Preparation of Nickel–Iron Concentrate from Low-Grade Laterite Nickel Ore by Solid-State Metalized Reduction and Magnetic Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Methods and Equipment
2.3. Thermodynamic Basis
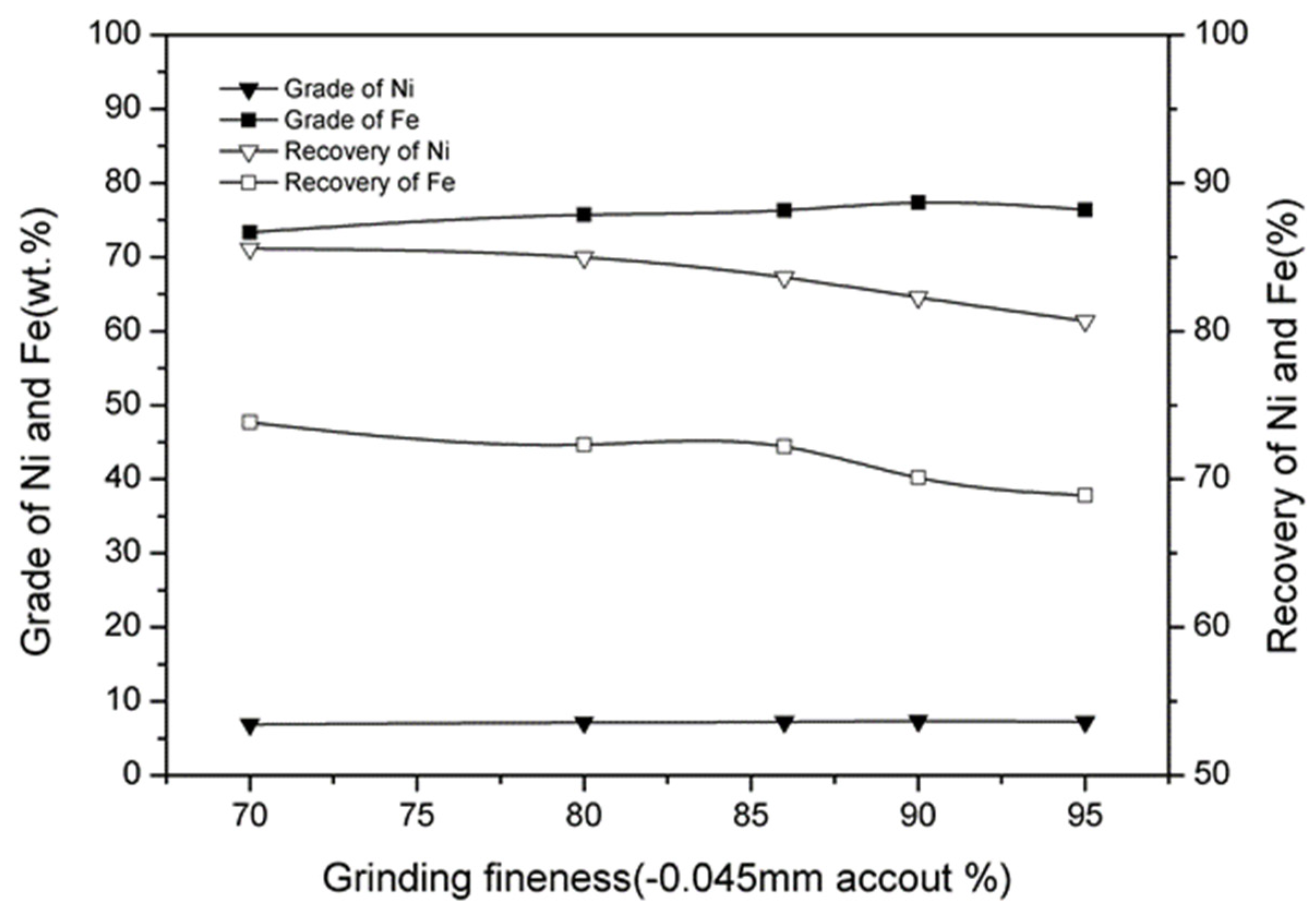
3. Results
3.1. Effect of Reduction Temperature
3.2. Effect of Reduction Time
3.3. Effect of Coal Addition
3.4. Effect of CaF2 Addition
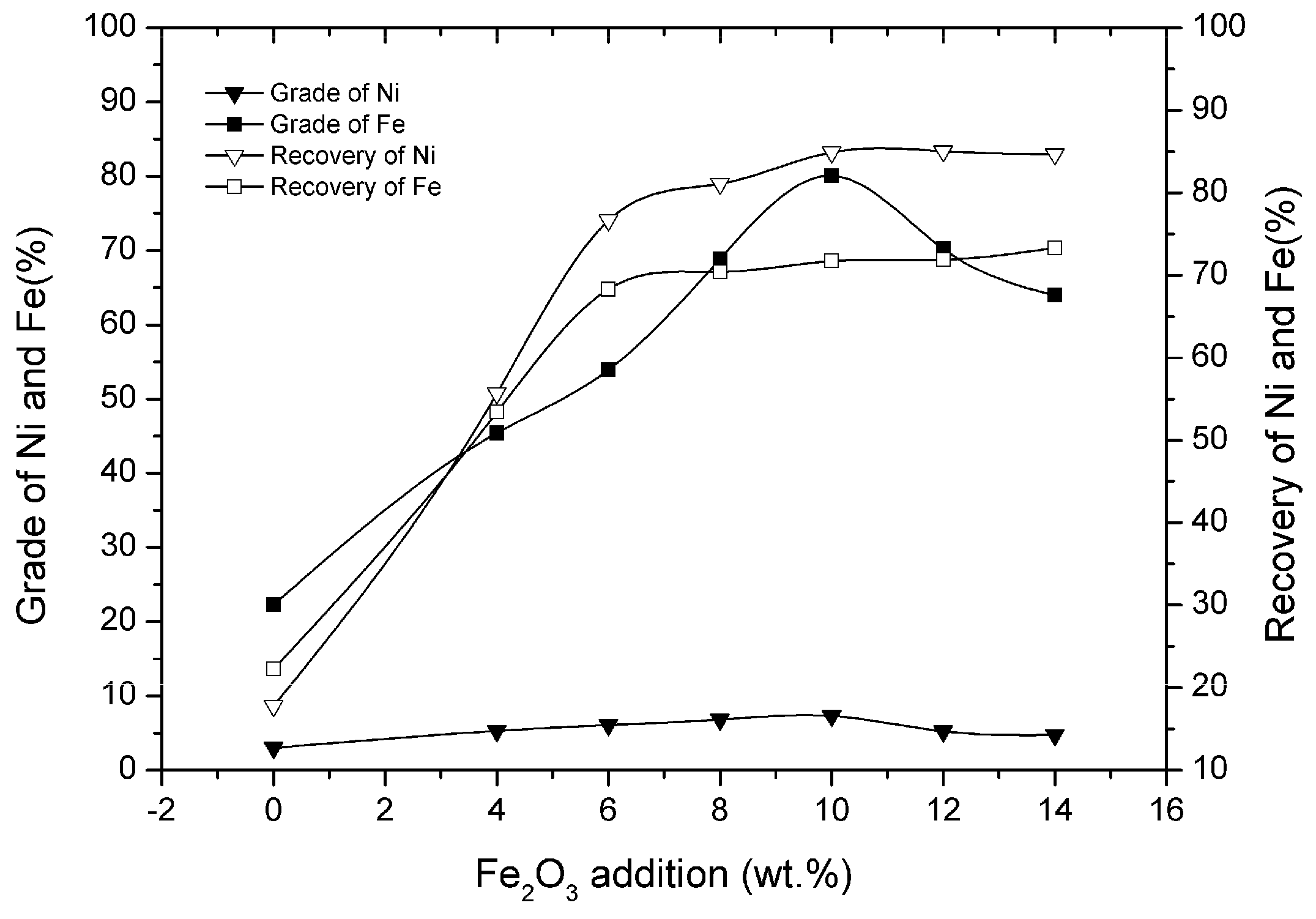
3.5. Effect of Fe2O3 Addition
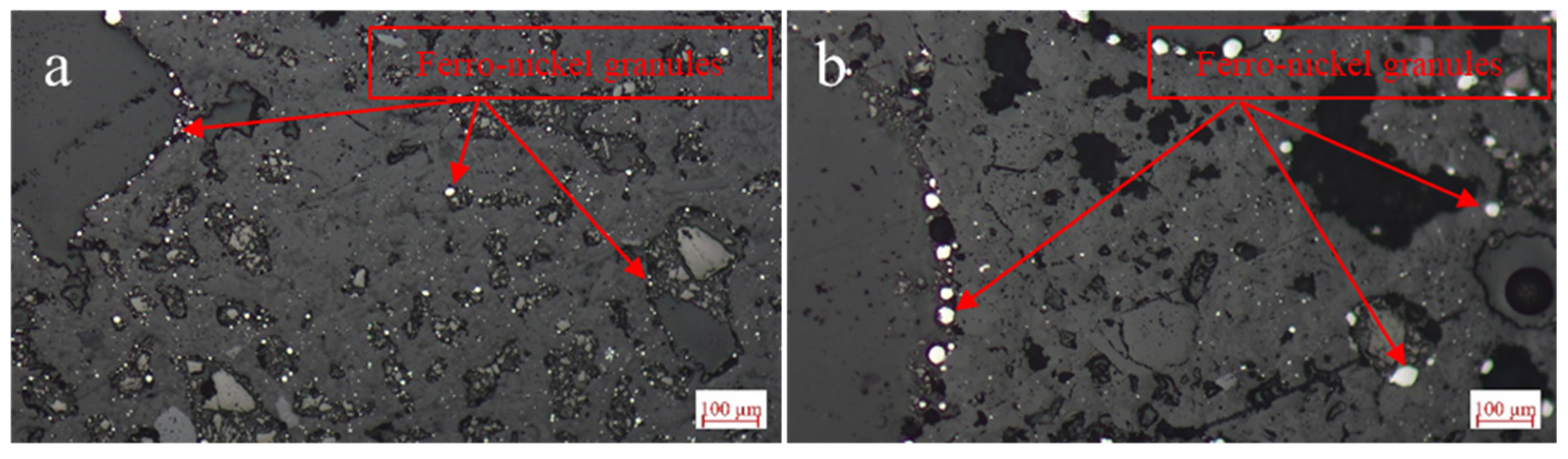
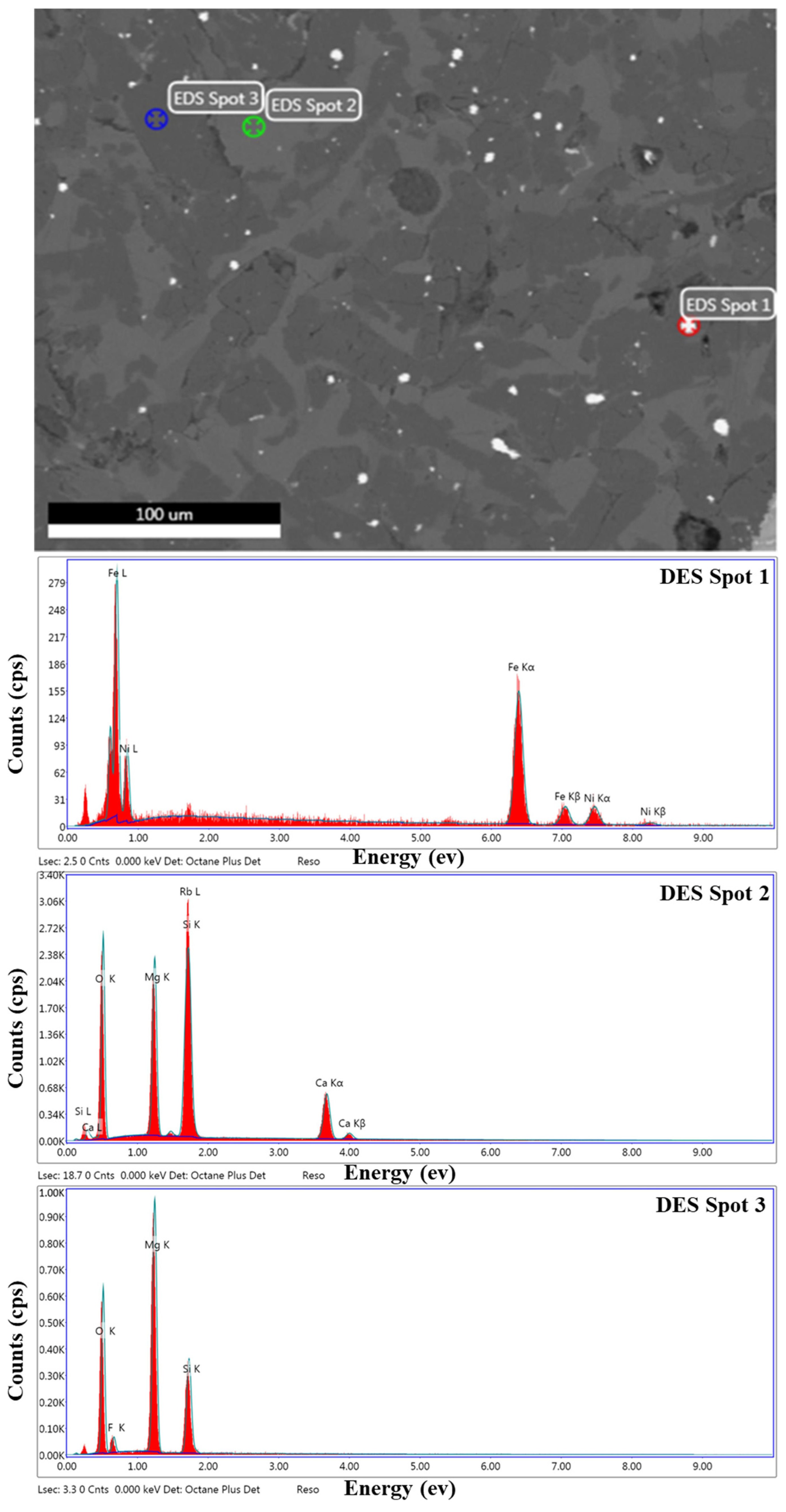
3.6. Elemental Composition of the Particular Regions in Reduction Product
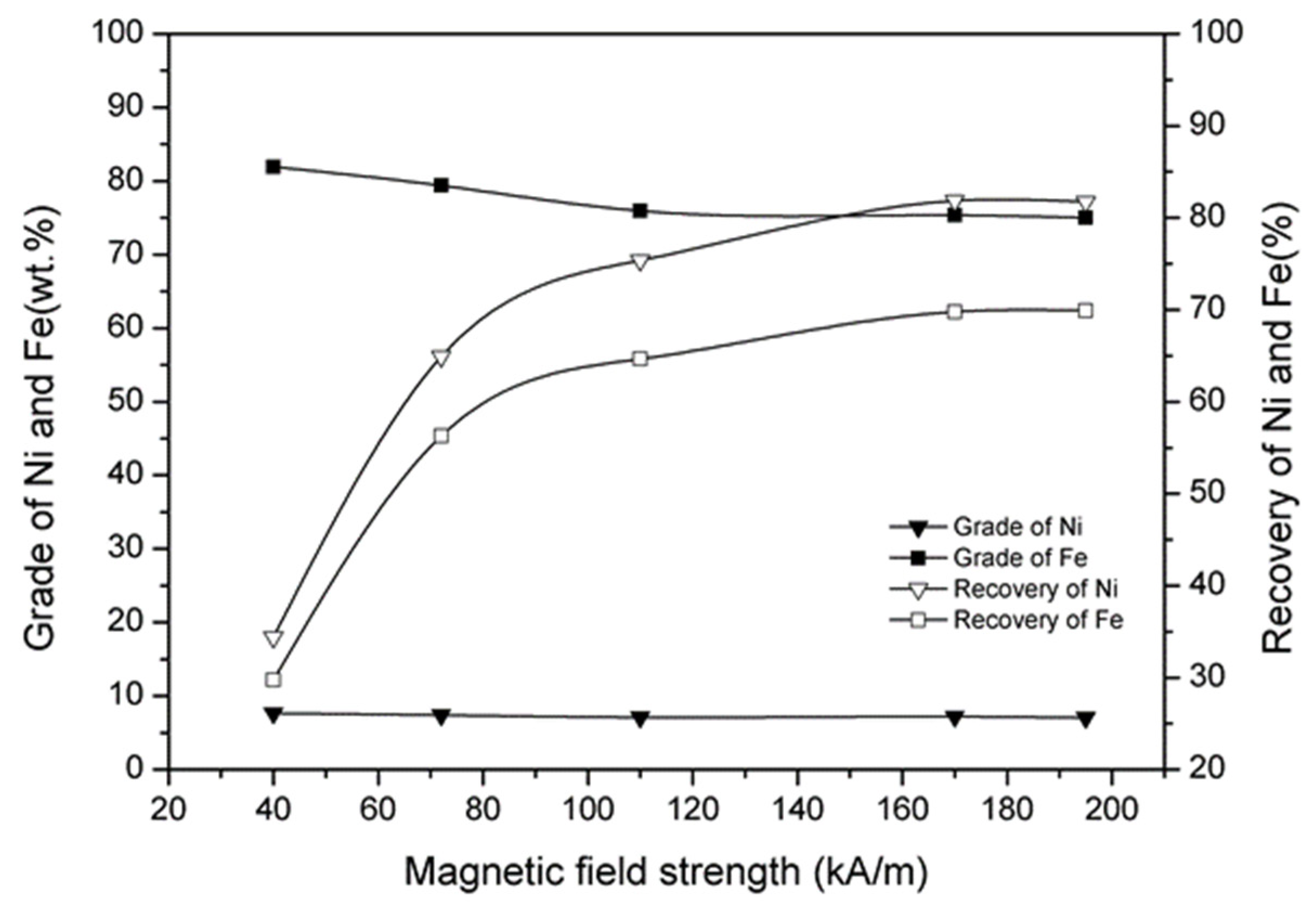
3.7. Effect of Magnetic Separation
4. Discussion
5. Conclusions
- The serpentinite, magnesium olivine, and chromite were the major constituent minerals in the laterite nickel ore investigated in this study, and the content of Ni and Fe were 0.98% and 6.99%, respectively. The particle size of serpentinite and magnesium olivine were mainly distributed below 150 μm and uniformly distributed in different particle size ranges. The particle size of chromite in the sample was mainly distributed between 38 μm and 150 μm, and the particle size of goethite was mainly distributed below 38 μm.
- The addition of CaF2 for the solid-state metallized reduction could dramatically improve the beneficiation of nickel and iron. When the addition of CaF2 was 12 wt.% of laterite nickel ore, the grades of nickel and iron in the nickel–iron concentrate increased from 2.01% and 21.92% to 7.32% and 78.74%, respectively; meanwhile, the recoveries of nickel and iron increased to 81.84% and 69.78%, respectively.
- Appropriate amounts of Fe2O3 addition for the solid-state metallized reduction could promote the generation of ferro-nickel granules. When the addition of Fe2O3 was 10 wt% of laterite nickel ore, the grade and recovery of nickel in the nickel–iron concentrate increased from 3.02% and 17.79% to 7.32% and 81.84%, respectively.
- A nickel–iron concentrate with a nickel grade of 7.32%, nickel recovery of 81.84%, iron grade of 78.74%, and iron recovery of 69.78% was obtained under the conditions of a reduction temperature of 1200 °C, reduction time of 120 min, calcium fluoride addition of 12%, ferric oxide addition of 10%, coal addition of 12%, and magnetic field strength of 170 kA/m.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tian, Q.H.; Li, Z.C.; Wang, Q.M.; Wang, S.S.; Guo, X.Y. Present situation of laterite nickel ore resources and research progress of smelting technology. Chin. J. Nonferr. Met. 2023, 33, 2975–2997. [Google Scholar]
- Yuan, S.; Zhou, W.T.; Li, Y.J.; Han, Y.X. Efficient enrichment of nickel and iron in laterite nickel ore by deep reduction and magnetic separation. Trans. Nonferrous Met. Soc. China 2020, 30, 812–822. [Google Scholar] [CrossRef]
- Li, G.H.; Shi, T.M.; Rao, M.J.; Jiang, T.; Zhang, Y.B. Beneficiation of nickeliferous laterite by reduction roasting in the presence of sodium sulfate. Miner. Eng. 2012, 32, 19–26. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, H.P.; Feng, A.S.; Zhao, J.W.; Cao, F. Study on Current Situation and Analysis of Supply and Demand of Global Nickel Resource. Conserv. Util. Miner. Resour. 2016, 1, 64–69. [Google Scholar]
- Whittingtion, B.I.; Muir, D. Pressure acid leaching of nickel laterites: A review. Miner. Process. Extr. Metall. Rev. 2000, 21, 527–600. [Google Scholar] [CrossRef]
- Whittingtion, B.I.; Muir, D. Pressure acid leaching of arid-region nickel laterite ore: Part II: Effect of ore type. Hydrometallurgy 2003, 70, 47–62. [Google Scholar] [CrossRef]
- He, F.; Ma, B.Z.; Wang, C.Y.; Ma, Y.T.; Asselin, E.; Cheng, Y.Q.; Zhang, W.J.; Zhao, J. Microwave pretreatment for enhanced selective nitric acid pressure leaching of limonitic laterite. J. Cent. South Univ. 2021, 28, 3050–3060. [Google Scholar] [CrossRef]
- Ma, B.Z.; Wang, C.Y.; Yang, W.J.; Yang, B.; Zhang, Y.L. Selective pressure leaching of Fe(II)-rich limonitic laterite ores from Indonesia using nitric acid. Miner. Eng. 2013, 45, 151–158. [Google Scholar] [CrossRef]
- Chander, S.; Sharma, V.N. Reduction roasting/ammonia leaching of nickeliferous laterites. Hydrometallurgy 1981, 7, 315–327. [Google Scholar] [CrossRef]
- Caron, M.H. Fundamental and practical factors in ammonia leaching of nickel and cobalt ores. Trans. AIME 1950, 180, 67–90. [Google Scholar] [CrossRef]
- Watanabe, T.; Ono, S.; Arai, H.; Matsumori, T. Direct reduction of garnierite ore for production of ferro-nickel with a rotary kiln at Nippon Yakin Kogyo Co.Ltd. oheyama works. Int. J. Min. Process. 1987, 19, 173–187. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Cui, Y.; Vining, K.; Hapugodda, S.; Douglas, J.; Pan, J.; Zheng, G.L. Upgrading low nickel content laterite ores using selective reduction followed by magnetic separation. Int. J. Min. Process. 2012, 106–109, 1–7. [Google Scholar] [CrossRef]
- Li, B.; Wang, H.; Wei, Y.G. The reduction of nickel from low-grade nickel laterite ore using a solid-state deoxidisation method. Miner. Eng. 2011, 24, 1556–1562. [Google Scholar] [CrossRef]
- Jiang, M.; Sun, T.C.; Liu, Z.G. Mechanism of sodium sulfate in promoting selective reduction of nickel laterite ore during reduction roasting process. Int. J. Min. Process. 2013, 123, 32–38. [Google Scholar] [CrossRef]
- Zhou, S.W.; Wei, Y.G.; Li, B.; Wang, H.; Ma, B.Z.; Wang, C.Y. Mechanism of sodium chloride in promoting reduction of high-magnesium low nickel oxide ore. Sci. Rep. 2016, 7, 29061. [Google Scholar]
- Zhou, S.W.; Wei, Y.G.; Li, B.; Wang, H.; Ma, B.Z.; Wang, C.Y. Chloridizing and reduction roasting of high-magnesium low-nickel oxide ore followed by magnetic separation of produce ferronickel concentrate. Metall. Mater. Trans. B 2016, 47, 145–153. [Google Scholar] [CrossRef]
- Sun, T.C.; Jiang, M.; Liu, Z.G.; Liu, N.; Zhang, S.Y.; Kou, J.; Xu, C.Y. Research on the effect of additive on selective reduction of the laterite ores with low nickel and high iron content. J. China Univ. Min. Technol. 2013, 42, 838–844. [Google Scholar]
- Saeed, F.; Lev, F. Challenges in processing nickel laterite ores by flotation. Int. J. Min. Process 2016, 151, 59–67. [Google Scholar]
- Huang, D.H.; Zhang, J.L.; Lin, C.C.; Mao, R. Production of ferro-nickel granules from nickel laterite ore/coal composite briquettes by direct reduction. J. Univ. Sci. Technol. Beijing 2011, 33, 1442–1447. [Google Scholar]
- Rao, M.J.; Li, G.H.; Zhang, X.; Luo, J.; Peng, Z.W.; Jiang, T. Reductive roasting of nickel laterite ore with sodium sulfate for Fe-Ni production. Part I: Reduction/sulfidation characteristics. Sep. Sci. Technol. 2016, 51, 1408–1420. [Google Scholar] [CrossRef]
- Ma, B.Z.; Xing, P.; Yang, W.J.; Wang, C.Y.; Chen, Y.Q.; Wang, H. Solid-state metalized reduction of magnesium-rich nickel oxide ores using coal as the reductant based on thermodynamic analysis. Metall. Mater. Trans. B 2017, 48, 2037–2046. [Google Scholar] [CrossRef]
- Pickles, C.A.; Forster, J.; Elliott, R. Thermodynamic analysis of the carbothermic reduction roasting of a nickeliferous limonitic laterite ore. Miner. Eng. 2014, 65, 33–40. [Google Scholar] [CrossRef]
- Li, J.C.; Bai, G.H.; Li, G.H. Solid-state reduction properties of carbon-bearing chromite pellets. Chin. J. Nonferr. Met. 2011, 21, 1159–1164. [Google Scholar]
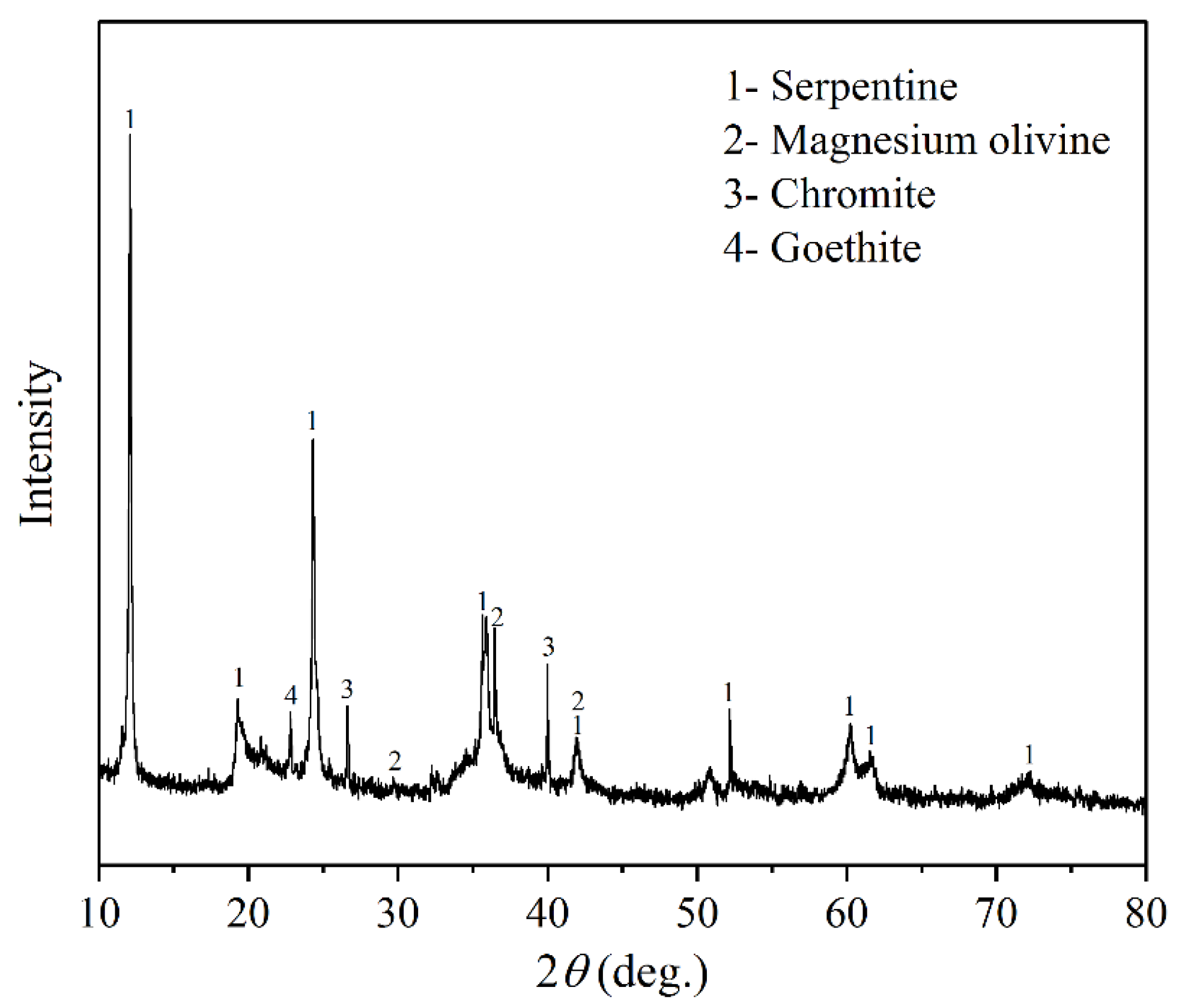

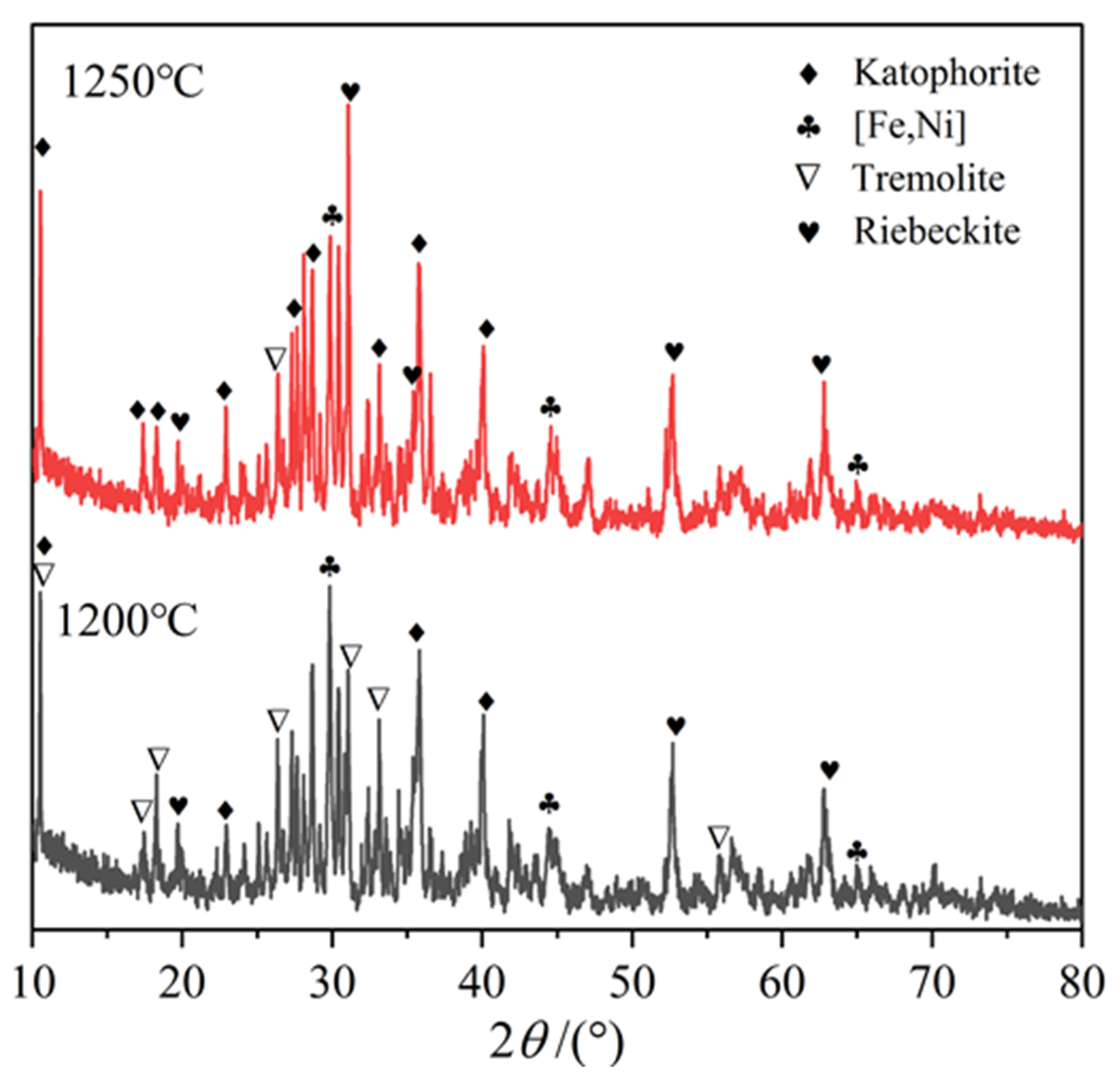
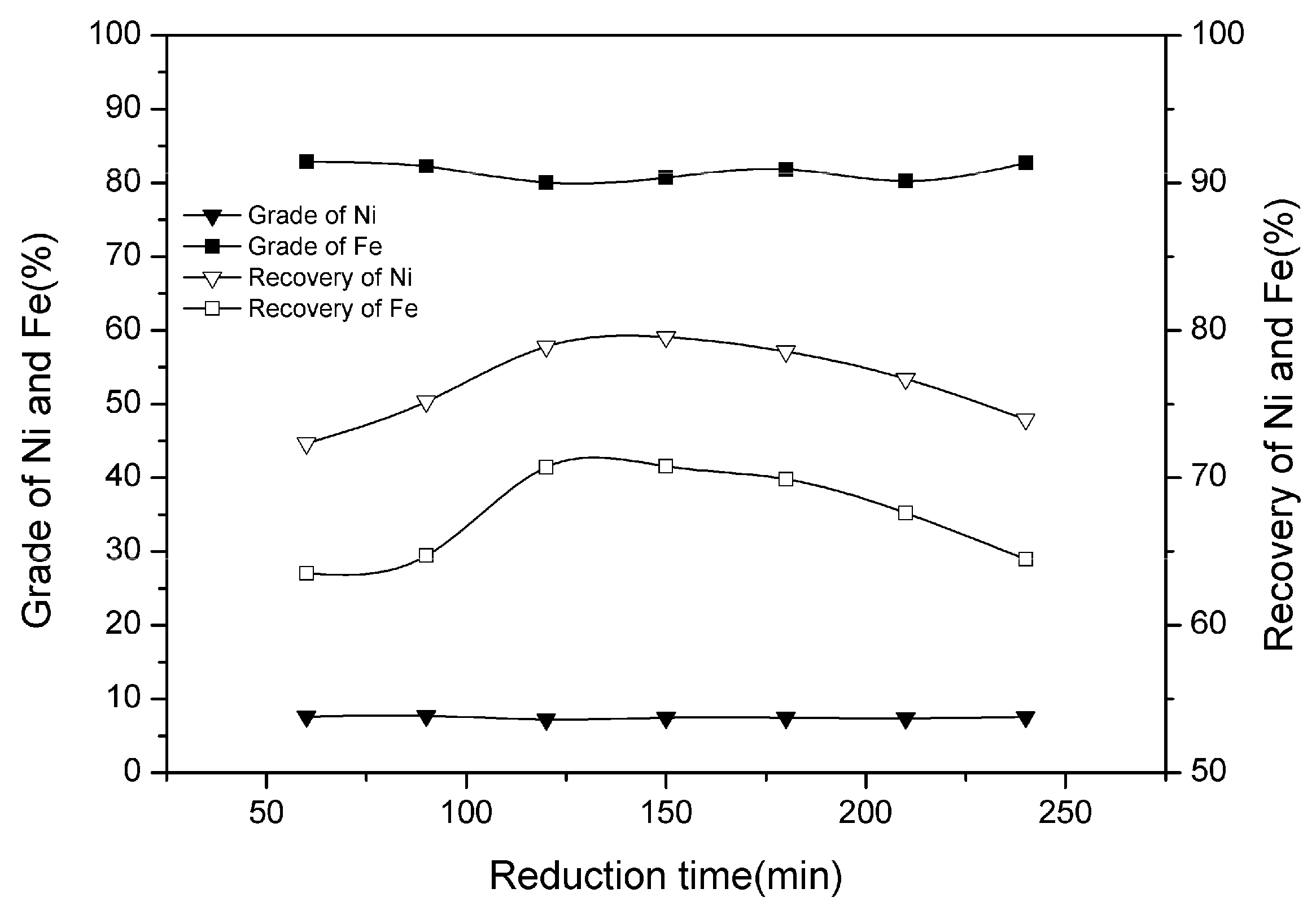
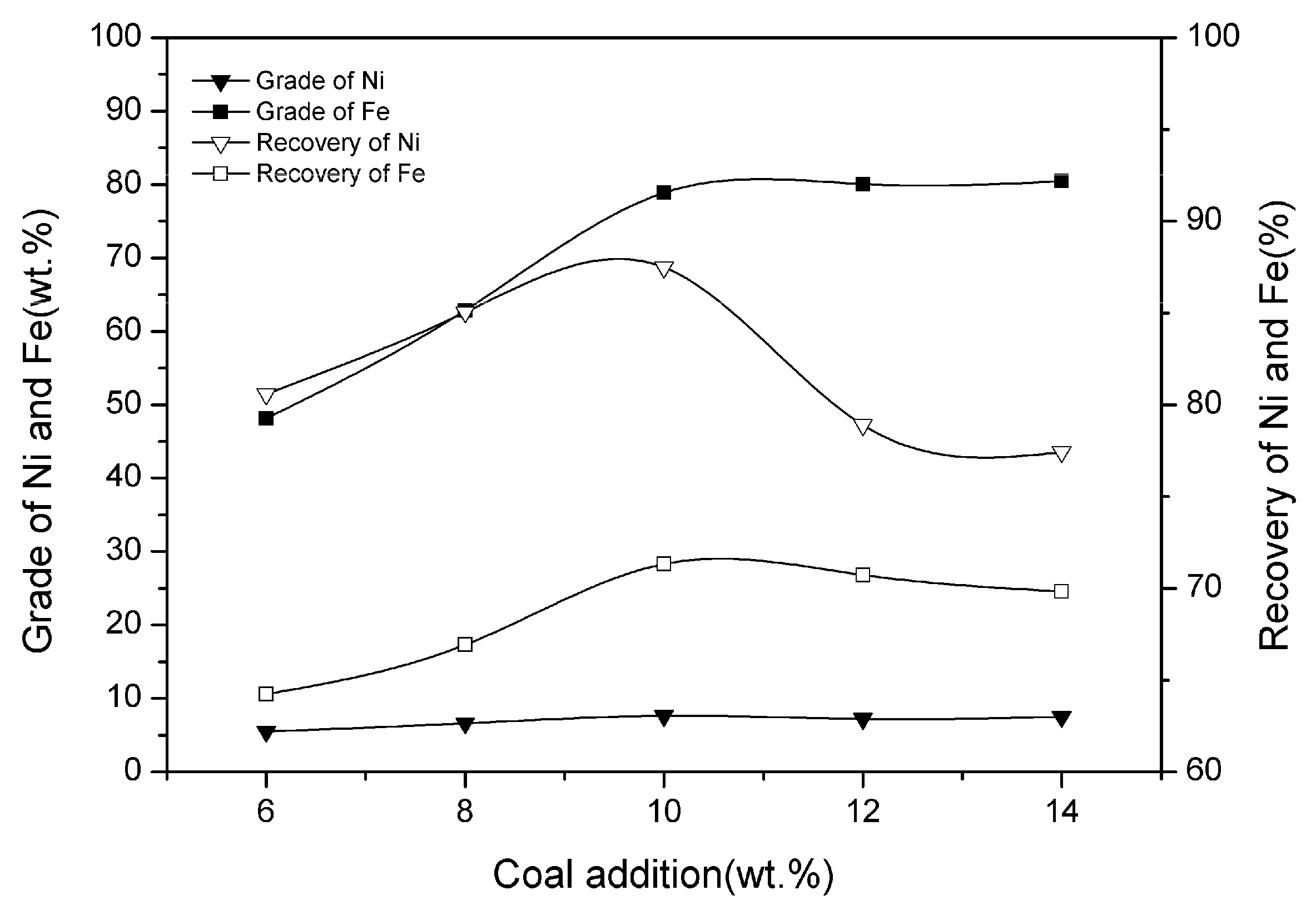



| Ni | SiO2 | MgO | TFe | Cr | Co | Cu |
|---|---|---|---|---|---|---|
| 0.98 | 39.65 | 32.45 | 6.99 | 0.26 | 0.013 | 0.01 |
| CaO | Al2O3 | MnO | TiO2 | K2O | S | P |
| 0.18 | 0.066 | 0.099 | 0.11 | 0.0049 | 0.050 | 0.0039 |
| Serpentinite | Magnesium Olivine | Chromite | Hornblende |
|---|---|---|---|
| 64.4 | 19.3 | 6.3 | 3.4 |
| Goethite | Quartz | Others | |
| 3.4 | 1.4 | 1.2 |
| Granularity (μm) | Serpentinite and Magnesium Olivine | Chromite | Goethite | Hornblende |
|---|---|---|---|---|
| 150–300 | 5.75 | 2.04 | 0.3 | 0 |
| 75–150 | 18.96 | 53.15 | 3.96 | 0.76 |
| 38–75 | 21.04 | 29.47 | 13.17 | 4.72 |
| 20–38 | 20.94 | 7.94 | 15.01 | 4.79 |
| 10–20 | 15.93 | 3.37 | 22.3 | 30.85 |
| <10 | 17.36 | 4.04 | 45.26 | 58.9 |
| Fixed Carbon | Volatile Matter | Ash | Moisture | S |
|---|---|---|---|---|
| 78.51 | 8.96 | 10.43 | 2.10 | 0.36 |
| Chemical Equation | (J/mol) | |
|---|---|---|
| 122,207–172.83 T | (1) | |
| −37,600–11.8 T | (2) | |
| 170,700–174.5 T | (3) | |
| 237,700–222 T | (4) | |
| −52,130–41.0 T | (5) | |
| 35,380–40.2 T | (6) | |
| 262,350–179.7 T | (7) | |
| −10,556.2–17.69 T | (8) | |
| 163,755–138.49 T | (9) |
| Co | Fe | Si | Ni | S | Total |
|---|---|---|---|---|---|
| 0.32 | 84.06 | 0.35 | 12.92 | 0.02 | 97.67 |
| F | MnO | TiO2 | SiO2 | Na2O | Fe2O3 | Cr2O3 |
|---|---|---|---|---|---|---|
| 0.086 | 0.11 | 0.004 | 60.00 | 0.01 | 1.74 | 0.32 |
| P2O5 | MgO | NiO | CaO | Al2O3 | Total | |
| 0.004 | 37.28 | 0.005 | 0.61 | 0.18 | 100.34 |
| F | MnO | TiO2 | SiO2 | Na2O | Fe2O3 | Cr2O3 |
|---|---|---|---|---|---|---|
| 1.421 | 0.098 | 0.02 | 54.52 | 0.018 | 0.20 | 0.25 |
| P2O5 | MgO | NiO | CaO | Al2O3 | Total | |
| 0.021 | 20.84 | 0.009 | 17.97 | 2.12 | 97.48 |
| Ni | TFe | Si | Cr | Co |
|---|---|---|---|---|
| 7.32 | 78.74 | 4.18 | 0.86 | 0.098 |
| Cu | C | S | P | |
| 0.082 | 0.78 | 0.21 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Hu, S.; Wang, S.; Liu, H.; Yu, D.; Liu, L.; Wang, H.; Wang, K. Preparation of Nickel–Iron Concentrate from Low-Grade Laterite Nickel Ore by Solid-State Metalized Reduction and Magnetic Separation. Minerals 2024, 14, 926. https://doi.org/10.3390/min14090926
Wang W, Hu S, Wang S, Liu H, Yu D, Liu L, Wang H, Wang K. Preparation of Nickel–Iron Concentrate from Low-Grade Laterite Nickel Ore by Solid-State Metalized Reduction and Magnetic Separation. Minerals. 2024; 14(9):926. https://doi.org/10.3390/min14090926
Chicago/Turabian StyleWang, Wei, Sichun Hu, Shoujing Wang, Hongzhao Liu, Deshui Yu, Lin Liu, Hongliang Wang, and Ke Wang. 2024. "Preparation of Nickel–Iron Concentrate from Low-Grade Laterite Nickel Ore by Solid-State Metalized Reduction and Magnetic Separation" Minerals 14, no. 9: 926. https://doi.org/10.3390/min14090926







