The Efficient Separation of Apatite from Dolomite Using Fucoidan as an Eco-Friendly Depressant
Abstract
:1. Introduction
2. Experiment
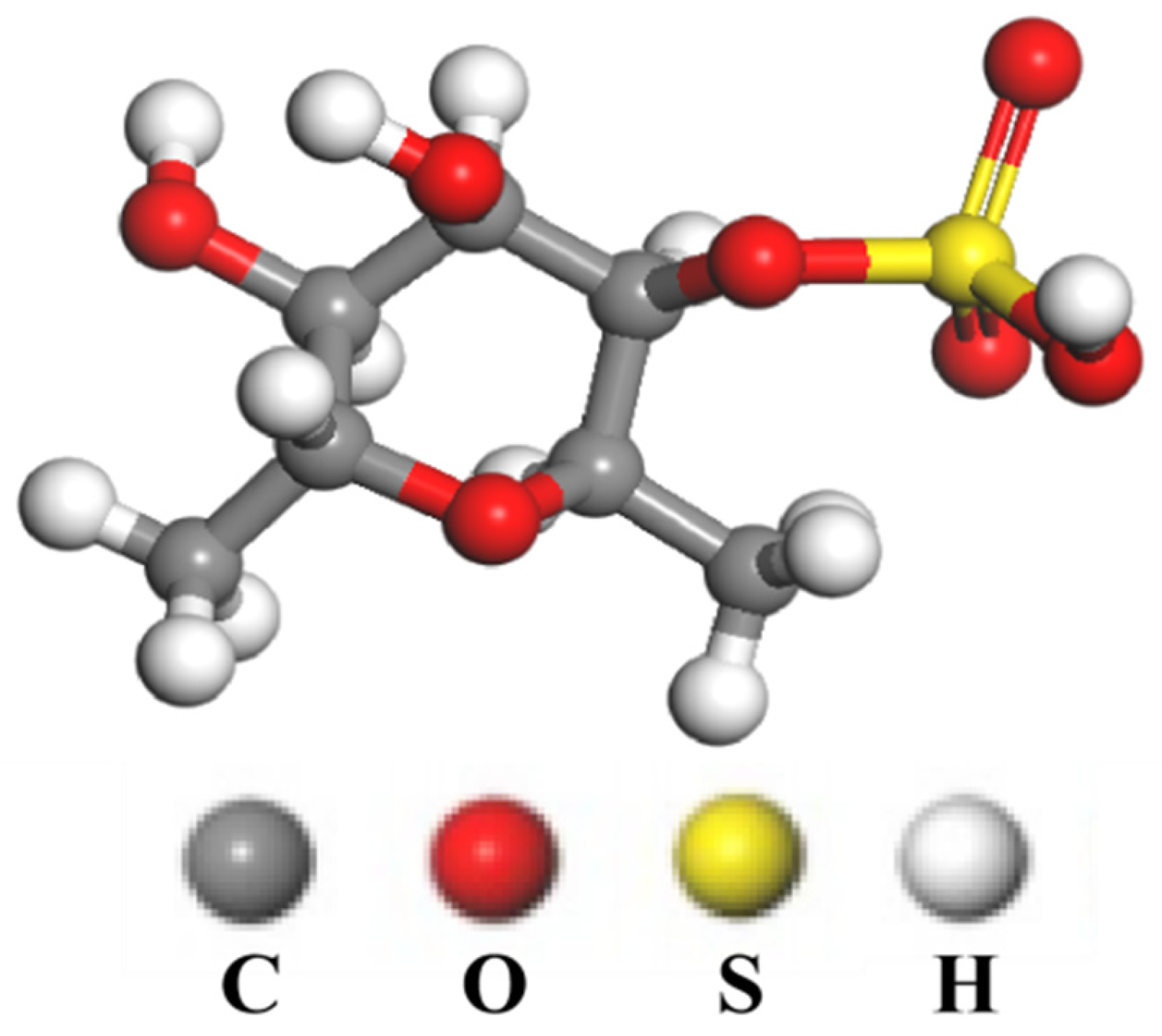
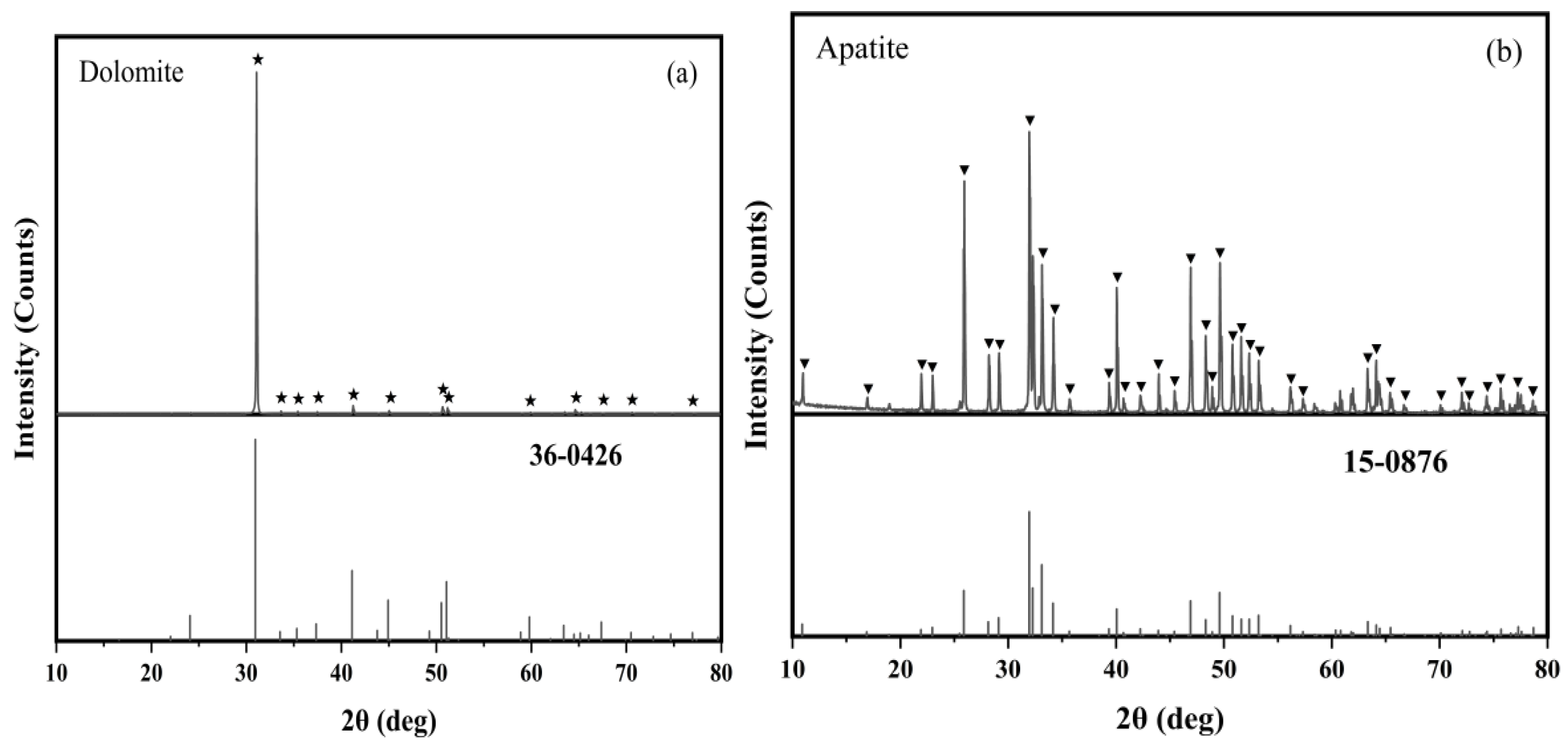
2.1. Materials and Reagents
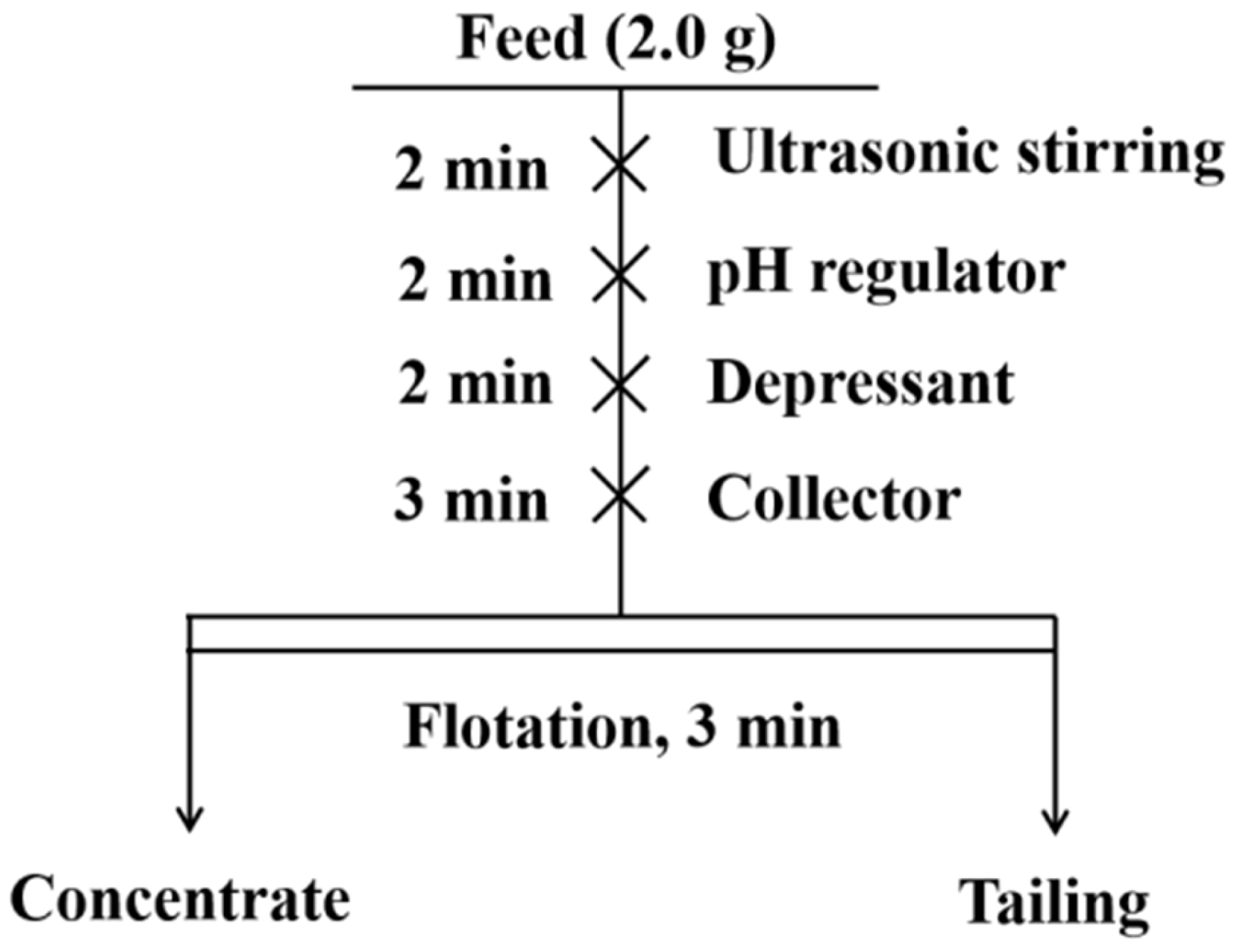
2.2. Flotation Tests
2.3. Contact Angle Measurement
2.4. Zeta Potential Determination
2.5. FTIR Determination
2.6. Microcalorimetry Analysis
2.7. XPS Analysis
3. Results and Discussion
3.1. Micro-Flotation of Single Mineral
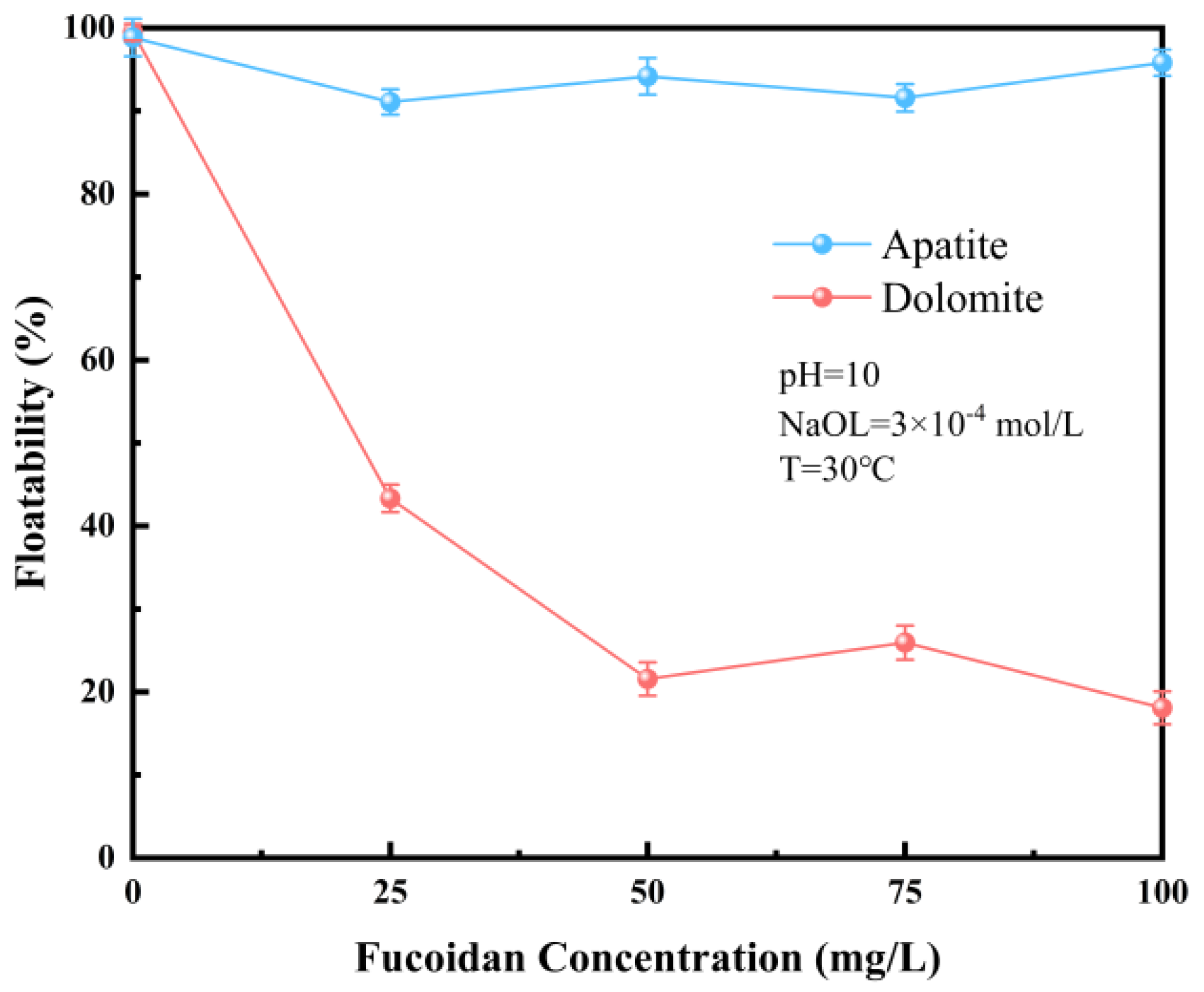
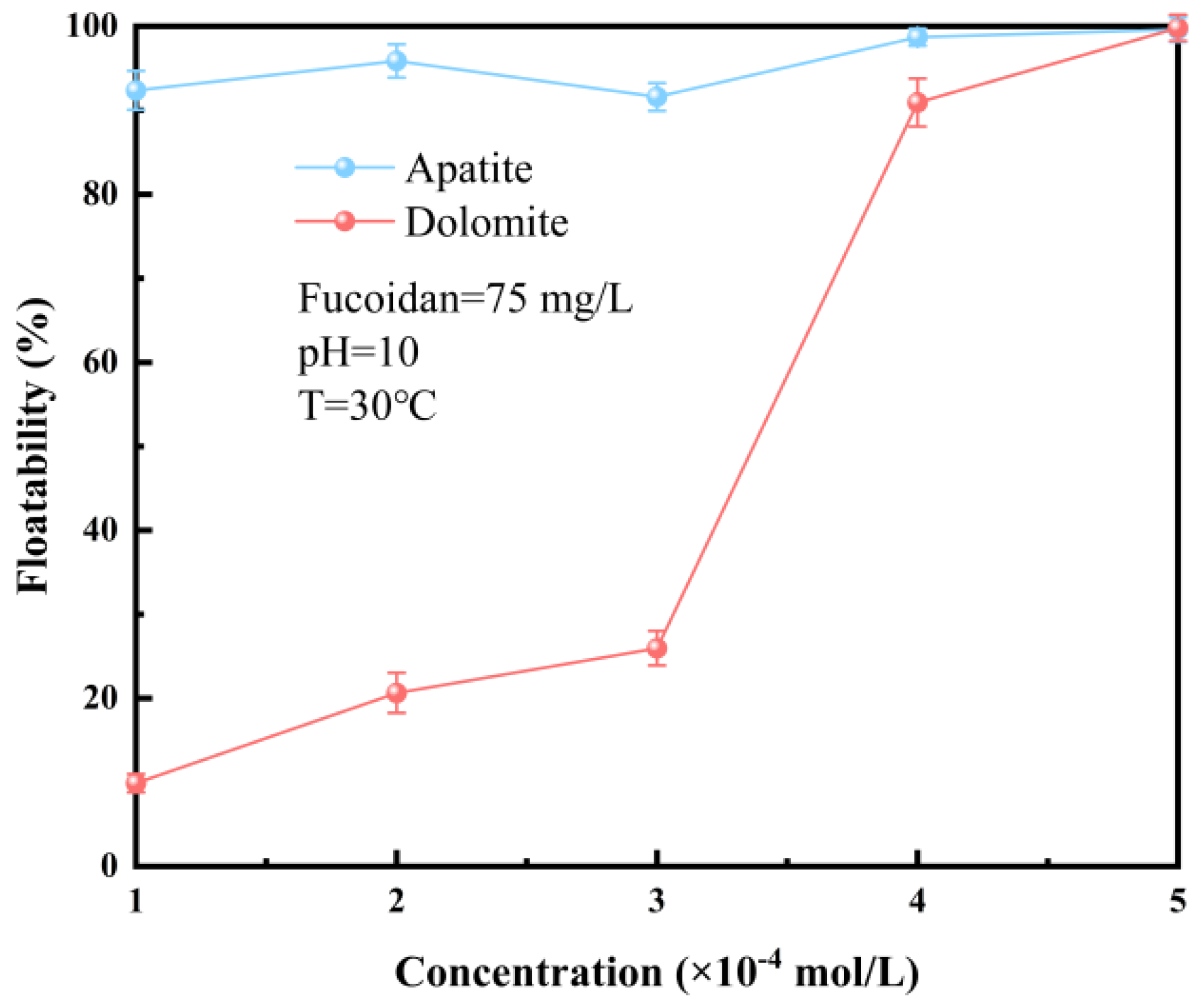
3.1.1. Influence of FD Concentration
3.1.2. Effect of pH
3.1.3. Effect of NaOL Concentration
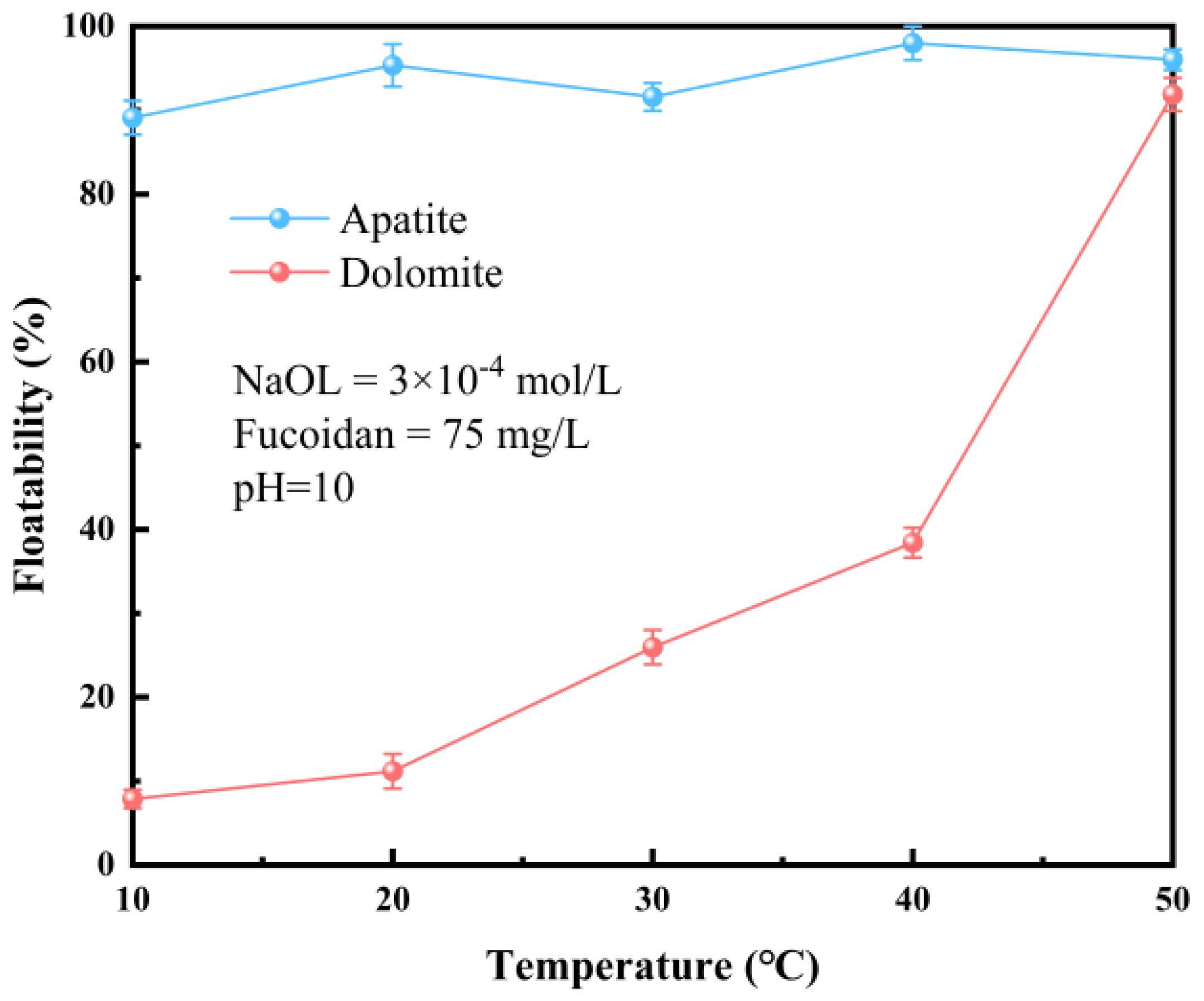
3.1.4. Effect of Temperature
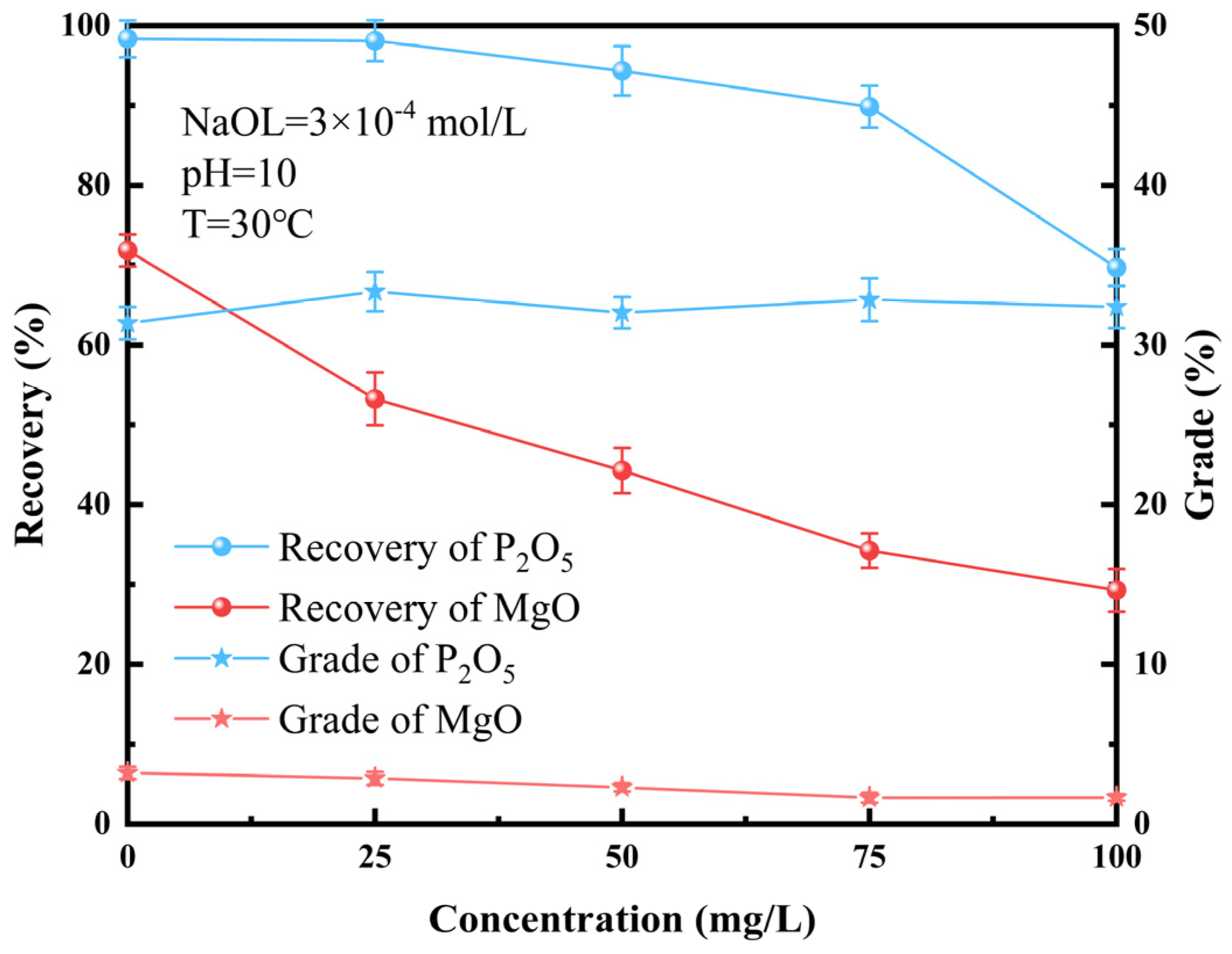
3.2. Flotation of Artificially Mixed Mineral
3.3. Contact Angle Results
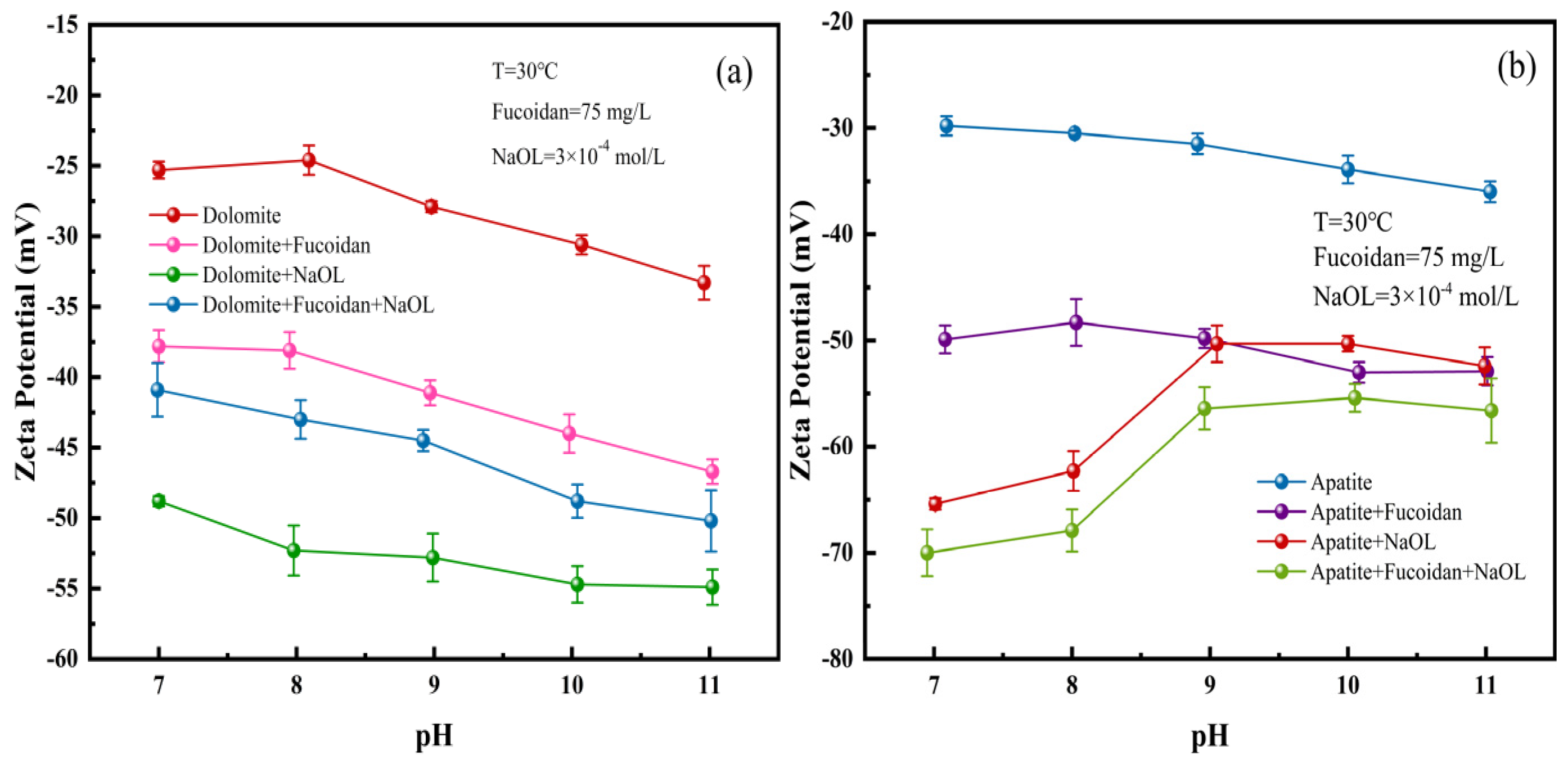
3.4. Zeta Potential Results
3.5. FTIR Analysis Results
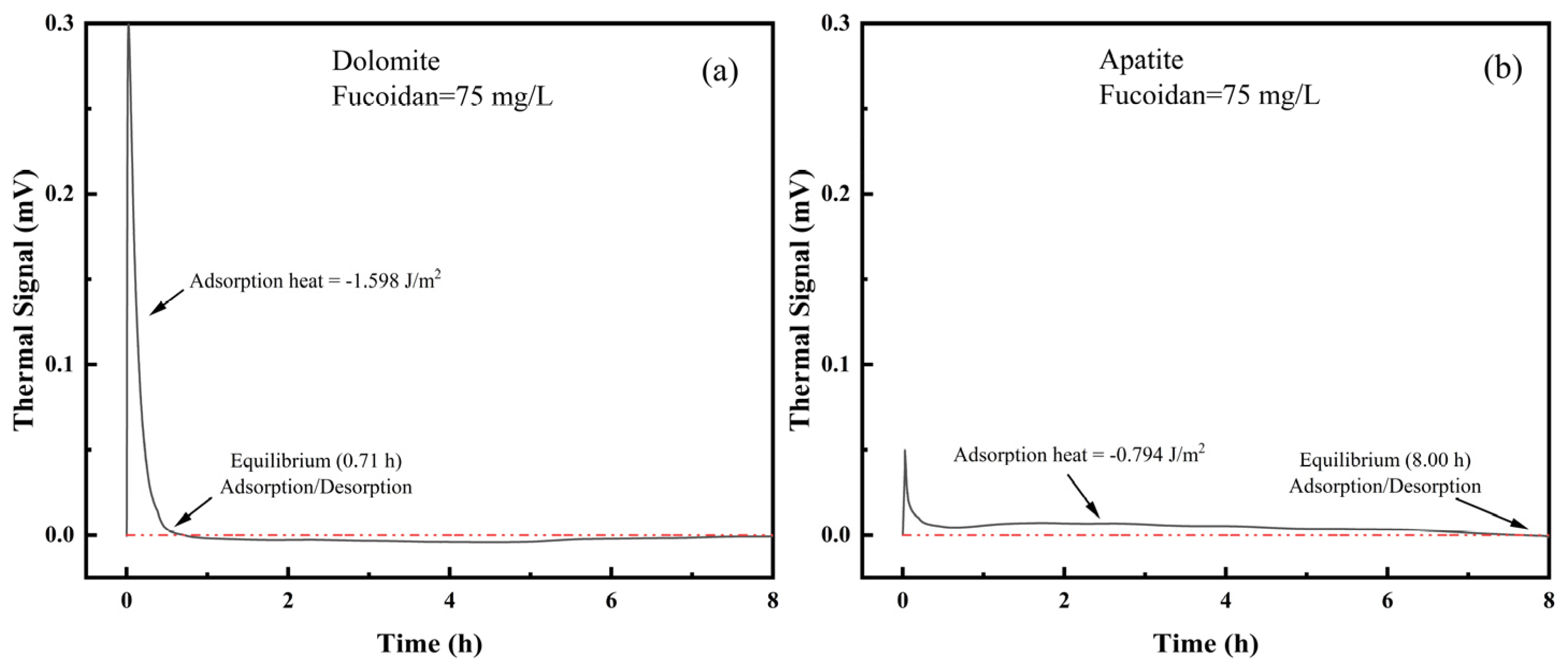
3.6. Microcalorimetry Determination
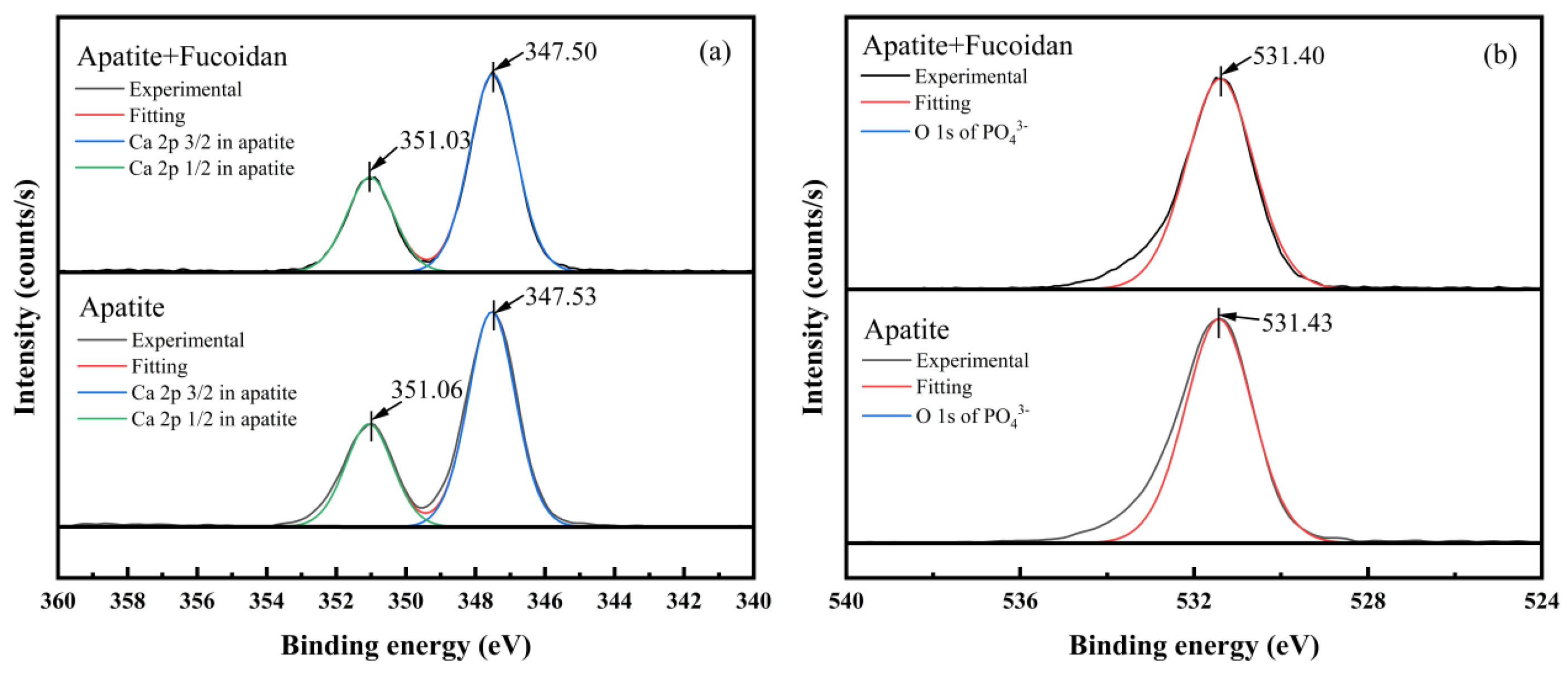
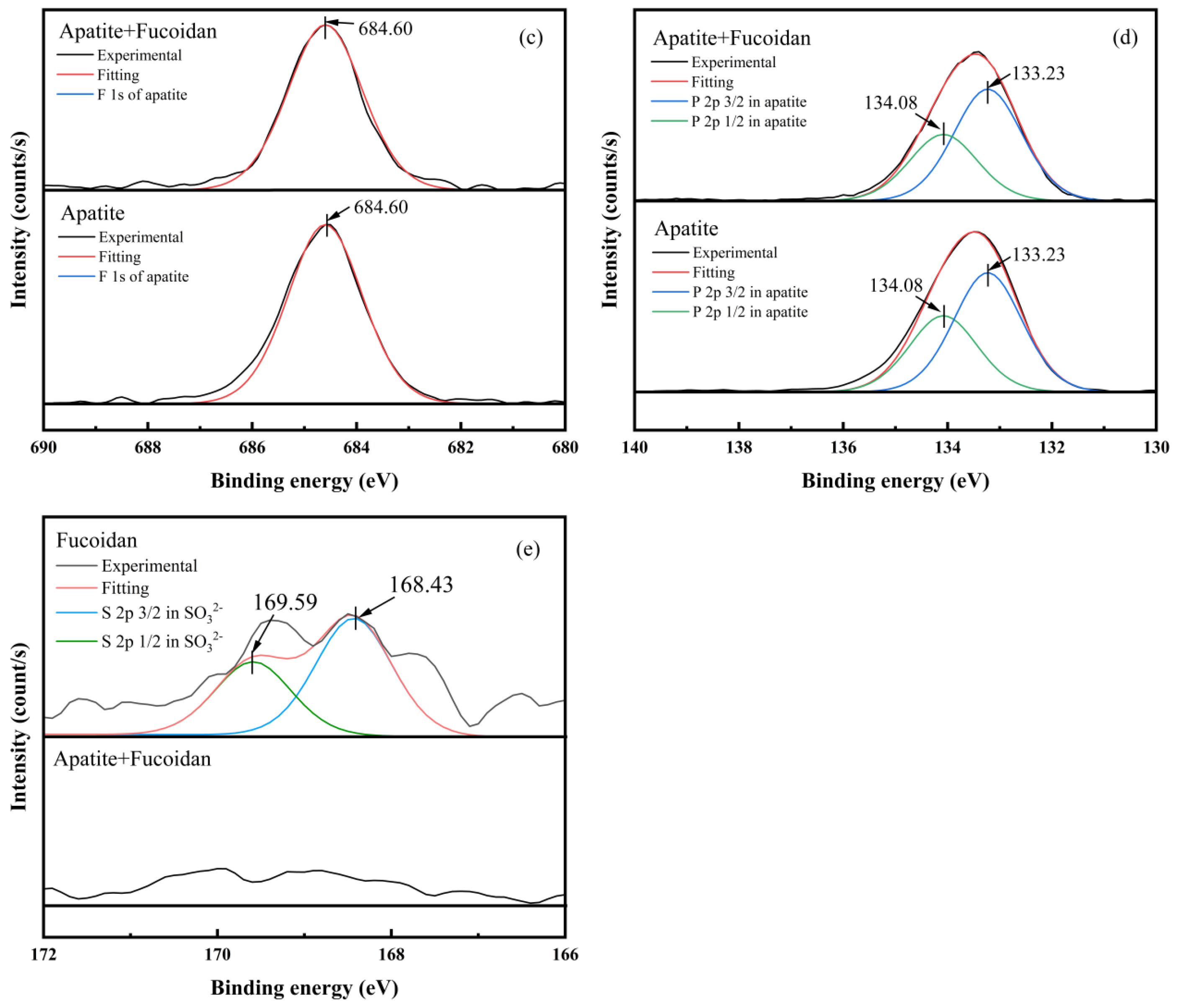
3.7. XPS Analysis Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abdel-Hakeem, M.; El-Habaak, G. The potential production of rock-based fertilizer and soil conditioner from phosphate mine wastes: A case study from Abu-Tartur plateau in the Western Desert of Egypt. J. Clean. Prod. 2021, 329, 129761. [Google Scholar] [CrossRef]
- Lopes, C.M.; Miranda Silva, A.M.; Estrada-Bonilla, G.A.; Ferraz-Almeida, R.; Vieira, J.L.V.; Otto, R.; Vitti, G.C.; Cardoso, E.J.B.N. Improving the fertilizer value of sugarcane wastes through phosphate rock amendment and phosphate-solubilizing bacteria inoculation. J. Clean. Prod. 2021, 298, 126821. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Q.; Wang, X. New insights on depressive mechanism of citric acid in the selective flotation of dolomite from apatite. Colloid Surface A 2022, 653, 130075. [Google Scholar] [CrossRef]
- Zeng, M.; Yang, B.; Guan, Z.; Zeng, L.; Luo, H.; Deng, B. The selective adsorption of xanthan gum on dolomite and its implication in the flotation separation of dolomite from apatite. Appl. Surf. Sci. 2021, 551, 149301. [Google Scholar] [CrossRef]
- Zhong, C.; Feng, B.; Zhang, W.; Zhang, L.; Guo, Y.; Wang, T.; Wang, H. The role of sodium alginate in the flotation separation of apatite and dolomite. Powder Technol. 2020, 373, 620–626. [Google Scholar] [CrossRef]
- Zeng, L.; Ding, K.; Zhang, X.; Zhou, Y.; Han, H. New insights into the influence of mineral surface transformation on the flotation behavior of anhydrite/apatite. Colloid Surface A 2024, 685, 133215. [Google Scholar] [CrossRef]
- Santos, L.H.; Santos, G.O.; Magalhães, L.F.; Silva, G.R.; Peres, A.E.C. Application of andiroba oil as a novel collector in apatite flotation. Miner. Eng. 2022, 185, 107678. [Google Scholar] [CrossRef]
- Jing, L.; Xu, L.; Xue, K.; Wang, D.; Ma, Z.; Meng, J.; Shi, X.; Liu, C. Selective depression by using environment-friendly depressant pectin in apatite and dolomite flotation system. Miner. Eng. 2023, 203, 108373. [Google Scholar] [CrossRef]
- Derhy, M.; Taha, Y.; El-Bahi, A.; Ait-Khouia, Y.; Benzaazoua, M.; Hakkou, R. Selective flotation of calcite and dolomite from apatite using bio-based alternatives to conventional collectors: Castor and mustard oils. Miner. Eng. 2024, 208, 108597. [Google Scholar] [CrossRef]
- Liu, X.; Ruan, Y.; Li, C.; Cheng, R. Effect and mechanism of phosphoric acid in the apatite/dolomite flotation system. Int. J. Miner. Process. 2017, 167, 95–102. [Google Scholar] [CrossRef]
- Wang, X.; Xie, R.; Liu, J.; Zhu, Y. The utilization of tamarind seed gum as a novel dolomite depressant in the selective flotation of apatite from dolomite. Adv. Powder Technol. 2023, 34, 104022. [Google Scholar] [CrossRef]
- Du, W.; Li, X. Insight into the inhibition mechanism of carboxymethyl cellulose for flotation of dolomite and fluorapatite: Experimental and DFT studies. Colloid Surface A 2023, 674, 131957. [Google Scholar] [CrossRef]
- Yang, B.; Yin, W.; Zhu, Z.; Sun, H.; Sheng, Q.; Fu, Y.; Yao, J.; Zhao, K. Differential adsorption of hydrolytic polymaleic anhydride as an eco-friendly depressant for the selective flotation of apatite from dolomite. Sep. Purif. 2021, 256, 117803. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Gomaa, M. Pretreated fucoidan and alginate from a brown seaweed as a substantial carbon source for promoting biomass, lipid, biochemical constituents and biodiesel quality of Dunaliella salina. Renew. Energy 2020, 157, 246–255. [Google Scholar] [CrossRef]
- Cholaraj, R.; Venkatachalam, R. Investigation of antioxidant and anticancer potential of fucoidan (in-vitro & in-silico) from brown seaweed Padina boergesenii. Algal. Res. 2024, 79, 103442. [Google Scholar]
- Lan, Y.; Qin, K.; Wu, S. The physiological activities of fucoidan and its application in animal breeding. Fish Shellfish Immun. 2024, 147, 109458. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, Q.; Farooqi, A.A.; Wang, J.; Yue, Y.; Geng, L.; Wu, N. Opportunities and challenges of fucoidan for tumors therapy. Carbohydr. Polym. 2024, 324, 121555. [Google Scholar]
- Wang, L.; Li, Z.; Zhang, H.; Huang, L.; Zhu, Y.; Li, F. Effect of Pulp Temperature on Separation of Magnesite from Dolomite in Sodium Oleate Flotation System. Miner. Eng. 2024, 212, 108718. [Google Scholar] [CrossRef]
- Cao, Q.; Zou, H.; Chen, X.; Wen, S. Flotation selectivity of N-hexadecanoylglycine in the fluorapatite–dolomite system. Miner. Eng. 2019, 131, 353–362. [Google Scholar] [CrossRef]
- Liu, M.; Li, H.; Jiang, T.; Liu, Q. Flotation of coarse and fine pyrochlore using octyl hydroxamic acid and sodium oleate. Miner. Eng. 2019, 132, 191–201. [Google Scholar] [CrossRef]
- Kuang, J.; Wang, X.; Yu, M.; Yuan, W.; Huang, Z.; Zhang, S.; Xiao, J. Flotation behavior and mechanism of calcium carbonate polymorphs with sodium oleate as collector. Powder Technol. 2022, 398, 117040. [Google Scholar] [CrossRef]
- Huang, W.; Liu, W.; Zhong, W.; Chi, X.; Rao, F. Effects of common ions on the flotation of fluorapatite and dolomite with oleate collector. Miner. Eng. 2021, 174, 107213. [Google Scholar] [CrossRef]
- Gong, X.; Yao, J.; Yang, B.; Guo, J.; Sun, H.; Yin, W. Study on the inhibition mechanism of guar gum in the flotation separation of brucite and dolomite in the presence of SDS. J. Mol. Liq. 2023, 380, 121721. [Google Scholar] [CrossRef]
- Gong, X.; Yao, J.; Yang, B.; Yin, W.; Wang, Y.; Fu, Y. Adsorption mechanism of green efficient chelating poly-L-aspartic acid in flotation separation of brucite and dolomite. Adv. Powder Technol. 2023, 34, 104207. [Google Scholar] [CrossRef]
- Ge, R.; Yang, B.; Martin, R.; Luo, H.; He, D.; Arroyo, M.A.C. The application of poly (sodium 4-styrene sulfonate) as an efficient dolomite depressant in the direct flotation of apatite from dolomite. Powder Technol. 2023, 425, 118583. [Google Scholar] [CrossRef]
- Liu, J.; Tao, Y.; Chang, T.; Ge, W.; Jiang, K.; Lv, L.; Zhu, Y.; Yuan, S. Study on flotation separation of barite fluorite by citric acid under new collector system. Colloid Surface A 2024, 692, 134058. [Google Scholar] [CrossRef]
- Lv, L.; Duan, H.; Liu, W.; Yue, T. Flotation separation of fluorite from calcite using bis hydroxamic acid collector. Miner. Eng. 2022, 187, 107803. [Google Scholar] [CrossRef]
- Ding, Z.; Li, J.; Bi, Y.; Yu, P.; Dai, H.; Wen, S.; Bai, S. The adsorption mechanism of synergic reagents and its effect on apatite flotation in oleamide-sodium dodecyl benzene sulfonate (SDBS) system. Miner. Eng. 2021, 170, 107070. [Google Scholar] [CrossRef]




| Minerals | Various Conditions | ||
|---|---|---|---|
| Ultrapure Water | 3 × 10−4 mol/L NaOL | 75 mg/L FD and 3 × 10−4 mol/L NaOL | |
| Dolomite | 28.58° ± 1.36° | 73.67° ± 1.65° | 30.08° ± 3.48° |
| Apatite | 26.75° ± 0.94° | 81.92° ± 0.72° | 60.50° ± 3.36° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yang, B.; Deng, B.; Luo, H.; Zhou, F. The Efficient Separation of Apatite from Dolomite Using Fucoidan as an Eco-Friendly Depressant. Minerals 2024, 14, 922. https://doi.org/10.3390/min14090922
Zhang Y, Yang B, Deng B, Luo H, Zhou F. The Efficient Separation of Apatite from Dolomite Using Fucoidan as an Eco-Friendly Depressant. Minerals. 2024; 14(9):922. https://doi.org/10.3390/min14090922
Chicago/Turabian StyleZhang, Yifan, Bingqiao Yang, Bing Deng, Huihua Luo, and Fang Zhou. 2024. "The Efficient Separation of Apatite from Dolomite Using Fucoidan as an Eco-Friendly Depressant" Minerals 14, no. 9: 922. https://doi.org/10.3390/min14090922







