Genesis of Fe-Ti-(V) Oxide-Rich Rocks by Open-System Evolution of Mafic Alkaline Magmas: The Case of the Ponte Nova Massif, SE Brazil
Abstract
:1. Introduction
2. Geologic Setting
3. Analytical Methods
4. Results
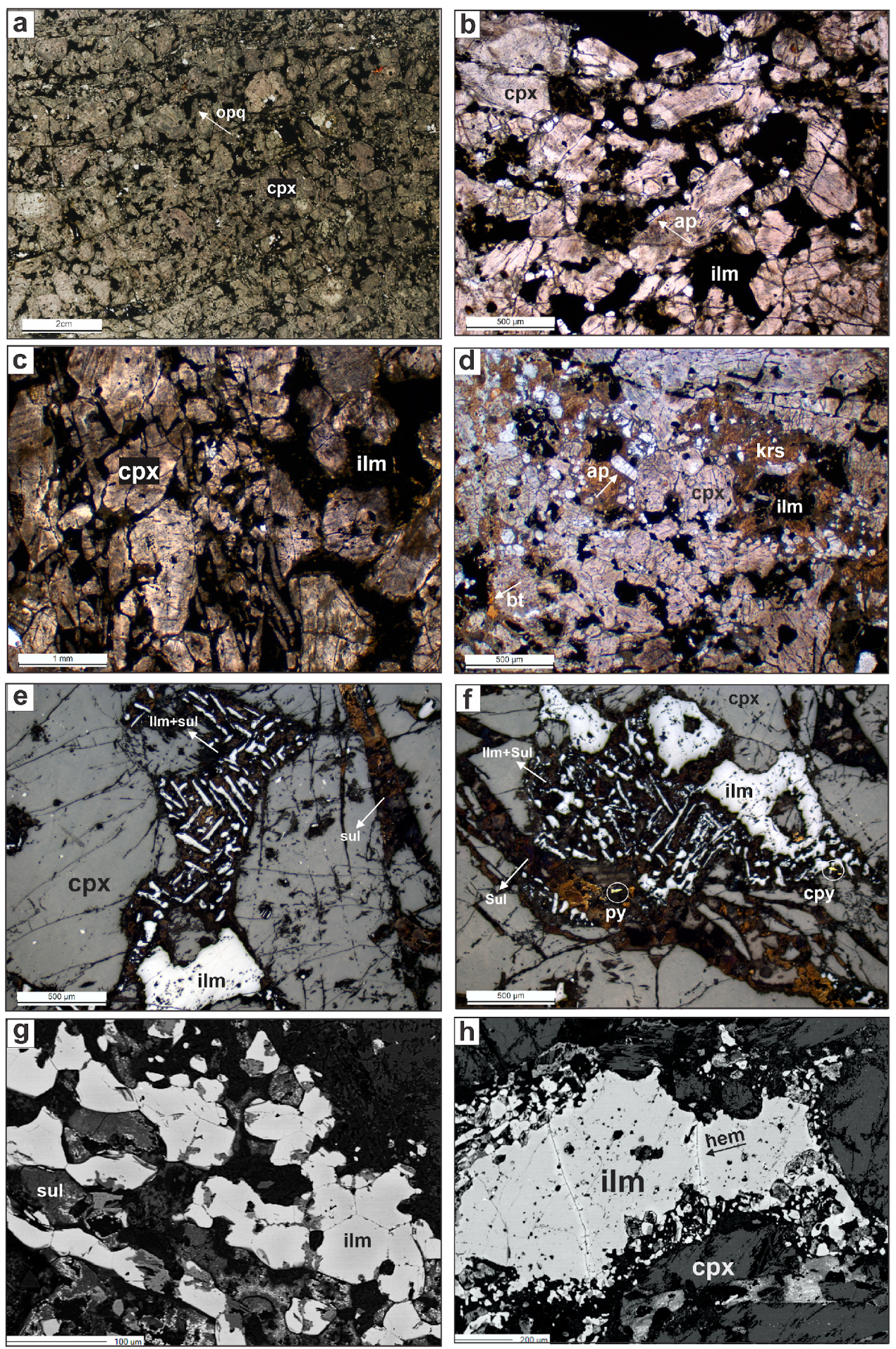
4.1. OCP and MTT Petrography
4.2. Major Elements
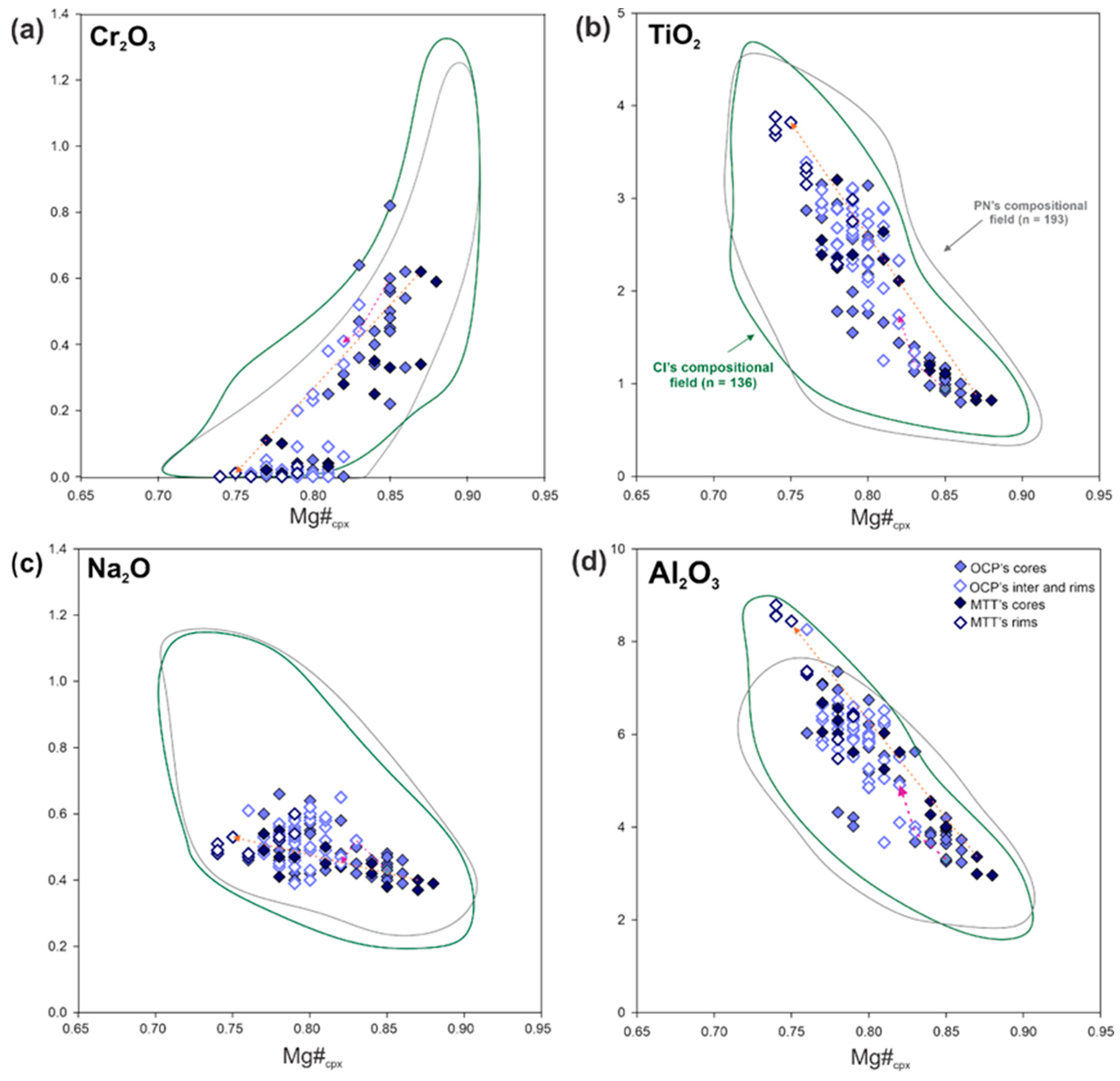
4.2.1. Clinopyroxene
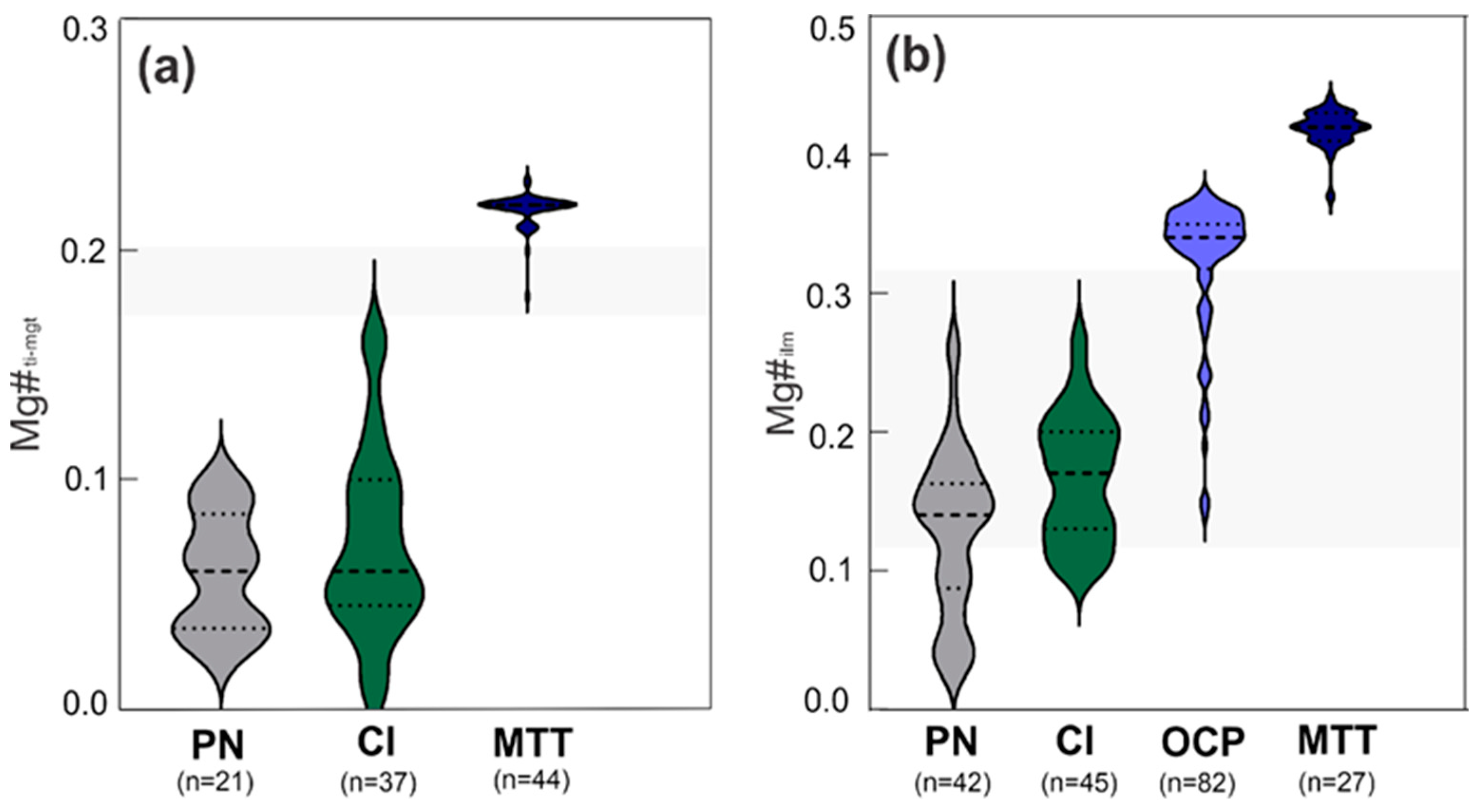
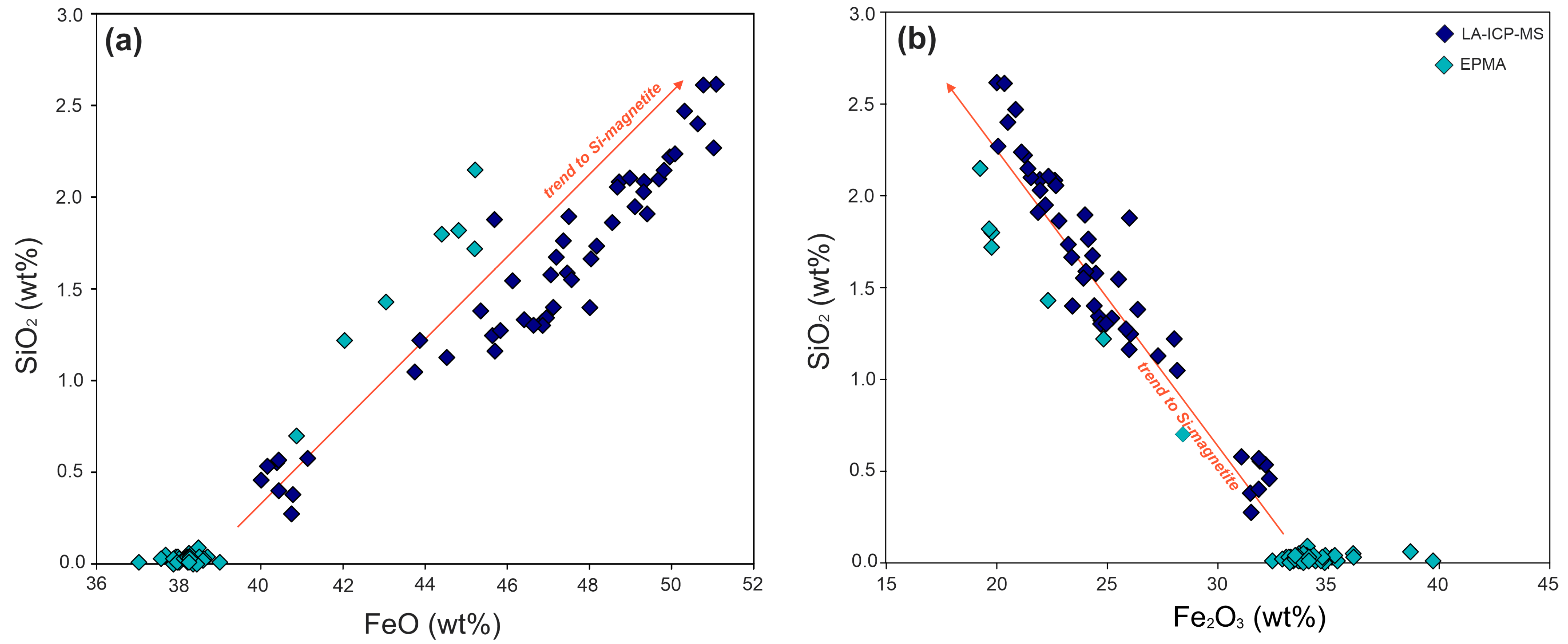
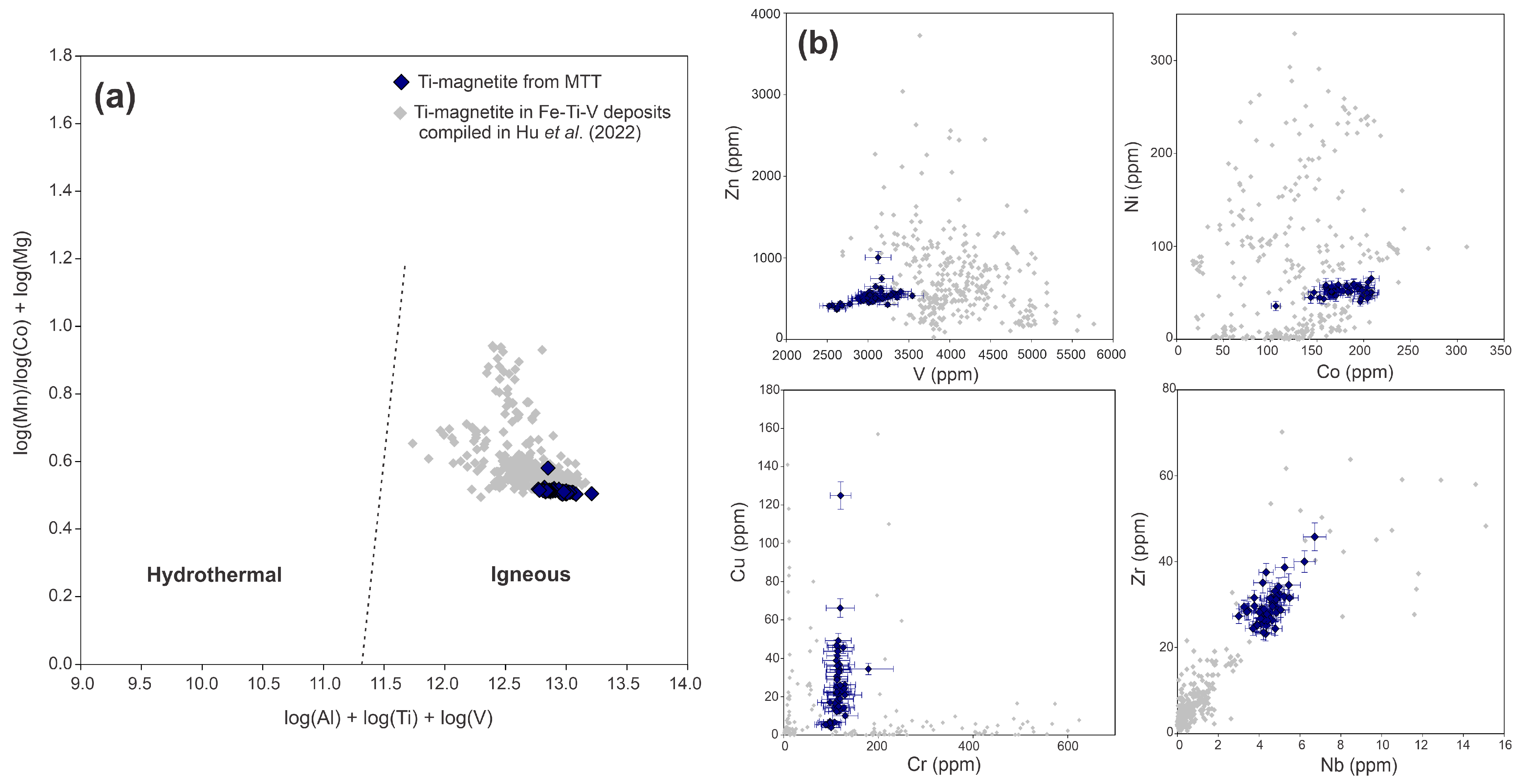
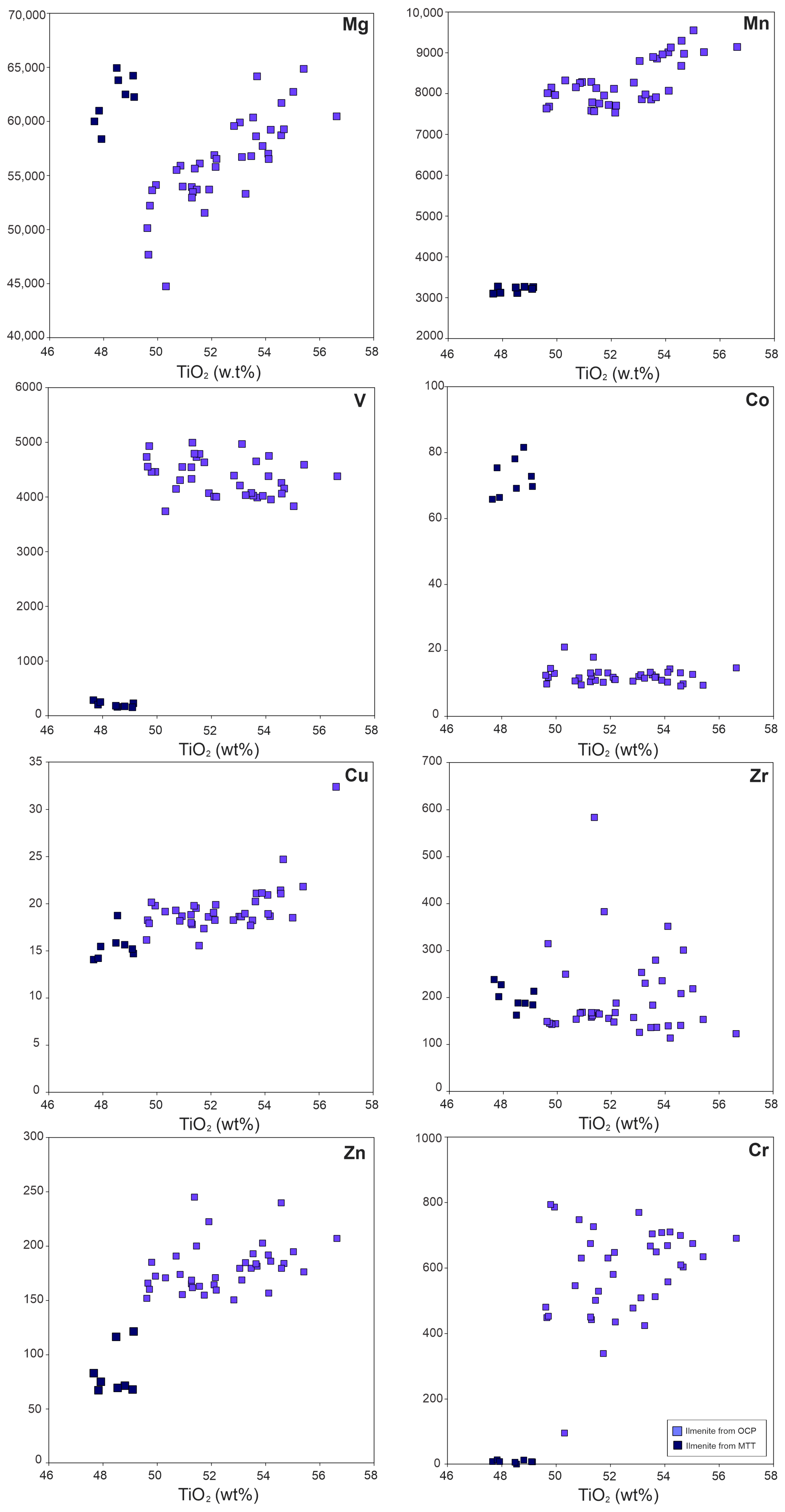
4.2.2. Fe-Ti Oxides
Ti-Magnetite
Ilmenite
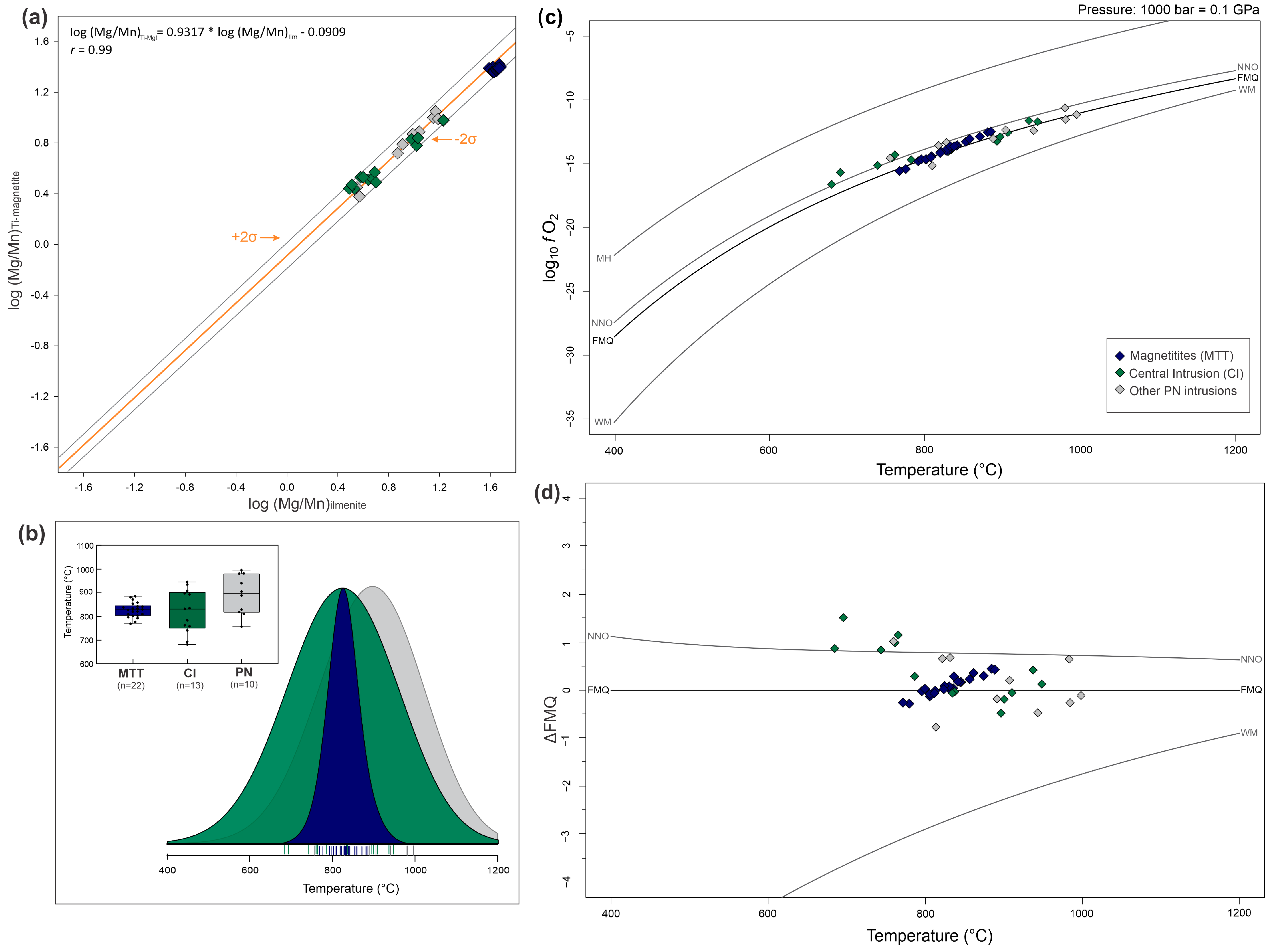
4.2.3. Temperature and Oxygen Fugacity Conditions
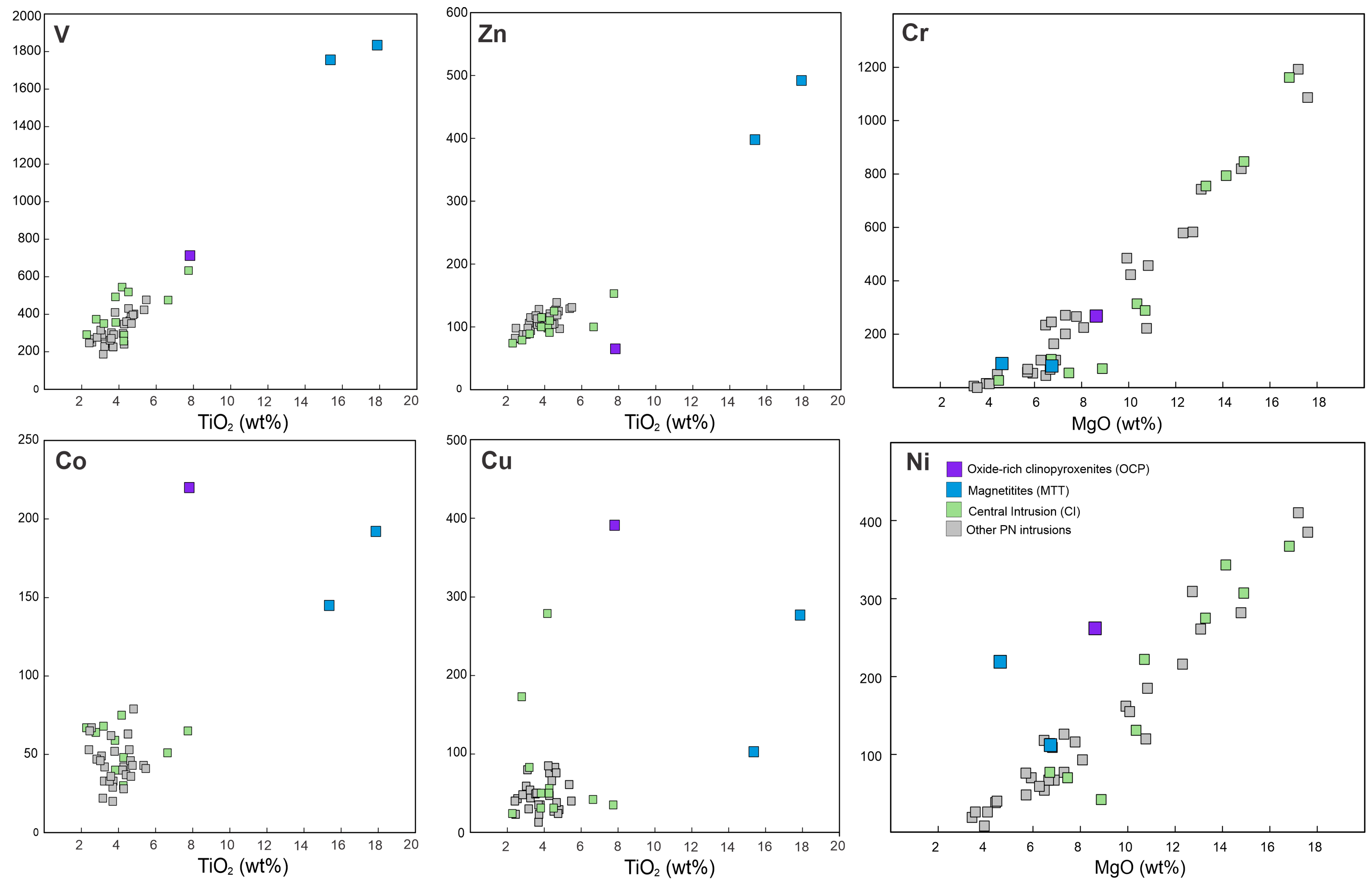
4.3. Whole-Rock Composition
4.3.1. Elemental Geochemistry
4.3.2. Sr Isotope Geochemistry
4.4. X-ray Diffraction
5. Discussion
5.1. Crustal Contamination
5.2. Magma Recharge
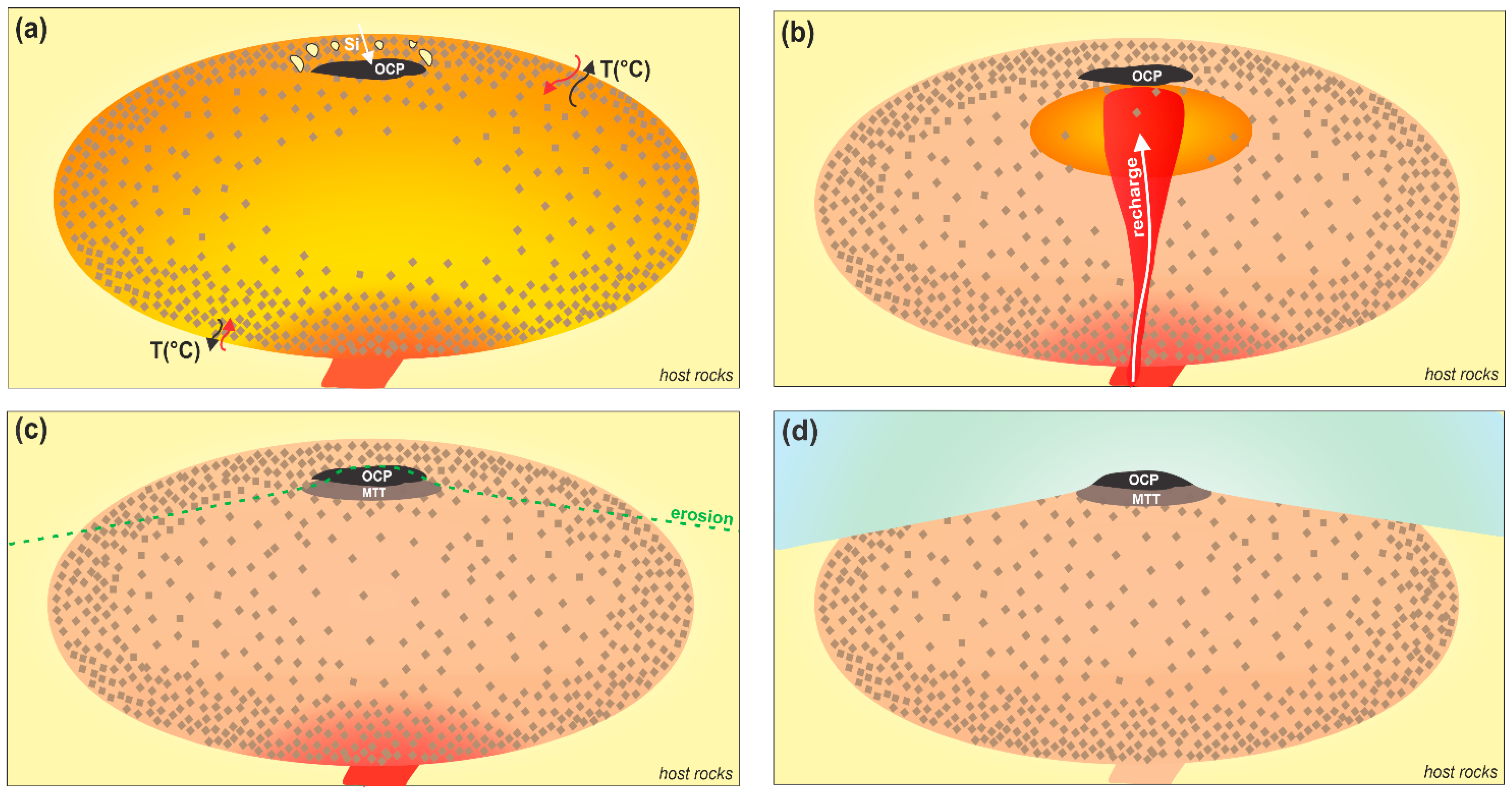
5.3. OCP and MTT Petrological Model
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cawthorn, R.G. Layered Intrusions, Developments in Petrology; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Latypov, R.M.; Namur, O.; Bai, Y.; Barnes, S.J.; Chistyakova, S.Y.; Holness, M.B.; Iacono-Marziano, G.; Kruger, W.A.J.; O’Driscoll, B.; Smith, W.D.; et al. Layered intrusions: Fundamentals, novel observations and concepts, and controversial issues. Earth-Sci. Rev. 2024, 249, 104653. [Google Scholar] [CrossRef]
- Lee, C.A. A review of mineralization in the Bushveld Complex and some other layered intrusions. In Layered Intrusions; Cawthorn, R.G., Ed.; Elsevier Science B.V: Amsterdam, The Netherlands, 1996; pp. 103–145. [Google Scholar] [CrossRef]
- Yao, Z.; Mungall, J.E. Magnetite layer formation in the Bushveld complex of South Africa. Nat. Commun. 2022, 13, 146. [Google Scholar] [CrossRef] [PubMed]
- Namur, O.; Higgins, M.D.; Vander Auwera, J. The Sept Iles intrusive suite, Quebec, Canada. In Layered Intrusions; Charlier, B., Namur, O., Latypov, R., Tegner, C., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 465–515. [Google Scholar] [CrossRef]
- Duchesne, J.-C.; Charlier, B. Geochemistry of cumulates from the Bjerkreim–Sokndal layered intrusion (S. Norway). Part I: Constraints from major elements on the mechanism of cumulate formation and on the jotunite liquid line of descent. Lithos 2005, 83, 229–254. [Google Scholar] [CrossRef]
- Zhou, M.F.; Robinson, P.T.; Lesher, C.M.; Keays, R.R.; Zhang, C.J.; Malpas, J. Geochemistry, petrogenesis and metallogenesis of the Panzhihua gabbroic layered intrusion and associated Fe–Ti–V oxide deposits, Sichuan Province, SW China. J. Petrol. 2005, 46, 2253–2280. [Google Scholar] [CrossRef]
- Chen, X.D.; Li, Y.G.; Luo, Z.H.; Fei, G.C.; Luo, W.; Zhang, T.J.; Peng, X.C.; Zou, Y.S. Formation of Fe-Ti oxide and Ni-Co sulfide ores by concentric tube flow and hydrous melt-rich recharge into the cooling crystal mush: Example from the Hongge intrusion in Panxi region, SW China. Ore Geol. Rev. 2023, 160, 105594. [Google Scholar] [CrossRef]
- Irvine, T.N. Origin of chromitite layers in the Muskox intrusion and other stratiform intrusions: A new interpretation. Geology 1977, 5, 273–277. [Google Scholar] [CrossRef]
- Ganino, C.; Arndt, N.T.; Zhou, M.-F.; Gaillard, F.; Chauvel, C. Interaction of magma with sedimentary wall rock and magnetite ore genesis in Panzhihua mafic intrusion, SW China. Mineral. Depos. 2008, 43, 677–694. [Google Scholar] [CrossRef]
- Irvine, T.N. Crystallization sequences in the Muskox intrusions and other layered intrusions: II Origin of chromitite layers and similar deposits of other magmatic ores. Geochim. Cosmochim. Acta 1975, 39, 991–1008. [Google Scholar] [CrossRef]
- Charlier, B.; Namur, O.; Toplis, M.J.; Schiano, P.; Cluzel, N.; Higgins, M.D.; Vander Auwera, J. Large-scale silicate liquid immiscibility during differentiation of tholeiitic basalt to granite and the origin of the Daly gap. Geology 2011, 39, 907–910. [Google Scholar] [CrossRef]
- Namur, O.; Charlier, B.; Holness, M.B. Dual origin of Fe-Ti-P gabbros by immiscibility and fractional crystallisation of evolved tholeiitic basalts in the Sept Iles layered intrusion. Lithos 2012, 154, 100–114. [Google Scholar] [CrossRef]
- Naslund, H.R.; McBirney, A.R. Mechanisms of formation of Igneous Layering. In Layered Intrusions; Cawthorn, R.G., Ed.; Elsevier Science B.V: Amsterdam, The Netherlands, 1996; pp. 1–43. [Google Scholar] [CrossRef]
- Derby, O.A. On the magnetite ore districts of Jacupiranga and Ipanema, São Paulo, Brazil. Am. J. Sci. 1891, 41, 311–321. [Google Scholar] [CrossRef]
- Krasnova, N.I.; Petrov, T.G.; Balaganskaya, E.G.; Garcia, D.; Moutte, J.; Zaitsev, A.N.; Wall, F. Introduction to phoscorites: Occurrence, composition, nomenclature and petrogenesis. In Phoscorites and Carbonatites from Mantle to Mine: The Key Example of the Kola Alkaline Province; Wall, F., Zaitsev, A.N., Eds.; Mineralogical Society Series; Mineralogical Society of Great Britain & Ireland: Middlesex, UK, 2004; pp. 45–79. [Google Scholar]
- Mikhailova, J.A.; Kalashnikov, A.O.; Sokharev, V.A.; Pakhomovosky, Y.A.; Konopleva, N.G.; Yakovenchuk, V.N.; Bazai, A.V.; Gor’yanov, P.; Ivanyuk, G.Y. 3D mineralogical mapping of the Kovdor phoscorite–carbonatite complex (Russia). Miner. Depos. 2016, 51, 131–149. [Google Scholar] [CrossRef]
- Azzone, R.G.; Ruberti, E.; Enrich, G.E.R.; Gomes, C.B. Geologia e geocronologia do maciço alcalino máfico-ultramáfico Ponte Nova (SP-MG). Geol. USP—Série Científica 2009, 9, 26–46. [Google Scholar] [CrossRef]
- Riccomini, C.; Velásquez, V.F.; Gomes, C.B. Tectonic controls of the Mesozoic and Cenozoic alkaline magmatism in the central-southeastern Brazilian Platform. In Mesozoic to Cenozoic Alkaline Magmatism in the Brazilian Platform; Comin-Chiaramonti, P., Gomes, C.B., Eds.; Edusp/Fapesp: São Paulo, Brazil, 2005; pp. 31–56. [Google Scholar]
- Azzone, R.G.; Munoz, P.M.; Enrich, G.E.R.; Alves, A.; Ruberti, E.; Gomes, C.B. Petrographic, geochemical and isotopic evidence of crustal assimilation processes in the Ponte Nova alkaline mafic-ultramafic massif, SE Brazil. Lithos 2016, 260, 58–75. [Google Scholar] [CrossRef]
- Azzone, R.G.; Chmyz, L.; Guarino, V.; Alves, A.; Gomes, C.B.; Ruberti, E. Isotopic clues tracking the open-system evolution of the Ponte Nova mafic-ultramafic alkaline massif, SE Brazil: The contribution of Pb isotopes. Geochemistry 2020, 80, 125648. [Google Scholar] [CrossRef]
- Ambrosio, M.R. The Crustal Assimilation in Alkaline Basic Magmas: The Ponte Nova Mafic-Ultramafic Massif and Dikes/Plugs of the Mantiqueira Range (SP-MG). Doctoral Thesis, University of São Paulo, Institute of Geosciences, São Paulo, Brazil, 2022. [Google Scholar] [CrossRef]
- Vaz, O.R.S.; Salomão, M.; Pedroso, E.; Pereira, R.; Neumann, R.; Garcia, P.; Tupinambá, M. Magnetita vanadífera do Maciço Ponte Nova: Uma Abordagem Exploratória. Anu. Inst. Geociênc.—UFRJ 2017, 40, 75–81. [Google Scholar] [CrossRef]
- de Almeida, F.F.M. Relações tectônicas das rochas alcalinas mesozóicas da região meridional da plataforma sul-americana. Rev. Bras. Geociênc. 1983, 13, 139–158. [Google Scholar] [CrossRef]
- de Almeida, F.F.M. Distribuição regional e relações tectônicas do magmatismo pós-paleozoico no Brasil. Rev. Bras. Geociênc. 1986, 16, 325–349. [Google Scholar] [CrossRef]
- Morbidelli, L.; Gomes, C.B.; Beccaluva, L.; Brotzu, P.; Conte, A.M.; Ruberti, E.; Traversa, G. Mineralogical, petrological and geochemical aspects of alkaline and alkaline-carbonatite associations from Brazil. Earth Sci. Rev. 1995, 39, 135–168. [Google Scholar] [CrossRef]
- Beccaluva, L.; Barbieri, M.; Born, H.; Brotzu, P.; Coltorti, M.; Conte, A.; Garbarino, C.; Gomes, C.B.; Macciotta, G.; Morbidelli, L.; et al. Fractional crystallization and liquid immiscibility processes in the alkaline-carbonatite complex of Juquiá, São Paulo, Brazil. J. Petrol. 1992, 33, 1371–1404. [Google Scholar] [CrossRef]
- Ruberti, E.; Gomes, C.B.; Comin-Chiaramonti, P. The alkaline magmatism from the Ponta Grossa arch. In Mesozoic to Cenozoic Alkaline Magmatism in the Brazilian Platform; Comin-Chiaramonti, P., Gomes, C.B., Eds.; Edusp/Fapesp: São Paulo, Brazil, 2005; pp. 473–521. [Google Scholar]
- Herz, N. Timing of spreading in the South Atlantic: Information from Brazilian alkalic rocks. Geol. Soc. Am. Bull. 1977, 88, 101–112. [Google Scholar] [CrossRef]
- Ulbrich, H.H.G.J.; Gomes, C.B. Alkaline rocks from continental Brazil. Earth-Sci. Rev. 1981, 17, 135–154. [Google Scholar] [CrossRef]
- Brotzu, P.; Barbieri, M.; Beccaluva, L.; Garbarino, C.; Gomes, C.B.; Macciotta, G.; Melluso, L.; Morbidelli, L.; Ruberti, E.; Sígolo, J.B.; et al. Petrology and geochemistry of the Passa Quatro alkaline complex (MG-SP-RJ), Brazil. J. S. Am. Earth Sci. 1992, 4, 237–252. [Google Scholar] [CrossRef]
- Brotzu, P.; Gomes, C.; Melluso, L.; Morbidelli, L.; Morra, V.; Ruberti, E. Petrogenesis of coexisting SiO2-undersaturated to SiO2-oversaturated felsic igneous rocks: The alkaline complex of Itatiaia, southeastern Brazil. Lithos 1997, 40, 133–156. [Google Scholar] [CrossRef]
- Ulbrich, H.H.G.J.; Vlach, S.R.F.; Demaiffe, D.; Ulbrich, M.N.C. Structure and origin of the Poços de Caldas Alkaline Massif, SE Brazil. In Mesozoic to Cenozoic Alkaline Magmatism in the Brazilian Platform; Comin-Chiaramonti, P., Gomes, C.B., Eds.; Edusp/Fapesp: São Paulo, Brazil, 2005; pp. 367–418. [Google Scholar]
- Bellieni, G.; Montes Lauar, C.R.; De Min, A.; Piccirillo, E.M.; Cavazzini, G.; Melfi, A.J.; Pacca, I.G. Early and Late Cretaceous magmatism from São Sebastião Island (SE-Brazil): Geochemistry and petrology. Geochim. Bras. 1990, 4, 59–83. [Google Scholar]
- Enrich, G.E.R.; Ruberti, E.; Gomes, C.B. Geology and geochronology of Monte de Trigo island alkaline suite, southeastern Brazil. Rev. Bras. Geociênc. 2009, 39, 67–80. [Google Scholar] [CrossRef]
- Azzone, R.G. Petrogênese do Maciço Alcalino Máfico-Ultramáfico Ponte Nova (SP-MG). Doctoral Thesis, University of São Paulo, Institute of Geosciences, São Paulo, Brazil, 2008. [Google Scholar] [CrossRef]
- Azzone, R.G.; Ruberti, E.; Enrich, G.E.R.; Gomes, C.B. Zr- and Ba-rich minerals from the Ponte Nova alkaline mafic-ultramafic massif, southeastern Brazil: Indication of an enriched mantle source. Can. Mineral. 2009, 47, 1087–1103. [Google Scholar] [CrossRef]
- Azzone, R.G.; Tarazona, L.M.C.; Ambrosio, M.R.; Guarino, V.; Chmyz, L.; Lima, N.M.; Ruberti, E. The Hidden Magmatic Chamber from the Ponte Nova Mafic-Ultramafic Alkaline Massif, SE. Brazil: Clues from Clinopyroxene and Olivine Antecrysts. Minerals 2022, 12, 775. [Google Scholar] [CrossRef]
- Bastin, G.F.; Van Loo, F.J.J.; Heijligers, H.J.M. Evaluation and use of gaussian ϕ(ρz) curves in quantitative electron probe microanalysis: A new optimization. X-ray Spectrom. 1984, 13, 91–97. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals, 2nd ed.; Longman Scientific & Technical: London, UK, 1992. [Google Scholar]
- Morimoto, N. Nomenclature of pyroxenes. Mineral. Petrol. 1988, 39, 55–76. [Google Scholar] [CrossRef]
- Droop, G.T.R. A general equation for estimating Fe3+ concentrations in ferromagnesian silicates and oxides from microprobe analyses, using stoichiometric criteria. Mineral. Mag. 1987, 51, 431–435. [Google Scholar] [CrossRef]
- Carmichael, I.S.E. The iron-titanium oxides of salic volcanic rocks and their associated ferromagnesian silicates. Contrib. Mineral. Petrol. 1967, 14, 36–64. [Google Scholar] [CrossRef]
- Griffin, W.L. GLITTER: Data reduction software for laser ablation ICP-MS. In Laser Ablation ICP-MS in the Earth Sciences; Sylvester, P., Ed.; Short Course Series; Mineralogical Association of Canada: Ottawa, ON, Canada, 2008; pp. 308–311. [Google Scholar]
- Mori, P.E.; Reeves, S.; Correia, C.T.; Haukka, M. Development of a fused glass disc XRF facility and comparison with the pressed powder pellet technique at Instituto de Geociências, São Paulo University. Rev. Bras. Geociênc. 1999, 29, 441–446. [Google Scholar] [CrossRef]
- Navarro, M.S.; Andrade, S.; Ulbrich, H.H.G.J.; Girardi, V.A.V. The analysis of rare earth elements with ICP-MS in basaltic and related rocks: Testing the efficiency of sample decomposition procedures. Geostand. Geoanal. Res. 2008, 32, 167–180. [Google Scholar] [CrossRef]
- Torquato, J.R.; Kawashita, K. Geologia nuclear—O método Rb/Sr. Rev. Geol. 1994, 7, 91–123. [Google Scholar]
- Sato, K.; Tassinari, C.C.G.; Kawashita, K.; Petronilho, L. O método geocronológico Sm-Nd no IG/USP e suas aplicações. An. Acad. Bras. Ciênc. 1995, 67, 313–336. [Google Scholar]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Gražulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quirós, M.; Lutterotti, L.; Le Bail, A. Crystallography Open Database–an open-access collection of crystal structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zeng, L.-P.; Liao, W.; Wen, G.; Hu, H.; Li, M.Y.H.; Zhao, X.-F. The Origin and Discrimination of High-Ti Magnetite in Magmatic-Hydrothermal Systems: Insight from Machine Learning Analysis. Econ. Geol. 2022, 117, 1613–1627. [Google Scholar] [CrossRef]
- Yavuz, F. WinMIgob: A Windows program for magnetite-ilmenite geothermometer and oxygen barometer. J. Geosci. 2021, 66, 51–69. [Google Scholar] [CrossRef]
- Ghiorso, M.S.; Evans, B.W. Thermodynamics of rhombohedral oxide solid solutions and a revision of the Fe-Ti two-oxide geothermometer and oxygen-barometer. Am. J. Sci. 2008, 308, 957–1039. [Google Scholar] [CrossRef]
- Sauerzapf, U.; Lattard, D.; Buchard, M.; Engelmann, R. The Titanomagnetite-Ilmenite equilibrium: New Experimental Data and Thermo-oxybarometric Application to the Crystallization of Basic to Intermediate Rocks. J. Petrol. 2008, 49, 1161–1185. [Google Scholar] [CrossRef]
- Bacon, C.R.; Hirschmann, M.M. Mg/Mn partitioning as a test for equilibrium between Fe-Ti oxides. Am. Mineral. 1988, 73, 57–61. [Google Scholar]
- Anenburg, M. Oxygen Fugacity Buffer Calculator. 2021. Available online: https://fo2.rses.anu.edu.au/fo2app/ (accessed on 25 July 2023).
- De la Roche, H.; Leterrier, J.; Grandclaude, P.; Marchal, M. A classification of volcanic and plutonic rocks using R1-R2 diagram and major-element analyses—Its relationships with current nomenclature. Chem. Geol. 1980, 29, 183–210. [Google Scholar] [CrossRef]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Wang, K.; Wang, C.Y.; Ren, Z.Y. Apatite-hosted melt inclusions from the Panzhihua gabbroic-layered intrusion associated with a giant Fe-Ti oxide deposit in SW China: Insights for magma unmixing within a crystal mush. Contrib. Mineral. Petrol. 2018, 173, 59. [Google Scholar] [CrossRef]
- Khedr, M.Z.; Takazawa, E.; Arai, S.; Stern, R.J.; Morishita, T.; El-Awady, A. Styles of Fe-Ti-V ore deposits in the Neoproterozoic layered mafic-ultramafic intrusions, south Eastern Desert of Egypt: Evidence for fractional crystallization of V-rich melts. J. Afr. Earth Sci. 2022, 194, 104620. [Google Scholar] [CrossRef]
- Mokchah, N.; Mathieu, L. Origin and evolution of the iron-rich upper unit and Fe-Ti-V mineralization of the Neoarchean Lac Doré Layered Intrusion, Chibougamau, Québec. J. Petrol. 2022, 63, egac006. [Google Scholar] [CrossRef]
- Shu, X.J.; Wang, X.L.; Chen, L.; Wang, D.; Dai, Z.Y. Open-system differentiation of mafic magmas before the formation of layered Fe-Ti(V) deposits in Southeast China. Ore Geol. Rev. 2023, 158, 105527. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Q.; Li, Z.; Wang, J.; Wang, J. In situ crystallization of Fe-Ti oxides in the Bijigou layered intrusion: Insights from crystal size distributions and compositions of magnetite and ilmenite. J. Asian Earth Sci. 2023, 255, 105773. [Google Scholar] [CrossRef]
- Spandler, C.; Mavrogenes, J.; Arculus, R. Origin of chromitites in layered intrusions: Evidence from chromite-hosted melt inclusions from the Stillwater complex. Geology 2005, 33, 893–896. [Google Scholar] [CrossRef]
- Boudreau, A.E. The Stillwater Complex—Overview and the significance of volatiles. Mineral. Mag. 2016, 80, 585–637. [Google Scholar] [CrossRef]
- Tornos, F.; Hanchar, J.M.; Steele-Macinnis, M.; Crespo, E.; Kamenetsky, V.S.; Casquet, C. Formation of magnetite-(apatite) systems by crystallizing ultrabasic iron-rich melts and slag separation. Miner. Depos. 2023, 59, 189–225. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.J.; Beaudoin, G.; Méric, J.; Boutroy, E.; Potvin-Doucet, C. Trace elements in magnetite as petrogenetic indicators. Miner. Depos. 2014, 49, 785–796. [Google Scholar] [CrossRef]
- Wang, Z.C.; Huang, X.W.; Liu, P.P.; Zhou, M.F. Trace element systematics of magnetite from alkaline mafic–ultramafic intrusions of the Permian Emeishan large igneous province, SW China. Contrib. Mineral. Petrol. 2024, 179, 8. [Google Scholar] [CrossRef]
- Vinagre, R.; Trouw, R.A.J.; Mendes, J.C.; Duffles, P.; Peternel, R.; Matos, G. New evidence of a Magmatic Arc in the Southern Brasília Belt, Brazil: The Serra da Água Limpa Batholith (Socorro-Guaxupé Nappe). J. S. Am. Earth. Sci. 2014, 54, 120–139. [Google Scholar] [CrossRef]
- White, W.M. Radiogenic Isotope Geochemistry. In Geochemistry; White, W.M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 312–360. [Google Scholar]
- Hibbard, M.J. Petrography to Petrogenesis; Prentice Hall: Hoboken, NJ, USA, 1995. [Google Scholar]
- Svetlitskaya, T.V.; Nevolko, P.A.; Fominykh, P.A. Fe-Ti Oxide Assemblages from the Contact-Metamorphosed Mafic-Ultramafic Rocks of the Sedova Zaimka Intrusion (Western Siberia, Russia): The Tracking of Metamorphic Transformations. Minerals 2020, 10, 253. [Google Scholar] [CrossRef]
- Pang, K.N.; Zhou, M.F.; Lindsley, D.H.; Zhao, D.; Malpas, J. Origin of Fe-Ti Oxide Ores in Mafic Intrusions: Evidence from the Panzhihua Intrusion, SW China. J. Petrol. 2008, 49, 295–313. [Google Scholar] [CrossRef]
- Qian, G.; Brugger, J.; Skinner, W.; Guorong, C.; Pring, A. An experimental study of the mechanisms of the replacement of magnetite by pyrite up to 300 °C. Geochim. Cosmochim. Acta 2010, 74, 5610–5630. [Google Scholar] [CrossRef]
- Jochum, K.P.; Nohl, U.; Herwig, K.; Lammel, E.; Stoll, B.; Hofmann, A.W. GeoRem: A New Geochemical Database for Reference Materials and Isotopic Standards. Geostand. Geoanalytical Res. 2005, 29, 333–338. [Google Scholar] [CrossRef]







| Intrusion | Lithologies | Color Index | Crystallization Stages | ||||
|---|---|---|---|---|---|---|---|
| Early-Magmatic (Primocrysts) | Principal | Late-Magmatic | Post-Magmatic | ||||
| CI | LS (regular) | (coarse- to medium-grained) (cumulatic) Olivine clinopyroxenites, Olivinel-bearing clinopyroxenites, Olivine melagabbros, Olivine-bearing melagabbros | 97–70 | Di, Ti-Aug, Ol, ±Ti-Mgt, ±Ap, ±Pl | Ti-Aug, Pl, Krs, Ti-Mgt, Ap, ±Ilm, ±Bdy | Ti-rich Bt, ±Afs, ±Nph | (incipient levels) Anl, Ab, Ms, Cb, Ep/Czo, Chl, Bt, Act, Ttn, Py, ±Po, ±Cpy, ±Sp |
| LS (Krs-rich) | (coarse- to medium-grained) (cumulatic) Olivine kaersutite clinopyroxenites, Kaersutite melagabbros | 95–80 | Di, Ti-Aug, Ol, ±Ti-Mgt, ±Ap, ±Pl | Krs, Ti-Aug, Pl, Ti-rich Bt, Ti-Mgt, Ap, ±Ilm, ±Bdy | Ti-rich Bt, ±Afs, ±Nph | ||
| US | (coarse- to medium-grained) (inequigranular to macrocrystic) Nepheline-bearing monzogabbros | 62–50 | Di, Ti-Aug, Pl, ±Ol | Pl, Ti-Aug, Bt, Ti-Mgt, Ti-rich Bt, Ap | Intergrowth of Afs + Nph + Pl | ||
| WI | LS | (coarse- to medium-grained) (cumulatic) Olivine melagabbros, Olivine-bearing melagabbros, Olivine-bearing clinopyroxenites | 99–75 | Di, Ti-Aug, Ol, ±Ti-Mgt, ±Ap, ±Pl | Ti-Aug, Pl, Krs, Ti-rich Bt, Ti-Mgt, Ap, ±Ilm, ±Bdy | Ti-rich Bt, ±Afs, ±Nph | |
| US | (coarse- to medium-grained) (inequigranular to equigranular, banded to massive) Nepheline-bearing monzogabbros | 52–36 | Pl, Ti-Aug, ±Ti-Mgt, ±Ap, ±Ol | Pl, ti-Aug, Ti-Mgt, Ap, Ti-rich Bt, Afs, Nph, Ba-rich Afs | Intergrowth of Afs + Nph + Pl | ||
| NI | (coarse- to medium-grained) (cumulatic) Olivine melamonzogabbros, Olivine-bearing melamonzogabbros | 76–67 | Di, Ti-Aug, Ol, ±Ti-Mgt, ±Ap, ±Pl | Ti-Aug, Pl, Ti-Mgt, Ap, Afs, Ilm, ±Bdy | Ti-rich Bt, ±Krs, intergrowth of Afs + Nph | -- | |
| EI | (medium- to fine-grained) (seriate to porphyritic) Nepheline monzodiorites, Nepheline-bearing monzodiorites | 58–51 | Di, Ol, Pl, ±Ap | Ti-Aug, Pl, Ba-Ti-rich Bt, Ti-Mgt, Ilm, Ap, ±Zrc | Afs, Ba-rich Afs, Nph, ±Sdl | (insipient levels) Anl, Ab, Prh, Cb, Ep/Czo, Chl, Bt, Py, ±Cpy, ±Sp | |
| CP | (medium-grained) (porphyritic to equigranular) microgabbros | 56–50 | Ti-Aug, Ol | Ti-Aug, Pl, Ti-Mgt, Bt, Ap | Bt, Afs, Nph | -- | |
| SSI | (porphyritic, medium-grained matrix) Nepheline-bearing melamonzogabbros | 67–60 | Di, Ti-Aug, Ol | Pl, Ti-Aug, Ti-Mgt, Ilm, Ap | Ti-rich Bt, Afs, Ba-rich Afs, Nph | (incipient levels) Cb, Anl, Ab, Ms, Ep/Czo, Chl, Bt, Ttn, Py, Fl | |
| (porphyritic, medium-grained matrix) Nepheline-bearing melamonzonites | 64–62 | Di, Ti-Aug | Pl, Ti-Aug, Ba-Ti-rich Bt, Afs, Ba-rich Afs, Ti-MgtIlm, Ap ± Zrc | Nph | |||
| coarse- to medium-grained (inequigranular, porphyritic) Nepheline-bearing monzonites | 46–40 | Pl, Afs, Ba-rich Afs, Ti-Aug | Ba-Ti-rich Bt, Ap, Prg, Hst, Ti-Mgt, Nph | Nph | |||
| OCP MTT | (coarse- to medium-grained) (cumulatic) Oxide-rich clinopyroxenites | 100 | Ti-Aug, Di, Ilm (±Ti-Mgt) | Ti-Aug, Ilm, (±Ti-Mgt), Ap, | Ti-rich Bt, ±Py, Ap, Krs | (low-levels) Ti-rich Bt, Hem, Py, ±Cpy, Mlnt, Rmb | |
| (coarse- to medium-grained) (cumulatic) Magnetitites | 100 | Ti-Mgt, Ilm, Ti-Aug | Ti-Aug, Ti-Mgt, Ilm | Ti-Mgt, Ilm | Hem, Ulv, Ant, Gth | ||
| MTT | OCP | ||
|---|---|---|---|
| wt% | R118 | SM82 | R119 |
| Ti-augite | 2.0 | 25.7 | 68.7 |
| Diopside | 1.8 | ||
| Ti-magnetite | 84.2 | 63.6 | |
| Ilmenite | 5.8 | 3.3 | 5.0 |
| Anatase | 0.9 | 0.5 | |
| Spinel | 5.1 | 4.3 | |
| Goethite | 1.9 | 2.6 | |
| Pyrite | 0.7 | ||
| Melantherite | 23.1 | ||
| Rhomboclase | 0.7 | ||
| GoF | 3.82 | 4.16 | 3.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, A.A.; Azzone, R.G.; Chmyz, L.; Tarazona, L.M.C.; de Andrade, F.R.D.; Martins, J.V.; Ruberti, E.; de Barros Gomes, C. Genesis of Fe-Ti-(V) Oxide-Rich Rocks by Open-System Evolution of Mafic Alkaline Magmas: The Case of the Ponte Nova Massif, SE Brazil. Minerals 2024, 14, 724. https://doi.org/10.3390/min14070724
de Souza AA, Azzone RG, Chmyz L, Tarazona LMC, de Andrade FRD, Martins JV, Ruberti E, de Barros Gomes C. Genesis of Fe-Ti-(V) Oxide-Rich Rocks by Open-System Evolution of Mafic Alkaline Magmas: The Case of the Ponte Nova Massif, SE Brazil. Minerals. 2024; 14(7):724. https://doi.org/10.3390/min14070724
Chicago/Turabian Stylede Souza, Amanda Andrade, Rogério Guitarrari Azzone, Luanna Chmyz, Lina Maria Cetina Tarazona, Fábio Ramos Dias de Andrade, José Vinicius Martins, Excelso Ruberti, and Celso de Barros Gomes. 2024. "Genesis of Fe-Ti-(V) Oxide-Rich Rocks by Open-System Evolution of Mafic Alkaline Magmas: The Case of the Ponte Nova Massif, SE Brazil" Minerals 14, no. 7: 724. https://doi.org/10.3390/min14070724








