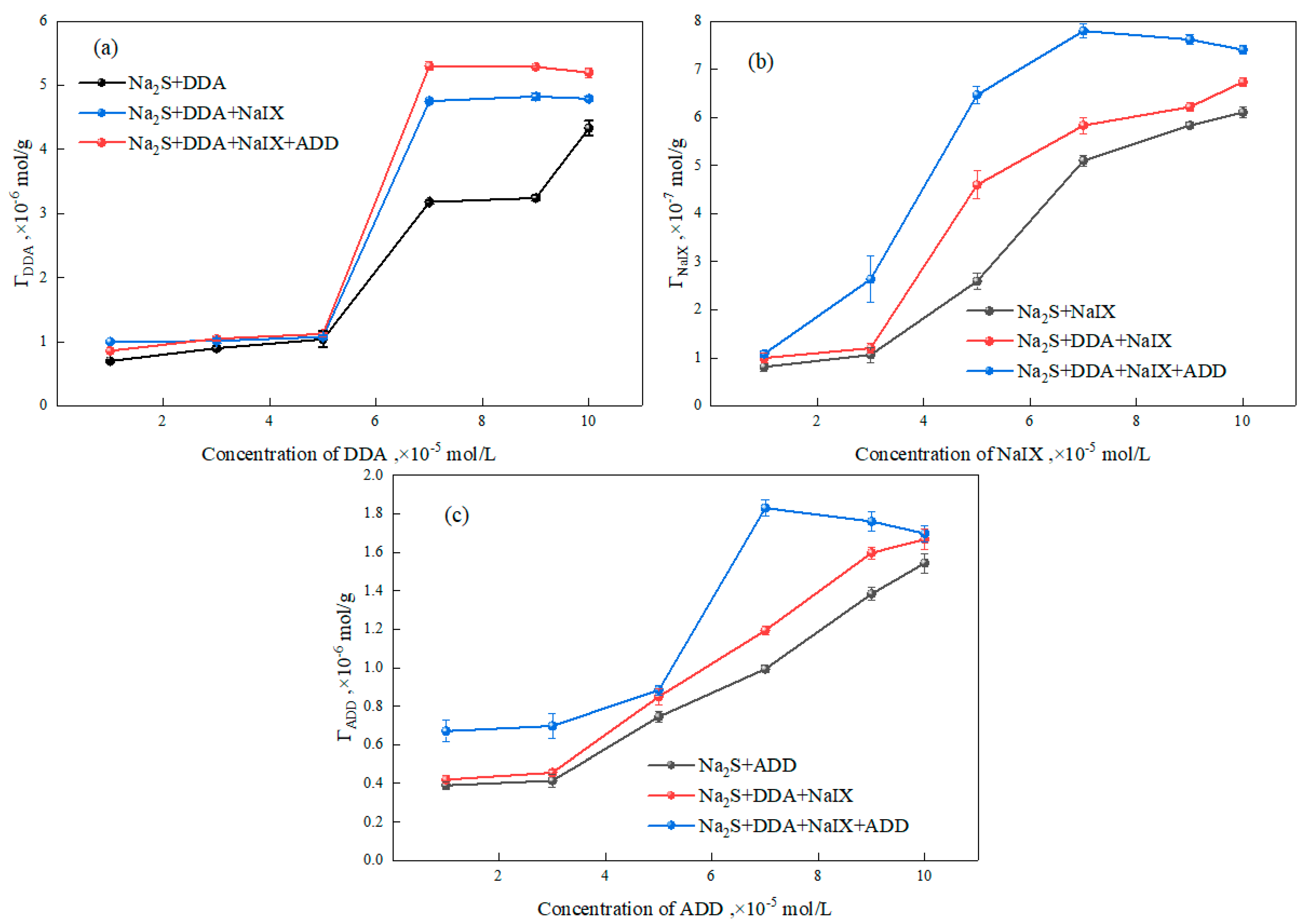
3.2.3. Adsorption Experiments
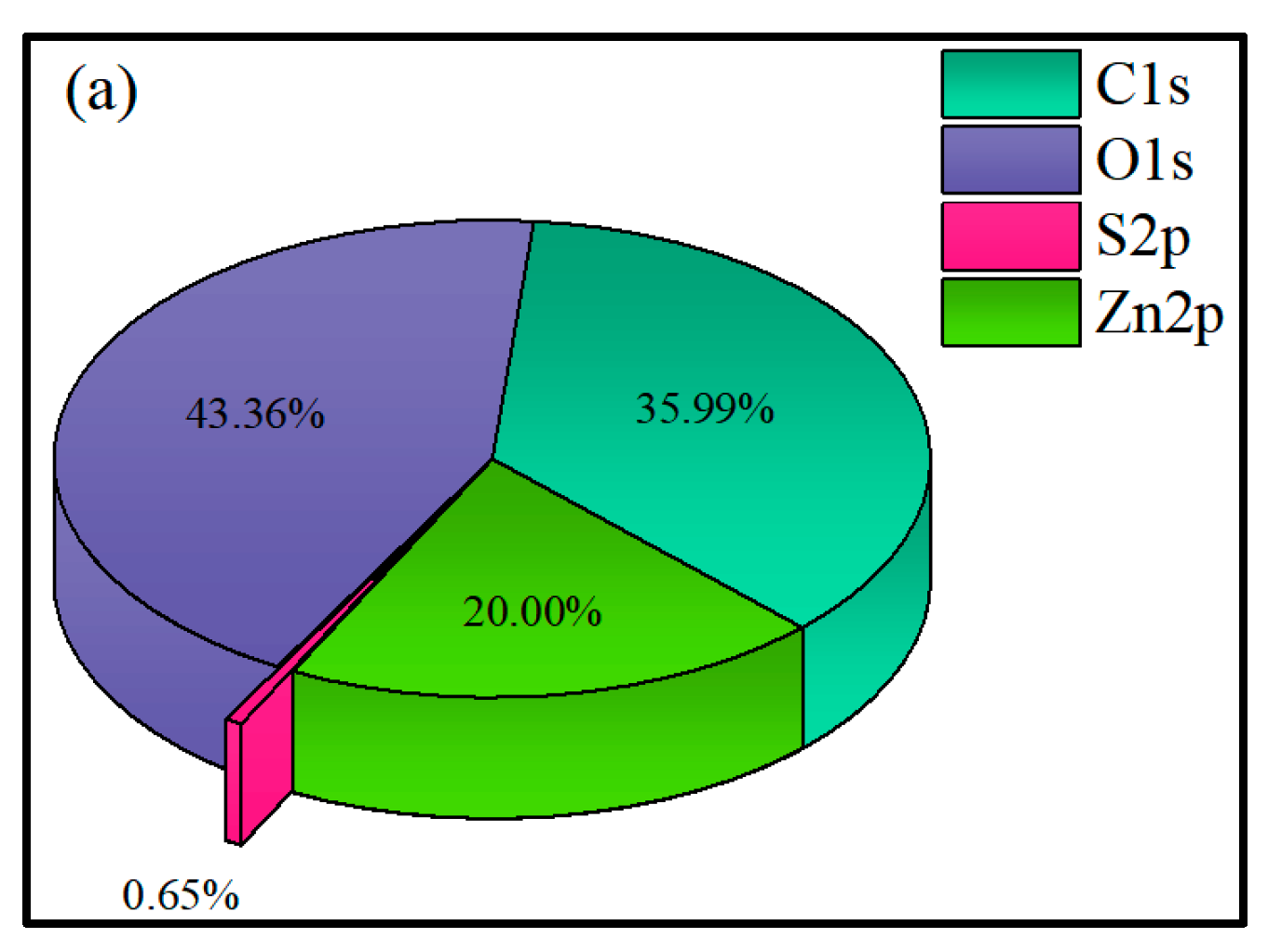
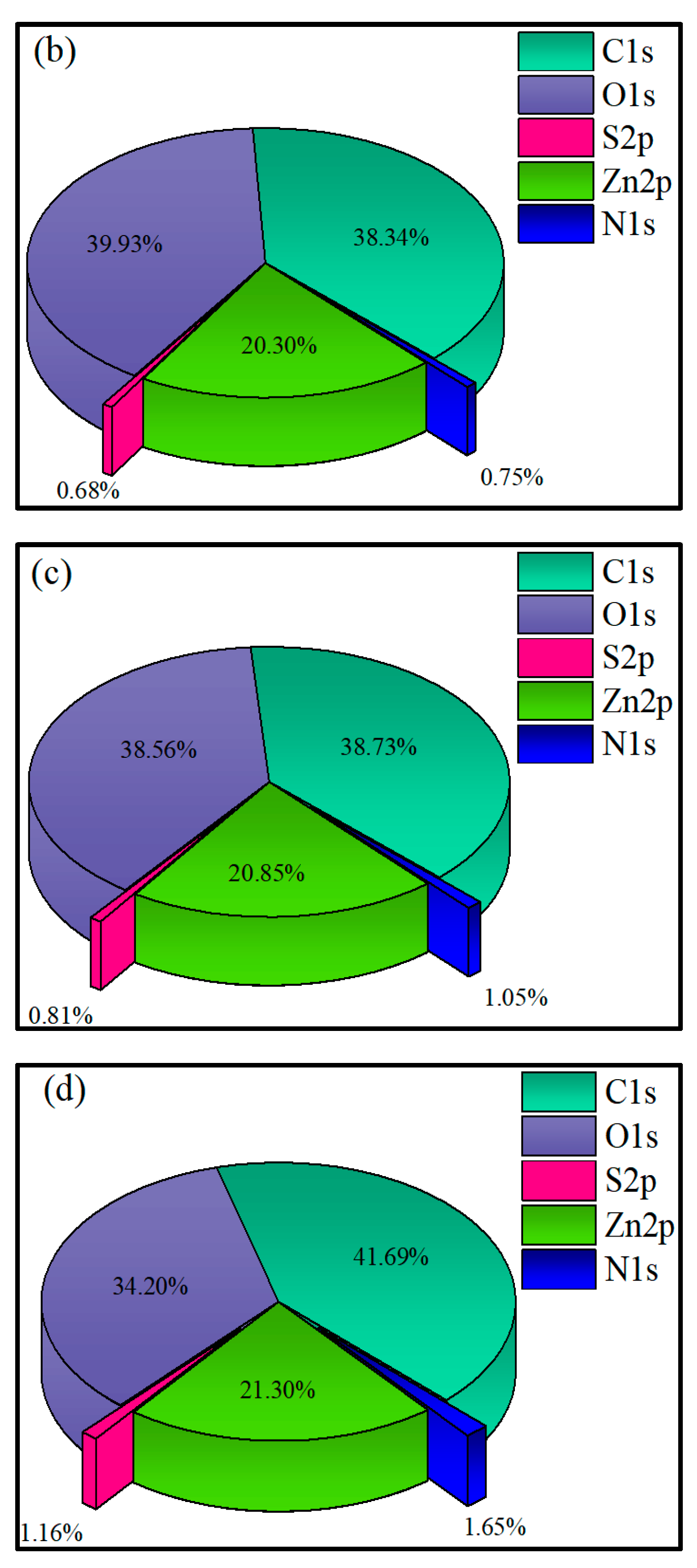
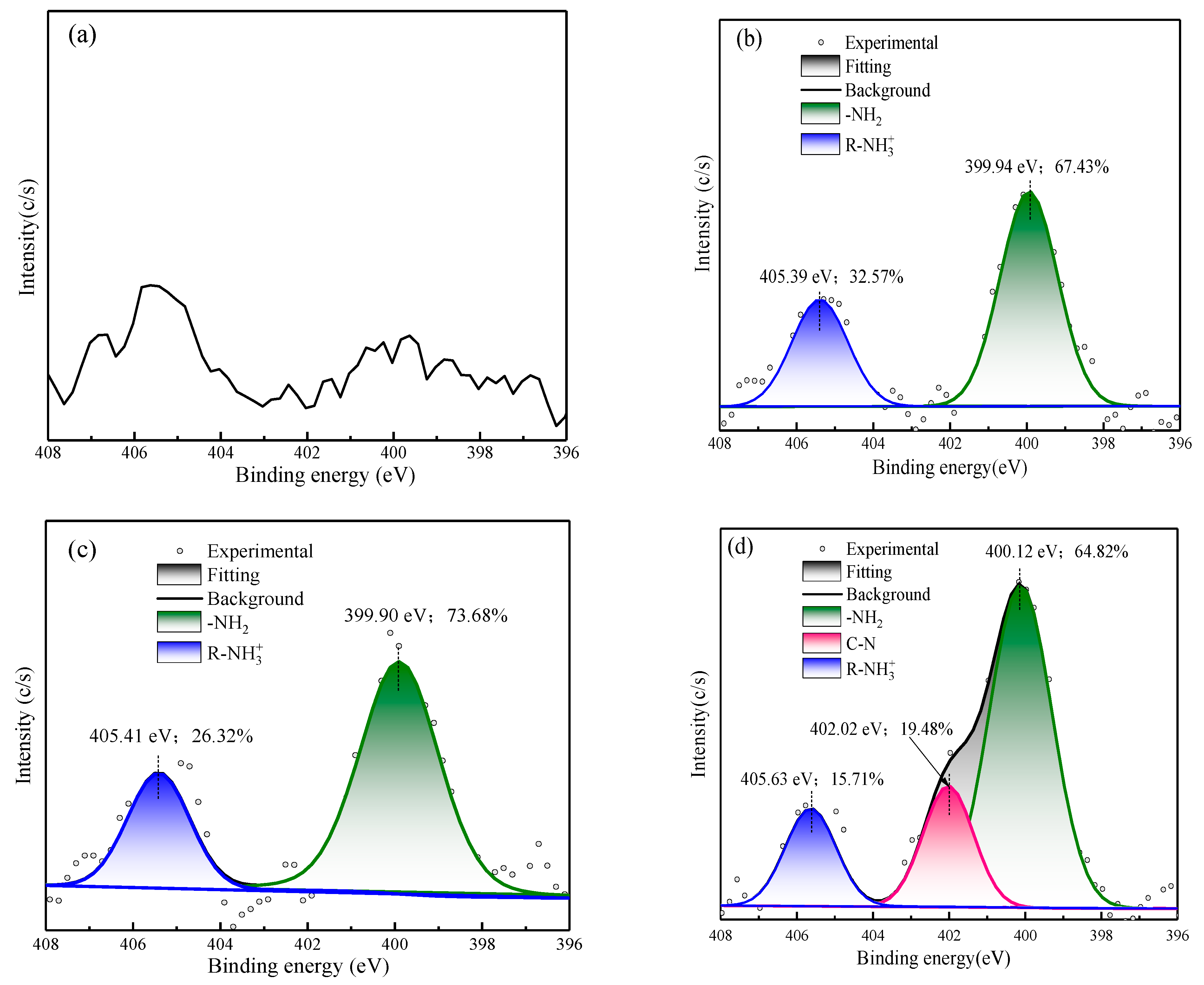
3.2.4. XPS Analysis
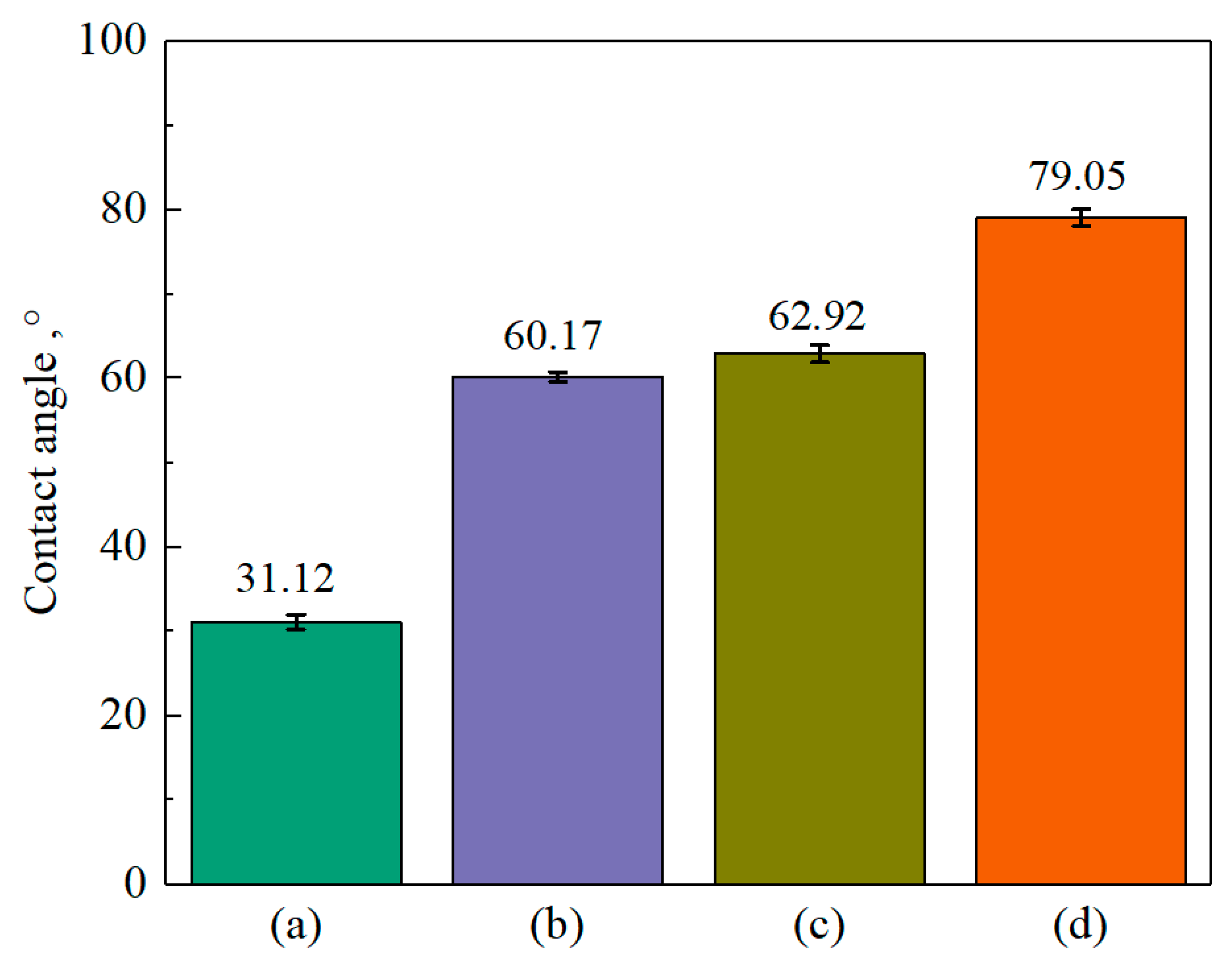
3.3. Contact-Angle Test
4. Conclusions
- By adopting the new open-circuit process and using a ternary-collector system to treat low-grade zinc oxide ore, favorable flotation indices of 28.71% and 86.24% were obtained for the zinc grade and recovery, respectively. Additionally, the open-circuit process effectively solved the problem of froth buildup caused by amine collectors during flotation.
- The results of microflotation experiments revealed that the floatability of smithsonite enhanced significantly by employing the ternary-collector treatment. Specifically, the recovery of smithsonite increased to 94.40%.
- The zeta-potential measurements and adsorption-capacity test results indicated that the ternary collector can be co-adsorbed onto the sulfidized smithsonite surface and thus reduce the total consumption of reagents.
- XPS results showed that the addition of NaIX promoted DDA adsorption on the mineral surface and that the adsorption of ADD increased the contact angle of the sulfidized smithsonite surface treated with the ternary collector, thus enhancing the hydrophobicity and floatability of the smithsonite.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Majid, E.; Mahdi, G.; Mehdi, I. A review of zinc oxide mineral beneficiation using flotation method. Adv. Colloid. Inter. Sci. 2014, 206, 68–78. [Google Scholar] [CrossRef]
- Hosking, N.C.; Ström, M.A.; Shipway, P.H.; Rudd, C.D. Corrosion resistance of zinc-magnesium coated steel. Corros. Sci. 2007, 49, 3669–3695. [Google Scholar] [CrossRef]
- Zhao, H.B.; Gan, X.W.; Wang, J.; Tao, L.; Qiu, W.Q.; Qiu, G.Z. Stepwise bioleaching of Cu-Zn mixed ores with comprehensive utilization of silver-bearing solid waste through a new technique process. Hydrometallurgy 2017, 171, 374–386. [Google Scholar] [CrossRef]
- Mudd, G.M.; Jowitt, S.M.; Werner, T.T. The world’s lead-zinc mineral resources: Scarcity, data, issues and opportunities. Ore Geol. Rev. 2017, 80, 1160–1190. [Google Scholar] [CrossRef]
- Irannajad, M.; Ejtemaei, M.; Gharabaghi, M. The effect of reagents on selective flotation of smithsonite–calcite–quartz. Miner. Eng. 2009, 22, 766–771. [Google Scholar] [CrossRef]
- Moradi, S.; Monhemius, A.J. Mixed sulphide-oxide lead and zinc ores: Problems and solutions. Miner. Eng. 2011, 24, 1062–1076. [Google Scholar] [CrossRef]
- Navidi Kashani, A.H.; Rashchi, F. Separation of oxidized zinc minerals from tailings: Influence of flotation reagents. Miner. Eng. 2008, 21, 967–972. [Google Scholar] [CrossRef]
- Li, B.B.; Zhang, G.F.; Shi, Q. Atomic insights into the interaction mechanism underpinning carbonate ion adsorption in smithsonite flotation. Process Saf. Environ. Prot. 2024, 190, 11–21. [Google Scholar] [CrossRef]
- Marabini, A.M.; Ciriachi, M.; Plescia, P.; Barbaro, M. Chelating reagents for flotation. Miner. Eng. 2007, 20, 1014–1025. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Z.; Wei, Q.; Jiao, F.; Yang, C.; Li, W.; Qin, W. Study on flotation recovery of typical carbon-bearing lead-zinc sulphide ore in Guizhou with pre-decarbonization. Geochemistry 2024, 84, 126096. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Forssberg, E. Smithsonite flotation using potassium amyl xanthate and hexylmercaptan. Trans. Inst. Min. Metall. Sect. C Miner. Process. Extr. Metall. 2006, 115, 107–112. [Google Scholar] [CrossRef]
- Nanda, S.; Kumar, S.; Mandre, N.R. Flotation behavior of a complex lead-zinc ore using individual collectors and its blends for lead sulfide. J. Dispers. Sci. Technol. 2023, 44, 1703–1710. [Google Scholar] [CrossRef]
- Wang, J.G.; Ji, Y.H.; Cheng, S.Y.; Liu, S.; Cao, J.; Chen, P. Selective flotation separation of galena from sphalerite via chelation collectors with different nitrogen functional groups. Appl. Surf. Sci. 2021, 568, 150956. [Google Scholar] [CrossRef]
- Shen, Z.H.; Zhang, Q.; Fang, J. Research Progress in Surface Sulfidization of Smithsonite. Nonferr. Met. Mieral Process. Sect. 2021, 01, 37–46+59. [Google Scholar] [CrossRef]
- Zhu, H.L.; Qin, W.Q.; Chen, C.; Chai, L.Y.; Li, L.S.; Liu, S.J.; Zhang, T. Selective flotation of smithsonite, quartz and calcite using alkyldiamineether as collector. Trans. Nonferr. Metals Soc. China 2018, 28, 163–168. [Google Scholar] [CrossRef]
- Luo, B.; Nie, W.L.; Dong, J.S. Effect of Lead Ions on the Sulfidization Flotation of Smithsonite Using Sodium Butyl Xanthate as a Collector. Miner. Eng. 2022, 185, 107710. [Google Scholar] [CrossRef]
- Ceylan, Y.; Altınışık, Y.; Cebeci, H.; Sis, L.K. A Process Mineralogy Approach to the Flotation of Complex Lead–Zinc Ores from Görgü (Malatya) Region. Min. Met. Explor. 2022, 39, 1219–1232. [Google Scholar] [CrossRef]
- Mehdilo, A.; Zarei, H.; Irannajad, M.; Arjmandfar, H. Flotation of zinc oxide ores by cationic and mixed collectors. Miner. Eng. 2012, 36, 331–334. [Google Scholar] [CrossRef]
- Feng, Q.C.; Wen, S.M. Formation of zinc sulfide specieson smithsonite surfaces and its response to flotation performance. J. Alloys Compd. 2017, 709, 602–608. [Google Scholar] [CrossRef]
- Mehdilo, A.; Irannajad, M.; Zarei, H. Smithsonite Flotation from Zinc Oxide Ore using Alkyl Amine Acetate Collectors. Sep. Sci. Technol. 2014, 49, 445–457. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Huang, K.H.; Yang, S.Y.; Liang, Z.A. Studies of benzyl hydroxamic acid/calcium lignosulphonate addition order in the flotation separation of smithsonite from calcite. Int. J. Min. Sci. Technol. 2021, 31, 1153–1158. [Google Scholar] [CrossRef]
- Barbery, G. Complex sulphide ores: Processing options. Miner. Process. A Crossroads 1986, 117, 157–194. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Forssberg, E. Adsorption studies of smithsonite flotation using dodecylamine and oleic acid. Min. Metall. Explor. 2006, 23, 87–96. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.M.; Zhang, G.F.; Chen, W.W.; Chen, Y.F. Effect of sodium carbonate on flotation behavior of smithsonite and its mechanism in presence of sodium oleate. Chin. J. Nonferrous Met. 2017, 27, 2379–2384. [Google Scholar] [CrossRef]
- Ferreira, P.H.T.; Fernandes Lima, R.M. Concentration of oxidized Brazilian zinc ore by flotation: Comparative study between anionic and cationic routes. Sep. Sci. Technol. 2022, 57, 2625–2634. [Google Scholar] [CrossRef]
- Sun, H.F.; Niu, F.S.; Zhang, J.X. Investigation on the flotation separation of smithsonite from calcite using calcium lignosulphonate as depressant. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127571. [Google Scholar] [CrossRef]
- Shi, Q.; Feng, Q.M.; Zhang, G.F.; Deng, H. Electrokinetic properties of smithsonite and its floatability with anionic collector. Colloids Surf. A Physicochem. Eng. Asp. 2012, 410, 178–183. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Forssberg, E. Physicochemical studies of smithsonite flotation using mixed anionic/cationic collector. Miner. Eng. 2007, 20, 624. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Forssberg, E. Studies on selective flotation of smithsonite from silicate minerals using mercaptans and one stage desliming. Miner. Process. Extr. Metall. 2011, 120, 79–84. [Google Scholar] [CrossRef]
- Majid, E.; Mehdi, I.; Mahdi, G. Influence of important factors on flotation of zinc oxide mineral using cationic, anionic and mixed (cationic/anionic) collectors. Miner. Eng. 2011, 24, 1402–1408. [Google Scholar] [CrossRef]
- Wang, L.; Hu, G.Y.; Sun, W.; Khoso, S.A.; Liu, R.Q.; Zhang, X.F. Selective flotation of smithsonite from dolomite by using novel mixed collector system. Trans. Nonferr. Met. Soc. China 2019, 29, 1082–1089. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, L.H.; Wang, J.M.; Wang, L.; Xiao, J.H. A comparison study of adsorption of benzohydroxamic acid and amyl xanthate on smithsonite with dodecylamine as co-collector. Appl. Surf. Sci. 2017, 426, 1141–1147. [Google Scholar] [CrossRef]
- Gao, Y.; Fu, X.Z.; Khoso, S.A.; Pan, Z.J.; Han, H.S.; Sun, W.; Yue, T. A quantitative innovation perspective on synergism and selectivity mechanism of mixed collectors in flotation. Miner. Eng. 2024, 206, 108474. [Google Scholar] [CrossRef]
- Deng, R.R.; Wang, Y.; Duan, W.T.; Xing, D.Q.; Hu, Y. Induced crystallization of Pb2+ on smithsonite surface during sulfidation-xanthate flotation. Colloids Surf. 2022, 650, 129576. [Google Scholar] [CrossRef]
- Wen, S.M. Thermodynamic theory of flotation for a complex multiphase solid–liquid system and high-entropy flotation. Int. J. Min. Met. Mater. 2024, 31, 1177–1197. [Google Scholar] [CrossRef]
- Jia, K.; Feng, Q.M.; Zhang, G.F.; Ji, W.Y.; Zhang, W.K.; Yang, B.Q. The role of S(II) and Pb (II) in xanthate flotation of smithsonite: Surface properties and mechanism. Appl. Surf. Sci. 2018, 442, 92–100. [Google Scholar] [CrossRef]
- Wang, M.T.; Zhang, G.F.; Chen, Y.F.; Zhao, L. Effect of surface oxidization on quartz slime coating in the sulfidization-amine flotation of smithsonite. Miner. Eng. 2022, 188, 107847. [Google Scholar] [CrossRef]
- Cao, S.M.; Zhang, X.L.; Zeng, S.Q. The present situation and progress of zinc oxide flotation. Adv. Mater. Res. 2013, 756, 68–71. [Google Scholar] [CrossRef]
- Liao, R.P.; Wen, S.M.; Liu, J.; Bai, S.J.; Feng, Q.C. Synergetic adsorption of dodecylamine and octyl hydroxamic acid on sulfidized smithsonite: Insights from experiments and molecular dynamics simulation. Sep. Purif. Technol. 2024, 329, 125106. [Google Scholar] [CrossRef]
- Krystian, C.; Cezary, R.; Grazyna, P. Flotation of zinc and lead oxide minerals from Olkusz region calamine ores. E3S Web Conf. 2016, 8, 01042. [Google Scholar] [CrossRef]
- Sun, Q.; Feng, Q.M.; Shi, Q. Adsorption mechanism of smithsonite by dodecyl phosphate ester potassium. J. Cent. South Univ. 2018, 49, 1845–1850. [Google Scholar] [CrossRef]
- Arturo, B.T.; Roberto, P.G.; Diego, M.C. Zeta potential of air bubbles conditioned with typical froth flotation reagents. Int. J. Min. Process 2015, 140, 50–57. [Google Scholar] [CrossRef]
- Wang, M.L.; Zhao, W.J.; Han, G.; Feng, Q.C. Utilization of lead ions to improve surface hydrophobicity and flotation recovery of sulfidized smithsonite. Colloids Surf. 2023, 663, 131126. [Google Scholar] [CrossRef]
- Zhang, G.F.; Cui, M.M.; Zhu, Y.G.; Shi, Q.; Luo, N. Effect of water glass on flotation separation of smithsonite and quartz. Chin. J. Nonferrous Met. 2012, 22, 3535–3541. [Google Scholar]
- Luo, B.; Liu, J.; Liu, Q.; Song, C.; Yu, L.; Li, S.; Lai, H. A Mechanism for the Adsorption of 2-(Hexadecanoylamino)Acetic Acid by Smithsonite: Surface Spectroscopy and Microflotation Experiments. Minerals 2019, 9, 15. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, J.; Dong, W.C.; Hao, J.M.; Wang, Y. Study on Sulfide Layer Attenuation Behavior of Smithsonite During Sulfidization Flotation. Front. Mater. 2019, 6, 347. [Google Scholar] [CrossRef]
- Zeng, Y.H.; Yao, X.; Liu, G.X.; He, G.M.; Yu, X.Y.; He, G.C.; Huang, Z.Q.; Zhang, R.R.; Cheng, C. Flotation behavior and mechanism of phenylpropenyl hydroxamic acid for the separation of smithsonite and calcite. J. Mol. Liq. 2021, 339, 116893. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, W.G.; Liu, W.B.; Zhou, S.J.; Peng, X.Y. Investigation on matching relationship between surface characters and collector properties: Achieving flotation separation of zinc oxide minerals from quartz. Colloids Surf. A 2021, 617, 126392. [Google Scholar] [CrossRef]

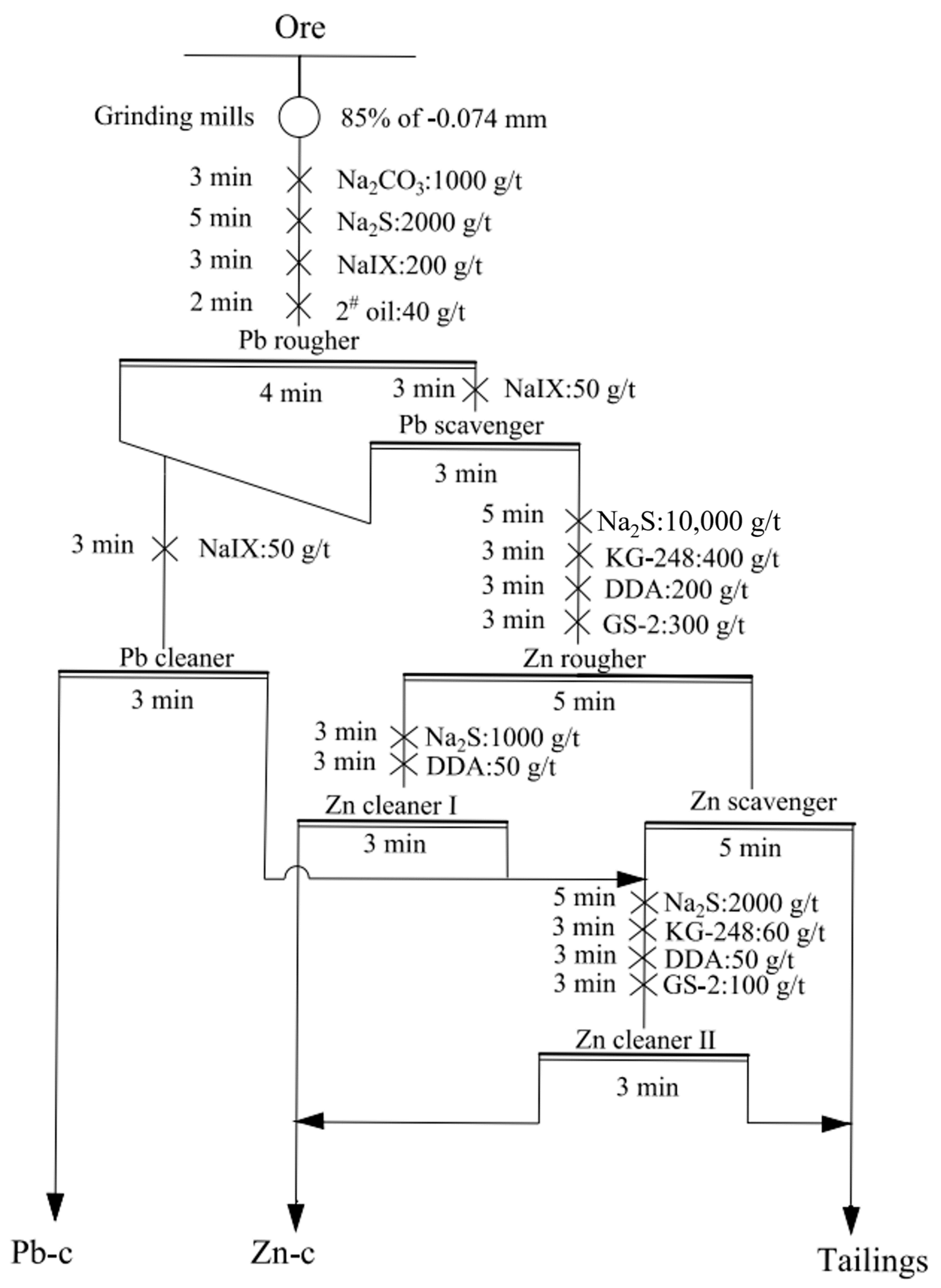

| Product | Yield, % | Zn Grade, % | Zn Recovery, % |
|---|---|---|---|
| Pb-c | 4.89 | 4.96 | 2.97 |
| Zn-c | 24.52 | 28.71 | 86.24 |
| Tailings | 70.59 | 1.25 | 10.79 |
| Sum | 100.00 | 8.16 | 100.00 |
| Name | Price, ¥/t | Dosage, g/t | Price, ¥ |
|---|---|---|---|
| Na2CO3 | 800 | 1000 | 0.8 |
| Na2S | 2200 | 15,000 | 33 |
| NaIX | 10,000 | 300 | 3 |
| KG-248 | 15,500 | 460 | 7.13 |
| DDA | 17,800 | 300 | 5.34 |
| GS-2 | 12,500 | 400 | 5 |
| 2# oil | 6000 | 40 | 0.24 |
| Sum | - | - | 54.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Feng, Q.; Zhang, Q.; Wen, S. Open-Circuit Technology of Zinc Oxide Ore Flotation with Ternary Collector and Its Adsorption Characteristics on Smithsonite Surface. Minerals 2024, 14, 902. https://doi.org/10.3390/min14090902
Li Z, Feng Q, Zhang Q, Wen S. Open-Circuit Technology of Zinc Oxide Ore Flotation with Ternary Collector and Its Adsorption Characteristics on Smithsonite Surface. Minerals. 2024; 14(9):902. https://doi.org/10.3390/min14090902
Chicago/Turabian StyleLi, Zhiwei, Qicheng Feng, Qian Zhang, and Shuming Wen. 2024. "Open-Circuit Technology of Zinc Oxide Ore Flotation with Ternary Collector and Its Adsorption Characteristics on Smithsonite Surface" Minerals 14, no. 9: 902. https://doi.org/10.3390/min14090902





