A Geochemical and Isotopic Investigation of Carbonatites from Huangshuian, Central China: Implications for Petrogenesis and Mantle Sources
Abstract
1. Introduction
2. Geological Setting and Sample Descriptions
3. Analytical Methods
4. Results
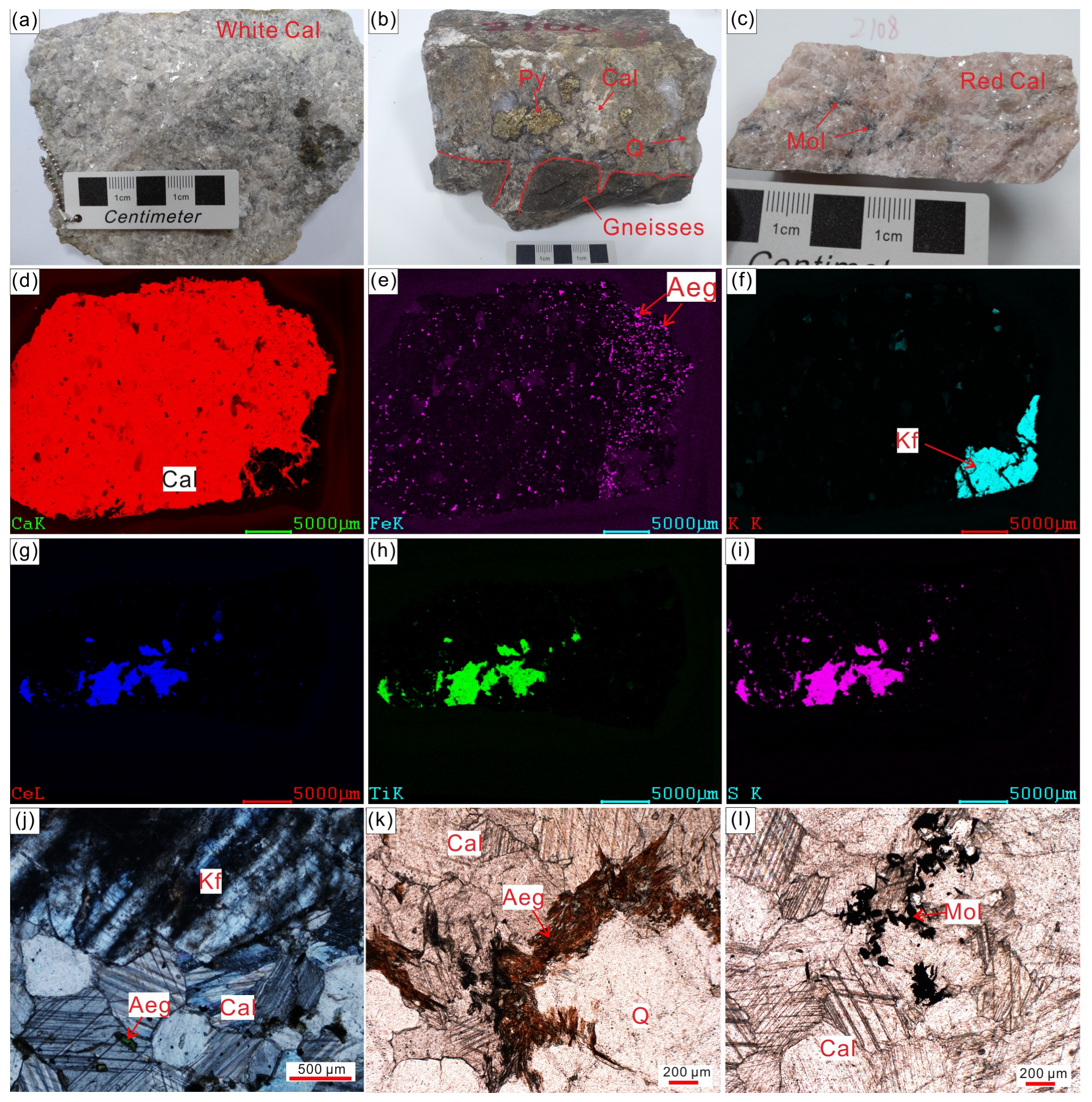
4.1. Whole-Rock Geochemistry
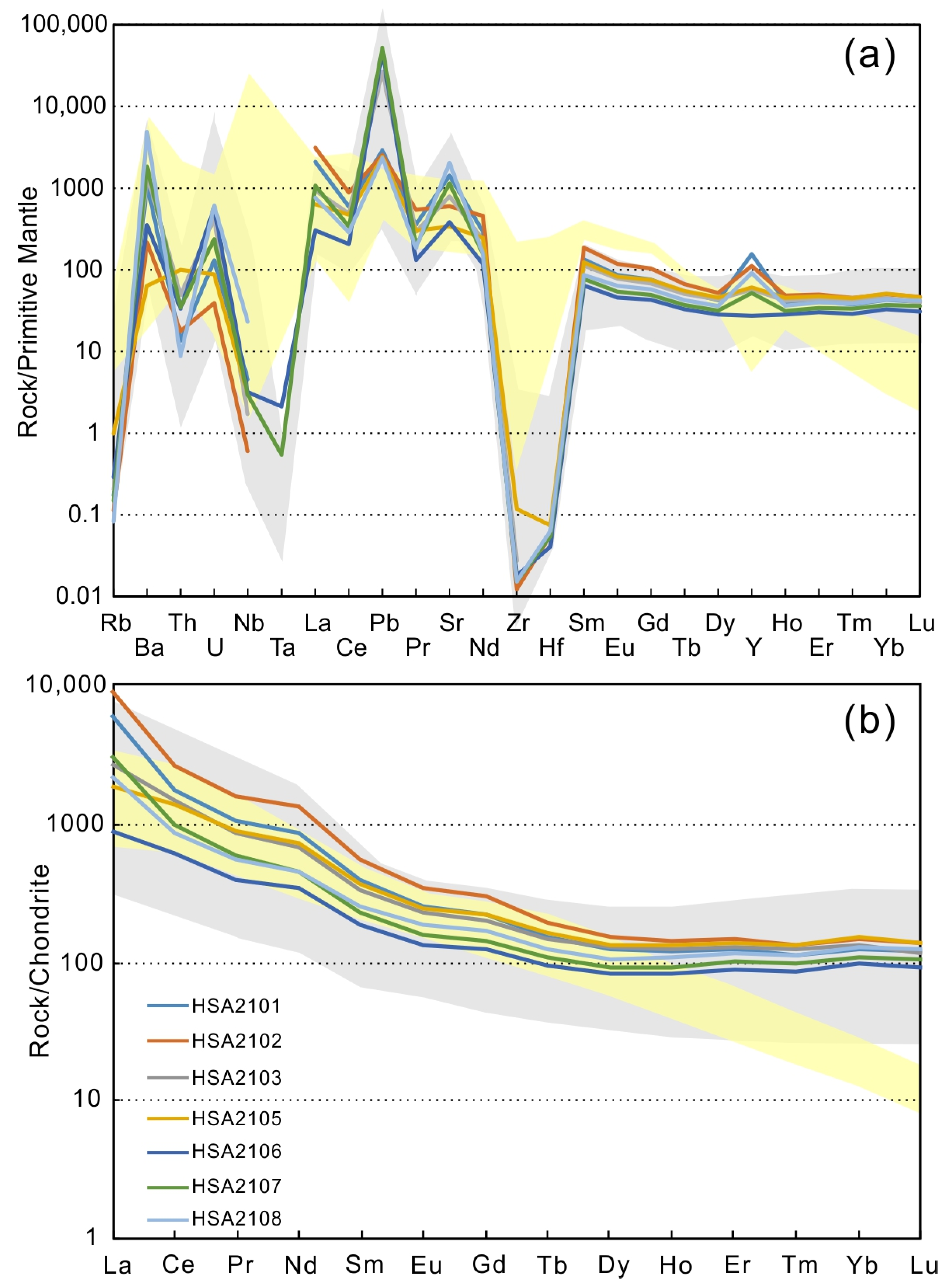
4.2. C and O Isotope Compositions
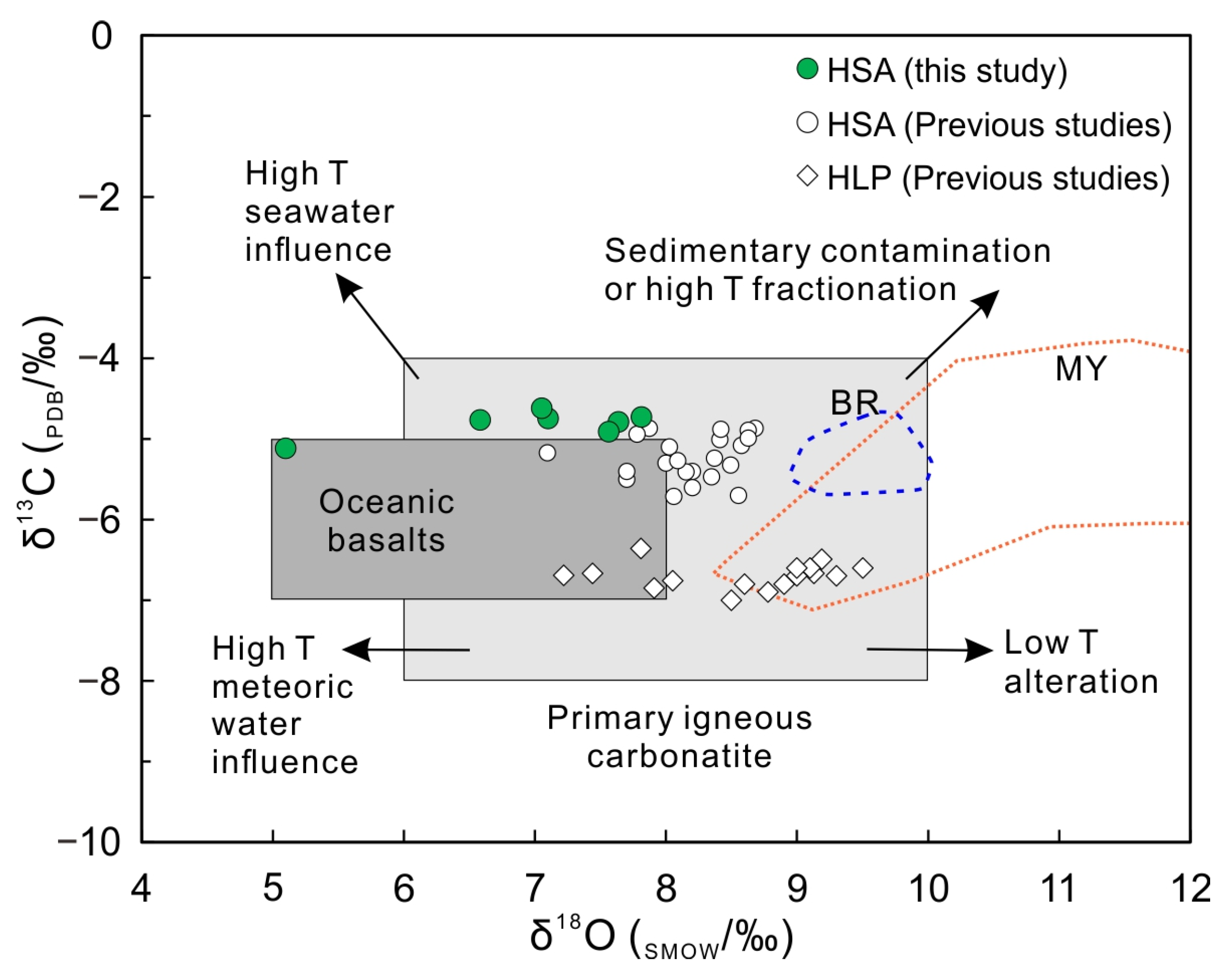
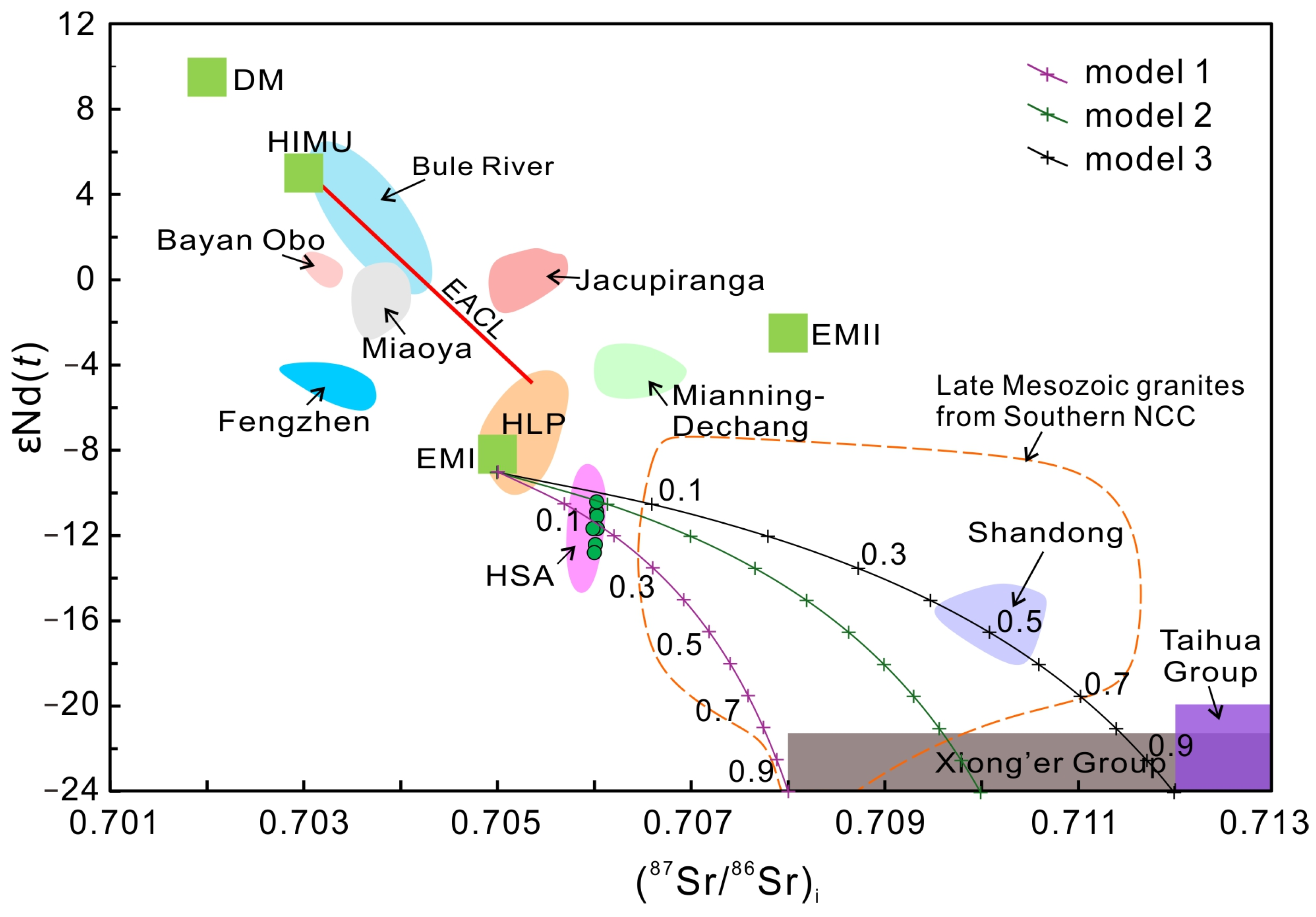
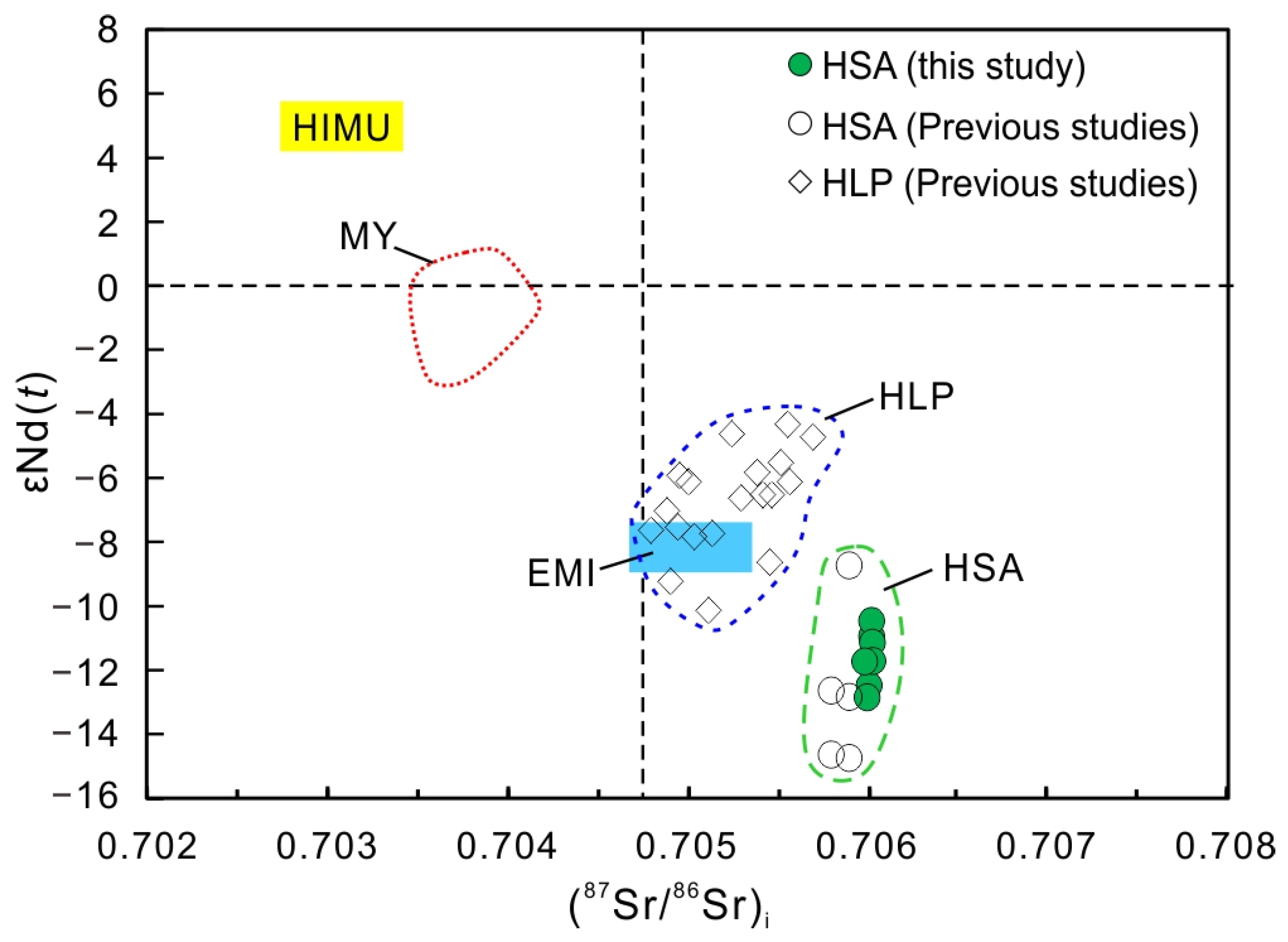
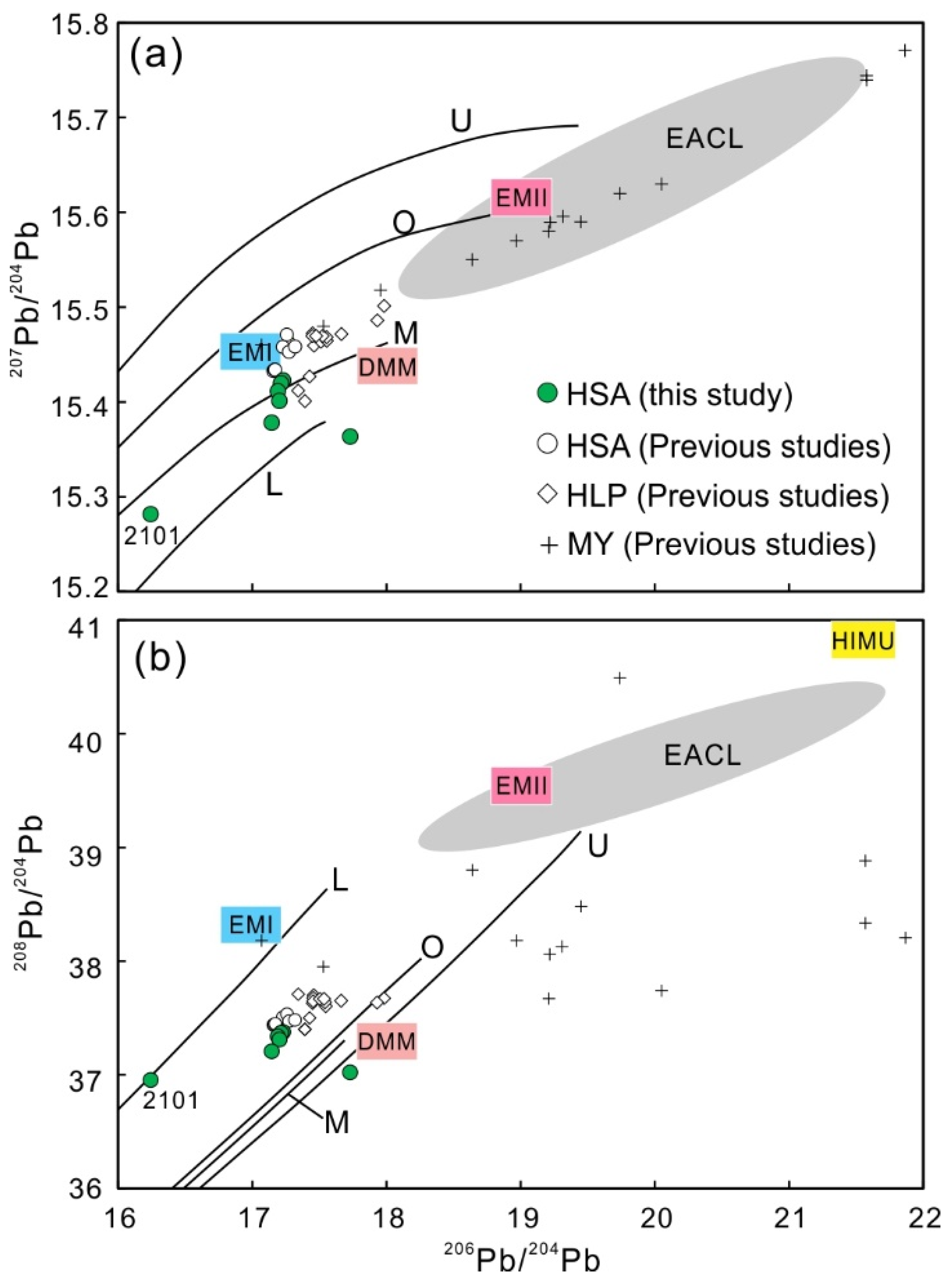
4.3. Sr, Nd, and Pb Isotope Compositions
5. Discussion
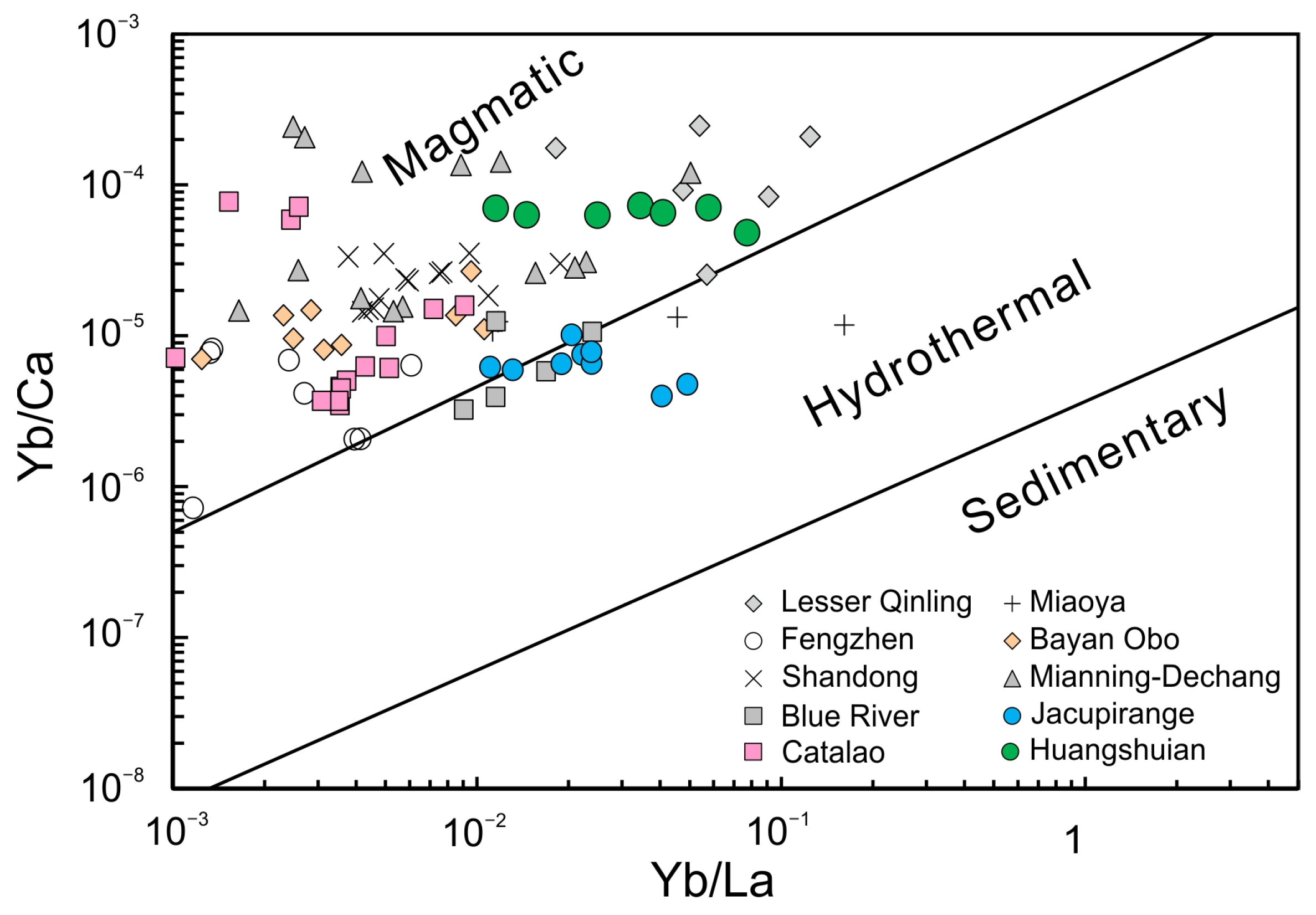
5.1. Petrogenesis of the HSA Carbonatites
5.2. Source of the Carbonatite and Deep Tectonic Processes
6. Conclusions
- (1)
- The Huangshuian carbonatites in Lesser Qinling Orogen display elevated CaO and low MgO and alkali contents, as well as significant enrichments of Pb, Mo, and HREE, compared to typical carbonatites. The stable C and O isotope compositions suggest their pristine nature and derivation from mantle source(s). It is postulated that Huangshuian carbonatites represent residual melts of late-stage magmatic differentiation from the parental carbonatite magma.
- (2)
- The combined Sr-Nd-Pb isotopic compositions for the carbonatites support the hypothesis that the Lesser Qinling carbonatites were derived from a heterogeneous upper mantle source involving an EMI-like mantle component and minor assimilation of the basement rocks.
- (3)
- We propose that the Lesser Qinling carbonatite magmas were derived directly from the melting of the carbonate-bearing subcontinental lithospheric mantle metasomatized by the subduction of the Mianlue Ocean during the early phase of the regional orogenic tectonism during the Early Triassic.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, K.; Simonetti, A. Source of parental melts to carbonatites–critical isotopic constraints. Mineral. Petrol. 2010, 98, 77–89. [Google Scholar] [CrossRef]
- Woolley, A.R.; Kjarsgaard, B.A. Carbonatite occurrences of the world: Map and database; geological survey of Canada. Open File 2008, 5796. [Google Scholar] [CrossRef]
- Song, W.L.; Xu, C.; Smith, M.P.; Kynicky, J.; Huang, K.J.; Wei, C.W.; Zhou, L.; Shu, Q.H. Origin of unusual HREE-Mo-rich carbonatites in the Qinling orogen, China. Sci. Rep. 2016, 6, 37377. [Google Scholar] [CrossRef] [PubMed]
- Woolley, A.R. Carbonatites: Nomenclature, average chemical compositions, and element distribution. In Carbonatites: Genesis and Evolution; Unwin Hyman: London, UK, 1989; pp. 1–13. [Google Scholar]
- Treiman, A.H.; Schedl, A. Properties of Carbonatite Magma and Processes in Carbonatite Magma Chambers. J. Geol. 1983, 91, 437–447. [Google Scholar] [CrossRef]
- Krafft, M.; Keller, J. Temperature Measurements in Carbonatite Lava Lakes and Flows from Oldoinyo Lengai, Tanzania. Science 1989, 245, 168–170. [Google Scholar] [CrossRef]
- Dawson, J.B.; Pinkerton, H.; Norton, G.E.; Pyle, D.M. Physicochemical properties of alkali carbonatite lavas; data from the 1988 eruption of Oldoinyo Lengai, Tanzania. Geology 1990, 18, 260–263. [Google Scholar] [CrossRef]
- Jones, A.P.; Genge, M.; Carmody, L. Carbonate Melts and Carbonatites. Rev. Mineral. Geochem. 2013, 75, 289–322. [Google Scholar] [CrossRef]
- Bell, K.; Blenkinsop, J. Neodymium and strontium isotope geochemistry of carbonatites. In Carbonatites--Genesis and Evolution; Bell, K., Ed.; Unwin Hyman: London, UK, 1989; pp. 278–300. [Google Scholar]
- Bell, K.; Blenkinsop, J.; Cole, T.J.S.; Menagh, D.P. Evidence from Sr isotopes for long-lived heterogeneities in the upper mantle. Nature 1982, 298, 251–253. [Google Scholar] [CrossRef]
- Bell, K.; Blenkinsop, J. Nd and Sr isotopic compositions of East African carbonatites; implications for mantle heterogeneity. Geology 1987, 15, 99–102. [Google Scholar] [CrossRef]
- Tilton, G.R.; Bell, K. Sr-Nd-Pb isotope relationships in Late Archean carbonatites and alkaline complexes, Applications to the geochemical evolution of Archean mantle. Geochim. Cosmochim. Acta 1994, 58, 3145–3154. [Google Scholar] [CrossRef]
- Hoernle, K.; Tilton, G.; Le Bas, M.J.; Duggen, S.; Garbe-Schönberg, D. Geochemistry of oceanic carbonatites compared with continental carbonatites, mantle recycling of oceanic crustal carbonate. Contrib. Mineral. Petrol. 2002, 142, 520–542. [Google Scholar] [CrossRef]
- Hulett, S.R.W.; Simonetti, A.; Rasbury, E.T.; Hemming, N.G. Recycling of subducted crustal components into carbonatite melts revealed by boron isotopes. Nat. Geosci. 2016, 9, 904–908. [Google Scholar] [CrossRef]
- Bell, K.; Simonetti, A. Carbonatite Magmatism and Plume Activity, Implications from the Nd, Pb and Sr Isotope Systematics of Oldoinyo Lengai. J. Petrol. 1996, 37, 1321–1339. [Google Scholar] [CrossRef]
- Doucelance, R.; Hammouda, T.; Moreira, M.; Martins, J.C. Geochemical constraints on depth of origin of oceanic carbonatites: The Cape Verde case. Geochim. Cosmochim. Acta 2010, 74, 7261–7282. [Google Scholar] [CrossRef]
- Xu, C.; Campbell, I.H.; Allen, C.M.; Huang, Z.L.; Qi, L.; Zhang, H.; Zhang, G.S. Flat rare earth element patterns as an indicator of cumulate processes in the Lesser Qinling carbonatites, China. Lithos 2007, 95, 267–278. [Google Scholar] [CrossRef]
- Nelson, D.R.; Chivas, A.R.; Chappell, B.W.; McCulloch, M.T. Geochemical and isotopic systematics in carbonatites and implications for the evolution of ocean-island sources. Geochim. Cosmochim. Acta 1988, 52, 1–17. [Google Scholar] [CrossRef]
- Bailey, D.K. Carbonate magmas. J. Geol. Soc. Lond. 1993, 150, 637–651. [Google Scholar] [CrossRef]
- Bell, K.; Tilton, G.R. Probing the mantle: The story from carbonatites. Eos Trans. Am. Geophys. Union 2002, 83, 273–277. [Google Scholar] [CrossRef]
- Bell, K. Carbonatites: Relationships to mantle-plume activity. Geol. Soc. Am. Spec. Pap. 2001, 352, 267–290. [Google Scholar]
- Bell, K.; Rukhlov, A.S. Carbonatites from the Kola Alkaline Province, origin, evolution and source characteristics. In Phoscorites and Carbonatites from Mantle to Mine, the Key Example of the Kola Alkaline Province; Mineralogical Society of Great Britain & Ireland: London, UK, 2004; Volume 10, pp. 421–455. [Google Scholar]
- Gerlach, D.C.; Cliff, R.A.; Davies, G.R.; Norry, M.; Hodgson, N. Magma sources of the Cape Verdes archipelago, Isotopic and trace element constraints. Geochim. Cosmochim. Acta 1988, 52, 2979–2992. [Google Scholar] [CrossRef]
- Simonetti, A.; Goldstein, S.L.; Schmidberger, S.S.; Viladkar, S.G. Geochemical and Nd, Pb, and Sr Isotope Data from Deccan Alkaline Complexes—Inferences for Mantle Sources and Plume-Lithosphere Interaction. J. Petrol. 1998, 39, 1847–1864. [Google Scholar] [CrossRef]
- Hart, S.R. Heterogeneous mantle domains, signatures, genesis and mixing chronologies. Earth Planet. Sci. Lett. 1988, 90, 273–296. [Google Scholar] [CrossRef]
- Hart, S.R.; Hauri, E.H.; Oschmann, L.A.; Whitehead, J.A. Mantle Plumes and Entrainment, Isotopic Evidence. Science 1992, 256, 517–520. [Google Scholar] [CrossRef]
- Xu, C.; Kynicky, J.; Chakhmouradian, A.R.; Qi, L.; Song, W.L. A unique Mo deposit associated with carbonatites in the Qinling orogenic belt, central China. Lithos 2010, 118, 50–60. [Google Scholar] [CrossRef]
- Smith, M.; Kynicky, J.; Xu, C.; Song, W.L.; Spratt, J.; Jeffries, T.; Brtnicky, M.; Kopriva, A.; Cangelosi, D. The origin of secondary heavy rare earth element enrichment in carbonatites: Constraints from the evolution of the Huanglongpu district, China. Lithos 2018, 308, 65–82. [Google Scholar] [CrossRef]
- Xue, S.; Ling, M.X.; Liu, Y.L.; Kang, Q.Q.; Huang, R.F.; Zhang, Z.K.; Sun, W.D. The formation of the giant Huayangchuan U-Nb deposit associated with carbonatite in the Qingling Orogenic Belt. Ore Geol. Rev. 2020, 122, 103498. [Google Scholar] [CrossRef]
- Xu, C.; Taylor, R.N.; Kynicky, J.; Chakhmouradian, A.R.; Song, W.L.; Wang, L.J. The origin of enriched mantle beneath North China block, Evidence from young carbonatites. Lithos 2011, 127, 1–9. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Yang, Z.M.; Li, Z.Q.; Xu, D.X. Geological and geochemical characteristics, metallogenetic mechanism and tectonic setting of carbonatite vein type Mo (Pb) deposits in the East Qinling molybdenum ore belt. Acta Geol. Sin. 2009, 83, 1968–1984, (In Chinese with English Abstract). [Google Scholar]
- Song, W.L.; Xu, C.; Qi, L.; Zhou, L.; Wang, L.J.; Kynicky, J. Genesis of Si-rich carbonatites in Huanglongpu Mo deposit, Lesser Qinling orogen, China and significance for Mo mineralization. Ore Geol. Rev. 2015, 64, 756–765. [Google Scholar] [CrossRef]
- Bai, T.; Chen, W.; Jiang, S.Y. Evolution of the carbonatite Mo-HREE deposits in the Lesser Qinling Orogen: Insights from in situ geochemical investigation of calcite and sulfate. Ore Geol. Rev. 2019, 113, 103069. [Google Scholar] [CrossRef]
- Stein, H.J.; Markey, R.J.; Morgan, J.W.; Du, A.; Sun, Y. Highly precise and accurate Re-Os ages for molybdenite from the East Qinling molybdenum belt, Shaanxi Province, China. Econ. Geol. 1997, 92, 827–835. [Google Scholar] [CrossRef]
- Cao, J.; Ye, H.S.; Li, H.Y.; Li, Z.Y.; Zhang, X.K.; He, W.; Li, C. Geological characteristics and molybdenite Re-Os isotopic dating of Huangshuian carbonatite vein-type Mo(Pb)deposit in Songxian County, Henan Province. Miner. Depos. 2014, 33, 53–69, (In Chinese with English Abstract). [Google Scholar]
- Zhang, W.; Chen, W.T.; Gao, J.F.; Chen, H.K.; Li, J.H. Two episodes of REE mineralization in the Qinling Orogenic Belt, Central China, in-situ U-Th-Pb dating of bastnäsite and monazite. Miner. Depos. 2019, 54, 1265–1280. [Google Scholar] [CrossRef]
- Chen, H.Y.; Wu, C.; Jiang, H.J.; Gao, C.; Kang, Q.Q.; Yang, C.S.; Wang, D.E.; Lai, C. Genesis of the supergiant Huayangchuan carbonatite-hosted uranium-polymetallic deposit in the Qinling Orogen, Central China. Gondwana Res. 2020, 86, 250–265. [Google Scholar]
- Mao, J.W.; Xie, G.Q.; Bierlein, F.; Qü, W.J.; Du, A.D.; Ye, H.S.; Pirajno, F.; Li, H.M.; Guo, B.J.; Li, Y.F.; et al. Tectonic implications from Re–Os dating of Mesozoic molybdenum deposits in the East Qinling–Dabie orogenic belt. Geochim. Cosmochim. Acta 2008, 72, 4607–4626. [Google Scholar] [CrossRef]
- Li, N.; Pirajno, F. Early Mesozoic Mo mineralization in the Qinling Orogen, An overview. Ore Geol. Rev. 2017, 81, 431–450. [Google Scholar] [CrossRef]
- Kynicky, J.; Smith, M.P.; Xu, C. Diversity of Rare Earth Deposits: The Key Example of China. Elements 2012, 8, 361–367. [Google Scholar] [CrossRef]
- Huang, G.W.; Pan, C.R.; Pan, J.Y.; Zhong, F.J.; Chen, Z.L.; Xia, F.; Yan, J.; Wu, D.H.; Min, Z. REE mineralization age and geodynamic setting of the Huanglongpu deposit in the East Qinling orogen, China: Evidence from mineralogy, U–Pb geochronology, and in-situ Nd isotope. Ore Geol. Rev. 2023, 152, 105255. [Google Scholar] [CrossRef]
- Wei, C.W.; Xu, C.; Chakhmouradian, A.R.; Brenna, M.; Kynicky, J.; Song, W.L. Carbon–Strontium Isotope Decoupling in Carbonatites from Caotan (Qinling, China): Implications for the Origin of Calcite Carbonatite in Orogenic Settings. J. Petrol. 2020, 61, egaa024. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Brey, G.P. Phase relations of carbonate-bearing eclogite as-semblages from 2.5 to 5.5 GPa: Implications for petrogenesis of carbonatites. Contrib. Mineral. Petrol. 2004, 146, 606–619. [Google Scholar] [CrossRef]
- Thomson, A.R.; Walter, M.J.; Kohn, S.C.; Brooker, R.A. Slab melting as a barrier to deep carbon subduction. Nature 2016, 529, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chakhmouradian, A.R.; Taylor, R.N.; Kynicky, J.; Li, W.B.; Song, W.L.; Fletcher, I.R. Origin of carbonatites in the South Qinling orogen, Implications for crustal recycling and timing of collision between the South and North China Blocks. Geochim. Cosmochim. Acta 2014, 143, 189–206. [Google Scholar] [CrossRef]
- Çimen, O.; Kuebler, C.; Monaco, B.; Simonetti, S.S.; Corcoran, L.; Chen, W.; Simonetti, A. Boron, carbon, oxygen and radiogenic isotope investigation of carbonatite from the Miaoya complex, central China, Evidences for late-stage REE hydrothermal event and mantle source heterogeneity. Lithos 2018, 322, 225–237. [Google Scholar] [CrossRef]
- Xu, C.; Kynický, J.; Song, W.L.; Tao, R.B.; Lü, Z.; Li, Y.X.; Yang, Y.H.; Pohanka, M.; Galiova, M.V.; Zhang, L.F.; et al. Cold deep subduction recorded by remnants of a Paleoproterozoic carbonated slab. Nat. Commun. 2018, 9, 2790. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Tian, S.H.; Yuan, Z.X.; Xie, Y.L.; Yin, S.P.; Yi, L.S.; Fei, H.C.; Yang, Z.M. The Himalayan collision zone carbonatites in western Sichuan, SW China, Petrogenesis, mantle source and tectonic implication. Earth Planet. Sci. Lett. 2006, 244, 234–250. [Google Scholar] [CrossRef]
- Zhang, G.W.; Meng, Q.G.; Lai, S.C. Tectonics and structure of Qinling Orogenic Belt. Sci. China Ser. B 1995, 38, 1379–1394. [Google Scholar]
- Kröner, A.; Compston, W.; Zhang, G.W.; Guo, A.L.; Todt, W. Age and tectonic setting of Late Archean greenstone-gneiss terrain in Henan Province, China, as revealed by single-grain zircon dating. Geology 1988, 16, 211–215. [Google Scholar] [CrossRef]
- Peng, P.; Zhai, M.G.; Ernst, R.E.; Guo, J.H.; Liu, F.; Hu, B. A 1.78 Ga large igneous province in the North China craton, The Xiong’er Volcanic Province and the North China dyke swarm. Lithos 2008, 101, 260–280. [Google Scholar] [CrossRef]
- Zhao, G.C.; He, Y.H.; Sun, M. The Xiong’er volcanic belt at the southern margin of the North China Craton: Petrographic and geochemical evidence for its outboard position in the Paleo-Mesoproterozoic Columbia Supercontinent. Gondwana Res. 2009, 16, 170–181. [Google Scholar] [CrossRef]
- Zhang, C.L.; Wang, T.; Wang, X.X. Origin and tectonic setting of the Early Mesozoic granitoids in Qinling orogenic belt. Geol. J. China Univ. 2008, 14, 304–316. [Google Scholar]
- Mao, J.W.; Xie, G.Q.; Pirajno, F.; Ye, H.S.; Wang, Y.B.; Li, Y.F.; Xiang, J.F.; Zhao, H.J. Late Jurassic-Early Cretaceous granitoid magmatism in Eastern Qinling, central-eastern China: SHRIMP zircon U-Pb ages and tec-tonic implications. Aust. J. Earth Sci. 2010, 57, 51–78. [Google Scholar] [CrossRef]
- Cao, J.; Ye, H.S.; Chen, X.D.; He, W.; Wang, P. Geochemistry, Zircon U–Pb Age, and Lu–Hf Isotope of the Granite Porphyry in Leimengou Mo Deposit in the East Qinling Molybdenum Ore Belt, China. Minerals 2018, 8, 293. [Google Scholar] [CrossRef]
- Jenner, G.A.; Longerich, H.P.; Jackson, S.E.; Fryer, B.J. ICP-MS—A powerful tool for high-precision trace-element analysis in Earth sciences: Evidence from analysis of selected U.S.G.S. reference samples. Chem. Geol. 1990, 83, 133–148. [Google Scholar] [CrossRef]
- McCrea, J.M. On the Isotopic Chemistry of Carbonates and a Paleotemperature Scale. J. Chem. Phys. 1950, 18, 849–857. [Google Scholar] [CrossRef]
- Balboni, E.; Jones, N.; Spano, T.; Simonetti, A.; Burns, P.C. Chemical and Sr isotopic characterization of North America uranium ores, Nuclear forensic applications. Appl. Geochem. 2016, 74, 24–32. [Google Scholar] [CrossRef]
- Tanaka, T.; Togashi, S.; Kamioka, H.; Amakawa, H.; Kagami, H.; Hamamoto, T.; Yuhara, M.; Orihashi, Y.; Yoneda, S.; Shimizu, H.; et al. JNdi-1, a neodymium isotopic reference in consistency with LaJolla neodymium. Chem. Geol. 2000, 168, 279–281. [Google Scholar] [CrossRef]
- Simonetti, A.; Gariépy, C.; Banic, C.M.; Tanabe, R.; Wong, H.K. Pb isotopic investigation of aircraft-sampled emissions from the Horne smelter (Rouyn, Québec), Implications for atmospheric pollution in northeastern North America. Geochim. Cosmochim. Acta 2004, 68, 3285–3294. [Google Scholar] [CrossRef]
- Manhes, G.; Minster, J.F.; Allègre, C.J. Comparative uranium-thorium-lead and rubidium-strontium study of the Saint Sèverin amphoterite, consequences for early solar system chronology. Earth Planet. Sci. Lett. 1978, 39, 14–24. [Google Scholar] [CrossRef]
- Baker, J.; Peate, D.; Waight, T.; Meyzen, C. Pb isotopic analysis of standards and samples using a 207Pb–204Pb double spike and thallium to correct for mass bias with a double-focusing MC-ICP-MS. Chem. Geol. 2004, 211, 275–303. [Google Scholar] [CrossRef]
- Kjarsgaard, B.A. The genesis of carbonatites by immiscibility. In Carbonatites: Genesis and Evolution; Unwin Hyman: London, UK, 1989; pp. 388–404. [Google Scholar]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts, implications for mantle composition and processes. Geol. Soc. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Huang, Y.M.; Hawkesworth, C.J.; van Calsteren, P.; McDermott, F. Geochemical characteristics and origin of the Jacupiranga carbonatites, Brazil. Chem. Geol. 1995, 119, 79–99. [Google Scholar] [CrossRef]
- Çimen, O.; Kuebler, C.; Simonetti, S.S.; Corcoran, L.; Mitchell, R.; Simonetti, A. Combined boron, radiogenic (Nd, Pb, Sr), stable (C, O) isotopic and geochemical investigations of carbonatites from the Blue River Region, British Columbia (Canada), Implications for mantle sources and recycling of crustal carbon. Chem. Geol. 2019, 529, 119240. [Google Scholar] [CrossRef]
- Keller, J.; Hoefs, J. Stable Isotope Characteristics of Recent Natrocarbonatites from Oldoinyo Lengai. In Carbonatite Volcanism; Springer: Berlin/Heidelberg, Germany, 1995; pp. 113–123. [Google Scholar]
- Demény, A.; Ahijado, A.; Casillas, R.; Vennemann, T.W. Crustal contamination and fluid/rock interaction in the carbonatites of Fuerteventura (Canary Islands, Spain), a C, O, H isotope study. Lithos 1998, 44, 101–115. [Google Scholar] [CrossRef]
- Zindler, A.; Hart, S.R. Chemical geodynamics. Annu. Rev. Earth Planet. Sci. 1986, 14, 493–571. [Google Scholar] [CrossRef]
- Zartman, R.E.; Doe, B.R. Plumbotectonics—The model. Tectonophysics 1981, 75, 135–162. [Google Scholar] [CrossRef]
- Möller, P.; Parekh, P.P.; Schneider, H.J. The application of Tb/Ca-Tb/La abundance ratios to problems of fluorspar genesis. Miner. Depos. 1976, 11, 111–116. [Google Scholar] [CrossRef]
- Kuebler, C.; Simonetti, A.; Chen, W.; Simonetti, S.S. Boron isotopic investigation of the Bayan Obo carbonatite complex, Insights into the source of mantle carbon and hydrothermal alteration. Chem. Geol. 2020, 557, 119859. [Google Scholar] [CrossRef]
- Ying, Y.F.; Zhou, X.H.; Zhang, H.F. Geochemical and isotopic investigation of the Laiwu-Zibo carbonatites from western Shandong Province, China, and implications for their petrogenesis and enriched mantle source. Lithos 2004, 75, 413–426. [Google Scholar] [CrossRef]
- Chmyz, L.; Arnaud, N.; Biondi, J.C.; Azzone, R.G.; Bosch, D.; Ruberti, E. Ar-Ar ages, Sr-Nd isotope geochemistry, and implications for the origin of the silicate rocks of the Jacupiranga ultramafic-alkaline complex (Brazil). J. S. Am. Earth Sci. 2017, 77, 286–309. [Google Scholar] [CrossRef]
- Guarino, V.; Wu, F.; Melluso, L.; de Barros Gomes, C.; Tassinari, C.C.G.; Ruberti, E.; Brilli, M. U–Pb ages, geochemistry, C–O–Nd–Sr–Hf isotopes and petrogenesis of the Catalão II carbonatitic complex (Alto Paranaíba Igneous Province, Brazil): Implications for regional-scale heterogeneities in the Brazilian carbonatite associations. Int. J. Earth Sci. 2017, 106, 1963–1989. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Huang, W. Influence of mantle CO2 in the generation of carbonatites and kimberlites. Nature 1975, 257, 297–299. [Google Scholar] [CrossRef]
- Green, D.H.; Wallace, M.E. Mantle metasomatism by ephemeral carbonatite melts. Nature 1988, 336, 459–462. [Google Scholar] [CrossRef]
- Moore, K.R.; Wood, B.J. The Transition from Carbonate to Silicate Melts in the CaO–MgO–SiO2–CO2 System. J. Petrol. 1998, 39, 1943–1951. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Lee, W.J. Model System Controls on Conditions for Formation of Magnesiocarbonatite and Calciocarbonatite Magmas from the Mantle. J. Petrol. 1998, 39, 1885–1893. [Google Scholar] [CrossRef]
- Grassi, D.; Schmidt, M.W. Melting of carbonated pelites at 8–13 GPa, generating K-rich carbonatites for mantle metasomatism. Contrib. Mineral. Petrol. 2011, 162, 169–191. [Google Scholar] [CrossRef]
- Lee, W.J.; Wyllie, P.J. Experimental Data Bearing on Liquid Immiscibility, Crystal Fractionation, and the Origin of Calciocarbonatites and Natrocarbonatites. Int. Geol. Rev. 1994, 36, 797–819. [Google Scholar] [CrossRef]
- Lee, W.J.; Wyllie, P.J. Processes of Crustal Carbonatite Formation by Liquid Immiscibility and Differentiation, Elucidated by Model Systems. J. Petrol. 1998, 39, 2005–2013. [Google Scholar] [CrossRef]
- Veksler, I.V.; Petibon, C.; Jenner, G.A.; Dorfman, A.M.; Dingwell, D.B. Trace Element Partitioning in Immiscible Silicate-Carbonate Liquid Systems: An Initial Experimental Study Using a Centrifuge Autoclave. J. Petrol. 1998, 39, 2095–2104. [Google Scholar] [CrossRef]
- Doroshkevich, A.G.; Veksler, I.V.; Klemd, R.; Khromova, E.A.; Izbrodin, I.A. Trace-element composition of minerals and rocks in the Belaya Zima carbonatite complex (Russia): Implications for the mechanisms of magma evolution and carbonatite formation. Lithos 2017, 284–285, 91–108. [Google Scholar] [CrossRef]
- Lee, W.J.; Wyllie, P.J. Liquid immiscibility between nephelinite and carbonatite from 1.0 to 2.5 GPa compared with mantle melt compositions. Contrib. Mineral. Petrol. 1997, 127, 1–16. [Google Scholar] [CrossRef]
- Veksler, I.V.; Nielsen, T.F.D.; Sokolov, S.V. Mineralogy of Crystallized Melt Inclusions from Gardiner and Kovdor Ultramafic Alkaline Complexes, Implications for Carbonatite Genesis. J. Petrol. 1998, 39, 2015–2031. [Google Scholar] [CrossRef]
- Lee, W.J.; Wyllie, P.J. The system CaO-MgO-SiO2-CO2 at 1 GPa, metasomatic wehrlites, and primary carbonatite magmas. Contrib. Mineral. Petrol. 2000, 138, 214–228. [Google Scholar] [CrossRef]
- Rosatelli, G.; Wall, F.; Stoppa, F. Calcio-carbonatite melts and metasomatism in the mantle beneath Mt. Vulture (Southern Italy). Lithos 2007, 99, 229–248. [Google Scholar] [CrossRef]
- Fischer, T.P.; Burnard, P.; Marty, B.; Hilton, D.R.; Füri, E.; Palhol, F.; Sharp, Z.D.; Mangasini, F. Upper-mantle volatile chemistry at Oldoinyo Lengai volcano and the origin of carbonatites. Nature 2009, 459, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Potter, N.J.; Kamenetsky, V.S.; Simonetti, A.; Goemann, K. Different types of liquid immiscibility in carbonatite magmas, A case study of the Oldoinyo Lengai 1993 lava and melt inclusions. Chem. Geol. 2017, 455, 376–384. [Google Scholar] [CrossRef]
- Cangelosi, D.; Smith, M.; Banks, D.; Yardley, B. The role of sulfate-rich fluids in heavy rare earth enrichment at the Dashigou carbonatite deposit, Huanglongpu, China. Mineral. Mag. 2020, 84, 65–80. [Google Scholar] [CrossRef]
- Harmer, R.E.; Gittins, J. The Case for Primary, Mantle-derived Carbonatite Magma. J. Petrol. 1998, 39, 1895–1903. [Google Scholar] [CrossRef]
- Brooker, R.A.; Kjarsgaard, B.A. Silicate–carbonate liquid immiscibility and phase relations in the system SiO2–Na2O–Al2O3–CaO–CO2 at 0·1–2·5 GPa with applications to carbonatite genesis. J. Petrol. 2011, 52, 1281–1305. [Google Scholar] [CrossRef]
- Wallace, M.E.; Green, D.H. An experimental determination of primary carbonatite magma composition. Nature 1988, 335, 343–346. [Google Scholar] [CrossRef]
- Dalton, J.A.; Presnall, D.C. The Continuum of Primary Carbonatitic-Kimberlitic Melt Compositions in Equilibrium with Lherzolite, Data from the System CaO-MgO-Al2O3-SiO2-CO2 at 6 GPa. J. Petrol. 1998, 39, 1953–1964. [Google Scholar] [CrossRef]
- Dasgupta, R.; Hirschmann, M.M.; Stalker, K. Immiscible transition from carbonaterich to silicate-rich melts in the 3 GPa melting interval of eclogite plus CO2 and genesis of sili-ca-under-saturated ocean island lavas. J. Petrol. 2006, 47, 647–671. [Google Scholar] [CrossRef]
- Martin, L.H.J.; Schmidt, M.W.; Mattsson, H.B.; Ulmer, P.; Hametner, K.; Günther, D. Element partitioning between immiscible carbonatite–kamafugite melts with application to the Italian ultrapotassic suite. Chem. Geol. 2012, 320–321, 96–112. [Google Scholar] [CrossRef]
- Nabyl, Z.; Massuyeau, M.; Gaillard, F.; Tuduri, J.; Iacono-Marziano, G.; Rogerie, G.; Le Trong, E.; Di Carlo, I.; Melleton, J.; Bailly, L. A window in the course of alkaline magma differentiation conducive to immiscible REE-rich carbonatites. Geochim. Cosmochim. Acta 2020, 282, 297–323. [Google Scholar] [CrossRef]
- Anenburg, M.; Mavrogenes, J.A.; Frigo, C.; Wall, F. Rare earth element mobility in and around carbonatites controlled by sodium, potassium, and silica. Sci. Adv. 2020, 6, eabb6570. [Google Scholar] [CrossRef]
- Wei, C.W.; Xu, C.; Song, W.L.; Chen, W.; Shi, A.G.; Li, Z.Q.; Fan, C.X. Heavy rare earth element and crustal-derived silicon enrichment in Huayangchuan carbonatites, Qinling orogenic belt. Lithos 2023, 436–437, 106987. [Google Scholar] [CrossRef]
- Li, F.C.; Zeng, Q.D.; Kang, Q.Q.; Fan, H.R.; Yang, K.F.; She, H.D.; Huang, L.L.; Yu, B.; Wu, J.J. Unusual HREE enrichment and mineralization age in the Jialu deposit from the Qinling Orogen, central China. Ore Geol. Rev. 2024, 166, 105932. [Google Scholar] [CrossRef]
- Stracke, A.; Hofmann, A.W.; Hart, S.R. FOZO, HIMU, and the rest of the mantle zoo. Geochem. Geophys. Geosyst. 2005, 6, Q05007. [Google Scholar] [CrossRef]
- Parai, R.; Mukhopadhyay, S.; Lassiter, J.C. New constraints on the HIMU mantle from neon and helium isotopic compositions of basalts from the Cook–Austral Islands. Earth Planet. Sci. Lett. 2009, 277, 253–261. [Google Scholar] [CrossRef]
- Hanyu, T.; Tatsumi, Y.; Senda, R.; Miyazaki, T.; Chang, Q.; Hirahara, Y.; Takahashi, T.; Kawabata, H.; Suzuki, K.; Kimura, J.; et al. Geochemical characteristics and origin of the HIMU reservoir, A possible mantle plume source in the lower mantle. Geochem. Geophys. Geosyst. 2011, 12, 1–30. [Google Scholar] [CrossRef]
- Xu, C.; Chakhmouradian, A.R.; Kynický, J.; Li, Y.X.; Song, W.L.; Chen, W. A Paleoproterozoic mantle source modified by subducted sediments under the North China craton. Geochim. Cosmochim. Acta 2019, 245, 222–239. [Google Scholar] [CrossRef]
- She, H.D.; Fan, H.R.; Yang, K.F.; Li, X.H.; Wang, Z.Y. REEs upgrading by post-carbonatite fluids in the Huangshui’an Mo-REE deposit, eastern Qinling Orogen (central China). Ore Geol. Rev. 2022, 150, 105177. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Liu, Y.; Tian, S.H.; Yang, Z.M.; Xie, Y.L. Formation of carbonatite-related giant rare-earth-element deposits by the recycling of marine sediments. Sci. Rep. 2015, 5, 10231. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hou, Z.Q. A synthesis of mineralization styles with an integrated genetic model of carbonatite-syenite-hosted REE deposits in the Cenozoic Mianning-Dechang REE metallogenic belt, the eastern Tibetan Plateau, southwestern China. J. Asian Earth Sci. 2017, 137, 35–79. [Google Scholar] [CrossRef]
- Woodard, J.; Huhma, H. Paleoproterozoic mantle enrichment beneath the Fennoscandian Shield: Isotopic insight from carbonatites and lamprophyres. Lithos 2015, 236, 311–323. [Google Scholar] [CrossRef]
- Xu, C.; Kynicky, J.; Chakhmouradian, A.R.; Li, X.H.; Song, W.L. A case example of the importance of multi-analytical approach in deciphering carbonatite petrogenesis in South Qinling orogen, Miaoya rare-metal deposit, central China. Lithos 2015, 227, 107–121. [Google Scholar] [CrossRef]
- Ling, M.X.; Liu, Y.L.; Williams, I.S.; Teng, F.Z.; Yang, X.Y.; Ding, X.; Wei, G.J.; Xie, L.H.; Deng, W.F.; Sun, W.D. Formation of the world’s largest REE deposit through protracted fluxing of carbonatite by subduction-derived fluids. Sci. Rep. 2013, 3, 1776. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Schilling, J.; Jonsson, E.; Kalvig, P.; Charles, N.; Tuduri, J.; Deady, E.A.; Sadeghi, M.; Schiellerup, H.; Müller, A.; et al. Europe’s rare earth element resource potential, An overview of REE metallogenetic provinces and their geodynamic setting. Ore Geol. Rev. 2016, 72, 838–856. [Google Scholar] [CrossRef]
- Yang, K.F.; Fan, H.R.; Pirajno, F.; Li, X.C. The Bayan Obo (China) giant REE accumulation conundrum elucidated by intense magmatic differentiation of carbonatite. Geology 2019, 47, 1198–1202. [Google Scholar] [CrossRef]
- Amsellem, E.; Moynier, F.; Bertrand, H.; Bouyon, A.; Mata, J.; Tappe, S.; Day, J. Calcium isotopic evidence for the mantle sources of carbonatites. Sci. Adv. 2020, 6, eaba3269. [Google Scholar] [CrossRef]
- Banerjee, A.; Chakrabarti, R.; Simonetti, A. Temporal evolution of δ44/40Ca and 87Sr/86Sr of carbonatites: Implications for crustal recycling through time. Geochim. Cosmochim. Acta 2021, 307, 168–191. [Google Scholar] [CrossRef]
- Huang, K.J.; Shen, B.; Lang, X.G.; Tang, W.B.; Peng, Y.; Ke, S.; Kaufman, A.J.; Ma, H.R.; Li, F.B. Magnesium isotopic compositions of the Mesoproterozoic dolostones, Implications for Mg isotopic systematics of marine carbonates. Geochim. Cosmochim. Acta 2015, 164, 333–351. [Google Scholar] [CrossRef]
- Horton, F. Rapid recycling of subducted sedimentary carbon revealed by Afghanistan carbonatite volcano. Nat. Geosci. 2021, 14, 508–512. [Google Scholar] [CrossRef]
- Meng, Q.R.; Zhang, G.W. Timing of collision of the North and South China blocks: Controversy and reconciliation. Geology 1999, 27, 123–126. [Google Scholar] [CrossRef]
- Meng, Q.R.; Zhang, G.W. Geologic framework and tectonic evolution of the Qin-ling orogen, central China. Tectonphyscs 2000, 323, 183–196. [Google Scholar] [CrossRef]
- Xu, C.; Song, W.L.; Qi, L.; Wang, L.J. Geochemical characteristic and tectonic setting of ore-bearing carbonatite in Huanglongpu Mo ore field. Acta Petrol. Sin. 2009, 25, 422–430, (In Chinese with English Abstract). [Google Scholar]
- Wu, Y.B.; Zheng, Y.F. Tectonic evolution of a composite collision orogen, An overview on the Qinling–Tongbai–Hong’an–Dabie–Sulu orogenic belt in central China. Gondwana Res. 2013, 23, 1402–1428. [Google Scholar] [CrossRef]
- Dong, Y.P.; Santosh, M. Tectonic architecture and multiple orogeny of the Qinling Orogenic Belt, Central China. Gondwana Res. 2016, 29, 1–40. [Google Scholar] [CrossRef]
- Chandra, J.; Paul, D.; Stracke, A.; Chabaux, F.; Granet, M. The Origin of Carbonatites from Amba Dongar within the Deccan Large Igneous Province. J. Petrol. 2019, 60, 1119–1134. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Simonetti, A.; Simonetti, S.; Cao, X.; Du, Y. A Geochemical and Isotopic Investigation of Carbonatites from Huangshuian, Central China: Implications for Petrogenesis and Mantle Sources. Minerals 2024, 14, 953. https://doi.org/10.3390/min14090953
Zhao H, Simonetti A, Simonetti S, Cao X, Du Y. A Geochemical and Isotopic Investigation of Carbonatites from Huangshuian, Central China: Implications for Petrogenesis and Mantle Sources. Minerals. 2024; 14(9):953. https://doi.org/10.3390/min14090953
Chicago/Turabian StyleZhao, Hao, Antonio Simonetti, Stefanie Simonetti, Xiaopeng Cao, and Yushan Du. 2024. "A Geochemical and Isotopic Investigation of Carbonatites from Huangshuian, Central China: Implications for Petrogenesis and Mantle Sources" Minerals 14, no. 9: 953. https://doi.org/10.3390/min14090953
APA StyleZhao, H., Simonetti, A., Simonetti, S., Cao, X., & Du, Y. (2024). A Geochemical and Isotopic Investigation of Carbonatites from Huangshuian, Central China: Implications for Petrogenesis and Mantle Sources. Minerals, 14(9), 953. https://doi.org/10.3390/min14090953










