Abstract
Variations in the recovery of platinum group metals (PGMs) are often attributed to mineralogical and other natural ore-type variations. To increase the recovery of PGMs by the flotation process, a comprehensive understanding of gangue and valuable minerals is essential for optimising the extraction and processing of metals. Recoveries may be improved if the questions of how, where, and why losses occur can be answered with a certain degree of confidence. A requirement is the availability of statistically reliable mineralogical data. The PGMs of MG-1–4 chromite tailings dumps of the western limb of the Bushveld complex (BC) were studied in detail to unravel the PGMs and the nature of the platinum group minerals in the sample. Characterisation of the chromite tailings via deportment analysis revealed that the sample contained a significant amount of 3E PGM + Au (Pt, Pd, Ru, and Au) and was concentrated in the -25 µm fraction. The results of automated mineralogical analysis showed that the sample was composed of the PGE-sulphides group, comprising 63.6 vol%, PGE-sulfarsenides 10.4 vol%, PGE-arsenides 1.3 vol%, PGE-bismuth tellurides 3.3 vol%, PGMs-alloy 4.1 vol%, and Laurite comprising 17.3 vol% of the total PGE population. The sample was composed of 66.5 vol% of liberated PGMs, 0.2 vol% attached to liberated BMS, 27.3 vol% of PGMs attached to or locked within silicate or oxide gangue composite particles, 0.2 vol% of PGMs associated with BMS attached to silicate or oxide gangue particles, and a low proportion (5.8 vol%) of PGMs reported being locked within gangue or oxide particles. The majority of PGM grains observed were reported in the fast-floating category (64.4 vol%), 27.6 vol% in the slow-floating 1 category, 2.2 vol% in the slow-floating 2 category, and 5.8 vol% to the non-floating category. The results of the study revealed that the PGMs of MG 1–4 chromite tailings were liberated; however, the low liberation index (<0.2) suggested that a significant portion of PGMs remained trapped within gangue, hindering their recovery. This highlights the need for effective comminution (crushing and grinding) to achieve better liberation. The sample contained fine particles that were more prone to being lost in the tailings and to lowering recovery due to the slimes coating valuable minerals. The recovery of the PGMs from this complex’s polymetallic bodies of low-grade and complex mineralogy will be insufficient with traditional methods and thus innovation is needed. Innovation like advanced comminution, novel flotation equipment or reagents, selective leaching and bioprocessing can overcome these challenges.
1. Introduction
The chromite layers found within the BC are widely recognised as potential sources of PGMs [1]. Historically, the low grades of PGMs in the main chromite layers have made their extraction and recovery economically unfeasible [2]. In the past, the focus of mining operations in the BC was primarily on the extraction of chromite for its use in the production of ferrochrome. PGMs were considered by-products with low economic value due to their relatively low concentrations in the chromite ores [3,4]. However, as PGM deposits with higher grades have become depleted over time, there has been a growing interest in finding alternative sources and developing beneficiation routes for the recovery of PGMs [5]. Chromite tailings, which are the by-products of chromite ore processing, have gained attention as potential sources of PGMs [6]. The beneficiation of PGMs from chromite tailings presents efficient recovery challenges. The beneficiation processes for chromite tailings require a comprehensive understanding of the ore characteristics, including the distribution of PGMs and associated minerals [7,8].
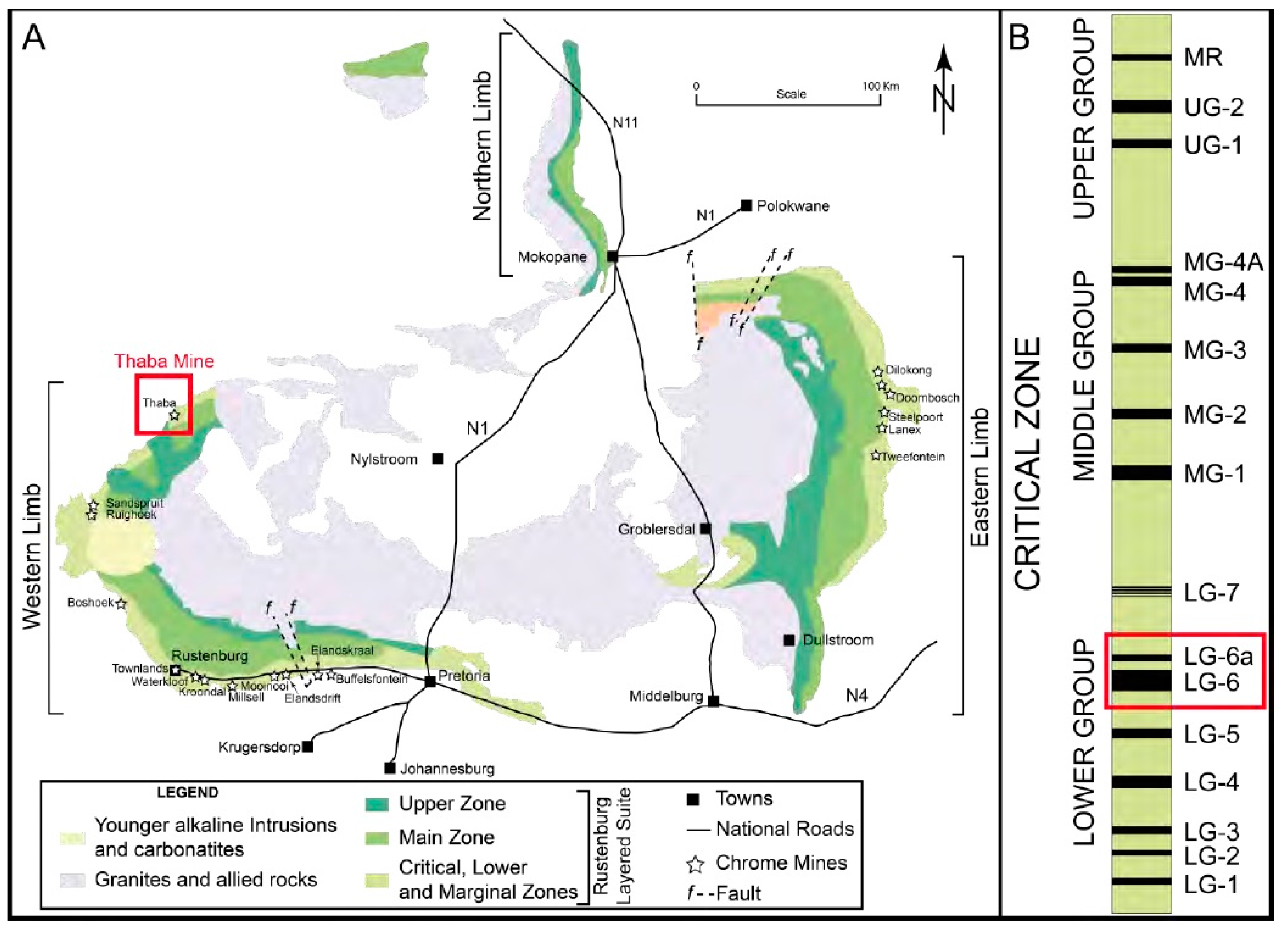
The BC is a highly layered igneous complex located in South Africa and is known for its rich deposits of PGMs, chromite, and vanadium [9,10,11]. It is divided into two main regions: the western limb and the eastern limb of the BC. Figure 1 shows the five stratigraphic sones that make up the western and eastern limbs. The ultramafic lower zone is followed by the critical, main, and upper zones. At the bottom is the marginal zone, which is primarily composed of mafic to ultramafic sills and chill zones [9,12]. The Upper Group 2 (UG2) chromitite layer and the Merensky Reef are the two significant PGM ore horizons. They are in the upper part of the critical zone (CS) [13,14]. Numerous other chromitite strata are also present, and they are separated into three groups based on their placement within the CS strata. These groups are the Lower, Middle, and Upper Groups (LG, MG, and UG, respectively). The Middle Group (MG) is located at the boundary between the Lower Critical Zone (LCS) and the Upper Critical Zone (UCS), where an ultramafic (pyroxenite) and a more mafic rock assemblage (pyroxenite, norite, and anorthosite) meet [15,16]. This boundary is located between the chromitite layers 2 and 3 of the MG on the eastern limb. It is distinguished by the first appearance of cumulus plagioclase [12,17].
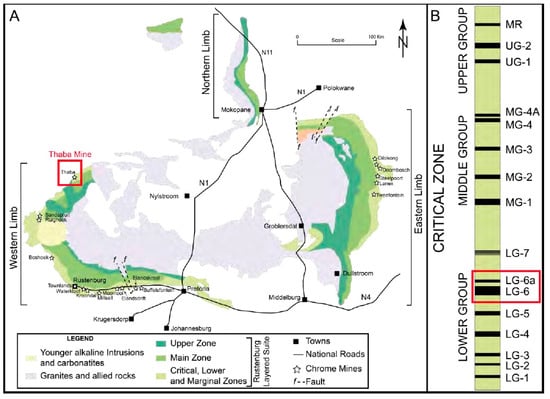
Figure 1.
General stratigraphic column for the western and the eastern Bushveld complex [9]. (A) General map for the western and the eastern Bushveld complex, (B) The Critical Zones.
It has been widely known that various chromitite seams of the BC contain elevated PGM content [18,19,20]. Nevertheless, except for the mainly massive UG2 chromitite seam with PGM contents ranging up to 10 ppm [2,11,21], the other LG, MG, and UG chromitites contain only slightly lower levels of PGMs ranging from 0.5 to 3 ppm and are sub-economic [20]. PGMs usually appear in MG ore as alloys, sulphides, and, to a lesser extent, tellurides and arsenides with finer grain sizes, usually less than 10 µm [22,23]. The degree of liberation has a significant impact on PGM recovery; the fastest-floating particles, freed base-metal sulphides, are linked to both liberated BMS minerals and PGM minerals themselves [24,25,26]. Table 1 presents an overview of the mineralisation of most of the more significant PGM ores processed in South Africa. The varying mineralogical characteristics and PGE grades of the different PGE reefs in the BIC present unique challenges for the optimisation of flotation processes to efficiently recover the PGMs. UG2 and Platreef are metallurgically complex and difficult to process as compared to Merensky, owing to their mineralogy, finer grids, and more complex circuit designs required to achieve suitable extraction and product suitable to be smelted [27].

Table 1.
PGM mineralisation of Merensky, UG2, and Platreef ores [6].
The recovery of PGMs is commonly achieved through flotation, which is a mineral processing technique that utilises the difference in hydrophobicity or surface wettability of minerals to achieve selective separation [28,29,30]. The issues related to low PGM recovery and grade on chromatites are linked to process mineralogy, which is a crucial factor in understanding an ore body and anticipating the challenges in processing it [31,32]. The floatability of PGM species in mineral processing, particularly during flotation, is a key factor in their recovery from ore [6]. To increase the recovery of PGMs by flotation, it is necessary to optimise the liberation of the key minerals in which the PGMs are contained which include sulphides, arsenides, tellurides, and ferroalloys among others, while at the same time ensuring the optimal depression of gangue minerals [6]. Bulatovic 2003 [33] said that the recovery and concertation grade from PGMs species depend largely on the mineralogy of the ore and the way the deposit is formed. Recent studies indicating the impact of PGM species on flotation have gained attention, especially in ores like Platreef and Great Dyke where PGE tellurides and arsenides are more predominant than sulphides. The floatability of PGMs is closely linked to their liberation and grain size; for instance, very fine PGMs (<3 μm) are generally slower to float due to lower probabilities of colliding with and attaching to air bubbles during the flotation process. It also means they have a lower mass, making them more susceptible to being carried away by water flow and less likely to remain attached to air bubbles.
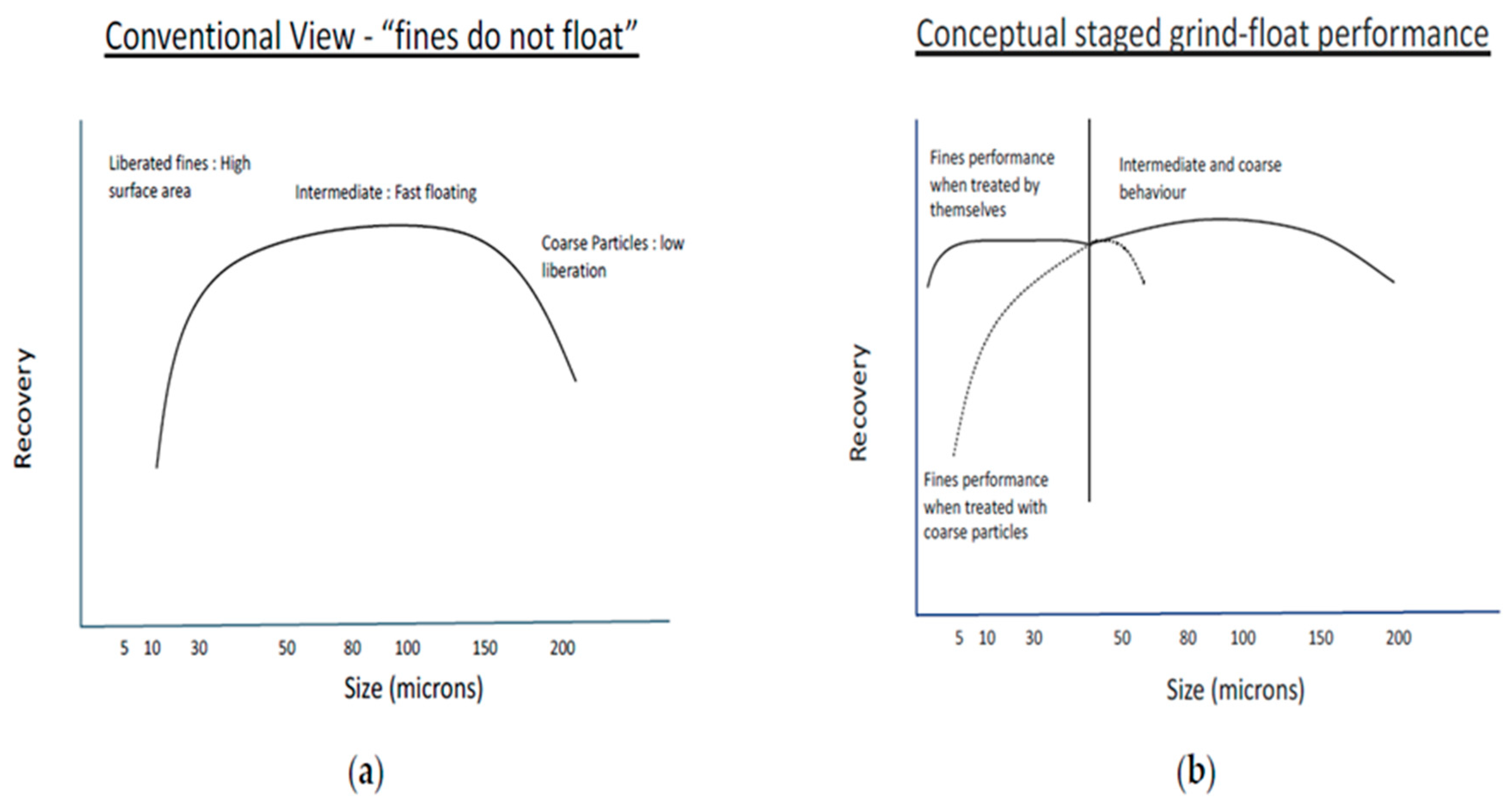
Particle size plays an important role in the recovery of PGMs by froth flotation. Jameson et al. [34] showed that large particles with a high percentage of surface liberation can float as fast as small particles with a lower percent of surface liberation [6]. Research has shown that intermediate particle sizes float far better than fine and coarse particles, as shown in Figure 2a [6]. However, with the treatment of fine particles separately, the same recovery profile remains, and the optimum size fraction shifts depending on the size range of the particles present in the system, as illustrated in Figure 2b. Many researchers have concluded that fine and coarse particles do not float well in many circuits under standard operational conditions. Fines require more energy, collector, and flotation time, but the flotation conditions are usually set to suit coarser fractions, and the energy and reagents cannot be optimised for both fines and coarse fractions simultaneously [35]. Understanding these factors and implementing appropriate strategies can improve the flotation recovery and grade of fine particles, leading to efficient separation and valuable product recovery [36,37]. When it comes to fines, their higher surface area-to-volume ratio makes them more prone to various challenges in flotation, such as increased slime coating, reduced bubble–particle collision probability, and decreased attachment to air bubbles [6,38].
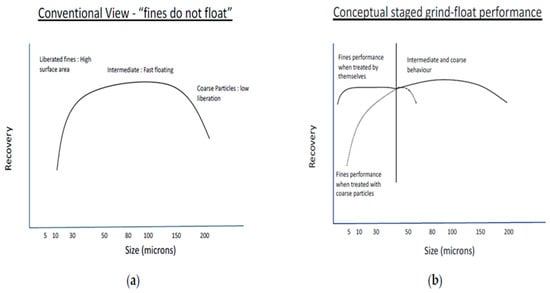
Figure 2.
(a) Recovery as a function of particle size, showing three defined regions, (b) Recovery of fines when treated separately from the other size fractions [6].
By treating fines separately, specific reagent conditions and flotation strategies can be employed to overcome these challenges and improve the recovery of fine particles [39]. Column flotation and intensified flotation cells are some techniques that can be utilised for treating fines effectively which provide a larger contact area between the air bubbles and the fine particles. The intensified flotation process enhances the probability of bubble–particle collision and attachment, leading to improved flotation performance for fines [40,41,42,43].
Studying PGM species’ flotation behaviour can enhance our understanding of the complex interplay between PGMs, reagents, and flotation conditions [30,44]. This knowledge can be applied to improve process efficiency, optimise recovery and grade, and develop predictive models for more effective mineral processing operations [28,45].
In this study, chromite tailings arising from MG 1–4 ore were studied, using cutting-edge and innovative mineralogical techniques to recover the chromite. This paper aimed to determine whether the recovery of PGMs can be adequately described by liberation index, size distribution, and base metal sulphide (BMS)/gangue relationships. This knowledge forms the basis for optimising mineral processing strategies, improving recovery and grade, and developing predictive models for the efficient extraction of PGMs.
2. Materials and Methods
2.1. Sample Preparation
A chromite tailing plant sample of MG 1–4 was obtained from one of the South African chromite beneficiation plants in the western limb. The chromite plant uses a gravity concentrator to beneficiate chromite using water as media. On receipt, the sample was air-dried, de-lumped, blended, and rifle split into 2 kg samples which were kept in sample bags. Particle size distribution (PSD) analysis of the received sample was carried out using a combination of wet and dry screening to separate the sample into various size classes from −212 µm to −25 µm based on a square root of 2 methods. Sub-samples were removed from each size fraction for chemical and mineralogical analysis. The head grade of platinum, palladium, and rhodium (3E) + Gold (Au) in the as-received sample determined using a fire assay and inductively coupled plasma mass spectrometry (ICP-OES) was 3.25 g/t.
2.2. Bulk Chemistry and Modal Mineralogy
The samples were analysed for their major and minor metal content. The chemical composition was measured with wavelength dispersive X-ray Fluorescence (WDXRF) using the Rigaku SSX Primus IV. The mineral identification and quantification were carried out using the Rigaku X-ray diffraction (XRD) Ultima IV (Bruker, Billerica, MA, USA). The 3E PGM+Au analysis was conducted using the fire assay nickel sulphide collection method, finishing with Agilent 5900 Inductively coupled plasma optical emission spectroscopy (ICP-OES) (Thermo Fisher Scientific, Waltham, MA, USA).
2.3. PGMs Characteristics
The quantitative evaluation of the minerals was conducted using automated scanning electron microscopy (FEI, Hillsboro, OR, USA) with a mineral liberation analyser (Quanta 650 FEG MLA). The software allows for the detailed analysis of mineralogical data and was instrumental in classifying the floatability of PGMs in our study [28].
3. Results
3.1. Particle Size Distribution (PSD) of the Chromite Tailings
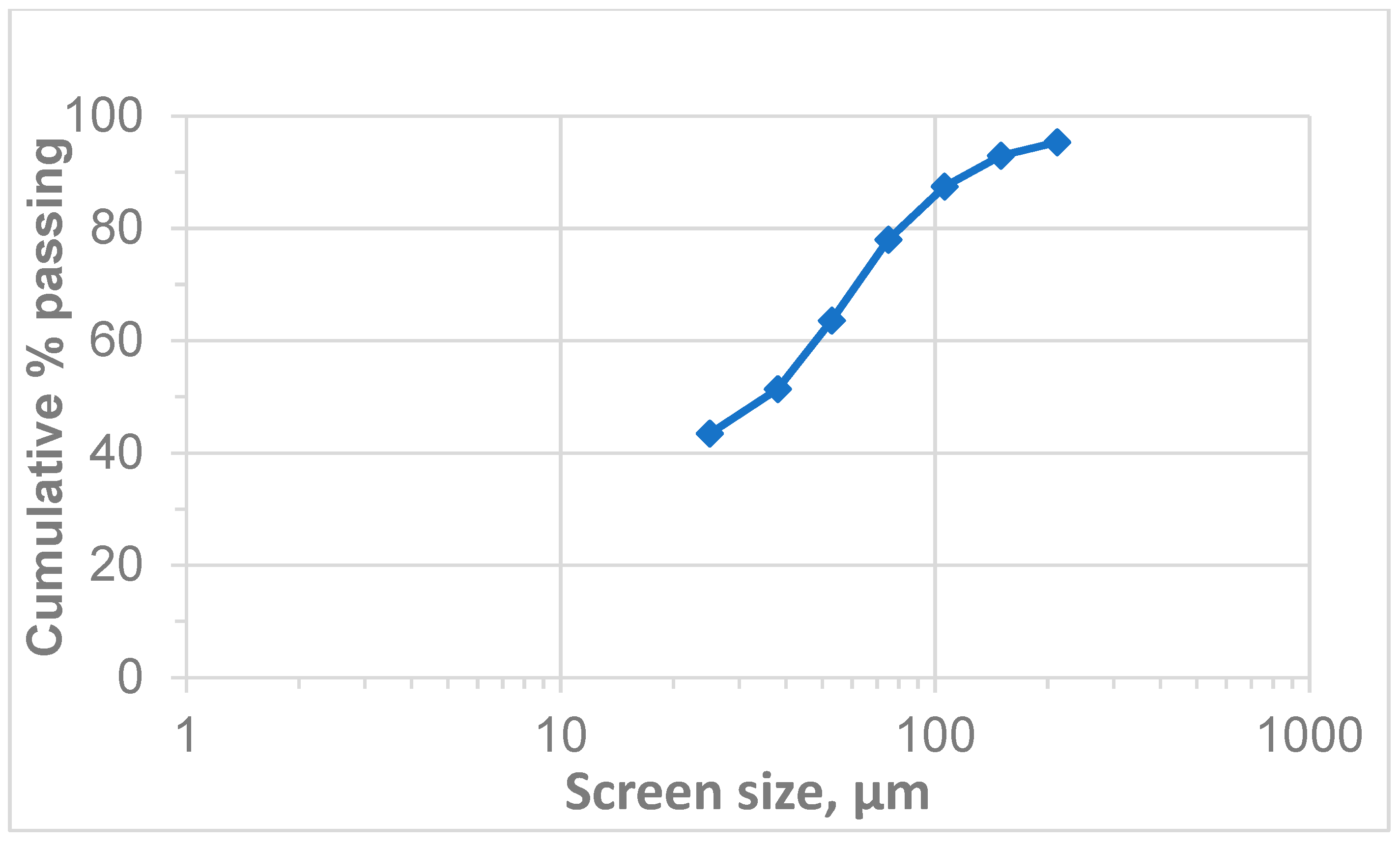
The PSD of the received chromite tailings was analysed, and the results are illustrated in Figure 3. The analysis results suggest that the sample contained a P80 of 80 µm, with a P50 of less than 38 µm; 43% of the material was less than 25 µm, representing the presence of fine and ultrafine particles. The presence of ultrafine particles in a sample can impact its performance, processing efficiency, and final product quality during froth flotation [6,46]. The −25 µm fraction was likely to contain a sizable amount of PGMs, although it may also have contained fine alteration minerals and other gangue [47]. Fine particles present challenges in flotation due to their low mass, high surface area, surface coatings, hindered particle–bubble collision, and increasing pulp viscosity which leads to poor gas dispersion. Several interrelated factors contribute to the difficulties encountered in the flotation of fine particles, ultimately affecting the recovery and grade of valuable product [42,48]. Corin et al. (2021) [6], found that higher impeller speeds are crucial for maximising the recovery of fine PGM particles. This is because increased power input creates more turbulent conditions, which improve the rate at which fine particles attach to bubbles. However, higher speeds can also lead to lower concentrate grades, as more impurities are collected alongside the valuable minerals. Therefore, it is essential to find the right balance between power input and bubble size to optimise both recovery and concentrate quality [49]. Safari et al. (2017) [26] demonstrated that using cavitation deceives to generate finer bubbles significantly improves the recovery of fine particles.
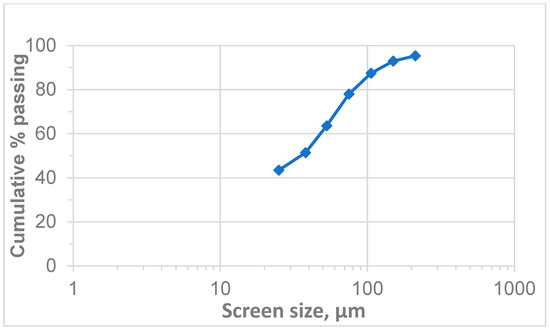
Figure 3.
Particle size distribution of the chromite tailings.
3.2. Bulk Chemical Composition of Chromite Tailings
The bulk chemistry of the chromite tailings was analysed by XRF, and the results are shown in Table 2. The results showed that the sample contained substantial amounts of Al, Mg, Cr, Si, and Fe, as well as moderate concentrations of Ca, along with the disseminated base metals Ni and Cu (BMS was analysed by ICP). The results demonstrated that the sample was dominated by silicate minerals such as olivine, pyroxene, and plagioclase feldspar [50]. PGMs often occur in association with sulphide minerals such as pentlandite (Ni,Fe)9S8 and chalcopyrite CuFeS2. These sulphide minerals can host PGMs in solid solutions or as discrete particles within their crystal structure [51].

Table 2.
Bulk XRF/ICP chemistry of chromite tailings.
PGM assays of individual size fractions were analysed using fire assaying and ICP-OES to determine the variation in bulk chemistry based on size and concentrations for Pt, Pd, Ru, and Au, and the results are shown in Table 3. The 3E PGM + Au values ranged between 1.06 and 5.06 g/t, the major PGMs present with platinum ranged between 0.65 and 3.85 g/t, representing between 68 and 71% of the total 3E PGM + Au, palladium ranged from 0.25 to 0.58 g/t (17 to 19% of the total 3E PGM + Au), rhodium between 0.10 and 0.58 g/t (10 to 11%of the total 3E PGM + Au), and gold between 0.03 and 0.06 g/t (2 to 3% of the total 3E PGM + Au), as shown in Table 3. With respect to the separation by sizing for examining the distribution of 3E PGM + Au, our findings indicated that PGMs were notably concentrated in the fraction below −25 µm, while other size fractions showed a random distribution. The results indicated that the PGM content remained locked in gangue minerals in size fractions ranging from −212 to +25 µm. Fines perform poorly when treated with coarse particles. If these particles were floated in a narrow size distribution, the flotation conditions could be tailored to them, and they would perform well. This explains the performance of the Mount Isa staged grind and float circuit [47].

Table 3.
The distribution (size-by-size assay) of the 3E PGM + Au of the chromite tailings.
3.3. Bulk Chemistry and Modal Mineralogy of Chromite Tailing
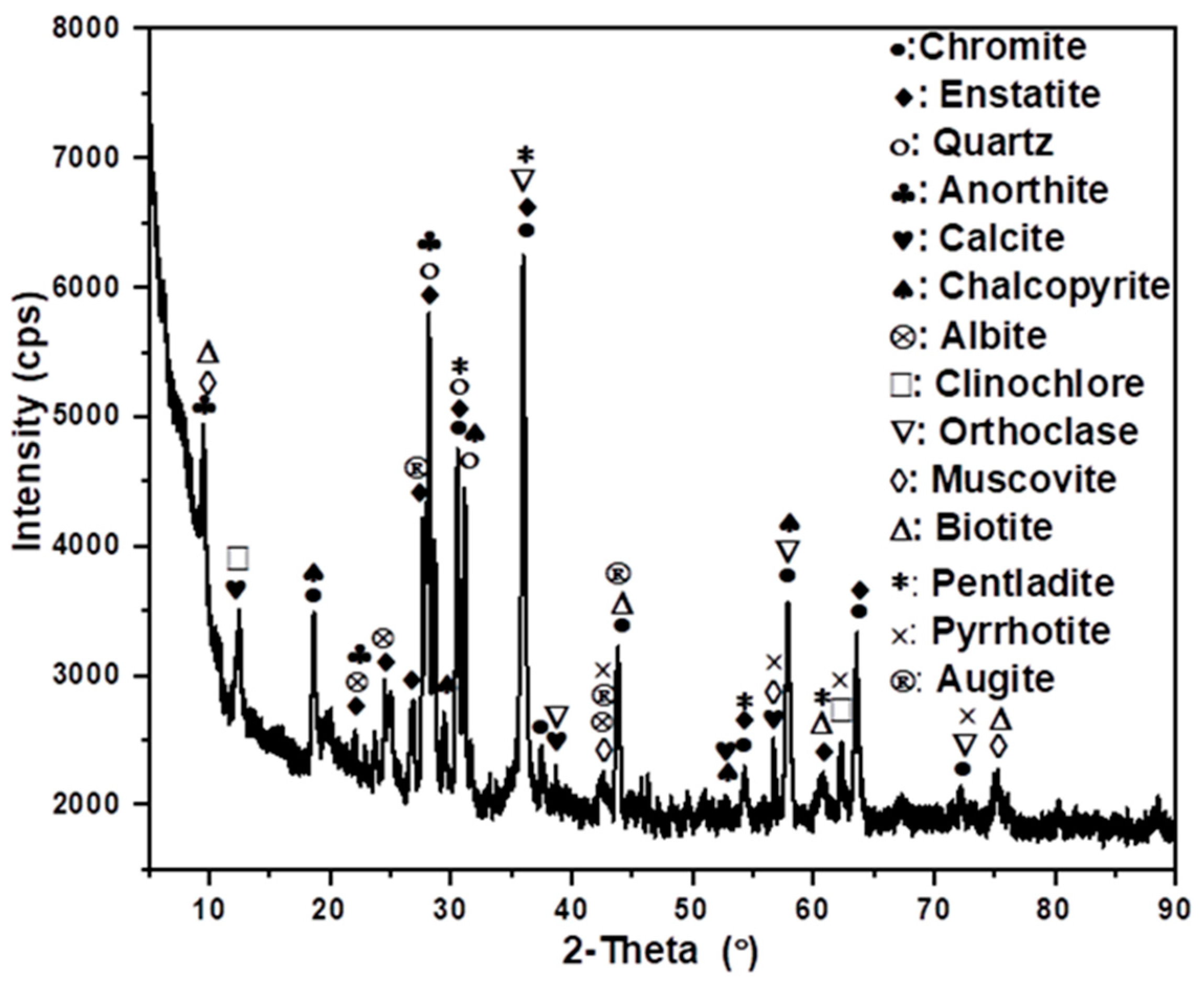
The sample was assessed for mineralogy, to investigate which factors would contribute to poor flotation responses and the results are shown in Figure 4. High phyllosilicate concentrations (particularly talc, augite, and clinochlore) are indicators of hydrothermal alteration in magmatic Ni-Cu-PGM ores. The presence of this gangue mineral suggests that the flotability of the PGMs in this sample would be quite poor [24,52]. These gangue minerals can depress the flotation of the valuable PGM-bearing minerals, leading to low recoveries in a conventional flotation circuit. The silicate and oxide minerals dominate the mineralogy of chromite tailings. The tailing sample also contained substantial quantities of naturally floatable gangue such as talc, clay, mica, and altered orthopyroxene, followed by minor valuable minerals contents of chalcopyrite, pentlandite, and pyrrhotite. The literature has showed that naturally floatable gangue minerals tend to adsorb flotation reagents and form stable froths, competing with valuable minerals like PGMs for reagent adsorption sites. This can reduce the recovery efficiency of PGMs during flotation. This can be addressed by understanding the surface charge characteristics of gangue minerals through zeta potential measurements, informed decisions regarding collector selection, gangue depression, pH control, and surface modification techniques [53,54].
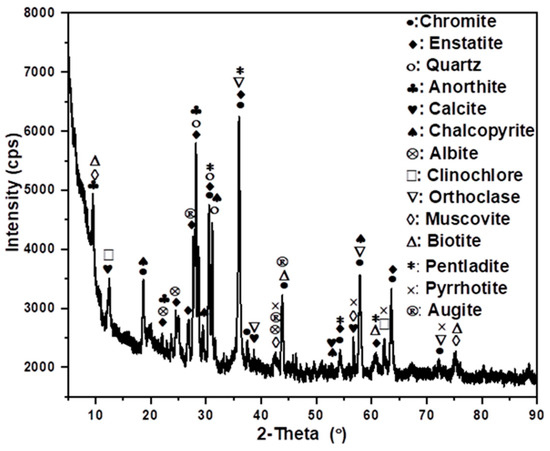
Figure 4.
Minerals characterisation of the chromite tailings using XRD.
3.4. Characterisation of PGM Species Found in the MG 1–4 Chromite Tailings
To investigate the PGM species for their theoretical flotation capabilities, grain size distribution, liberation, and association, the sample was analysed using MLA.
3.4.1. PGM Species of the MG 1–4 Chromite Tailings
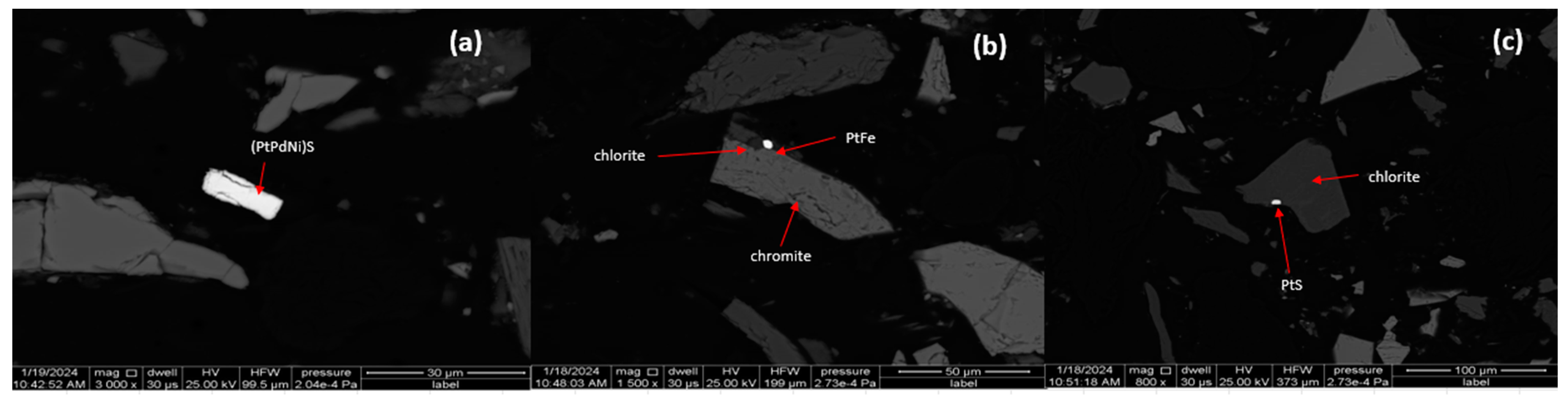
Based on 160 intersected grains, fifteen PGMs species were categorised from the X-ray spectra of encountered PGMs grains. Table 4 displays the PGM species present. The PGE grouping results showed that the PGE-sulphides group comprised 63.6 vol% of the total PGMs population, followed by Laurite comprising 17.3 vol%, PGE sulfarsenides 10.4 vol%, PGE-alloy 4.1 vol%, PGE-bismuth tellurides 3.3 vol%, and PGE-arsenides 1.3 vol%. The PGE sulphides dominated the population; however, if PGE sulphides are not adequately liberated, their recovery through flotation can be hindered [6]. If the PGE sulphides are finely disseminated or locked within the gangue matrix, it becomes challenging for the flotation process to effectively separate them [23]. In such cases, achieving optimal liberation becomes crucial to enhancing the floatability and subsequent recovery of PGE sulphides. According to Chetty et. al. [22], PGM flotability in terms of species has recently been given attention in ores like Platreef and Great Dyke, where PGE tellurides and arsenides are more abundant compared to sulphides. Meanwhile, PGMs in the UG2 reef are characterised by abundant PGE sulphide, laurite, cooperite, and braggite [6,28]. Shackleton et al. [55] said that the type of PGM species is largely only relevant to flotability if liberated PGMs are considered, in which case species can be directly correlated with flotation response [22]. However, Candice et al. [28] tested the flotation behaviour of liberated PGMs, and the results indicated that based on volume percentage, PGE arsenides and PGE sulphides dominated the liberated PGE population due to the presence of large PtAs2 and PtS grains. The liberated results indicated that the flotation of the chromite tailings would be dominated by (Pt,Pd,Ni)S, RuS2, and PtS based on volume percentage and large grains. However, Nel et. al. [56] asserted that the “hierarchy” is not clear in mineral floatability. This is consistent with similar research on the Lac Des Iles concentrator [56]. Figure 5 serves to illustrate that the PGMs were found to liberated, and they are also on the grain boundaries of silicate and oxide gangue. Complete liberation of the values is not an economically viable practise, as it is highly energy intensive. An important contributor to overall recovery is the recovery of locked and partially liberated particles.

Table 4.
PGM species found in the MG 1–4 chromite tailings.

Figure 5.
MLA backscattered electron image of a liberated PGM grain (a), PGM grain attached to chromite and chlorite (b), and PGM grain attached to chlorite (c).
3.4.2. Liberated PGMs Grain Size Distribution in the Chromite Tailings
The liberated PGMs grain size distribution plays a significant role in flotation processes [28]. The distribution of liberated PGM grains refers to the range of particle sizes that are effectively liberated from the gangue minerals and can be targeted for flotation recovery. This distribution can impact the efficiency and effectiveness of the flotation process [6]. Based on the volume percentages shown in Table 5, PGE sulphides and arsenides dominated the liberated PGMs population. This was due to the large presence of large PtS, (RhPtPd)AsS, and Cu(PtRhIr)2S4 sulphide grains. PGM grains influence the flotability of PGM-bearing material as they provide information about the range of sizes of the liberated PGM grains and are important for understanding their behaviour downstream [23,28]. Table 6 and Table 7 represent the size distribution of PGMs and grain mode of occurrence of PGMs grains. The majority of PGM grains observed were in the fast-floating category (64.4 vol%), 27.6 vol% in the slow-floating 1 category, 2.2 vol% in the slow-floating 2 category, and 5.8 vol% in the non-floating category. While the majority of the grains (49) were represented by 41.5% of the total between 0 and 3 µm, 46 grains represented 39% of the total between 3 and 6 µm, whereas only 1 grain (0.8%) fell within the 15–18 µm grain size range. Generally, larger grains (to a certain limit) have a higher likelihood of collision and attachment to bubbles, resulting in faster flotation kinetics. In contrast, smaller grains may exhibit slower flotation rates due to reduced collision efficiency [27,57]. Optimal grain size distribution is essential for maximising flotation recovery [58].

Table 5.
Distribution of PGM type grain sizes of chromite tailings.

Table 6.
PGM grain Floatability Index.

Table 7.
PGM grain size distribution of PGMs in chromite tailings.
3.4.3. PGMs Mineral Mode of Occurrence in the MG 1–4 Chromite Tailings
All PGMs observed in the sample were classified according to their texture setting into one of the following categories, as shown in Table 8. It should be noted that the mode of occurrence of the PGMs was considered for the PGM population as a whole, and no species was distinguished. The mode of occurrence of PGMs considers their associations with base metal sulphides (BMS) and gangue minerals, such as silicate and chromite [22]. These associations provide insights into how PGMs are distributed within the ore deposit. Corin et al. [6] stated that the liberation of PGMs from their associated minerals is a critical factor in achieving efficient flotation recovery.

Table 8.
Grain mode of occurrence of PGMs.
The majority of PGMs in the sample were 66.5% liberated, which is advantageous for flotation. The particle size distribution indicates that 13 of 51 liberated PGMs had an equivalent circular diameter (ECD) of <3 µm. Since most of the PGMs in the sample were 66.5% liberated, this was advantageous since two-thirds of the PGM grains were free and would have a higher probability of being selectively captured by air bubbles during flotation, leading to improved recovery rates. However, the finer particle size may require careful consideration during flotation, as it can affect the kinetics and efficiency of the flotation process.
PGMs attached to or locked within silicate or oxide gangue composite particles (AG) (27.3%) represented a significant portion of the sample. The particle size distribution indicates that 11 of the PGMs grains had a size range of <3 µm. The presence of particles containing both PGMs and gangue minerals can pose selectivity challenges during flotation. The gangue minerals may interfere with the attachment of PGMs to air bubbles, leading to a decrease in selectivity and the potential loss of valuable PGMs in the tailings.
PGMs associated with BMS attached to silicate or oxide gangue particles (SAG) (0.2%) constituted a minor proportion of the sample. The particle size distribution indicates that only one PGM grain had a size range of <3 µm.
PGMs locked within gangue or oxide particles (G) (5.8%) represented a relatively low proportion of the sample. The particle size distribution indicates that 23 of the PGM grains had a size range of <3 µm. This indicates that only a small fraction of the PGMs was tightly bound or enclosed within gangue or oxide particles. The majority of PGMs were liberated and attached to silicate or oxide gangue particles. The majority of PGMs identified in the sample were associated with gangue in the form of enstatite, chlorite, diopside, magnetite, plagioclase, quarts, and hornblende. The presence of these gangue minerals can significantly impact the flotation of PGMs in several ways. Plagioclase and enstatite are generally hydrophilic, making them less likely to attach to air bubbles. However, their surface chemistry can vary. If they are coated with hydrophobic materials or have a high iron content, they can compete with PGMs for collector reagents, reducing the effectiveness of PGM flotation. Chlorite can form coatings on the surfaces of PGMs, preventing collectors from attaching effectively. This further hinders PGM flotation. While hornblende can be both hydrophilic and hydrophobic, its tendency to form fine particles can increase pulp viscosity. This makes it harder for air bubbles to rise and carry PGMs, reducing flotation efficiency. To overcome these challenges, additional processing steps might be required, such as regrinding, re-flotation, or chemical leaching, which increase the cost of the recovery of PGMs, according to James et al. (2012) [34].
3.4.4. Liberation Index of the PGM in the Chromite Tailings
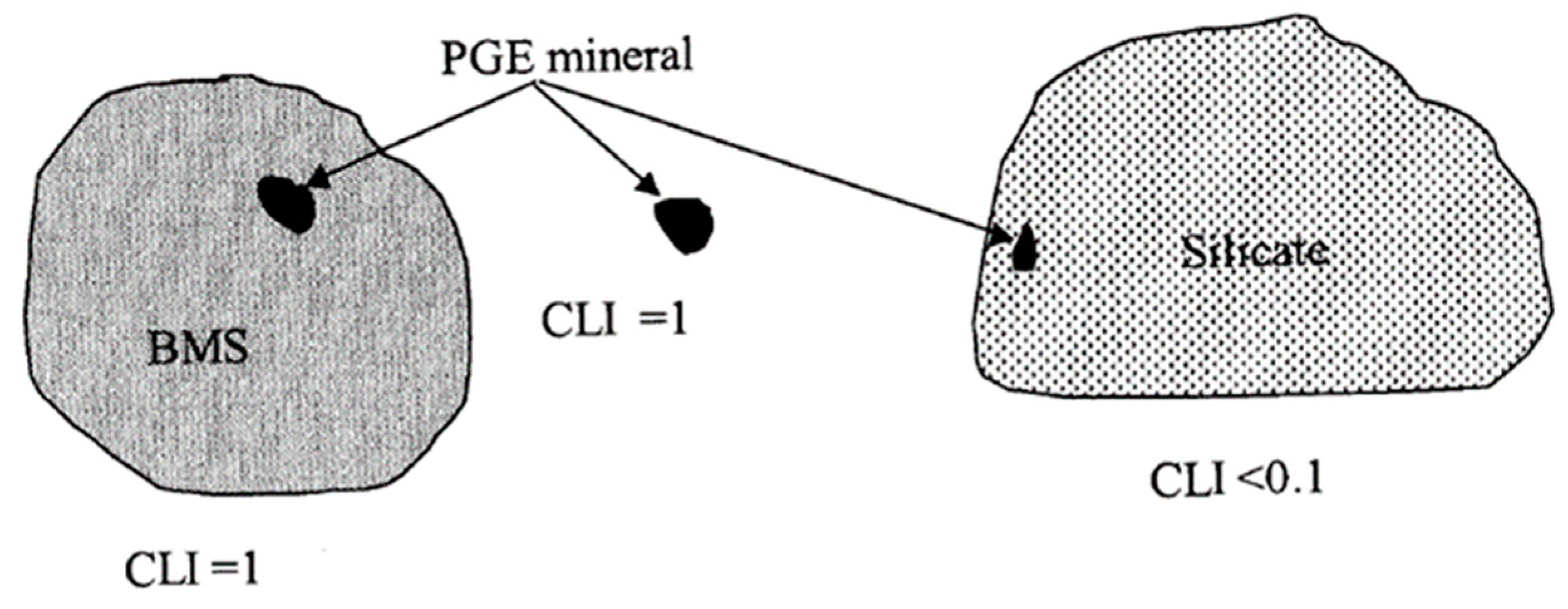
In addition, a combined liberation index (CLI) was calculated for all the mineral-bearing grains of the PGMs (Figure 6 and Table 9) using Equation (1).
where floatable mineral = BMS and PGM. This calculation yields a value between 0 and 1, with 1 indicating a particle consisting of a liberated PGM mineral grain, or a PGM attached to or locked in a liberated base-metal sulphide grain [22]. A particle consisting of a large silicate grain with a small inclusion of PGMs will be characterised by a combined liberation index approaching 0 [24]. A liberation index based on area measurements does not always give a true reflection of floatability, which depends on the exposed surface area. For instance, a large PGM grain rimmed by silicate may have a high CLI but will not be recoverable by flotation. However, in the case of small grains, perimeter measurements necessary for the calculation of exposed surface area are inaccurate, which explains the decision to base the liberation index on area measurements [22].
Combined liberation index = area of floatable mineral in particle/total area of the particle
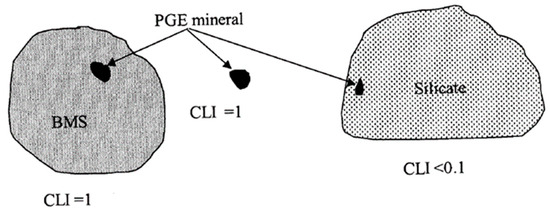
Figure 6.
Graphical representation of three PGE mineral-bearing particles illustrating the combined liberation index (CLI) principle [24].

Table 9.
Liberation index (PGMs and BMS area fraction).
The sample was characterised by 66.7 vol% with a liberation index of 0.8, indicating that the PGMs particles were fully liberated. However, the sample also exhibited a low liberation index of <0.2 that it represented by 30.8 vol% of the sample. The low liberation index suggests that a smaller proportion of PGMs were attached to silicate or oxide particles. This is illustrated by Figure 5; the PGM particles were attached to chromite and chlorite. The low liberation index limits their accessibility for efficient recovery. A higher liberation index suggests a higher likelihood of successful recovery, as a larger proportion of PGMs are in a favourable state for flotation. A low liberation index presents a challenge for efficient PGM recovery. Gibson et al. (2023) [23] and Corin et al. (2021) [6] have emphasized strategies to improve liberation, such as finer grinding or alternative comminution methods, which might be necessary to enhance PGM recovery.
3.4.5. PGM Grain Distributions in Different Size Fractions of the Chromite Tailings
The distribution of PGM particles within different size fractions can also have implications for their flotability, which refers to the susceptibility of particles to be recovered by flotation [6,59]. Table 10 represents the results of PGM distribution in different size fractions of the chromite tailings. The fine fraction of <20 µm had a high percentage of PGM particles (74%), suggesting that a significant proportion of the PGMs existed as fine particles. Fine particles often exhibit lower flotability due to several factors, such as sliming effects, surface oxidation, low particle–bubble collision, and poor flotation techniques available to float ultrafine liberated particles [60,61]. Fine particles tend to have a larger surface area relative to their volume, which can result in increased surface oxidation or the formation of hydrophilic coatings. These factors can reduce the hydrophobicity of the PGM particles and make them less amenable to attachment to air bubbles during the flotation process.

Table 10.
PGM distributions in different size fractions of the chromite tailings.
The medium fraction (20–50 µm) contained 18% of the PGM particles. The flotability of the particles in this size range can vary depending on factors such as mineral associations and surface properties. Generally, medium-sized particles may exhibit moderate flotability compared to fine particles. These particles can have a better balance between surface area and volume, which may enhance their hydrophobicity and attachment to air bubbles [54]. However, the flotability of particles in this size range can still be influenced by other factors such as mineralogy, gangue mineral associations, and the presence of surface coatings [57,62]. Optimal flotation conditions and suitable reagents may be required to maximise the recovery of PGMs in this fraction.
Coarse fraction (>50 µm) contained the smallest proportion of PGM particles (7.53%). This fraction of particles generally has higher flotability compared to fine particles [6]. They have a smaller surface area relative to their volume, which can result in reduced surface oxidation and hydrophilic coatings. Coarser particles (to an optimum particle size) are more likely to be readily attached to air bubbles during the flotation process, leading to higher flotation recovery. However, the flotability of coarse PGM particles can still be influenced by factors such as mineral associations, particle shape, and hydrodynamic conditions within the flotation cell. The optimisation of flotation parameters, such as bubble size, agitation, and froth characteristics, can help maximise the recovery of PGMs in this fraction. It is important to note that the flotability of PGM particles is not solely determined by their size distribution. Other factors, such as mineralogy, surface chemistry, and the presence of valuable or detrimental elements can also influence the flotability.
3.4.6. PGMs–BMS–Gangue Associations in the MG 1–4 Chromite Tailings
Table 11 represents the PGM–BMS–gangue associations. The flotability of PGMs can be influenced by their associations with different minerals within a sample. The PGMs identified in the sample were associated with plagioclase, enstatite, hornblende, chlorite, chromite, magnetite, rutile, ilmenite, diopside, and pyrrhotite. Plagioclase, enstatite, and hornblende are common silicate minerals and are generally hydrophilic, meaning they tend to repel water and air bubbles. Their association with PGMs can potentially decrease their flotability by inhibiting their attachment to air bubbles during flotation [27].

Table 11.
PGM–BMS–Gangue associations in the MG 1–4 chromite tailings.
Chromite minerals and their association with PGMs can enhance their flotability, as chromite particles tend to readily attach to air bubbles [63,64]. However, it is important to note that chromite is essentially recovered by entrainment, which may affect the final grade of PGMs during flotation. Pyrrhotite associations (compared to BMS associations) with PGMs can potentially decrease their flotability and hinder their attachment to air bubbles during flotation [65,66,67].
The flotability of PGMs associated with these minerals can be influenced by various factors, including specific mineralogy, particle size, surface chemistry, and the presence of valuable or detrimental elements. The overall flotability of PGMs will depend on the interplay of these factors and the optimisation of flotation conditions, such as reagent selection and dosages, pH, energy input, and flotation kinetics, to maximise their recovery.
4. Conclusions
This study has provided a comprehensive mineralogical characterisation of chromite tailings from the Middle Group (1–4) of the Bushveld complex, South Africa, that can guide the optimisation of the recovery of PGMs from MG chromite tailings. Our findings indicated that the sample had a P80 of 80 μm and a high proportion mass percentage of 43% of particles finer than 25 μm, indicating the presence of a significant amount of fine and ultrafine particles. The presence of these fine and ultrafine particles can pose several challenges in the flotation process, including reduced flotation kinetics and recovery of valuable minerals due to their small size and high surface area. Increased entrainment of these fine particles in froth leads to lower concentrate grades and potential issues with particle stabilisation and bubble–particle attachment.
The sample contained 20% altered minerals, such as talc and serpentine, which can have significantly different surface properties compared to valuable minerals. These altered minerals can interfere with the selective flotation of valuable minerals, leading to reduced recoveries and concentrate grades. Specialised reagent schemes and optimisation of the flotation conditions may be required to address the detrimental effects of altered minerals. A substantial 74% of PGM grains were found to be below 20 µm, with only 18% in the 20–50 µm range and 7.5% exceeding 50 µm. This fine grain size distribution presents a major challenge for conventional flotation processes, as liberation and efficient recovery become increasingly difficult with decreasing particle size. The sample had a relatively high proportion (27.3%) of PGM grains that were attached to or locked within silicate or oxide gangue composite particles. Additionally, a small percentage (5.8%) of the PGM grains were locked within gangue or oxide particles. These associations and the locking of valuable minerals within gangue can limit their liberation and accessibility during the flotation process, reducing their recovery.
The majority of the PGM grains (64.4%) were reported to be in the fast-floating category, which is favourable. However, a significant portion (27.6%) was in the slow-floating 1 category, and a small proportion (2.2%) was in the slow-floating 2 category. The presence of these slow-floating PGM grains can pose challenges in achieving high overall recoveries, as they may require longer flotation times. By leveraging this detailed mineralogical characterisation, the flotation process can be designed and optimised to achieve the efficient recovery of PGMs from Middle Group chromite tailings of the Bushveld Complex.
Author Contributions
Conceptualisation, N.P.B., M.S., W.N. and V.S.; methodology, N.P.B. and M.S.; formal analysis, N.P.B. and M.S.; investigation, N.P.B. and M.S.; resources, N.P.B., W.N. and M.S.; data curation, N.P.B. and M.S.; writing—original draft preparation, N.P.B.; writing—review and editing, N.P.B., M.S., W.N. and V.S.; visualisation, N.P.B. and M.S.; supervision, M.S., W.N. and V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a University of Johannesburg research grant (URC 2024), the University capacity development programme (USDP/DHET 2024), and Mintek 2024.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
The authors acknowledge and appreciate the contributions of the University of Johannesburg extraction metallurgy laboratory staff and Mintek mineral processing laboratory staff. Colleagues in the Mineralogy Division are acknowledged for conducting the mineralogical analysis.
Conflicts of Interest
M.S. is an employee of Mintek. This paper reflects the views of the scientists and not the company.
References
- Yudovskaya, M.A.; Kinnaird, J.A. Chromite in the Platreef (Bushveld Complex, South Africa): Occurrence and evolution of its chemical composition. Miner. Depos. 2010, 45, 369–391. [Google Scholar] [CrossRef]
- Junge, M.; Oberthür, T.; Osbahr, I.; Gutter, P. Platinum-group elements and minerals in the lower and middle group chromitites of the western Bushveld Complex, South Africa. Miner. Depos. 2016, 51, 841–852. [Google Scholar] [CrossRef]
- Oberthür, T.; Junge, M.; Rudashevsky, N.; de Meyer, E.; Gutter, P. Platinum-group minerals in the LG and MG chromitites of the eastern Bushveld Complex, South Africa. Miner. Depos. 2016, 51, 71–87. [Google Scholar] [CrossRef]
- Langa, M.M.; Jugo, P.J.; Leybourne, M.I.; Grobler, D.F.; Adetunji, J.; Skogby, H. Chromite chemistry of a massive chromitite seam in the northern limb of the Bushveld Igneous Complex, South Africa: Correlation with the UG-2 in the eastern and western limbs and evidence of variable assimilation of footwall rocks. Miner. Depos. 2021, 56, 31–44. [Google Scholar] [CrossRef]
- Sefako, R.; Sekgarametso, K.; Sibanda, V. Potential Processing Routes for Recovery of Platinum Group Metals from Southern African Oxidized PGM Ores: A Review. J. Sustain. Metall. 2017, 3, 797–807. [Google Scholar] [CrossRef]
- Corin, K.C.; McFadzean, B.J.; Shackleton, N.J.; O’connor, C.T. Challenges related to the processing of fines in the recovery of platinum group minerals (PGMs). Minerals 2021, 11, 533. [Google Scholar] [CrossRef]
- Murthy, Y.R.; Tripathy, S.K.; Kumar, C.R. Chrome ore beneficiation challenges & opportunities—A review. Miner. Eng. 2011, 24, 375–380. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Zhang, S.E.; Nwaila, G.T.; Bourdeau, J.E.; Safari, M.; Hoseinie, S.H.; Nwaila, P.; Ruuska, J. Dry laboratories—Mapping the required instrumentation and infrastructure for online monitoring, analysis, and characterization in the mineral industry. Miner. Eng. 2023, 191, 107971. [Google Scholar] [CrossRef]
- Cawthorn, R.G.; Cawthorn, R.G. Geological interpretations from the PGE distribution in the Bushveld Merensky and UG2 chromitite reefs. J. S. Afr. Inst. Min. Metall. 2017, 111, 11–14. [Google Scholar]
- Cawthorn, R.G. The platinum and palladium resources of the Bushveld Complex. S. Afr. J. Sci. 1999, 95, 481–489. [Google Scholar]
- Kottke-Levin, J. A Geochemical Study of the Middle Group Chromitites, Helena Mine, Bushveld Complex, South Africa. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2011. [Google Scholar]
- Bachmann, K.; Menzel, P.; Tolosana-Delgado, R.; Schmidt, C.; Hill, M.; Gutzmer, J. Multivariate geochemical classification of chromitite layers in the Bushveld Complex, South Africa. Appl. Geochem. 2019, 103, 106–117. [Google Scholar] [CrossRef]
- Magson, J.; Tredoux, M.; Roelofse, F. Association of platinum-group elements with chromitite within the Merensky reef, Western Limb, Bushveld Complex: Results of a high resolution mineralogical and geochemical study. J. Afr. Earth Sci. 2018, 144, 161–175. [Google Scholar] [CrossRef]
- Maier, W.D.; Bowen, M.P. The UG2—Merensky Reef interval of the Bushveld Complex northwest of Pretoria. Miner. Depos. 1996, 31, 386–393. [Google Scholar] [CrossRef]
- Von Gruenewaldt, G.; Hatton, C.J. Platinum-Group Metals—A Resource in the Tailings of Chromium Mines in South Africa. J. S. Afr. Inst. Min. Metall. 1987, 87, 265–268. [Google Scholar]
- Hoeve, T.J.V.; Scoates, J.S.; Wall, C.J.; Weis, D.; Amini, M. A temperature-composition framework for crystallisation of fractionated interstitial melt in the Bushveld Complex from trace element systematics of sircon and rutile. J. Petrol. 2018, 59, 1383–1416. [Google Scholar] [CrossRef]
- Scoon, R.N.; Viljoen, M.J. Geoheritage of the Eastern Limb of the Bushveld Igneous Complex, South Africa: A Uniquely Exposed Layered Igneous Intrusion. Geoheritage 2019, 11, 1723–1748. [Google Scholar] [CrossRef]
- McIntosh, R. Petrogenesis of the LG-6 Chromitite at Ruighoek Mine Western Limb of the Bushveld Complex. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2017. [Google Scholar]
- Langa, M.M.; Jugo, P.J.; Leybourne, M.I.; Grobler, D.F. Stratigraphic variations in chromite chemistry through the UG-2 and UG-2E chromitites, bushveld igneous complex: Implications for chromitite petrogenesis. Int. Geol. Rev. 2023, 65, 2961–2979. [Google Scholar] [CrossRef]
- Cawthorn, R.G.; Lee, C.A.; Schouwstra, R.P.; Mellowship, P. Relationship between PGE and PGM in the bushveld complex. Can. Miner. 2002, 40, 311–328. [Google Scholar] [CrossRef]
- Meima, J.A.; Rammlmair, D.; Junge, M. The use of Laser Induced Breakdown Spectroscopy for the mineral chemistry of chromite, orthopyroxene and plagioclase from Merensky Reef and UG-2 chromitite, Bushveld Complex, South Africa. Chem. Geol. 2021, 589, 120686. [Google Scholar] [CrossRef]
- Chetty, D.; Gryffenberg, L.; Lekgetho, T.B.; Molebale, I.J. Automated SEM study of PGM distribution across a UG2 flotation concentrate bank: Implications for understanding PGM floatability. J. S. Afr. Inst. Min. Metall. 2009, 109, 587–593. [Google Scholar]
- Gibson, B.A.; Nwaila, G.; Manzi, M.; Ghorbani, Y.; Ndlovu, S.; Petersen, J. The valorisation of platinum group metals from flotation tailings: A review of challenges and opportunities. Miner. Eng. 2023, 201, 108216. [Google Scholar] [CrossRef]
- Penberthy, C.J. The Effect of Mineralogical Variation in the UG2 Chromitite on Recovery of Platinum-Group Elements. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2006. [Google Scholar]
- Naldrett, A.J.; Wilson, A.; Kinnaird, J.; Yudovskaya, M.; Chunnett, G. The origin of chromitites and related PGE mineralization in the Bushveld Complex: New mineralogical and petrological constraints. Miner. Depos. 2011, 47, 209–232. [Google Scholar] [CrossRef]
- Safari, M.; Harris, M.; Deglon, D. The effect of energy input on the flotation of a platinum ore in a pilot-scale oscillating grid flotation cell. Miner. Eng. 2017, 110, 69–74. [Google Scholar] [CrossRef]
- Ramonotsi, M. Characterisation of the Effect of Alteration on the PPM Platinum Ore and Evaluation of Selected Strategies to Improve Metallurgical Performance. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2011. [Google Scholar]
- Carelse, C.; Manuel, M.; Chetty, D.; Taguta, J.; Safari, M.; Youlton, K. The flotation behaviour of liberated Platinum Group minerals in Platreef ore under reduced reagent conditions. Miner. Eng. 2022, 190, 107913. [Google Scholar] [CrossRef]
- Sibanda, V.; Khan, R.; Danha, G. The effect of chemical reagents on flotation performance of a pentlandite ore: An attainable region approach. Powder Technol. 2019, 352, 462–469. [Google Scholar] [CrossRef]
- Safari, M.; Hoseinian, F.; Deglon, D.; Filho, L.L.; Pinto, T.S. Impact of flotation operational parameters on the optimization of fine and coarse Itabirite iron ore beneficiation. Powder Technol. 2022, 408, 117772. [Google Scholar] [CrossRef]
- Graham, S.D.; Brough, C.P.; Cropp, A. An introduction to SEISS Mineralogic Mining and the correlation of light mi-croscopy with automated mineralogy: A case study using BMS and PGM analysis of samples from a PGE-bearing chromitite prospect. Precious Metall. 2015, 15, 10. [Google Scholar]
- Bradshaw, D. The role of ‘process mineralogy’ in improving the process performance of complex sulphide ores. In Proceedings of the IMPC 2014—27th International Mineral Processing Congress, Santiago, Chile, 20–24 October 2014. [Google Scholar]
- Bulatovic, S. Evaluation of alternative reagent schemes for the flotation of platinum group minerals from various ores. Miner. Eng. 2003, 16, 931–939. [Google Scholar] [CrossRef]
- Jameson, G.J. The effect of surface liberation and particle size on flotation rate constants. Miner. Eng. 2012, 36–38, 132–137. [Google Scholar] [CrossRef]
- Safari, M.; Hoseinian, F.S.; Deglon, D.; Filho, L.L.; Souza, T.C. Investigation of the reverse flotation of hematite in three different types of laboratory flotation cells. In Proceedings of the XXIX International Mineral Processing Congress 2019 (IMPC 2018), Moscow, Russia, 17–21 September 2018; pp. 1376–1383. [Google Scholar]
- Hoseinian, F.S.; Bahram, R.; Elaheh, K.; Mehdi, S. The effect of water recovery on the ion flotation process efficiency. Physicochem. Probl. Miner. Process. 2020, 56, 919–927. [Google Scholar] [CrossRef]
- Sajjad, M.; Otsuki, A. Correlation between Flotation and Rheology of Fine Particle Suspensions. Metals 2022, 12, 270. [Google Scholar] [CrossRef]
- Safari, M.; Deglon, D. An Attachment-Detachment Kinetic Model for the Effect of Energy Input on Flotation. Miner. Eng. 2018, 117, 8–13. [Google Scholar] [CrossRef]
- Pease, J.D.; Young, M.F.; Curry, D.; Johnson, N.W. Improving fines recovery by grinding finer. Miner. Process. Extr. Metall. 2010, 119, 216–222. [Google Scholar] [CrossRef]
- Tsave, P.K.; Kostoglou, M.; Karapantsios, T.D.; Lazaridis, N.K. A hybrid device for enhancing flotation of fine particles by combining micro-bubbles with conventional bubbles. Minerals 2021, 11, 561. [Google Scholar] [CrossRef]
- Sadovskiy, D.; Rulyova, N.; Filippov, L. Column flotation of fine glass beads enhanced by their prior heteroaggregation with microbubbles. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126398. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Safari, M.; Khoshdast, H.; Güner, M.K.; Hoang, D.H.; Sambrook, T.; Kowalczuk, P.B. Introducing key advantages of intensified flotation cells over conventionally used mechanical and column cells. Physicochem. Probl. Miner. Process. 2022, 58, 155101. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Safari, M.; Hoang, D.H.; Khoshdast, H.; Albijanic, B.; Kowalczuk, P.B. Technological assessments on recent developments in fine and coarse particle flotation systems. Miner. Eng. 2022, 180, 107509. [Google Scholar] [CrossRef]
- Sefako, R.; Sibanda, V.; Sekgarametso, K. PGM extraction from oxidized ores using flotation and leaching. J. S. Afr. Inst. Min. Metall. 2019, 119, 929–936. [Google Scholar] [CrossRef]
- Soufiabadi, A.M.; Dehghan, R.; Nejadaria, M.; Safari, M.; Hassanzadeh, A.; Khoshdast, H. Effect of different process water sources on rougher flotation efficiency of a copper ore: A case study at Sarcheshmeh Copper Complex (Iran). Physicochem. Probl. Miner. Process. 2023, 59, 184087. [Google Scholar] [CrossRef]
- Murthy, Y.R.; Tripathy, S. Process optimization of a chrome ore gravity concentration plant for sustainable development. J. S. Afr. Inst. Min. Metall. 2020, 120, 26–268. [Google Scholar] [CrossRef]
- Pease, J.; Curry, D.; Young, M. Designing flotation circuits for high fines recovery. Miner. Eng. 2005, 19, 831–840. [Google Scholar] [CrossRef]
- Malenga, E.N.; Mulaba-Bafubiandi, A.; Nheta, W. Application of the response surface method (RSM) based on central composite design (CCD) and design space (DS) to optimize the flotation and the desliming conditions in the recovery of PGMs from mine sludge. Sep. Sci. Technol. 2022, 57, 2960–2983. [Google Scholar] [CrossRef]
- Singh, A. Enhanced flotation of platinum mineral fines through feed cavitation: ‘Bringing the mountain to Mohamed’. In Proceedings of the IMPC 2016: XXVIII International Mineral Processing Congress Proceedings, Quebec City, QC, Canada, 11–15 September 2016; Canadian Institute of Mining, Metallurgy and Petroleum: Montreal, QC, Canada, 2016. [Google Scholar]
- Baloyi, N.; Nheta, W.; Sibanda, V. Characterisation of PGMs-Bearing Chromite Plant Tailings. In Proceedings of the 39th JOHANNESBURG International Conference on “Chemical, Biological and Environmental Engineering” (JCBEE-23), Johannesburg, South Africa, 16–17 November 2023. [Google Scholar] [CrossRef]
- Amaral, L.F.S. The Distribution of Platinum-Group Elements and Other Chalcophile Elements among Sulfide Minerals from the Ovoid Ore Body of the Voisey’s Bay Ni-Cu Sulfide Deposit. Ph.D. Thesis, Université du Québec à Chicoutimi, Chicoutimi, QC, Canada, 2017. [Google Scholar]
- Edwards, R. Ore Deposit Geology and Its Influence on Mineral Exploration; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Nazari, S.; Hoseinian, F.S.; Li, J.; Safari, M.; Khoshdast, H.; Li, J.; He, Y. Synergistic effect of grinding time and submicron (nano) bubbles on the zeta potential state of spent lithium-ion batteries: A gene expression programming approach. J. Energy Storage 2023, 70, 107942. [Google Scholar] [CrossRef]
- Martinovic, J.; Bradshaw, D.J.; Harris, P.J. Investigation of surface properties of gangue minerals in platinum bearing ores. J. S. Afr. Inst. Min. Metall. 2005, 105, 349–356. [Google Scholar]
- Shackleton, N.; Malysiak, V.; O’connor, C. Surface characteristics and flotation behaviour of platinum and palladium tellurides. Miner. Eng. 2007, 20, 1232–1245. [Google Scholar] [CrossRef]
- Nel, E.; Martin, C. Ore Processing at Impala’s UG-2 concentrator in Rustenburg, South Africa. Tech-Bull 2004, 2. [Google Scholar]
- Taguta, J.; Safari, M.; Govender, V.; Chetty, D. Investigating the Amenability of a PGM-Bearing Ore to Coarse Particle Flotation. Minerals 2023, 13, 698. [Google Scholar] [CrossRef]
- Safari, M.; Hoseinian, F.; Deglon, D.; Filho, L.L.; Pinto, T.S. Investigation of the reverse flotation of iron ore in three different flotation cells: Mechanical, oscillating grid and pneumatic. Miner. Eng. 2020, 150, 106283. [Google Scholar] [CrossRef]
- Safari, M.; Deglon, D. Evaluation of an Attachment–Detachment Kinetic Model for Flotation. Minerals 2020, 10, 978. [Google Scholar] [CrossRef]
- Junge, M.; Oberthür, T.; Kraemer, D.; Melcher, F.; Piña, R.; Derrey, I.T.; Manyeruke, T.; Strauss, H. Distribution of platinum-group elements in pristine and near-surface oxidized Platreef ore and the variation along strike, northern Bushveld Complex, South Africa. Miner. Depos. 2019, 54, 885–912. [Google Scholar] [CrossRef]
- Hoseinian, F.S.; Safari, M.; Deglon, D. Ion flotation kinetic predictions using empirical and phenomenological models. Miner. Eng. 2024, 210, 108645. [Google Scholar] [CrossRef]
- Anzoom, S.J.; Bournival, G.; Ata, S. Coarse particle flotation: A review. Miner. Eng. 2024, 206, 108499. [Google Scholar] [CrossRef]
- Mailula, T.D.; Bradshaw, D.J.; Harris, P.J. The effect of copper sulphate addition on the recovery of chromite. J. S. Afr. Inst. Min. Metall. 2003, 103, 143–146. [Google Scholar]
- Pownceby, M.I.; McCallum, D.A.; Bruckard, W.J. Automated and Quantitative Mineralogy Applied to Chromite Ore Characterization and Beneficiation. Minerals 2023, 13, 440. [Google Scholar] [CrossRef]
- McCallum, D.A.; Bruckard, W.J.; Pownceby, M.I. Chromite ore—Characterisation through to processing. In Proceedings of the MetPlant 2008, Perth, Australia, 18–19 August 2008. [Google Scholar]
- Mokadze, A.M.; Ndlovu, S.; Shemi, A.; Dworzanowski, M. The Reduction of Chrome in UG-2 Flotation Concentrate by Hydrometallurgical Means. Int. J. Miner. Process. Extr. Metall. 2021, 6, 41. [Google Scholar] [CrossRef]
- Ramlall, N. Measuring and modelling entrainment in rougher and cleaner batch flotation. J. S. Afr. Inst. Min. Metall. 2020, 120, 233–242. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).