Surface Chemistry and Flotation of Gold-Bearing Pyrite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Flotation Tests
2.2.2. Electrochemical Studies
3. Results
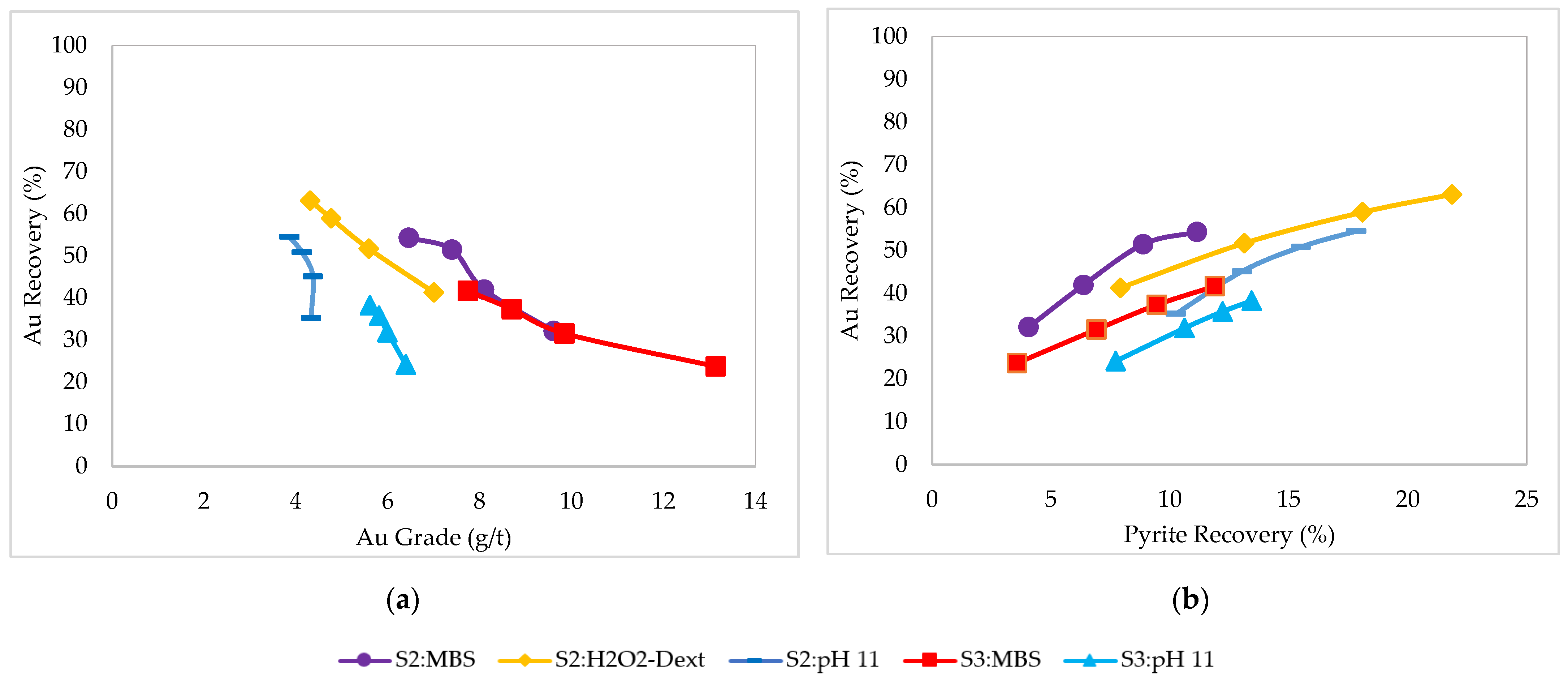
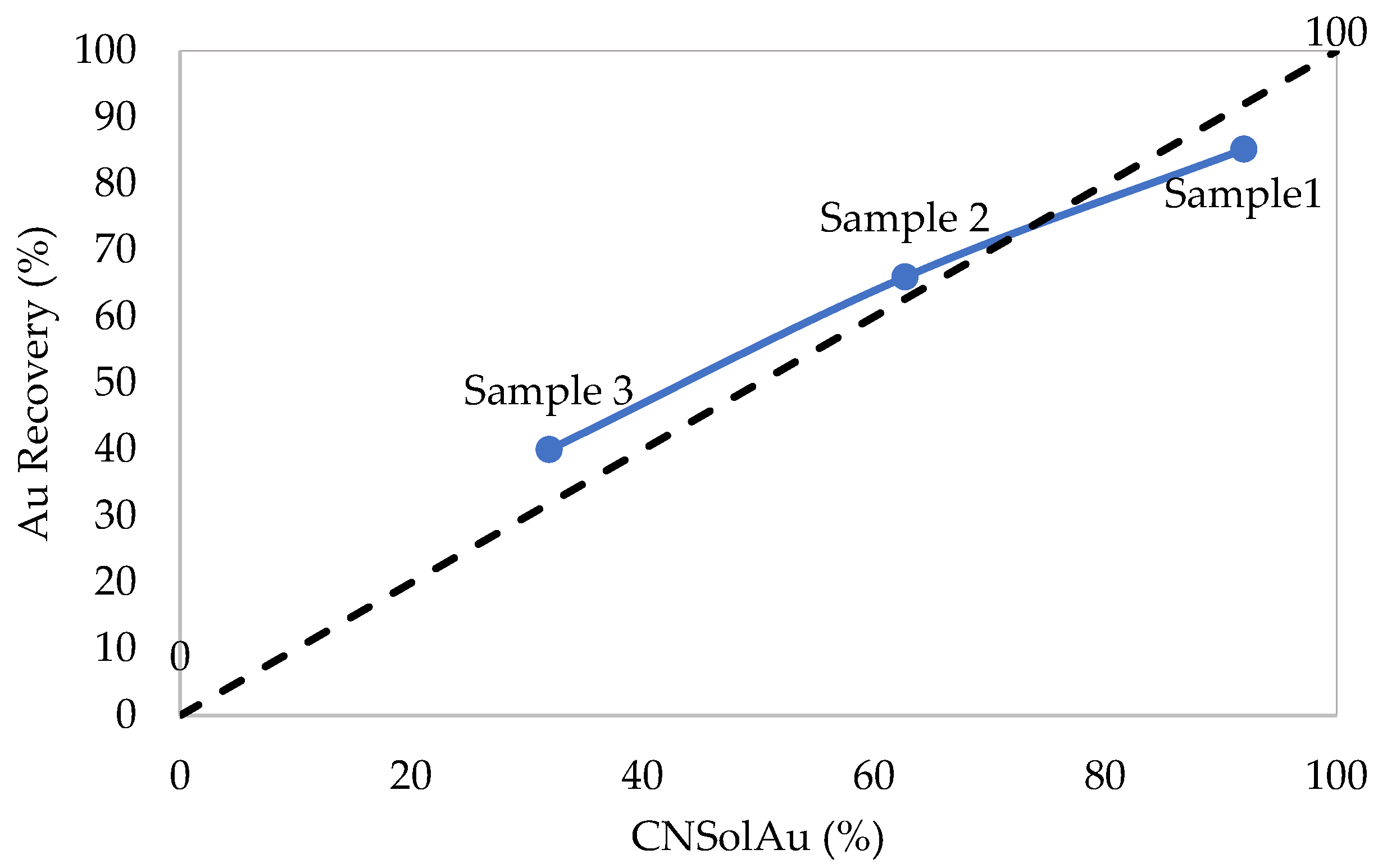
3.1. Flotation Tests
3.1.1. Flotation Concentrate (Sample 1)
3.1.2. Massive Pyrite Ore Samples (Sample 2 and Sample 3)
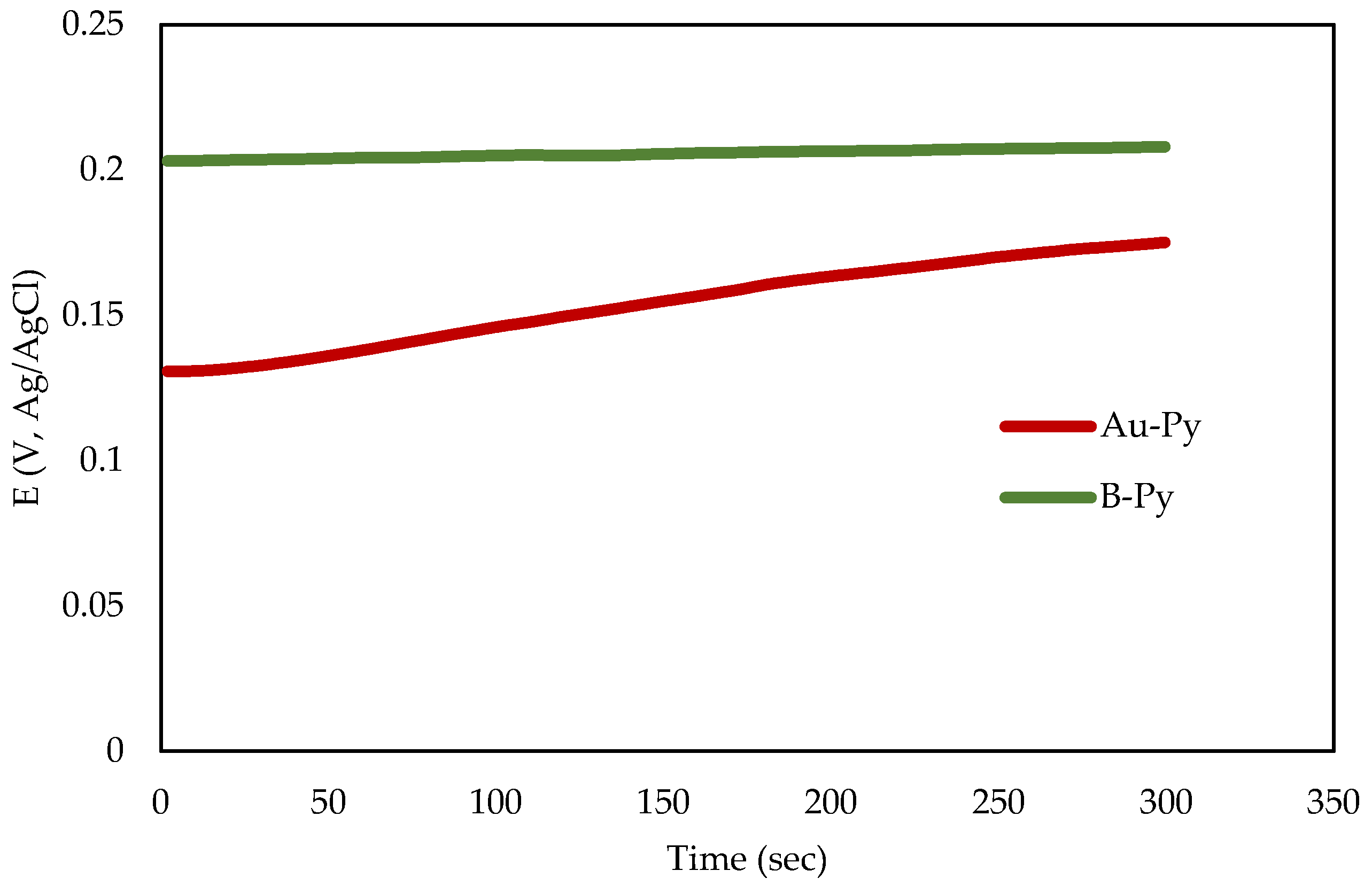
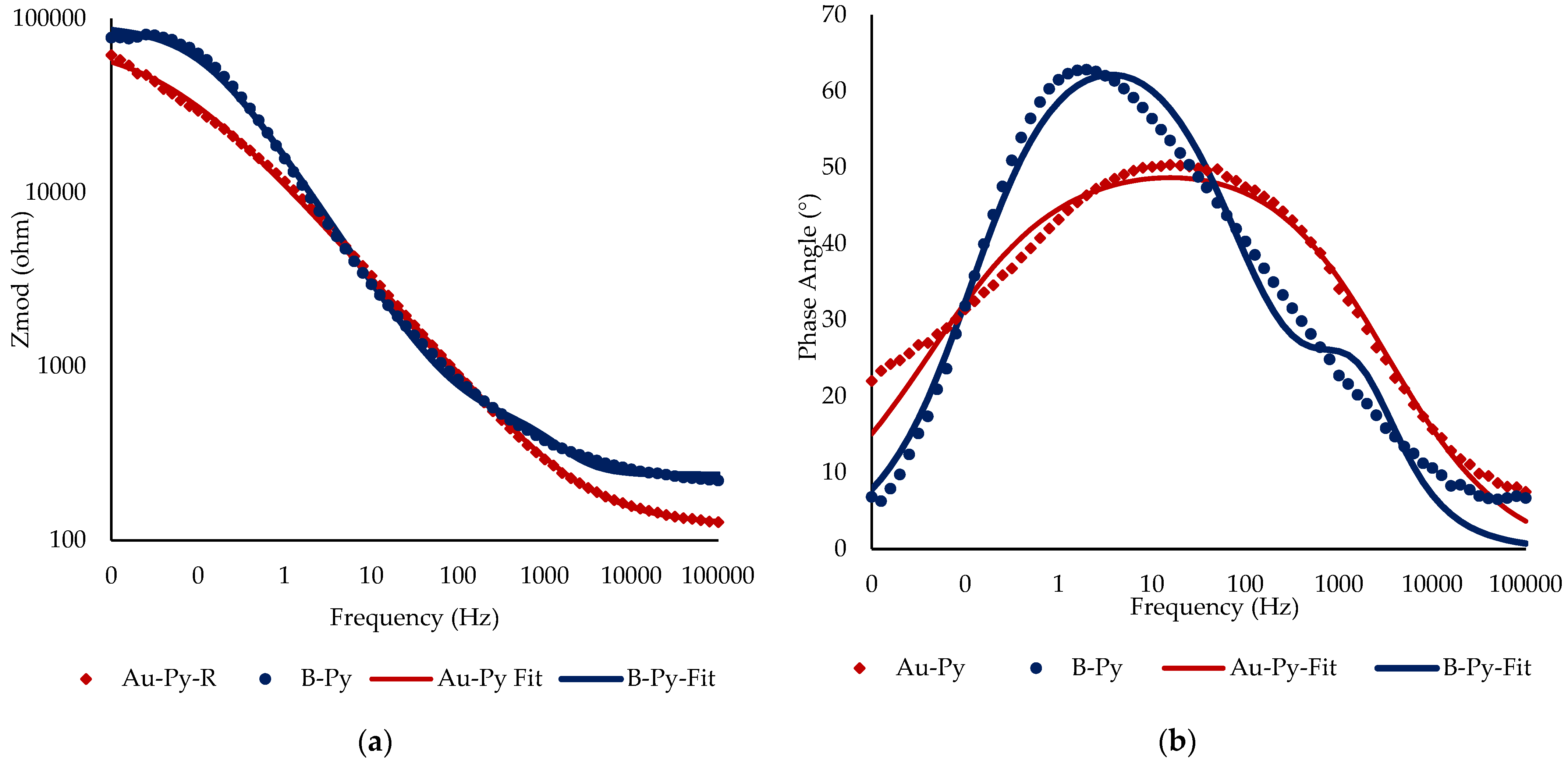
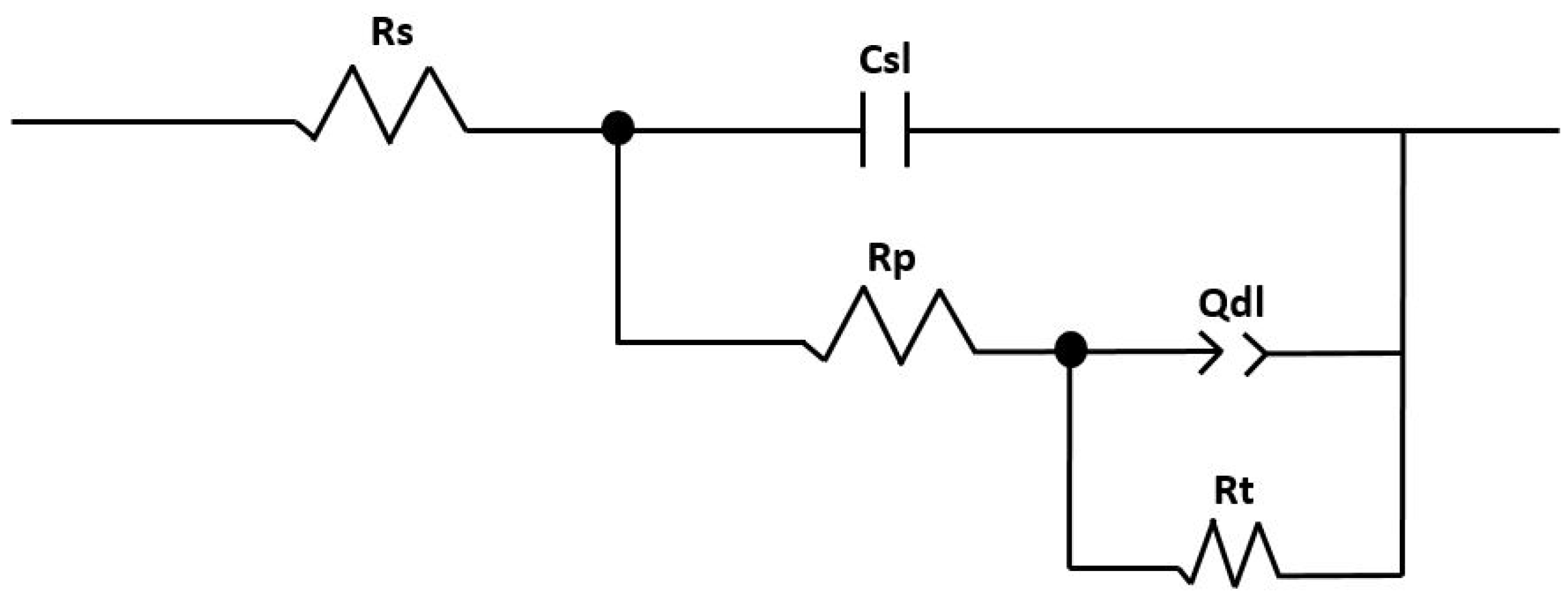
3.2. Electrochemical Experiments
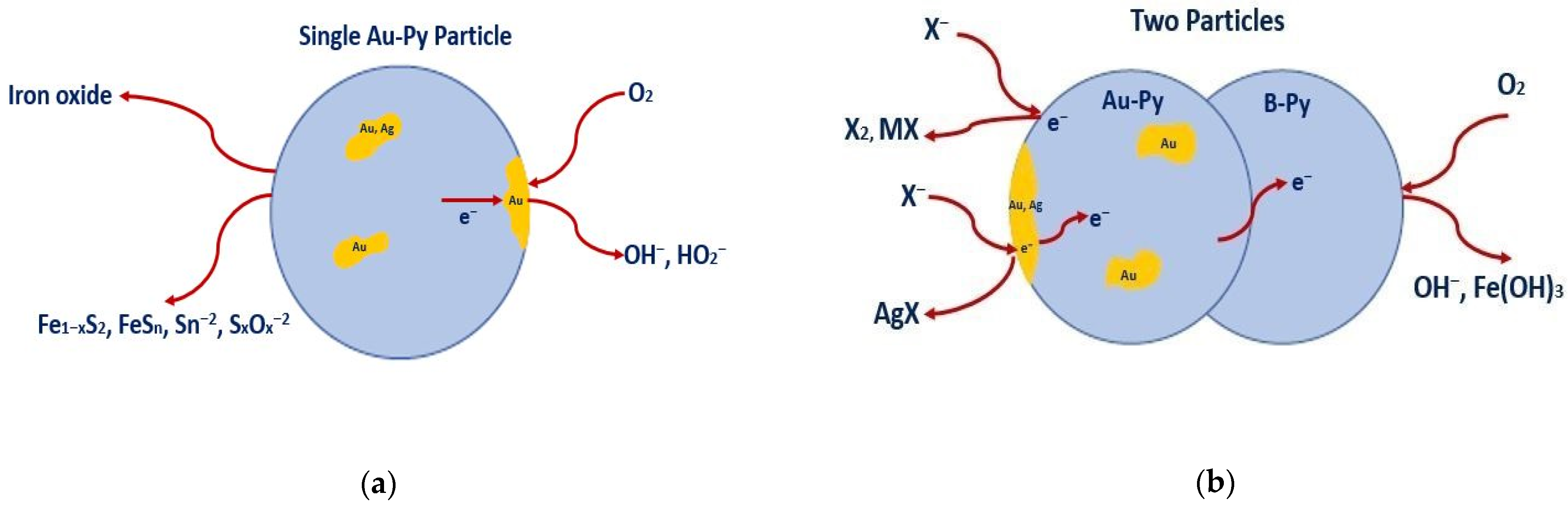
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marsden, J.; House, I. The Chemistry of Gold Extraction, 2nd ed.; SME: Littleton, CO, USA, 2006. [Google Scholar] [CrossRef]
- Chang, Z.; He, B.; Luo, Y.; Shen, Z.; Zou, L.; Wang, Q.; Sun, Z. Effects of Au dopping on the adsorption of xanthate on pyrite surface in presence of H2O: A DFT Study. Miner. Eng. 2024, 210, 08667. [Google Scholar] [CrossRef]
- Gao, Z.; Yang, Z.; Li, H.; Luo, T.; Yao, L.; Rao, W. Genesis and characteristics of gold hosted by pyrite. Geol. J. China Univ. 2000, 6, 156–162. [Google Scholar]
- Nagaraj, D.R.; Brinen, J.; Farinato, R.; Lee, J. A study of interactions of dicresyl monothiophosphate with noble metals by electrochemical and spectroscopic methods. Langmuir 1992, 8, 1943–1949. [Google Scholar] [CrossRef]
- Forrest, K.; Yan, D.; Dune, R. Optimization of gold recovery by selective gold flotation for copper-gold-pyrite ores. Miner. Eng. 2001, 14, 227–241. [Google Scholar] [CrossRef]
- Mu, Y.; Peng, Y.; Lauten, R.A. The depression of pyrite in selective flotation by different reagent systems—A literature review. Miner. Eng. 2016, 96–97, 143–156. [Google Scholar] [CrossRef]
- Özçelik, S.; Ekmekçi, Z. Surface Chemistry and Flotation of Au Containing Pyrites with Different Textures. Flotation 23. In Proceedings of the MEI Conferences, Cape Town, South Africa, 17–20 April 2023. [Google Scholar]
- Klimpel, R.R.; Isherwood, S. Some new flotation products for improved recovery of gold and platinum. In Randol Gold Forum; Beaver Creesk, CO, USA, 1993; pp. 105–111. Available online: https://scholar.google.com/scholar_lookup?title=Some%20new%20flotation%20products%20for%20improved%20recovery%20of%20gold%20and%20platinum&publication_year=1993&author=R.R.%20Klimpel&author=S.%20Isherwood (accessed on 1 September 2024).
- Monte, M.B.M.; Lins, F.F.; Oliveira, J.F. Selective flotation of gold from pyrite under oxidizing conditions. Int. J. Miner. Process. 1997, 51, 255–267. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Y.-J.; Xian, Y.-J.; Wen, S. Electronic structure and flotability of gold-bearing pyrite: A density functional theory study. Cent. S. Univ. Press 2017, 24, 2288–2293. [Google Scholar] [CrossRef]
- Xian, H.; He, H.; Zhu, J.; Du, R.; Wu, X.; Tang, H.; Tan, W.; Liang, X.; Zhu, R.; Teng, H. Crystal habit-directed gold deposition on pyrite: Surface chemical interpretation of the pyrite morphology indicative of gold enrichment. Geochim. Cosmochim. Acta 2019, 264, 191–204. [Google Scholar] [CrossRef]
- Moslemi, H.; Gharabaghi, M. A review on electrochemical behavior of pyrite in the froth flotation process. J. Ind. Eng. Chem. 2017, 47, 1–18. [Google Scholar] [CrossRef]
- Pauporte, T.; Schuhmann, D. An electrochemical study of natural enargite under conditions relating to those used in flotation of sulphide minerals. Colloids Surf. A Physicochem. Eng. Asp. 1996, 111, 1–19. [Google Scholar] [CrossRef]
- Huai, Y.; Plackowski, C.; Peng, Y. The surface properties of pyrite coupled with gold in the presence of oxygen. Miner. Eng. 2017, 111, 131–139. [Google Scholar] [CrossRef]
- Huai, Y.; Plackowski, C.; Peng, Y. The effect of gold coupling on the surface properties of pyrite in the presence of ferric ions. Appl. Surf. Sci. 2019, 488, 277–283. [Google Scholar] [CrossRef]
- Lin, S.; Wang, C.; Liu, R.; Sun, W.; Jing, G. Surface characterization of molybdenite, bismuthinite, and pyrite to identify the influence of pH on the mineral floatability. Appl. Surf. Sci. 2022, 577, 151756. [Google Scholar] [CrossRef]
- Öztürk, Y.; Bıçak, Ö.; Ekmekçi, Z. Effects of residual xanthate on flotation efficiency of a Cu-Zn Sulfide ore. Minerals 2022, 12, 279. [Google Scholar] [CrossRef]
- Shen, Y.; Nagaraj, D.R.; Farinato, R.; Somasundaran, P. Study of xanthate decomposition in aqueous solution. Miner. Eng. 2016, 93, 10–15. [Google Scholar] [CrossRef]
- Park, I.; Hong, S.; Jeon, S.; Ito, M.; Hiroyoshi, N. A review of recent advances in depression techniques for flotation separation of Cu-Mo sulfides in porphyry copper deposits. Metals 2020, 10, 1269. [Google Scholar] [CrossRef]
- Rao, S.R. Surface Chemistry of Froth Flotation: Volume 1: Fundamentals, 2nd ed.; Springer: New York, NY, USA, 2013; p. 507. [Google Scholar] [CrossRef]
- Niu, X.; Chen, J.; Li, Y.; Xia, L.; Li, L.; Sun, H.; Ruan, R. Correlation of surface oxidation with xanthate adsorption and pyrite flotation. Appl. Surf. Sci. 2019, 495, 143411. [Google Scholar] [CrossRef]
- Yin, W.; Xue, J.; Li, D.; Sun, Q.; Yao, J.; Huang, S. Flotation of heavily oxidized pyrite in the presence of fine digenite particles. Miner. Eng. 2018, 115, 142–149. [Google Scholar] [CrossRef]
- Wang, C.; Liu, R.; Khoso, S.A.; Lu, H.; Sun, W.; Ni, Z.; Lyu, F. Combined inhibitory effect of calcium hypochlorite and dextrin on flotation behavior of pyrite and galena sulphides. Miner. Eng. 2020, 150, 106274. [Google Scholar] [CrossRef]
- Sun, X.; Huang, L.; Wu, D.; Tong, X.; Yang, S.; Hu, B. The selective depression effect of dextrin on pyrite during the Zn-Fe sulfides flotation under low alkaline conditions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129573. [Google Scholar] [CrossRef]
- Allan, G.C.; Woodcock, J.T. A review of the flotation of native gold and electrum. Miner. Eng. 2001, 14, 931–962. [Google Scholar] [CrossRef]
- Moslemi, H.; Shamsi, P.; Habashi, F. Pyrite and pyrrhotite open circuit potentials study: Effects on flotation. Miner. Eng. 2011, 24, 1038–1045. [Google Scholar] [CrossRef]
- Ertekin, Z.; Pekmez, K.; Kappes, R.; Ekmekçi, Z. Application of EIS technique to investigate the adsorption of different types of depressants on pyrite. Physicochem. Probl. Miner. Process. 2021, 57, 112–126. [Google Scholar] [CrossRef]
- Mu, Y.; Li, L.; Peng, Y. Surface properties of fractured and polished pyrite in relation to flotation. Miner. Eng. 2017, 101, 10–19. [Google Scholar] [CrossRef]
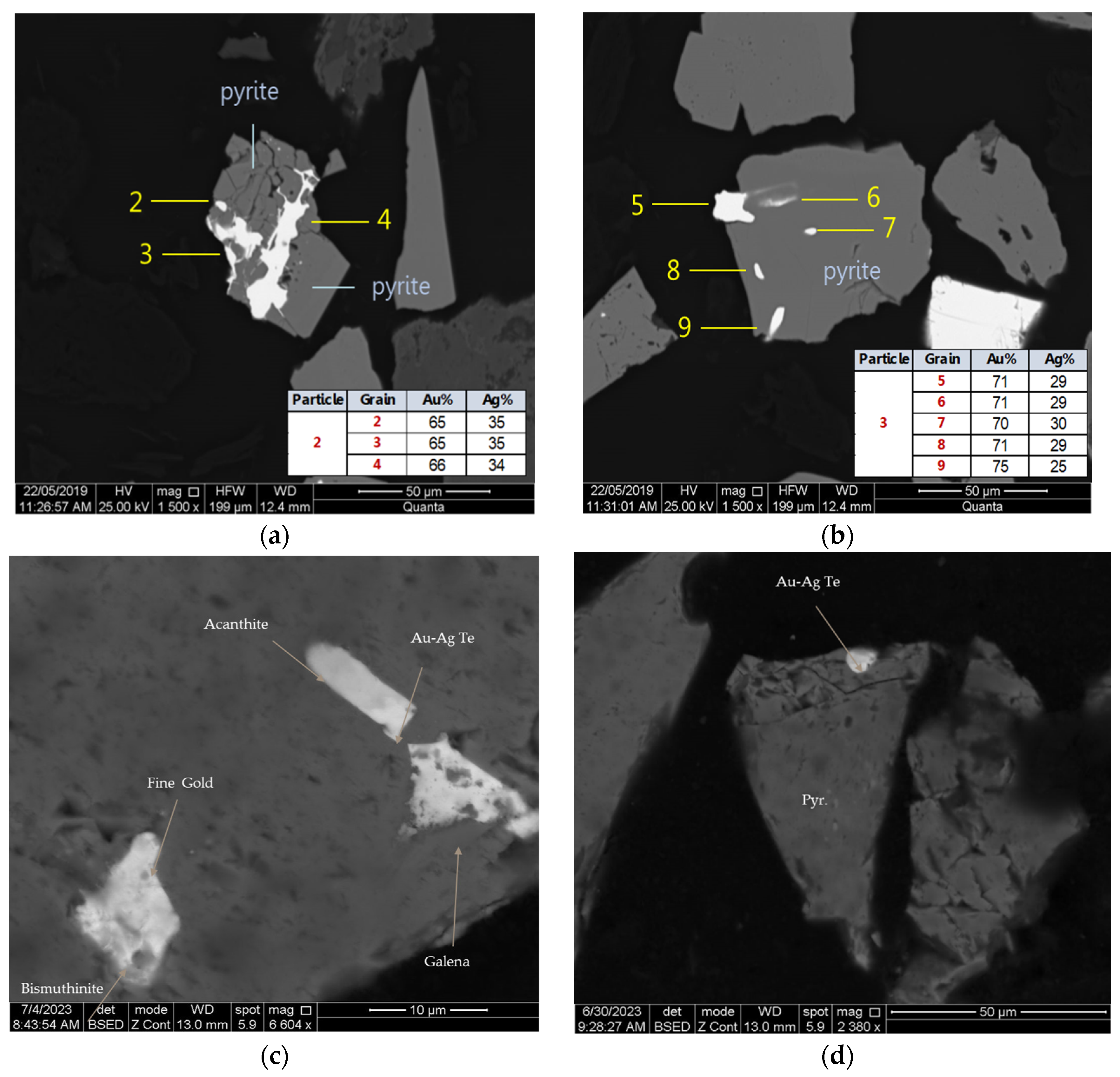
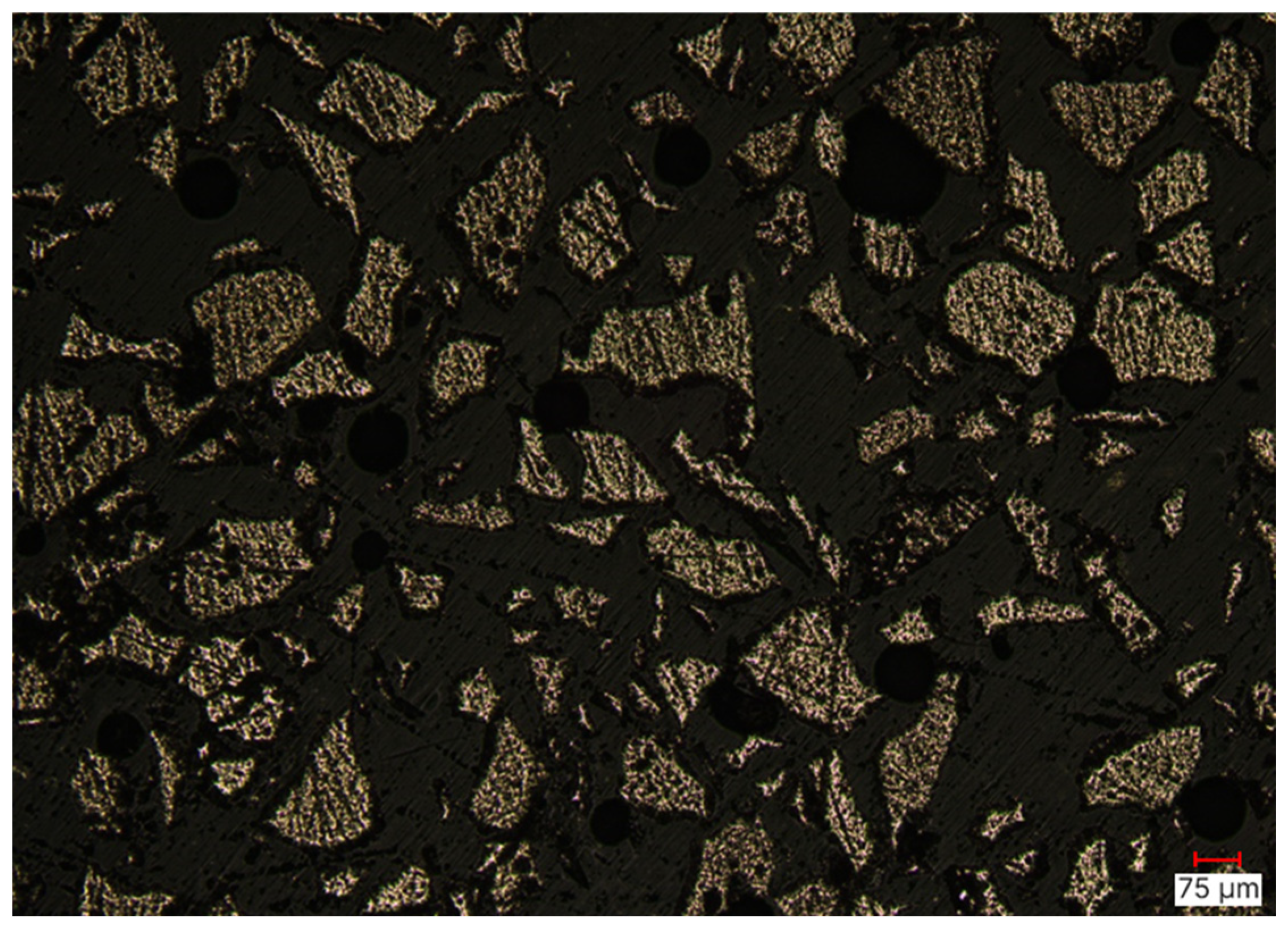


| Cu | Fe | Pb | Zn | S | Au | Ag | As | CNSolAu | |
|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | g/t | g/t | % | % | |
| Sample 1 | 0.52 | 25.8 | 10.4 | 10.55 | 33.8 | 85 | 211 | 2.5 | 92.0 |
| Sample 2 | 0.1 | 23.63 | 0.05 | 0.12 | 25.19 | 1.42 | 7.0 | 0.09 | 62.72 |
| Sample 3 | 0.07 | 25.82 | 0.06 | 0.06 | 26.54 | 2.40 | 12.0 | 0.02 | 31.93 |
| Mineral Group | Sample 1 | Mineral Group | Sample 2 | Sample 3 |
|---|---|---|---|---|
| Pyrite | 44.9 | Pyrite | 52.63 | 57.28 |
| Arsenopyrite | 1.03 | Galena | 0.06 | 0.11 |
| Galena | 19.7 | Sphalerite | 0.15 | 0.07 |
| Sphalerite | 8.47 | Chalcopyrite | 0.05 | 0.16 |
| Chalcopyrite | 0.70 | Quartz | 15.79 | 12.90 |
| Cerussite | 1.28 | Feldspar | 1.59 | 0.71 |
| Coronadite and other Pb | 2.91 | Muscovite | 4.46 | 1.60 |
| Quartz | 7.97 | Si-Al Clays | 1.95 | 1.41 |
| K-feldspar | 1.62 | Calcite | 17.05 | 15.63 |
| Muscovite/biotite/illite | 0.73 | Rhodochrosite | 0.00 | 5.35 |
| Mn-Ca-silicates | 3.30 | Sulfide/Silicate Texture | 2.67 | 1.52 |
| Mn-Fe-(Ca)-carbonates | 1.85 | Other Minerals | 3.6 | 3.26 |
| Mn-Ca-carbonates | 1.42 | Total | 100.00 | 100.00 |
| Other Minerals | 4.12 | |||
| Total | 100.00 |
| Rs (Ω) | Rp (Ω) | Rt (kΩ) | Csl (F) | Qdl (S*sn) | n (°) | Chi-Squared χ2 | |
|---|---|---|---|---|---|---|---|
| Au-Py | 126.6 | 19.39 | 74.21 | 1.650 × 10−7 | 2.87 × 10−5 | 0.57 | 2.47 × 10−3 |
| B-Py | 238.8 | 315.2 | 91.04 | 5.24 × 10−7 | 1.38 × 10−5 | 0.76 | 5.84 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özçelik, S.; Ekmekçi, Z. Surface Chemistry and Flotation of Gold-Bearing Pyrite. Minerals 2024, 14, 914. https://doi.org/10.3390/min14090914
Özçelik S, Ekmekçi Z. Surface Chemistry and Flotation of Gold-Bearing Pyrite. Minerals. 2024; 14(9):914. https://doi.org/10.3390/min14090914
Chicago/Turabian StyleÖzçelik, Seda, and Zafir Ekmekçi. 2024. "Surface Chemistry and Flotation of Gold-Bearing Pyrite" Minerals 14, no. 9: 914. https://doi.org/10.3390/min14090914






