Perspectives for High-Purity Quartz from European Resources
Abstract
1. Introduction
2. Quartz Production and Uses
2.1. Silica Sand and Gravel
2.2. High-Purity Quartz
2.3. Global Producers and EU Position
2.4. Evaluation Rules Used
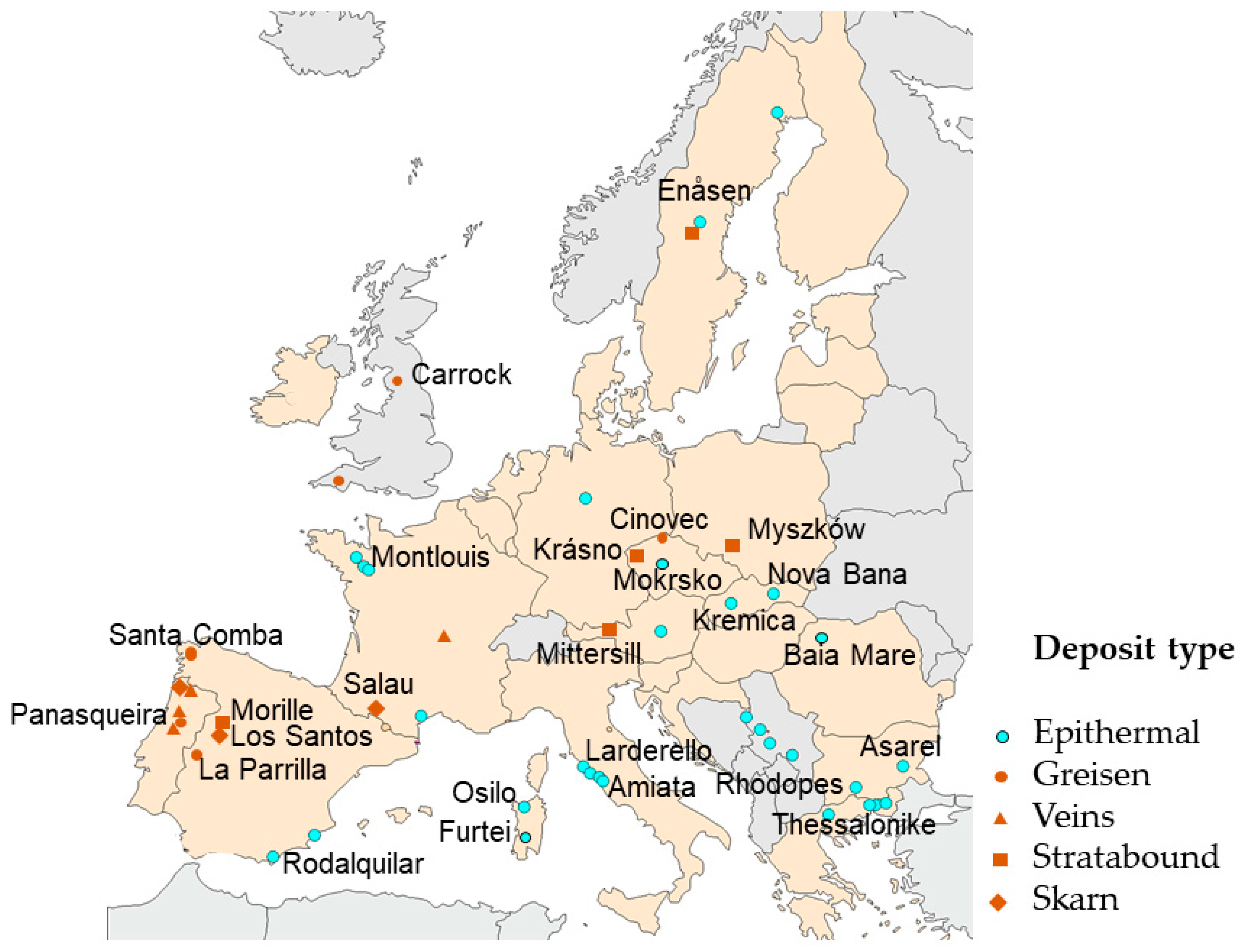
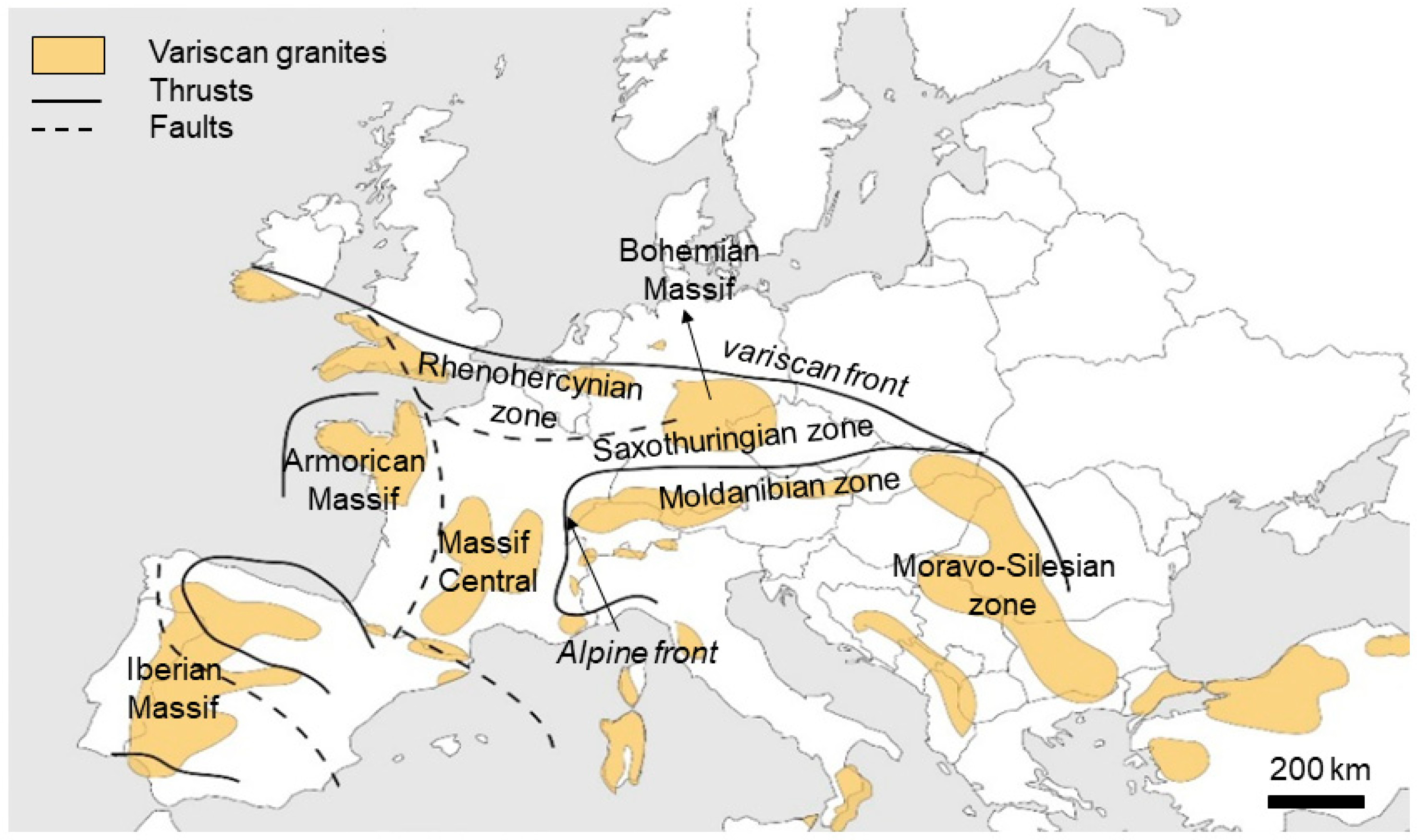
3. Types of Quartz Deposits in Europe
3.1. Magmatic Quartz
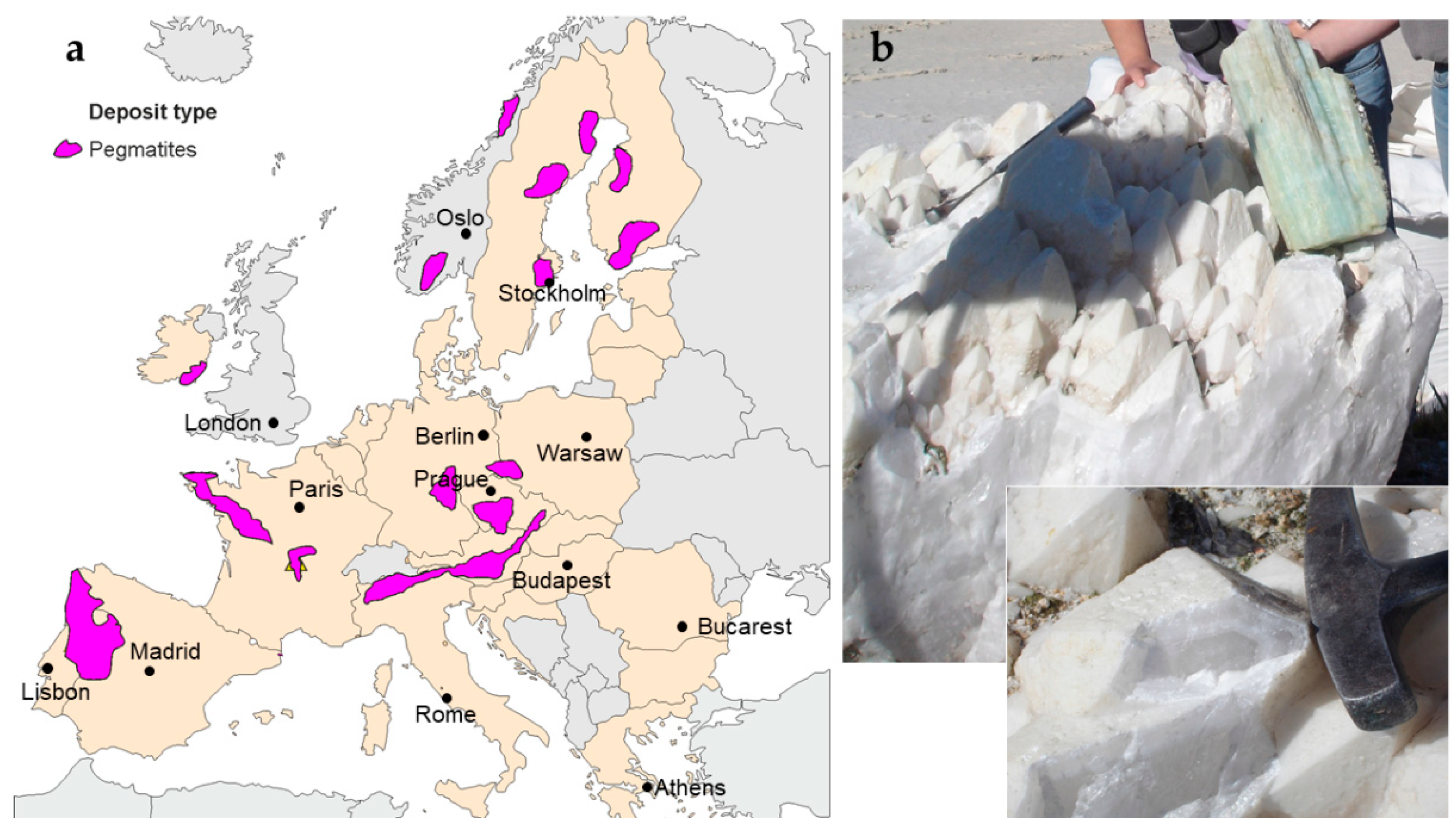
3.2. Pegmatitic Quartz
3.3. Hydrothermal Quartz
3.4. Metamorphic Deposits
3.5. Sedimentary Quartz
3.6. Case Studies
| Parameter | Evje–Iveland Pegmatites (Norway) | Panasqueira Greisen Veins (Portugal) |
|---|---|---|
| Deposit type | Feldspar–quartz–mica pegmatite field | Quartz–muscovite–topaz greisen veins in a tungsten deposit |
| Geological age | Precambrian pegmatites | Variscan hydrothermal system |
| Primary minerals | Quartz, feldspar, mica | Quartz, muscovite, topaz, wolframite |
| Quartz characteristics | Coarse-grained, relatively low Fe/Ti impurities | Fine- to medium-grained, low Fe/Al/Ti |
| Historic mining focus | Feldspar and rare mineral extraction | Tungsten (wolframite); quartz treated as gangue |
| Beneficiation studies | Magnetic separation + acid leaching → quartz > 99.9% SiO2 | Flotation + acid leaching → upgraded quartz suitable for high-purity trials |
| HPQ potential | Proximity to Norway’s drag refining plants enhances viability | By-product valorization aligns with EU circular economy goals |
| Strategic relevance | Possible domestic European HPQ feedstock | Adds value to waste streams from active tungsten mine |
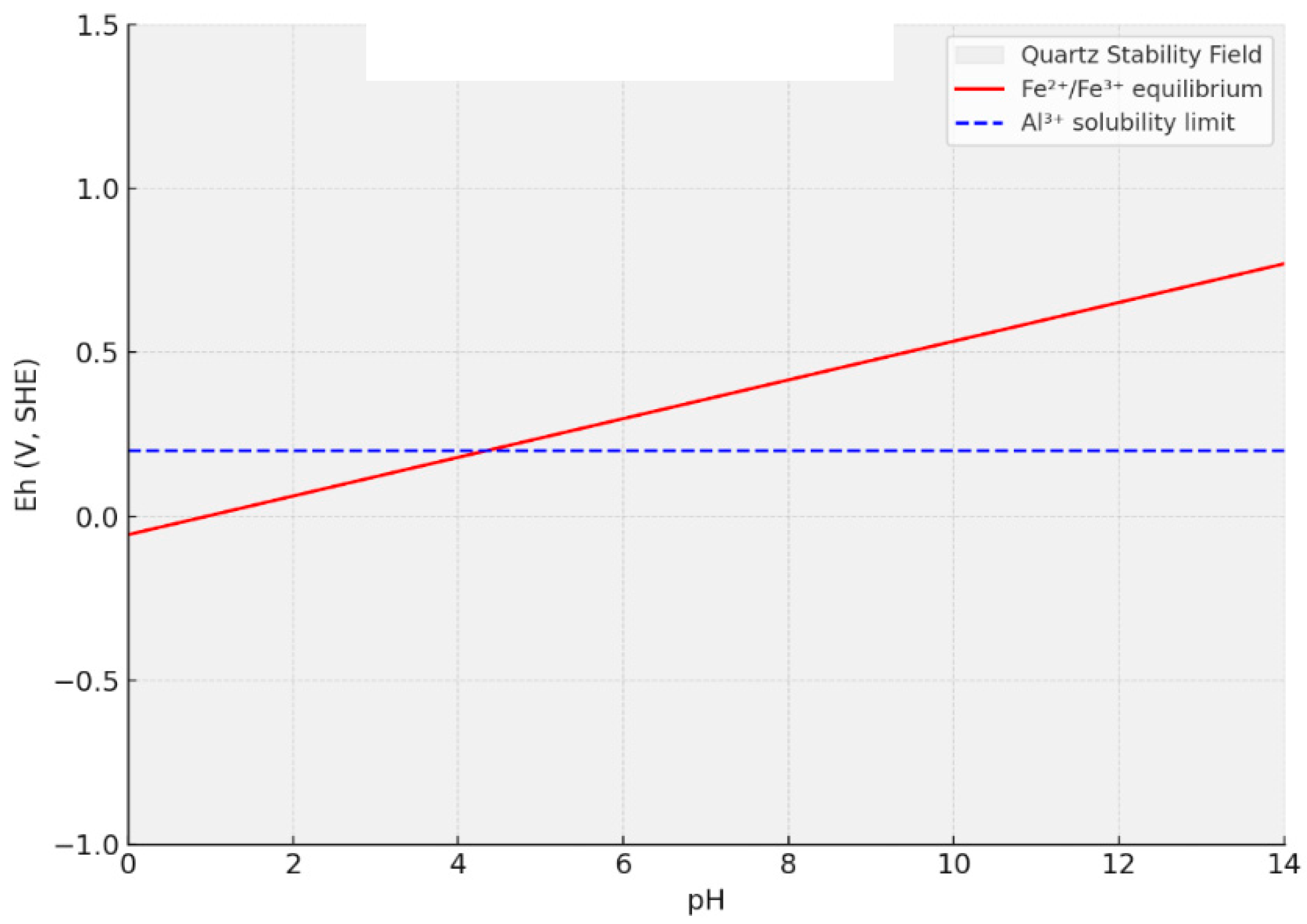
4. Quartz Purification Methods
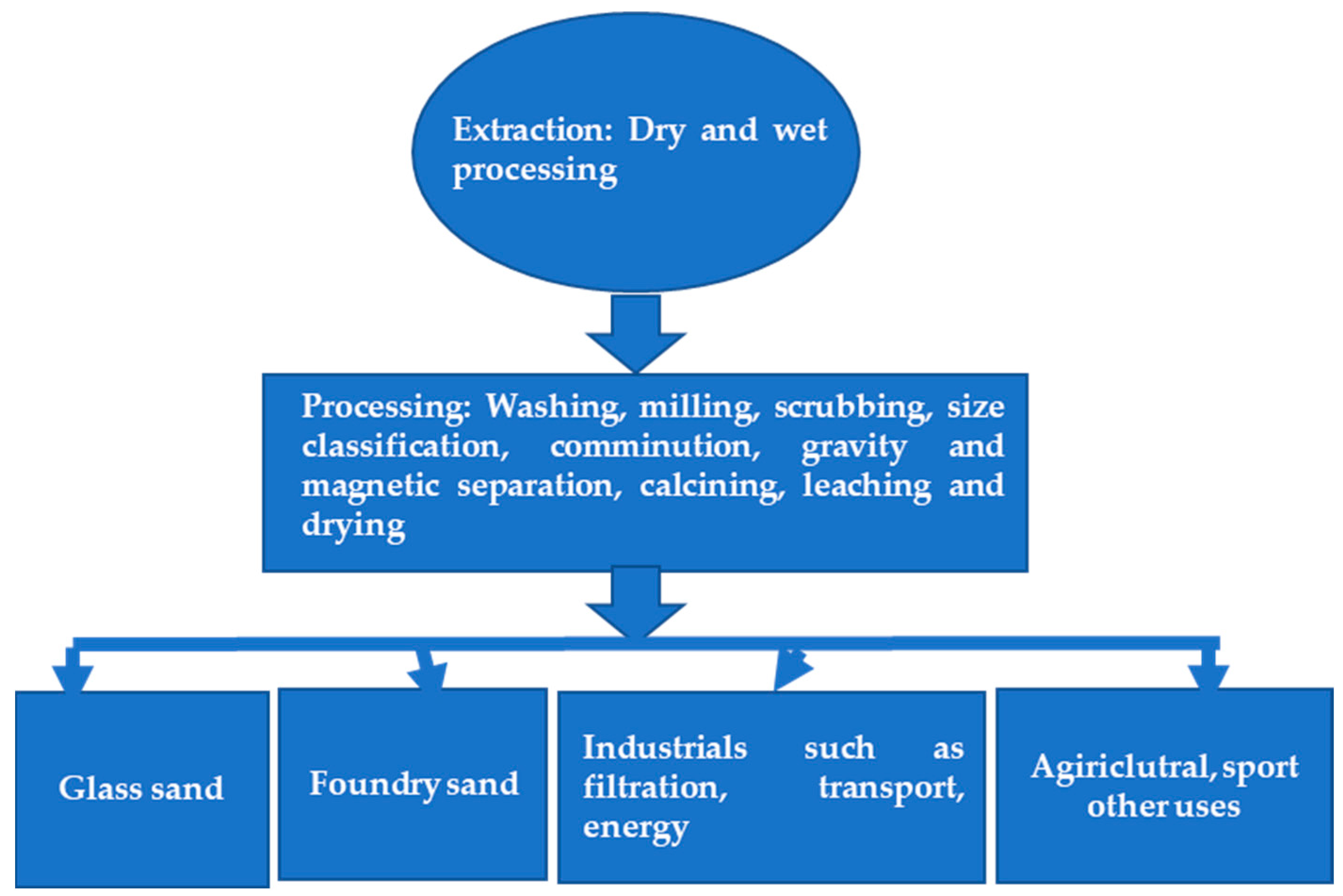
4.1. Extraction Methods
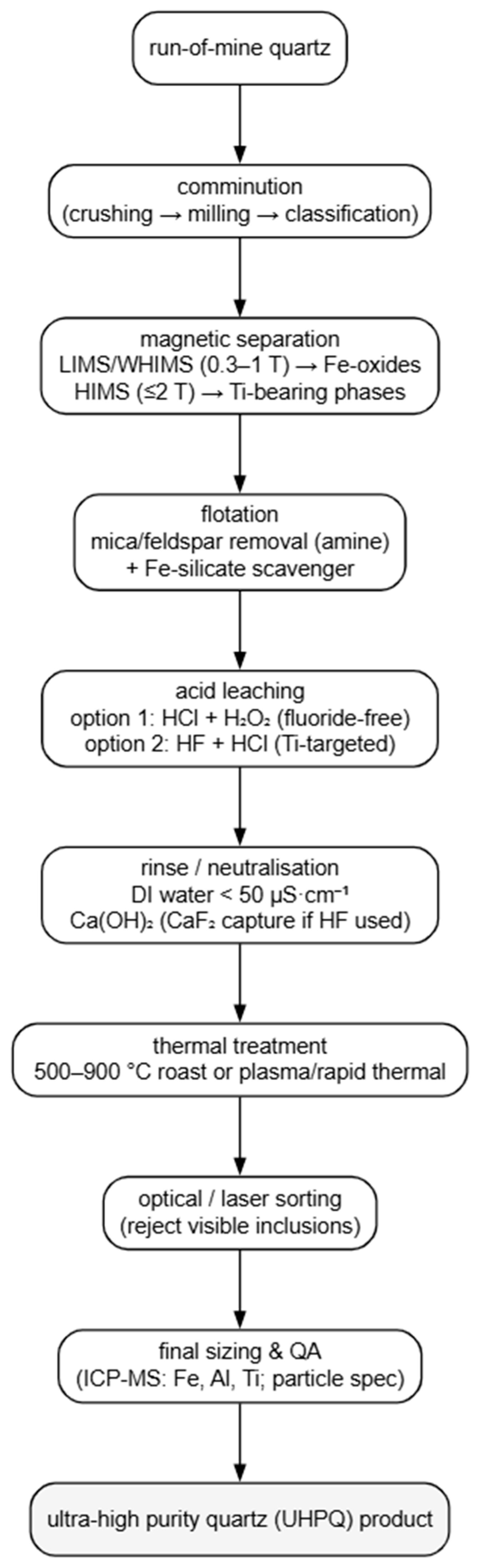
4.2. The Main Purification Process of Quartz Sand
4.2.1. Deposit-Specific Suitability of Purification Methods
4.2.2. Waste Management
4.2.3. Sustainability Impacts
- Mining and comminution—Energy-intensive blasting and crushing represent up to 40%–50% of total energy use in mineral processing LCAs [131]. Dust and land disturbance contribute to ecosystem impacts.
- Acid leaching—Hydrometallurgical purification contributes most to water consumption and chemical waste, especially with HF- and HCl-based systems. LCAs of comparable leaching processes report acid effluent volumes of 1–2 m3 per ton of concentrate, highlighting the importance of recycling circuits [133].
- Thermal and high-temperature processing—High-temperature refining (>1800 °C) is the dominant contributor to carbon footprint, with electricity consumption translating into 3–5 t CO2-eq per ton of ultrapure quartz depending on the energy mix [134,135]. Plasma purification, while more selective, may increase electricity use but reduce chemical waste.
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, M.; Yang, X.; Hou, Z. Preparation of High-Purity Quartz Sand by Vein Quartz Purification and Characteristics of Products. Minerals 2024, 14, 727. [Google Scholar] [CrossRef]
- Aulich, H.A.; Römer, U.; Lange, R.; Heymer, H. Preparation of High Purity Starting Materials for the Production of Solar Grade Silicon. Siemens Forsch.-Entwicklungsber 1982, 11, 327–331. [Google Scholar]
- Long, H.; Zhu, D.; Pan, J.; Li, S.; Yang, C.; Guo, Z. Advanced Processing Techniques and Impurity Management for High-Purity Quartz in Diverse Industrial Applications. Minerals 2024, 14, 571. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, W.G.; Shim, T.H.; Park, J.G. Fumed Silica-Based Ultra High Purity Synthetic Quartz Powder via Sol–Gel Process for Advanced Semiconductor Process Beyond Design Rule of 3 nm. Nanomaterials 2023, 13, 390. [Google Scholar] [CrossRef]
- Di Sabatino, M.; Hendawi, R.; Garcia, A. Silicon Solar Cells: Trends, Manufacturing Challenges, and AI Perspectives. Crystals 2024, 14, 167. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, N. Study on the Preparation of High-Quality Quartz and Its Mechanism by Combining Pretreatment with Metallurgy. Minerals 2024, 14, 1229. [Google Scholar] [CrossRef]
- Zhang, H.; Chiao, M. Anti-fouling Coatings of Poly(dimethylsiloxane) Devices for Biological and Biomedical Applications. J. Med. Biol. Eng. 2015, 35, 143–155. [Google Scholar] [CrossRef]
- Kareem, A.; Rajan, T.V.; Kumar, A.; Kumar, R.; Singh, A. A Review on AA 6061 Metal Matrix Composites Produced by Stir Casting. Materials 2021, 14, 175. [Google Scholar] [CrossRef]
- Manjunath, R.; Kumar, D.; Kumar, A. A Review on the Significance of Hybrid Particulate Reinforcements on the Mechanical and Tribological Properties of Stir-Casted Aluminum Metal Matrix Composites. J. Bio-Tribo-Corros. 2021, 7, 122. [Google Scholar] [CrossRef]
- Raja, R.; Siva, I.; Balan, K.P.; Ramesh, M.; Kumar, A.M.; Saravanakumar, K. Development of Al-Mg2Si Alloy Hybrid Surface Composites by Friction Stir Processing: Mechanical, Wear, and Microstructure Evaluation. Materials 2023, 16, 4131. [Google Scholar] [CrossRef]
- Sahu, S.; Behera, B.K.; Rout, M.; Mishra, P.C. Mechanical and Tribological Behavior of LM26/SiC/Ni-Gr Hybrid Metal Matrix Composites. J. Compos. Sci. 2023, 7, 159. [Google Scholar] [CrossRef]
- Toli, A.; Tsaousi, G.M.; Balomenos, E.; Panias, D.; Heuer, M.; Philipson, H.; Tranell, G. Sustainable Silicon and High Purity Alumina Production from Secondary Silicon and Aluminium Raw Materials through the Innovative SisAl Technology. Mater. Proc. 2021, 5, 85. [Google Scholar] [CrossRef]
- Li, X.; Li, T.; Gao, J.; Huang, H.; Li, L.; Li, J. A Novel “Green” Solvent to Deeply Purify Quartz Sand with High Yields: A Case Study. J. Ind. Eng. Chem. 2016, 35, 383–387. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Ma, T.; Zhu, D.; Dong, D.; Yang, X.; Liu, L.; Wang, G. Evolution of Primary and Eutectic Si Phase and Mechanical Properties of Al2O3/Al-20Si Composites under High Pressure. Crystals 2021, 11, 364. [Google Scholar] [CrossRef]
- Lewis, P.; Tarrant, A.; Frehn, A.; Grensing, F.; Nicholson, J.; Farrah, N.; Acreman, M. Aluminium–Silicon Lightweight Thermal Management Alloys with Controlled Thermal Expansion. Crystals 2024, 14, 455. [Google Scholar] [CrossRef]
- Dash, S.S.; Chen, D. A Review on Processing–Microstructure–Property Relationships of Al–Si Alloys: Recent Advances in Deformation Behavior. Metals 2023, 13, 609. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials Act. European Commission. 2023. Available online: https://en.wikipedia.org/wiki/Critical_Raw_Materials_Act (accessed on 10 October 2025).
- European Commission. Report on the List of Critical Raw Materials for the EU. European Commission. 2017. Available online: https://ec.europa.eu/docsroom/documents/25421/attachments/1/translations/en/renditions/native2017 (accessed on 10 October 2025).
- European Commission. Europe 2020: A Strategy for Smart, Sustainable and Inclusive Growth. COM (2010) 2020 Final. 2010. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2010:2020:FIN:en:PDF (accessed on 10 October 2025).
- European Commission. Critical Raw Materials: Resilience of Critical Raw Materials in the EU. European Commission. 2023. Available online: https://single-market-economy.ec.europa.eu/sectors/raw-materials/areas-specific-interest/critical-raw-materials_en (accessed on 10 October 2025).
- Müller, A.; Wanvik, J.E.; Ihlen, P. Quartz: Deposits, Mineralogy and Analytics. In Quartz: Deposits, Mineralogy and Analytics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 69–112. [Google Scholar] [CrossRef]
- Wang, H.; Gao, H.; Zhang, X.-Y.; Yan, Q.-H.; Xu, Y.; Zhou, K.; Dong, R.; Li, P. Geology and geochronology of the super-large Bailongshan Li–Rb–(Be) rare-metal pegmatite deposit, West Kunlun orogenic belt, NW China. Lithos 2020, 360, 105449. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Chen, H.; Liu, X.; Ma, S. Purification of Quartz via Low-Temperature Microwave Chlorinated Calcination Combined with Acid Leaching and Its Mechanism. Silicon 2023, 15, 971–981. [Google Scholar] [CrossRef]
- Götze, J.; Pan, Y.; Müller, A. Mineralogy and Mineral Chemistry of Quartz: A Review. Mineral. Mag. 2021, 85, 639–664. [Google Scholar] [CrossRef]
- Zhan, L.; Wang, Q.; Ku, J.; Shang, H.; Shen, Z. Purification Technologies for High-Purity Quartz: From Mineralogy to Applications. Sep. Purif. Rev. 2025, 1–18. [Google Scholar] [CrossRef]
- Götze, J. Chemistry, Textures and Physical Properties of Quartz—Geological Interpretation and Technical Application. Miner. Mag. 2009, 73, 645–671. [Google Scholar] [CrossRef]
- Gui, Y.; Chen, X.; Zhu, X.; Fu, H.; Jiang, X.; Sun, J.; Wang, L.; Ban, B.; Chen, J. Quartz Lattice Al Impurity Removal: A Simple Annealing−Leaching Process and Analysis. Materials Letters 2025, 373, 138478. [Google Scholar] [CrossRef]
- Larsen, R.B.; Polve, M.; Juve, G. Granite pegmatite quartz from Evje-Iveland: Trace element chemistry and implications for the formation of high-purity quartz. Nor. Geol. Unders. 2000, 436, 57–66. [Google Scholar]
- Müller, A.; Ihlen, P.M.; Wanvik, J.E.; Flem, B. High-purity quartz mineralisation in kyanite quartzites, Norway. Mineral. Depos. 2007, 42, 523–535. [Google Scholar] [CrossRef]
- Wang, S.; Yu, D.; Ma, C.; Wei, F.; Zhang, H. A New Insight into the Influence of Fluid Inclusions in High-Purity Quartz Sand on the Bubble Defects in Quartz Glass: A Case Study from Vein Quartz in the Dabie Mountain. Minerals 2024, 14, 794. [Google Scholar] [CrossRef]
- ReportLinker. Global Quartz Market Dataset; Report ID: ea738bfb24294521e6074f7cba4a72ffe1e14bcd; ReportLinker. 2024. Available online: https://www.reportlinker.com/dataset/ea738bfb24294521e6074f7cba4a72ffe1e14bcd31 (accessed on 10 October 2025).
- Mordor Intelligence. Asia-Pacific Silica Sand Market—Growth, Trends, COVID-19 Impact, and Forecasts (2025–2030). Mordor Intelligence. 2024. Available online: https://www.mordorintelligence.com/industry-reports/asia-pacific-silica-sand-market (accessed on 10 October 2025).
- Faure, M.; Ferrière, J. Reconstructing the Variscan Terranes in the Alpine Basement: Facts and Arguments for an Alpidic Orocline. Geosciences 2022, 12, 65. [Google Scholar] [CrossRef]
- Gupta, A.; Yan, D.S. Mineral Processing Design and Operation: An Introduction; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Nie, H.; Liu, Q.; Li, P.; Li, P.; Ding, J.; Sun, C.; Zhai, C.; Zhao, J.; Jin, Z.; Dang, W. Quartz Types, Formation Mechanism, and Its Effect on Shale Oil and Gas Enrichment: A Review. Earth-Sci. Rev. 2025, 261, 105011. [Google Scholar] [CrossRef]
- VRX Silica. Silica Sand Processing Plant Design and Costs. Investor Insight. 27 March 2019. Available online: https://investorinsight.com.au/silica-sand-processing-plant-design-and-costs-vrx-silica/ (accessed on 10 October 2025).
- Welche, P.; Heine, V.; Dove, M. Negative Thermal Expansion in Beta-Quartz. Phys. Chem. Miner. 1998, 26, 63–77. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2025: Quartz (High-Purity and Industrial Cultured Crystal); U.S. Geological Survey: Reston, VA, USA, 2025. [CrossRef]
- Simcoa. History and Silicon Production. Simcoa. 2023. Available online: https://www.simcoa.com.au/history-silicon (accessed on 10 October 2025).
- Harben, P.W. The Industrial Mineral Hand Book—A Guide to Markets, Specifications and Prices, 4th ed.; Industrial Mineral Information: London, UK, 2002; 412p, ISBN 978-1900663519. [Google Scholar]
- Korkmaz, A.; Demir, I.; Özçelik, H. A Life-Cycle Assessment of Silica Sand: Comparing Electrostatic, Flotation, and Gravity Concentration. Sustainability 2016, 8, 11. [Google Scholar] [CrossRef]
- The Quartz Corp. High Purity Quartz. The Quartz Corp. 2023. Available online: https://www.thequartzcorp.com/high-purity-quartz (accessed on 10 October 2025).
- Lin, M.; Liu, Z.; Wei, Y.; Meng, Y.; Qiu, H.; Lei, S. A Critical Review on the Mineralogy and Processing for High-Grade Quartz. Min. Metall. Explor. 2020, 37, 1627–1639. [Google Scholar] [CrossRef]
- MarketsandMarkets. High Purity Quartz Market by Application: Semiconductor, Solar, Optics, Specialty Glass; MarketsandMarkets Research: Pune, India, 2023; Available online: https://www.marketsandmarkets.com (accessed on 10 October 2025).
- Götze, J.; Möckel, R. (Eds.) Quartz: Deposits, Mineralogy and Analytics; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Cognitive Market Research. Global High Purity Quartz Market Analysis (2025–2033); Cognitive Market Research: Pune, India, 2025; Available online: https://www.cognitivemarketresearch.com/high-purity-quartz-market-report? (accessed on 10 October 2025).
- Pishahang, M.; Kelsall, C.C.; Henry, A. Purification of Metallurgical-Grade Silicon up to Solar Grade. Semicond. Sci. Technol. 2015, 30, 104024. [Google Scholar] [CrossRef]
- Safarian, J.; Tranell, G.; Tangstad, M. Processes for Upgrading Metallurgical-Grade Silicon to Solar Grade Silicon. Energy Procedia 2012, 20, 88–97. [Google Scholar] [CrossRef]
- Bons, P.D.; Elburg, M.A.; Gomez-Rivas, E. A Review of the Formation of Tectonic Veins and Their Microstructures. J. Struct. Geol. 2012, 43, 33–62. [Google Scholar] [CrossRef]
- Franz, L.; Romer, R.L.; Neubauer, F. Polyphase Metamorphism in the Bohemian Massif: Petrology, Geochronology and Implications for Crustal Evolution. Int. J. Earth Sci. 1996, 85, 683–703. [Google Scholar] [CrossRef]
- Vatalis, K.I.; Charalambides, G.; Benetis, N.P. Market of high-purity quartz innovative applications. Procedia Econ. Financ. 2015, 24, 734–742. [Google Scholar] [CrossRef]
- Ali, A.; Khan, M.S.; Hussain, S.; Ahmad, N.; Nawaz, M.; Hussain, I. Research on 4N8 High-Purity Quartz Purification Technology from Hunza District, Pakistan. Minerals 2023, 14, 1049. [Google Scholar] [CrossRef]
- Shaukat, H.; Tariq, M.; Khan, S.A.; Nazir, S.; Ahmad, M. A Review of the Recent Advances in Piezoelectric Materials, Energy Harvester Structures, and Their Applications in Analytical Chemistry. Appl. Sci. 2023, 13, 1300. [Google Scholar] [CrossRef]
- Shen, J.; Li, X.; Zhao, W.; Wang, Y.; Guo, Q. The Force–Frequency Characteristics of Quartz Wafers under a Cantilever Beam Structure. Sensors 2024, 24, 3359. [Google Scholar] [CrossRef]
- Bai, J.; Chen, B.; Liao, S.; Wang, X.; Ye, X.; Xiao, Y. Optimized Scheduling Model Considering the Demand Response and Sequential Requirements of Polysilicon Production. Energies 2024, 17, 6048. [Google Scholar] [CrossRef]
- Sibelco. Sibelco to Double High Purity Quartz Capacity at Spruce Pine; Sibelco Newsroom: Antwerp, Belgium, 2023. [Google Scholar]
- PV-Tech. China Quartz Crucible Raw Material Prices Collapse in 2023. PV-Tech News 2023. Available online: https://www.pv-tech.org (accessed on 10 October 2025).
- Jiangsu Pacific Quartz. Expansion Plan for Semiconductor-Grade Quartz; Company Disclosure via Business Press; Jiangsu Pacific Quartz: Lianyungang, China, 2024. [Google Scholar]
- Mordor Intelligence. High Purity Quartz Market—Growth, Trends, Forecasts (2025–2030); Mordor Intelligence: Hyderabad, India, 2023; Available online: https://www.mordorintelligence.com (accessed on 10 October 2025).
- Plissart, G.; Diot, H.; Monnier, C.; Mărunţiu, M. New insights into the building of the Variscan Belt in eastern Europe (Romania, Serbia, Bulgaria). Geol. Soc. Lond. Spec. Publ. 2019, 478, 389–426. [Google Scholar] [CrossRef]
- Topuz, G. Variscan orogeny in the Black Sea region. Int. J. Earth Sci. 2017, 106, 569–592. [Google Scholar]
- Azevedo, M.R.; Valle Aguado, B.; Nolan, J.; Martins, M.; Medina, J. Origin and emplacement of syn-orogenic Variscan granitoids in Iberia the Beiras massif. South. Variscan Belt J. Virtual Explor. 2005, 19, 7. [Google Scholar] [CrossRef]
- Bellido, F.; Monteserín, V.; Gumiel, P.; Ferrero, A.; Baltuille, J.M.; López, M.T. Características petrológicas y geoquími-cas de las principales variedades de granitos ornamentales del macizo de “O Porriño” (SO de Galicia). Bol. Geol. Min. 2005, 116, 331–349. [Google Scholar]
- Chappell, B.W.; Hine, R. The Cornubian Batholith: An example of magmatic fractionation on a crustal scale. Resour. Geol. 2006, 56, 203–244. [Google Scholar] [CrossRef]
- Putzolu, F.; Seltmann, R.; Dolgopolova, A.; Armstrong, R.N.; Shail, R.K.; Spratt, J.; Buret, Y.; Broderick, C.; Brownscombe, W. Influence of magmatic and magmatic-hydrothermal processes on the lithium endowment of micas in the Cornubian Batholith (SW England). Mineral. Depos. 2024, 59, 1067–1088. [Google Scholar] [CrossRef]
- Marcoux, É.; Barré, B.; Pichavant, M.; Poujol, M. Âge et genèse de la coupole granitique à métaux rares (Sn, Li, Nb-Ta, W) de Montebras (Creuse, Massif central français). BSGF-Earth Sci. Bull. 2021, 192, 16. [Google Scholar] [CrossRef]
- Franceschelli, M.; Puxeddu, M.; Cruciani, G. Variscan metamorphism in Sardinia, Italy: Review and discussion. J. Virtual Explor. 2005, 19, 2–36. [Google Scholar] [CrossRef]
- Breiter, K.; Ďurišová, J.; Dosbaba, M. Quartz chemistry–A step to understanding magmatic-hydrothermal processes in ore-bearing granites: Cínovec/Zinnwald Sn-W-Li deposit, Central Europe. Ore Geol. Rev. 2017, 90, 25–35. [Google Scholar] [CrossRef]
- Spear, F.S.; Wark, D.A. Cathodoluminescence imaging and titanium thermometry in metamorphic quartz. J. Metamorph. Geol. 2009, 27, 187–205. [Google Scholar] [CrossRef]
- Müller, A. Cathodoluminescence and Characterisation of Defect Structures in Quartz with Applications to the Study of Granitic Rocks; Geosciences Dissertation Series, No. 2438; Universität Göttingen: Göttingen, Germany, 2000. [Google Scholar] [CrossRef]
- Černý, P. Rare-element granitic pegmatites. Part I: Anatomy and internal evolution of pegmatite deposits. Geosci. Can. 1991, 18, 49–67. [Google Scholar]
- Roda-Robles, E.; Gil-Crespo, P.P.; Pesquera, A.; Lima, A.; Garate-Olave, I.; Merino-Martínez, E.; Cardoso-Fernandes, J.; Errandonea-Martin, J. Compositional variations in apatite and petrogenetic significance: Examples from peraluminous granites and related pegmatites and hydrothermal veins from the Central Iberian Zone (Spain and Portugal). Minerals 2022, 12, 1401. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Pesquera, A.; Gil-Crespo, P.P.; Vieira, R.; Lima, A.; Garate-Olave, I.; Martins, T.; Torres-Ruiz, J. Geology and mineralogy of Li mineralization in the Central Iberian Zone (Spain and Portugal). Mineral. Mag. 2016, 80, 103–126. [Google Scholar] [CrossRef]
- Martins, T.; Roda-Robles, E.; Lima, A.; de Parseval, P. Geochemistry and evolution of micas in the Barroso–Alvão peg-matite field, Northern Portugal. Can. Mineral. 2012, 50, 1117–1129. [Google Scholar]
- Roda Robles, E.; Pesquera Perez, A.; Velasco Roldan, F.; Fontan, F. The granitic pegmatites of the Fregeneda area (Salamanca, Spain): Characteristics and petrogenesis. Mineral. Mag. 1990, 63, 535–558. [Google Scholar] [CrossRef]
- Barros, R.; Kaeter, D.; Menuge, J.F.; Škoda, R. Controls on chemical evolution and rare element enrichment in crystal-lising albite-spodumene pegmatite and wallrocks: Constraints from mineral chemistry. Lithos 2021, 352, 105289. [Google Scholar]
- Dill, H.G. Pegmatitic Rocks and Economic Geology. In The Hagendorf-Pleystein Province: The Center of Pegmatites in an En-Sialic Orogen; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–54. [Google Scholar]
- Müller, A.; Roda Robles, M.; Romer, R.L.; Augland, L.E.; Zhou, H.; Rosing Schow, N.; Spratt, J.; Husdal, T. Two stage re-gional rare element pegmatite formation at Tysfjord, Norway: Implications for the timing of late Svecofennian and late Caledonian high temperature events. Int. J. Earth Sci. 2022, 111, 987–1007. [Google Scholar] [CrossRef]
- Husdal, T.; Müller, A.; Olerud, S.; Thoresen, Ø. Pegmatites of the Tysfjord–Hamarøy Area, Northern Norway. In Norwegian Pegmatites I: Tysfjord–Hamarøy, Evje–Iveland, Langesundsfjord; (NGF Geological Guides, Vol. 6); 2017; pp. 3–47. ISBN 978-82-8347-020-8. Available online: http://geologi.no/images/GeologiskeGuider/PEG2017_Excursion_Guide_NGF_Series_2017-6_red.pdf (accessed on 10 October 2025).
- Segalstad, T.V.; Eggleston, T.L. Pegmatittene i Tørdal, Telemark. Tidsskr. Norges Geol. Undersøk. 1993, 20, 190–195. [Google Scholar]
- Lin, M.; Pei, Z.; Lei, S. Mineralogy and Processing of Hydrothermal Vein Quartz from Hengche, Hubei Province (China). Minerals 2017, 7, 161. [Google Scholar] [CrossRef]
- Filipe, A.; de Oliveira, D.P. Tungsten: An historical perspective of this critical raw material in Portugal. Geonovas 2017, 30, 51–66. [Google Scholar]
- Launay, G.; Branquet, Y.; Sizaret, S.; Guillou Frottier, L.; Gloaguen, E. How Greisenization Could Trigger the Formation of Large Vein and Greisen Sn–W Deposits: A Numerical Investigation Applied to the Panasqueira Deposit. Ore Geol. Rev. 2023, 153, 105299. [Google Scholar] [CrossRef]
- Werner, A.B.T.; Sinclair, W.D.; Amey, E.B. International Strategic Mineral Issues Summary Report—Tungsten; U.S. Geo-logical Survey Circular 930-0; U.S. Government Printing Office: Washington, DC, USA, 1998.
- Cheilletz, A.; Pelleter, E.; Martin-Izard, A.; Tornos, F. World skarn deposits: Skarns of Western Europe. In Economic Geology 100th Anniversary Volume; Society of Economic Geologists, Inc.: Littleton, CO, USA, 2005; pp. 1–10. [Google Scholar]
- Tornos, F.; Galindo, C.; Crespo, J.L.; Spiro, B.F. Geochemistry and origin of calcic tungsten-bearing skarns, Los Santos, Central Iberian Zone, Spain Can. Mineral. 2008, 46, 87–109. [Google Scholar]
- Zachariáš, J.; Morávek, P.; Gadas, P.; Pertoldová, J. The Mokrsko-West gold deposit, Bohemian Massif, Czech Republic: Mineralogy, deposit setting and classification. Ore Geol. Rev. 2014, 58, 238–263. [Google Scholar] [CrossRef]
- Grancea, L.; Bailly, L.; Leroy, J.; Banks, D.; Marcoux, E.; Milési, J.; Cuney, M.; André, A.S.; Istvan, D.; Fabre, C. Fluid evo-lution in the Baia Mare epithermal gold/polymetallic district, Inner Carpathians, Romania. Mineral. Depos. 2002, 37, 630–647. [Google Scholar] [CrossRef]
- Vlasáč, J.; Mikuš, T.; Majzlan, J.; Števko, M.; Tuček, P. Bonanza-type epithermal Ag-Au mineralisation in Nová Baňa (Western Carpathians, Slovakia). Ore Geol. Rev. 2025, 178, 106493. [Google Scholar] [CrossRef]
- Koděra, P.; Jaroslav, L.; Fallick, A.E.; Wälle, M.; Biroň, A. Hydrothermal fluids in epithermal and porphyry Au deposits in the Central Slovakia Volcanic Field. Geol. Soc. Lond. Spec. Publ. 2014, 402, 177–206. [Google Scholar] [CrossRef]
- Pierfranco, L. Epithermal Precious Metal Deposits of Italy—An Overview. Miner. Depos. 1999, 34, 373–385. [Google Scholar] [CrossRef]
- Arribas, A.; Cunningham, C.G.; Rytuba, J.J.; Rye, R.O.; Kelly, W.C.; Podwysocki, M.H.; McKee, E.H.; Tosdal, R.M. Ge-ology, Geochronology, Fluid Inclusions, and Isotope Geochemistry of the Rodalquilar Gold Alunite Deposit, Spain. Econ. Geol. 1995, 90, 795–822. [Google Scholar] [CrossRef]
- Marcoux, E.; Le Berre, P.; Cocherie, A. The Meillers Autunian hydrothermal chalcedony: First evidence of a ~295 Ma au-riferous epithermal sinter in the French Massif Central. Ore Geol. Rev. 2014, 25, 69–87. [Google Scholar] [CrossRef]
- Hallberg, A. The Enåsen gold deposit, central Sweden: 1. A Palaeoproterozoic high-sulphidation epithermal gold min-eralization. Mineral. Depos. 1994, 29, 150–162. [Google Scholar] [CrossRef]
- Mohanty, K.; Oliva, J.; Alfonso, P.; Sampaio, C.H.; Anticoi, H. A Comparative Study of Quartz and Potassium Feldspar Flotation Process Using Different Chemical Reagents. Minerals 2024, 14, 167. [Google Scholar] [CrossRef]
- Götze, J. Application of Cathodoluminescence (CL) Microscopy and Spectroscopy in Geosciences. Minerals 2012, 2, 365–397. [Google Scholar] [CrossRef]
- Wagner, T.; Boyce, A.J.; Erzinger, J. Fluid-rock interaction during formation of metamorphic quartz veins: A REE and stable isotope study from the Rhenish Massif, Germany. Am. J. Sci. 2010, 310, 977–1023. [Google Scholar] [CrossRef]
- Hartenfels, S.; Hartkopf-Fröder, C.; Königshof, P. The Rhenish Massif: More than 150 years of research in a Variscan mountain chain. Palaeobiodivers. Palaeoenviron. 2022, 102, 493–502. [Google Scholar] [CrossRef]
- Müller, A.; Wanvik, J.E.; Ihlen, P.M. Petrological and Chemical Characterisation of High Purity Quartz Deposits with Examples from Norway. Mineral. Depos. 2007, 42, 523–532. [Google Scholar] [CrossRef]
- Dapiaggi, M.; Pagliari, L.; Pavese, A.; Sciascia, L.; Francescon, F.; Merli, M. The formation of silica high-temperature polymorphs from quartz: Influence of grain size and mineralising agents. J. Eur. Ceram. Soc. 2015, 35, 4547–4555. [Google Scholar] [CrossRef]
- Capek, P.; Vesely, M.; Svoboda, M.; Remzova, M.; Zouzelka, R.; Kocirik, M.; Brabec, L.; Rathousky, J. Bohemian Sandstone for Restoration of Cultural Heritage Sites: 3D Microstructure and Mass Transport Properties. Herit. Sci. 2023, 11, 36. [Google Scholar] [CrossRef]
- Klein, R.; Pesonen, L.J.; Salminen, J.; Mertanen, S. Paleomagnetism of Mesoproterozoic Satakunta sandstone, Western Finland. Precambrian Res. 2014, 244, 156–169. [Google Scholar] [CrossRef]
- Woronko, B.; Zieliński, P.; Sokołowski, R.J. Climate evolution during the Pleniglacial and Late Glacial as recorded in quartz grain morphoscopy of fluvial to aeolian successions of the European Sand Belt. Geologos 2015, 21, 89–103. [Google Scholar] [CrossRef]
- Van der Meulen, M.J.; Westerhoff, W.E.; Menkovic, A.; Gruijters, S.H.L.L.; Dubelaar, C.W.; Maljers, D. Silica Sand Resources in the Netherlands. Neth. J. Geosci. 2009, 88, 147–160. [Google Scholar] [CrossRef]
- Koster, E.A. The European Aeolian Sand Belt: Geoconservation of Drift Sand Landscapes. Geoheritage 2009, 1, 93–110. [Google Scholar] [CrossRef]
- Bašistová, L.; Vontorová, J.; Zlá, S.; Kawuloková, M.; Lichý, P.; Dvorský, T. Variability in the Distinctive Features of Silica Sands in Central Europe. Buildings 2024, 14, 279. [Google Scholar] [CrossRef]
- Kenig, K. Depositional environments of loesses from the Sandomierz section, SE Poland: Lithological and SEM quartz grain analysis. Geol. Q. 2022, 68, 321–338. [Google Scholar]
- Tan, D.; Ma, Y.; Li, Z.; Jin, J.; Liu, R.; Zou, X.; Tao, T. Characteristics of Quartz Grains in the Middle and Lower Reaches of the Hutubi River, NW China, and Its Paleo-Environmental Significance. Sediment. Geol. 2023, 441, 106551. [Google Scholar] [CrossRef]
- Moral Cardona, J.P.; Gutiérrez Mas, J.M.; Sánchez Bellón, Á.; Domínguez-Bella, S.; Martínez López, J. Surface Textures of Heavy-Mineral Grains: A New Contribution to Provenance Studies. Sediment. Geol. 2005, 174, 223–235. [Google Scholar] [CrossRef]
- Müller, H.; Ihlen, P.M.; Larsen, R.B.; Bjerkgård, T. Geological and Mineralogical Characterization of the Evje–Iveland Pegmatite Field, Southern Norway. In Rare Element Geochemistry and Mineral Deposits; Springer: Cham, Switzerland, 2018; pp. 205–227. [Google Scholar] [CrossRef]
- The Quartz Corp. High Purity Quartz from Spruce Pine (USA) and Drag (Norway). Company Website. Available online: https://www.thequartzcorp.com (accessed on 10 October 2025).
- Marignac, C.; Cuney, M.; Cathelineau, M.; Lecomte, A.; Carocci, E.; Pinto, F. The Panasqueira Rare Metal Granite Suites and Their Involvement in the Genesis of the World-Class Panasqueira W–Sn–Cu Vein Deposit: A Petrographic, Mineralogical, and Geochemical Study. Minerals 2020, 10, 562. [Google Scholar] [CrossRef]
- Launay, G.; Sizaret, S.; Lach, P.; Melleton, J.; Guillou-Frottier, L.; Gloaguen, E. Genetic Relationship between Greisenization and Sn–W Mineralization in Vein and Greisen Deposits: Insights from the Panasqueira Deposit (Portugal). Bull. Soc. Géol. Fr. 2021, 192, 2. [Google Scholar] [CrossRef]
- London, D. Pegmatites; Mineralogical Society of America: Chantilly, VA, USA, 2008. [Google Scholar]
- Larsen, E.; Kleiv, R.A. Flotation of Quartz from Quartz–Feldspar Mixtures by the HF Method. Miner. Eng. 2016, 98, 44–51. [Google Scholar] [CrossRef]
- Jennings, A.; Senior, A.; Guerin, K.; Main, P.; Walsh, J. A Review of High-Purity Quartz for Silicon Production in Australia. Aust. J. Earth Sci. 2024, 71, 1085–1097. [Google Scholar] [CrossRef]
- Müller, A.; Ihlen, P.M.; Snook, B.; Larsen, R.B.; Flem, B.; Bingen, B.; Williamson, B.J. The Chemistry of Quartz in Granitic Pegmatites of Southern Norway: Petrogenetic and Economic Implications. Econ. Geol. 2015, 110, 1737–1757. [Google Scholar] [CrossRef]
- London, D. Pegmatites. Can. Mineral. Spec. Publ. 2008, 10, 347–400. [Google Scholar]
- Gonçalves, J.P.; Fernández, J.F.; Tello, M.J.; Ribeiro, M.J. Comminution and Purification of Quartz for Advanced Applications. Appl. Geochem. 2019, 102, 56–67. [Google Scholar] [CrossRef]
- Janelidze, I.; Jandieri, G.; Tsertsvadze, T. Thermodynamic Analysis of Interaction of Components in the SiO2–C System: Improvement of Technical Silicon Production Technological Process. Phys. Chem. Solid State 2021, 22, 345–352. [Google Scholar] [CrossRef]
- Li, F.; Jiang, X.; Zuo, Q.; Li, J.; Ban, B.; Chen, J. Purification Mechanism of Quartz Sand by Combination of Microwave Heating and Ultrasound Assisted Acid Leaching Treatment. Silicon 2020, 13, 531–541. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Y.; Zhang, Y.; Liu, W.; Li, Y.; Deng, H. Plasma-Based Isotropic Etching Polishing of Synthetic Quartz. J. Mater. Process. Technol. 2021, 289, 116984. [Google Scholar] [CrossRef]
- Habashi, F. Principles of Extractive Metallurgy, Volume 2: Hydrometallurgy; Gordon & Breach: New York, NY, USA, 1980. [Google Scholar]
- Fuerstenau, D.W. (Ed.) Froth Flotation 50th Anniversary Volume; Society of Mining Engineers of AIME, Minerals Beneficiation Division: New York, NY, USA, 1962; 677p. [Google Scholar]
- Kossoff, D.; Dubbin, W.E.; Alfredsson, M.; Edwards, S.J.; Macklin, M.G.; Hudson-Edwards, K.A. Mine Tailings Dams: Characteristics, Failure, Environmental Impacts, and Remediation. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef]
- Ribeiro, A.B.; Mateus, E.P.; Ottosen, L.M.; Bech, J. Electrokinetic and Thickening Technologies for Sustainable Mine Tailings Management. J. Clean. Prod. 2020, 261, 121129. [Google Scholar] [CrossRef]
- Zulu, E.; Ramasamy, S.; Sikhwivhilu, K.K.; Syampungani, S. A Comprehensive Review of Recent Advances in Membrane Innovations for Efficient Heavy Metal Removal from Mine Effluents. Sci. Afr. 2024, 18, e02510. [Google Scholar] [CrossRef]
- European Commission. The European Green Deal; European Union: Brussels, Belgium, 2020. [Google Scholar]
- Kinnunen, P.; Obenaus-Emler, R.; Raatikainen, J.; Guignot, S.; Guimerà, J.; Ciroth, A.; Heiskanen, K. Review of Closed Water Loops with Ore Sorting and Tailings Valorization for a More Sustainable Mining Industry. J. Clean. Prod. 2021, 288, 125633. [Google Scholar] [CrossRef]
- Jeswiet, J.; Szekeres, A. Energy Consumption in Mining Comminution. Procedia CIRP 2016, 48, 140–145. [Google Scholar] [CrossRef]
- Ballantyne, G.R.; Powell, M.S. Benchmarking Comminution Energy Consumption for the Processing of Copper and Gold Ores. Miner. Eng. 2014, 65, 109–114. [Google Scholar] [CrossRef]
- Norgate, T.E.; Jahanshahi, S.; Rankin, W.J. Assessing the Environmental Impact of Metal Production Processes. J. Clean. Prod. 2007, 15, 838–848. [Google Scholar] [CrossRef]
- Jiao, L.; Huang, Y.; Zhang, Y.; Li, S.; Liu, Y.; Wei, G.; Wei, L. Preparation of High-Purity Quartz by Roasting–Water Quenching and Ultrasound-Assisted Acid Leaching Process. Minerals 2025, 15, 1028. [Google Scholar] [CrossRef]
- Rong, K.; Luo, D.; Deng, J.; Sun, S.; Song, S.; Jiang, B.; Yu, Z.; Zhao, K. An Environmentally Friendly Strategy for the Preparation of High-Purity Quartz Using Combined Collector Reverse Flotation Coupled with Acid-Leaching Technology. Miner. Eng. 2025, 211, 109274. [Google Scholar] [CrossRef]
- Khalifa, M.; Ouertani, R.; Hajji, M.; Ezzaouia, H. Innovative Technology for the Production of High-Purity Sand Silica by Thermal Treatment and Acid Leaching Process. Hydrometallurgy 2019, 185, 204–209. [Google Scholar] [CrossRef]

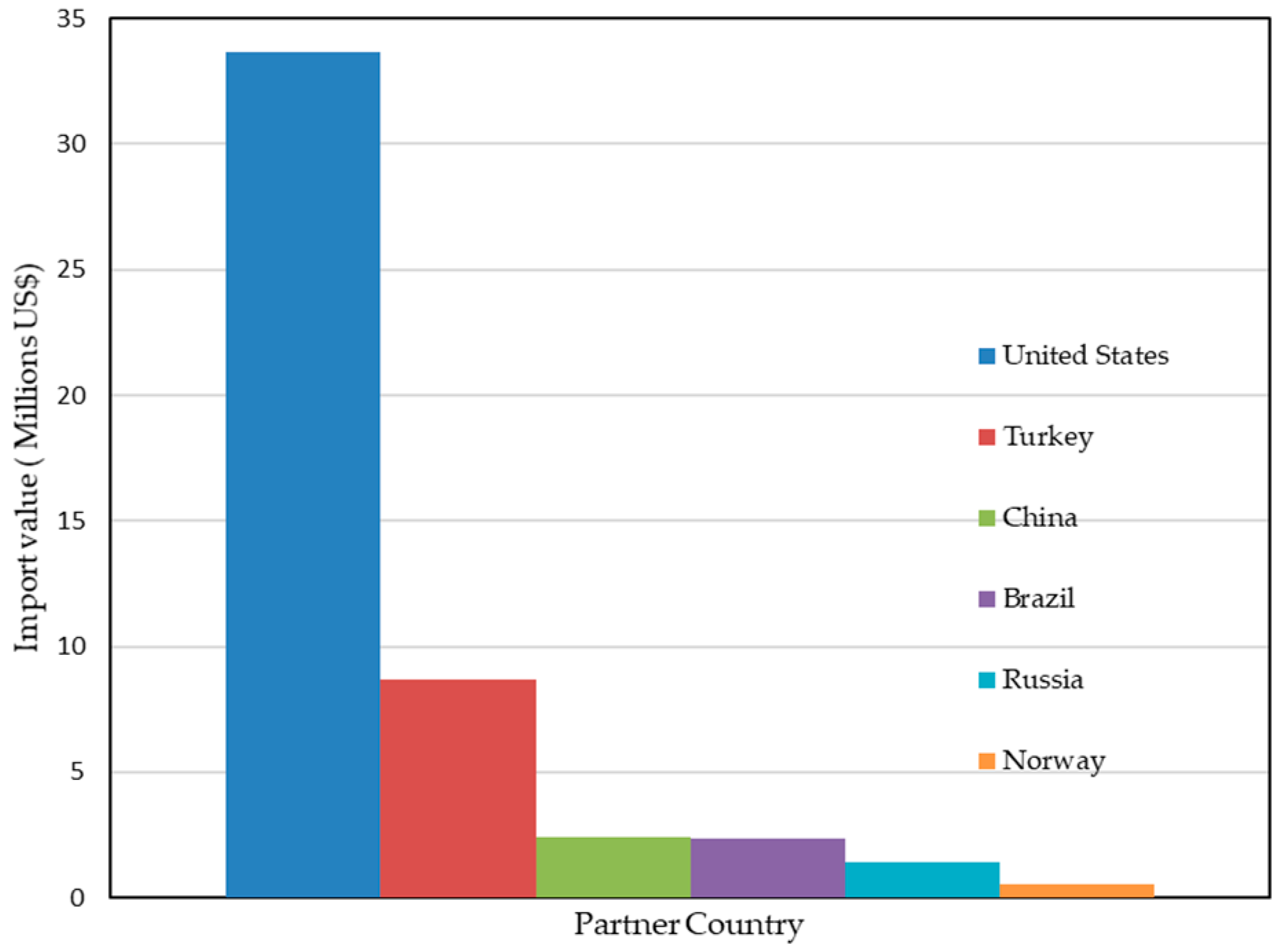

| Country | Main Companies | Capacity | EU Trade (2023, HS 250610) | Indicative Pricing (USD/t) | References |
|---|---|---|---|---|---|
| United States | Sibelco (Spruce Pine, NC, IOTA®); The Quartz Corp (Spruce Pine, NC) | Sibelco is investing ~USD 200 M to double capacity; TQC also operates in Drag, Norway | EU imports: USD 33.6 M (7153 t) | Ultrapure HPQ > 20,000; crucible-grade 4900–30,800 | [56,57,58] |
| Norway | The Quartz Corp (Drag) | Two refining plants; >USD 35 M upgrades | EU imports: USD 0.53 M (12,660 t) | Ultrapure 2800–5500 | [56] |
| China | Jiangsu Pacific Quartz (Donghai) | New project: 60,000 t/yr HPQ sand, 150,000 t/yr semiconductor-grade | EU imports: USD 2.4 M (1786 t) | Crucible sand: 1750–4900 (outer/inner layers) | [58,59] |
| Turkey | Multiple, Aydln | n/a (not ultrapure) | EU imports: USD 8.7 M (38,758 t) | Mostly medium-purity 300–600 | [56] |
| Russia | Kyshtym Mining, Kyshytm | n/a | EU imports: USD 1.4 M (221 t) | Medium-purity 400–800 | [56] |
| Brazil | Unimin/Sibelco JV, Jaguaruna | n/a | EU imports: USD 2.3 M (2322 t) | Medium-purity 300–600 | [56] |
| India | Gujarat Mineral Dev. Corp., Ahmedabad | ~8–10 kt est. | Limited to regional trade | 2000–3500 | [60] |
| Deposit (Country) | Purity (1–5) | Tonnage (1–5) | Processing Feasibility (1–5) | Total (Max 15) | Remarks |
|---|---|---|---|---|---|
| Evje–Iveland (Norway) | 4 | 3 | 5 | 12 | Low impurities; close to drag refining plants) [111]. |
| Panasqueira (Portugal) | 3 | 4 | 4 | 11 | By-product quartz; beneficiation potential demonstrated [113]. |
| Beauvoir (France) | 3 | 3 | 3 | 9 | Lithium-bearing granite; moderate impurities [28]. |
| Zinnwald (Germany) | 2 | 3 | 2 | 7 | Fine-grained greisen quartz; higher impurities [28]. |
| Spain (Galicia pegmatites) | 3 | 2 | 3 | 8 | Small pegmatite bodies [28]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanty, K.; Alfonso, P.; Oliva, J.; Sampaio, C.H.; Anticoi, H. Perspectives for High-Purity Quartz from European Resources. Minerals 2025, 15, 1080. https://doi.org/10.3390/min15101080
Mohanty K, Alfonso P, Oliva J, Sampaio CH, Anticoi H. Perspectives for High-Purity Quartz from European Resources. Minerals. 2025; 15(10):1080. https://doi.org/10.3390/min15101080
Chicago/Turabian StyleMohanty, Kalyani, Pura Alfonso, Josep Oliva, Carlos Hoffmann Sampaio, and Hernan Anticoi. 2025. "Perspectives for High-Purity Quartz from European Resources" Minerals 15, no. 10: 1080. https://doi.org/10.3390/min15101080
APA StyleMohanty, K., Alfonso, P., Oliva, J., Sampaio, C. H., & Anticoi, H. (2025). Perspectives for High-Purity Quartz from European Resources. Minerals, 15(10), 1080. https://doi.org/10.3390/min15101080