Abstract
Conditions of high-temperature volcano-related mineral formation are a source of the new and rare minerals and their associations; they are rather fragmentarily described for volcanic systems as a whole, except for several objects characterized in this regard. The study aim is to present the first results of the mineralogical study of atypical suprasubduction zone neoformation encountered from the Taketomi flank eruption (1933–1934) of the Alaid volcano (Kuril Islands), which has been studied through electron microprobe analyses and powder and single-crystal X-ray diffraction. The following mineral paragenesis is described: diopside, andradite, anorthite, wollastonite, esseneite, wadalite, rhönite-like mineral, fluorite, calcite, apatite, and atacamite. The parageneses of calcium silicates found in volcanic systems are usually interpreted as reworked crustal xenoliths and commonly associated with volcanoes that have a carbonate basement. However, carbonates have not been previously described at the base of the Alaid volcano. Even though the skarn nature of such a mineral paragenesis is possible, we suggest the important role of high-temperature volcanic gases along with the pyrometamorphic effect in the mineral-forming process at depth or in near-surface conditions (fumarole-like type in the form of a system of cracks and burrows). The described mineral paragenesis has not been previously documented, at least for the North Kuril Islands. A detailed mineralogical study of such formations is one of the important steps in understanding the functioning of magmatic systems, the circulation and transformation of natural matter, and mineral-forming processes.
Keywords:
volcano; basalt; Kuril; garnet; calcium; wollastonite; fluorite; andradite; clinopyroxene; chemical composition 1. Introduction
Volcanic systems are of interest to a wide range of specialists because they contain unique information about the formation of the Earth’s crust [1], the structure of the deep layers of the Earth [2], the processes of concentration and transformation of primary matter [3], ore formation [4,5], mineral formation at elevated temperatures [6,7,8,9], and gas transport of chemical elements [10] and because of their hazardous nature [11]. From a mineralogical perspective, occurring phases that reflect the specificity of a volcanic or post-volcanic process are of particular interest. In addition to rock-forming minerals, fumarole sublimates are significant. Furthermore, untypical mineral paragenesis may be formed as a result of the interaction of xenoliths with magmas (hornfelsing and pyrometamorphism) [12]. World-class mineralogical localities with dozens of new and rare minerals formed due to the interaction of xenolith with magma include the Verkhnechegemskaya or Upper Chegem volcanic caldera (Northern Caucasus Mountains, Russia), where the large carbonate xenoliths interacted with ignimbrites [13], and the Laacher See Volcanic Complex (Eifel, Germany), where tuffs interacted with xenoliths of quartz veins, mica schist, crystalline schist, metasomatites, subvolcanites, sanidinites, and tephrites [14]. Among volcanoes with fumarole mineralization, the absolute record holder for the number of new minerals is the Tolbachik volcano (Kamchatka, Russia) [15]. The most famous and well-studied objects with fumarole mineralization include Vulcano (Lipari Islands, Italy) [16], Vesuvius (southern Italy) [17,18,19], and some other [20]
This paper reports for the first time on the paragenesis of calcium minerals (silicates, phosphates, carbonates, and fluorides) found in unaltered basalts of Alaid volcano, which is part of the Kuril-Kamchatka volcanic belt, and their structural and chemical characteristics.
2. Geological Setting
The Kuril island arc system is located in the northwestern part of the Pacific Ocean and associated with the subduction of the oceanic plate beneath the continental margin. It is one of the world’s most active seismic and volcanic regions [21,22], where different types of modern volcanism are widely represented.
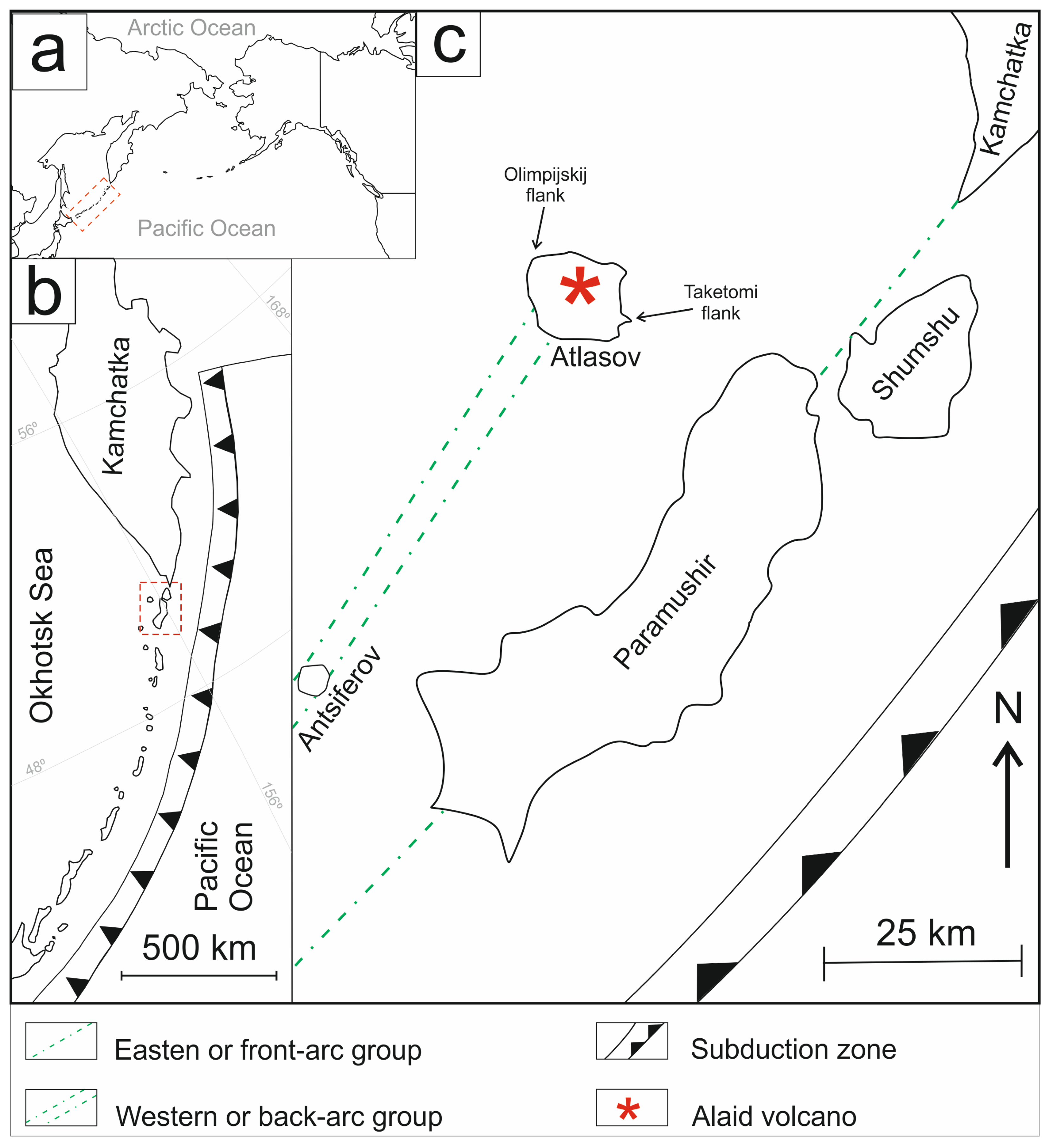
The Alaid volcano forms Atlasov Island, part of the North Kurils (Figure 1). Alaid is a stratovolcano with an underwater section approximately 600 m high and an above-water regular cone section, rising 2285 m above the Sea of Okhotsk (Figure 2a). Alaid is one of the most active volcanos in the Kuril segment of the Kuril–Kamchatka volcanic belt; it is located west of the Greater Kuril Ridge and belongs to the so-called western or back-arc group (Figure 1). Unlike other volcanoes in the Northern Kurils, Alaid has a lot of side cones, totaling more than 30 [23]. Both terminal and lateral eruptions are typical for the volcano. Historical records indicate that summit eruptions occurred in 1793, 1854, 1860, 1894, 1981, 2015–2016, and 2022 [24,25,26,27]. Lateral eruptions took place in 1933-1934, forming an east submarine flank called Taketomi, and in 1972, creating a feature named Olimpijskij (or Olympic) [28]. Taketomi originated from an underwater eruption that created a new island; however, subsequent erosion formed a land spit [21] that connected it to Atlasov Island (Figure 2b).
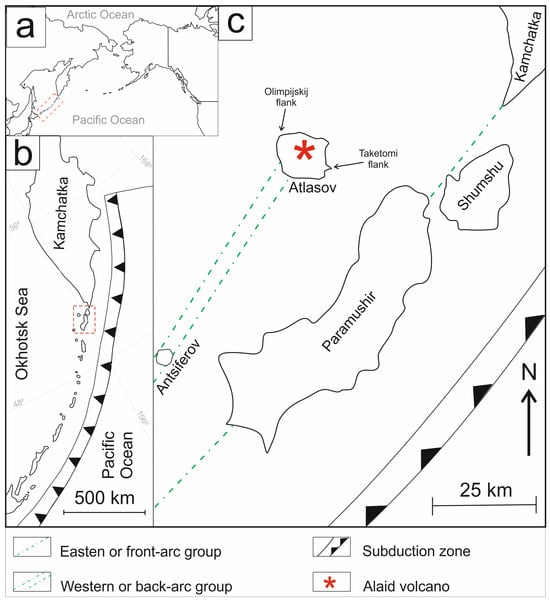
Figure 1.
The scheme shows the location of the Alaid volcano and its east submarine Taketomi flank (a) in the North Pacific, (b) within the Kuril–Kamchatka Island arc, and (c) as part of the western island group. The red dash area shown in (a) and (b) corresponds to a section that is enlared in (b) and (c), respectively.

Figure 2.
(a) Alaid volcano; (b) Taketomi flank of the Alaid volcano.
The Alaid volcano primarily consists of plagioclase and pyroxene–plagioclase, with limited occurrences of augite–olivine andesite basalts [29]. Previous studies of the volcanic eruptions—both summit and lateral—have shown a relatively homogeneous composition among the volcanic rocks, predominantly featuring high-potassium basalts [26,28,30]. Information regarding the basement structure in this area is quite limited. Dredging of the supposed projection of the Alaid basement has detected terrigenous and volcano–terrigenous rocks, as well as fragments of medium- and acidic-composition effusive rocks [31,32]. Similar rocks have been described as xenoliths in the Olympic breakthrough lavas (Figure 1) [28].
3. Materials and Methods
3.1. Materials
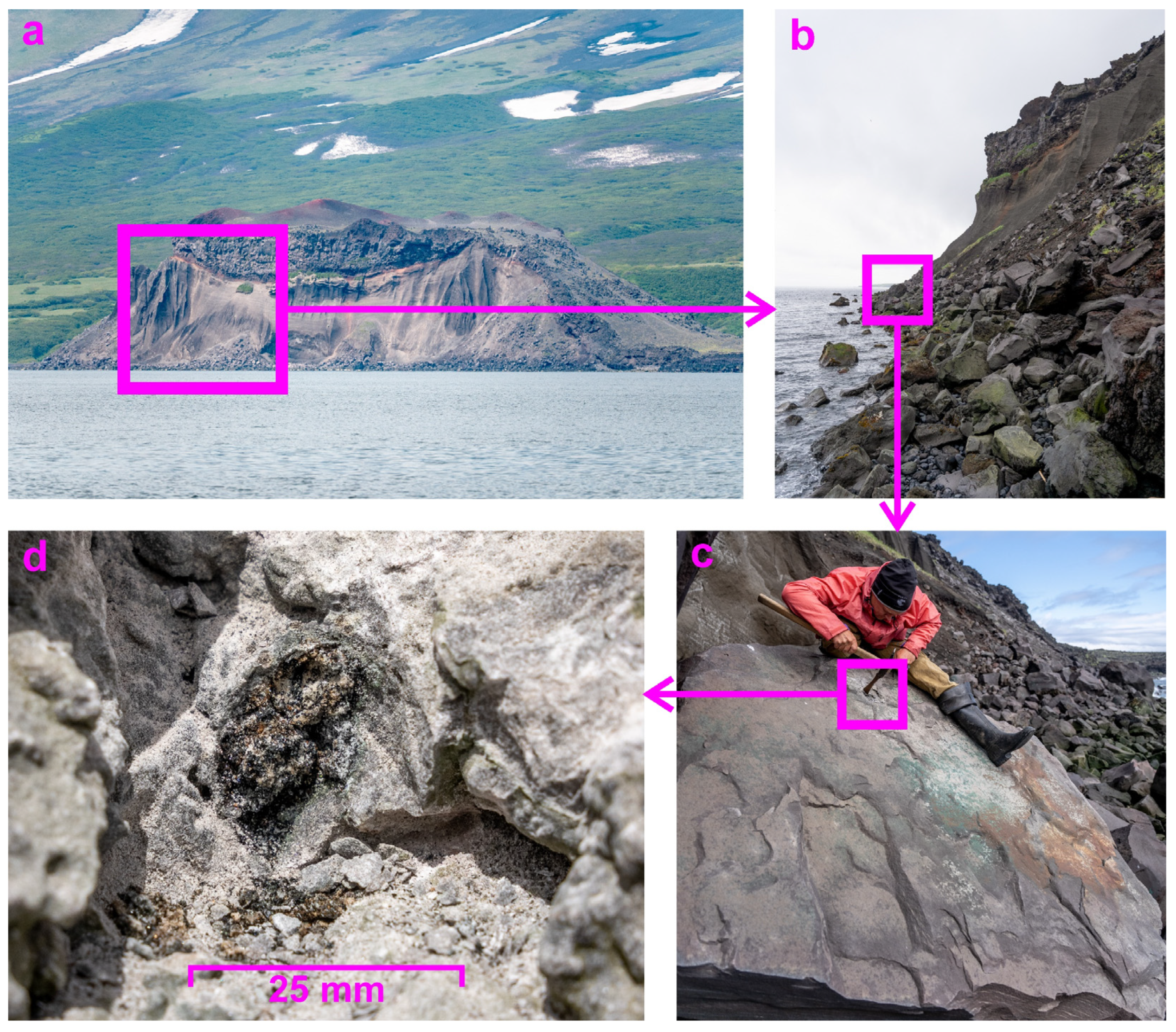
The sample described in this work was collected during fieldwork undertaken in June and July 2023 [24]. The volcanological routes that included sampling were undertaken in the southeast sector of Atlasov Island, the Alaid summit, and the Taketomi flank (Figure 1). The products of geological sampling of the listed routes are mainly represented by basalts, which may include a coating of a thin layer of variegated fumarole and/or hypergene minerals or voids filled by newly formed hydrothermal and/or hypergene sulphates, phosphates. Among the samples selected, there was one completely atypical neoformation extracted from basalts and represented by a geode (about 3 cm long) with walls encrusted with well-developed crystals, which is the subject of the current study. The neoformation sample studied therein was found in the basalt block (originally from the lava flow at the top) at the Taketomi shoreline (Figure 3) and consisted of well-developed, shiny crystals lining a cavity that was unusual for igneous rocks (Figure 3 and Figure 4). Sampling was carried out using a hammer and chisel through long tapping, and then it was wrapped in paper and placed in a plastic box. The neoformation-hosted basalt block was partially covered by turquoise minerals visually identified as atacamite in accordance with previous research [33]. This study is devoted to the mineralogical investigation of the neoformation from the Taketomi flank.
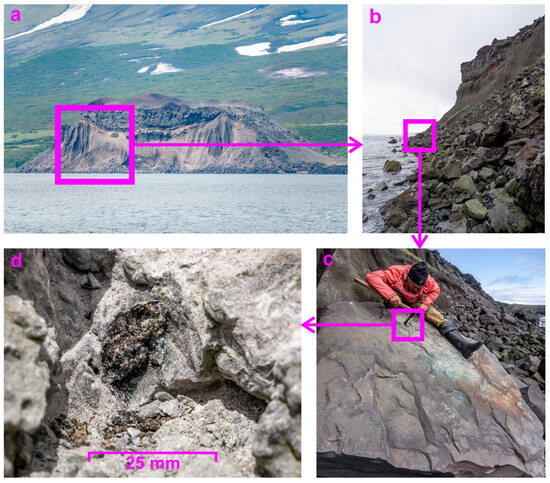
Figure 3.
The location of the neoformation-hosted basalt block: (a) the Taketomi flank of the Alaid volcano; (b) blocks of erupted rock; (c) the basalt block where the neoformation was visually identified; and (d) the sample studied in this work.
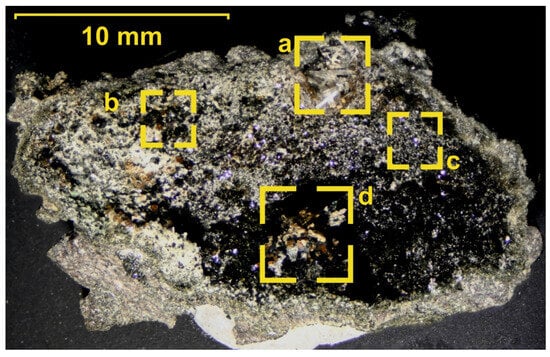
Figure 4.
The neoformation (or the geode lined by crystals) studied therein.
The neoformation extracted from basalts and studied in this work is shown in Figure 4. The minerals were identified using a set of analytical methods.
3.2. Methods
3.2.1. Scanning Electron Microscopy and Electron–Microprobe Analysis of Unpolished Grains
The crystals and their intergrowth were selected from the sample and deposited onto carbon tape and coated with carbon. Their morphology and primary elemental composition were studied using a Hitachi S–3400N (Hitachi High-Tech Corporation, Tokyo, Japan) scanning electron microscope equipped with an energy-dispersive spectrometer Oxford X–Max 20 (Oxford Instruments, Abingdon, England) at an accelerating voltage of 20 kV and a probe current of 1 nA. The energy-dispersive spectra were processed using the AzTec Energy software (version 6.1) package (Oxford Instruments Group, St. Petersburg, Russia). The standards used were CaSO4 (Ca), Cu (Cu), InP (P), BaF2 (F), NaCl (Cl), Zn (Zn), Mn (Mn), FeS2 (Fe), and Al2O3 (O).
3.2.2. Scanning Electron Microscopy and Electron–Microprobe Analysis of Polished Grains
The morphology and chemical composition of the minerals prepared as polished sections and coated with carbon were studied using (a) a Hitachi S–3400N scanning electron microscope equipped with an energy-dispersive spectrometer Oxford X–Max 20 at an accelerating voltage of 20 kV and a probe current of 1 nA and (b) a Tescan Vega 3 scanning electron microscope with a tungsten cathode equipped with an X-Max 80 mm2 energy dispersion detector at an accelerating voltage of 20 keV, a beam current of 1 nA, and an accumulation time of 20 s. The energy-dispersive spectra were processed using the AzTec Energy software package in both cases. The standards used were for (a) CaSO4 (Ca), Cu (Cu), InP (P), BaF2 (F), NaCl (Cl), Zn (Zn), Mn (Mn), FeS2 (Fe), and Al2O3 (O) and for (b) AlPO4 (P), CaF2 (F), K(AlSi3)O8 (Si, K, Na), Al2O3 (O, Al), PbCl2 (Cl), MgCaSi2O6 (Ca), MnTiO3 (Ti, Mn), FeS2 (Fe, S), and Cu (Cu). The elements were determined using spectral lines of K-series–O, F, Na, Mg, Al, P, Cl, K, Ca, Ti, Mn, Fe, and Cu.
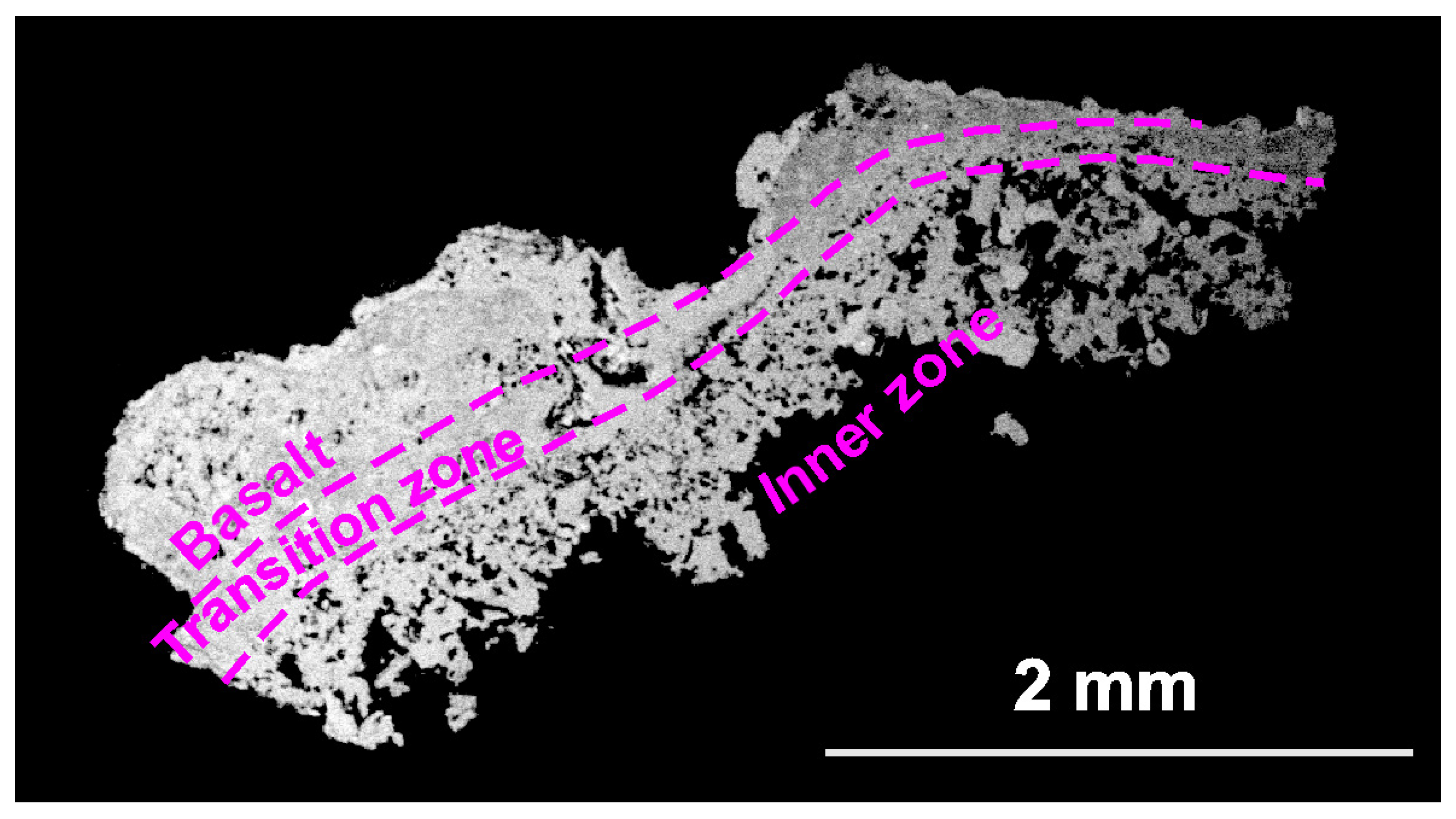
Some analyses are obtained from a cross section (Figure 5) and include basalt, including the transition zone and the inner zone (with minerals from the void); however, analyses of the inner zone were mainly obtained (Figure 5).
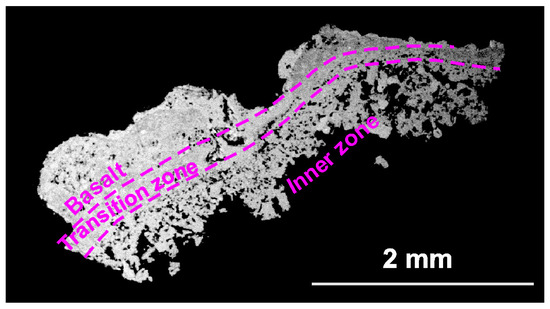
Figure 5.
The zones of neoformation.
3.2.3. Powder X-Ray Diffraction
Powder X-ray diffraction (XRD) data were collected for mineral aggregates using a Rigaku R-Axis Rapid II diffractometer (Debye-Scherrer geometry, d = 127.4 mm) equipped with a rotating anode X-ray source (CoKα, λ = 1.79021 Å) and a curved image plate detector. The data were integrated using the software package Osc2Tab/SQRay [34] and processed using the International Centre for Diffraction Data (ICDD) database incorporated into the PDXL program.
The unit cell parameters of minerals were refined using the Pawley method implemented in Topas version 5 software [35]. Refinement was based on the reflections in the 2θ region from 10 to 80°. The background was modeled using a Chebyshev polynomial approximation of the 12th order.
3.2.4. Single-Crystal X-Ray Diffraction
Single-crystal X–ray diffraction analyses were undertaken for esseneite, andradite (garnet), and wollastonite using a Rigaku XtaLAB Synergy–S diffractometer (at 50 kV and 1.0 mA, MoKα radiation, room temperature, frame width of 0.5° in ω, and exposure times of 1.48 and 0.8 s per frame for garnet and wollastonite, respectively) with a high-stability, sharp-focus X-ray source, PhotonJet–S, and a high-speed, direct-action detector, HyPix–6000HE. The data were processed using the CrysAlisPro software package [36]; an empirical absorption correction was calculated based on spherical harmonics using the SCALES ABSPACK algorithm. The crystal structures were solved using Shelx (version 2014) [37] and Olex2 (version Olex2-1.5) software [38].
4. Results
For correct mineral identification, the structure type and the chemical composition of the minerals were determined using X-ray diffraction and electron–microprobe analysis, respectively.
4.1. Powder X-Ray Diffraction
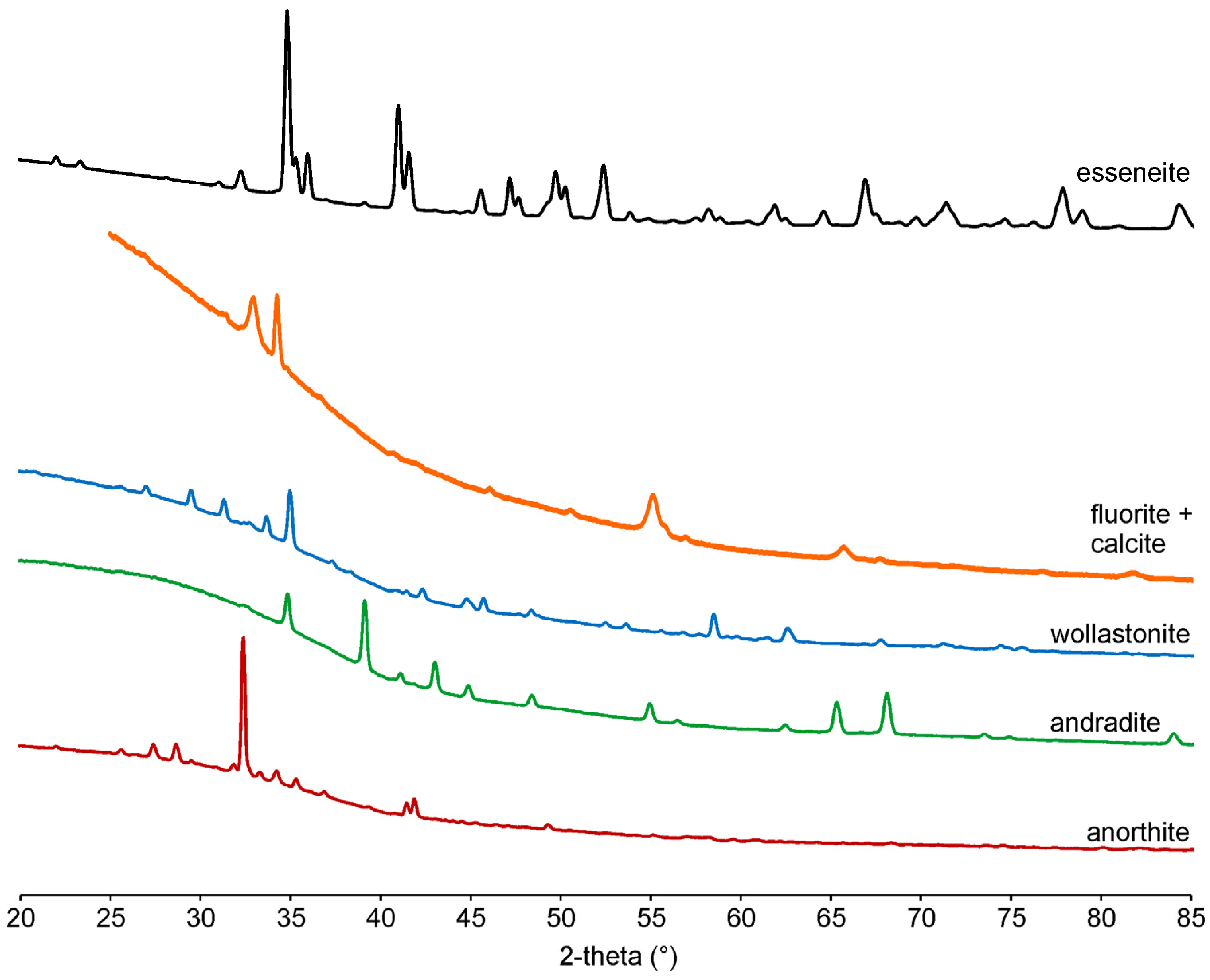
In total, 15 powder X-ray diffraction patterns of visually different crystals or their intergrowth were collected to identify the phases by structure type. The powder X-ray diffraction data (Figure 6) allowed for the identification of minerals of plagioclase (Table S1), garnet (Table S2), and clinopyroxene groups (Table S3), wollastonite (Table 1), and fluorite–calcite intergrowth (Table S4). The refined unit cell parameters using powder X-ray diffraction data are given in Table 1.
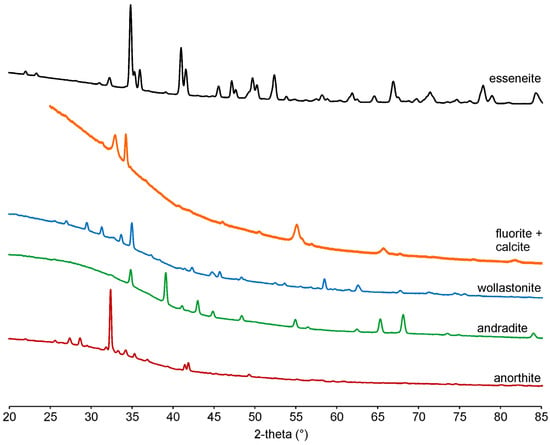
Figure 6.
Powder X-ray diffraction patterns.

Table 1.
The unit cell parameters refined from powder X-ray diffraction patterns.
4.2. Scanning Electron Microscopy and Electron–Microprobe Analysis
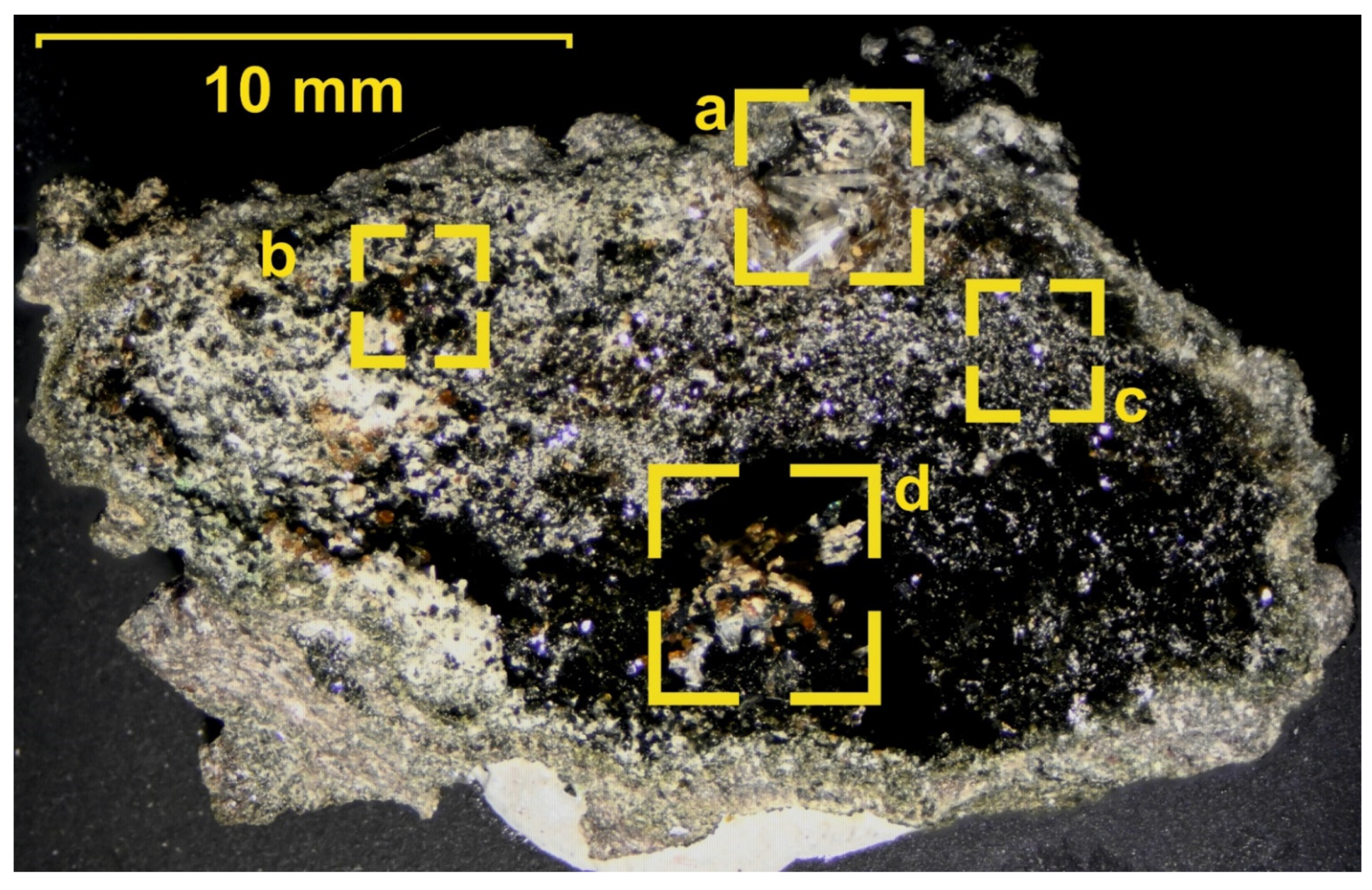
The obtained chemical analyses are recalculated and assigned to mineral species (Figure 7); the results are given in Table 2 and Table 3.
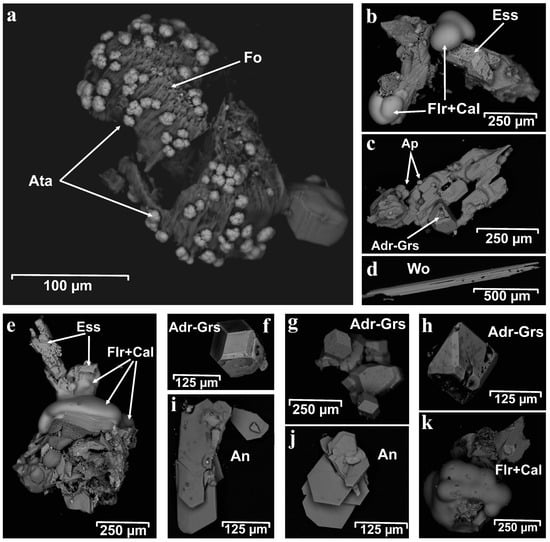
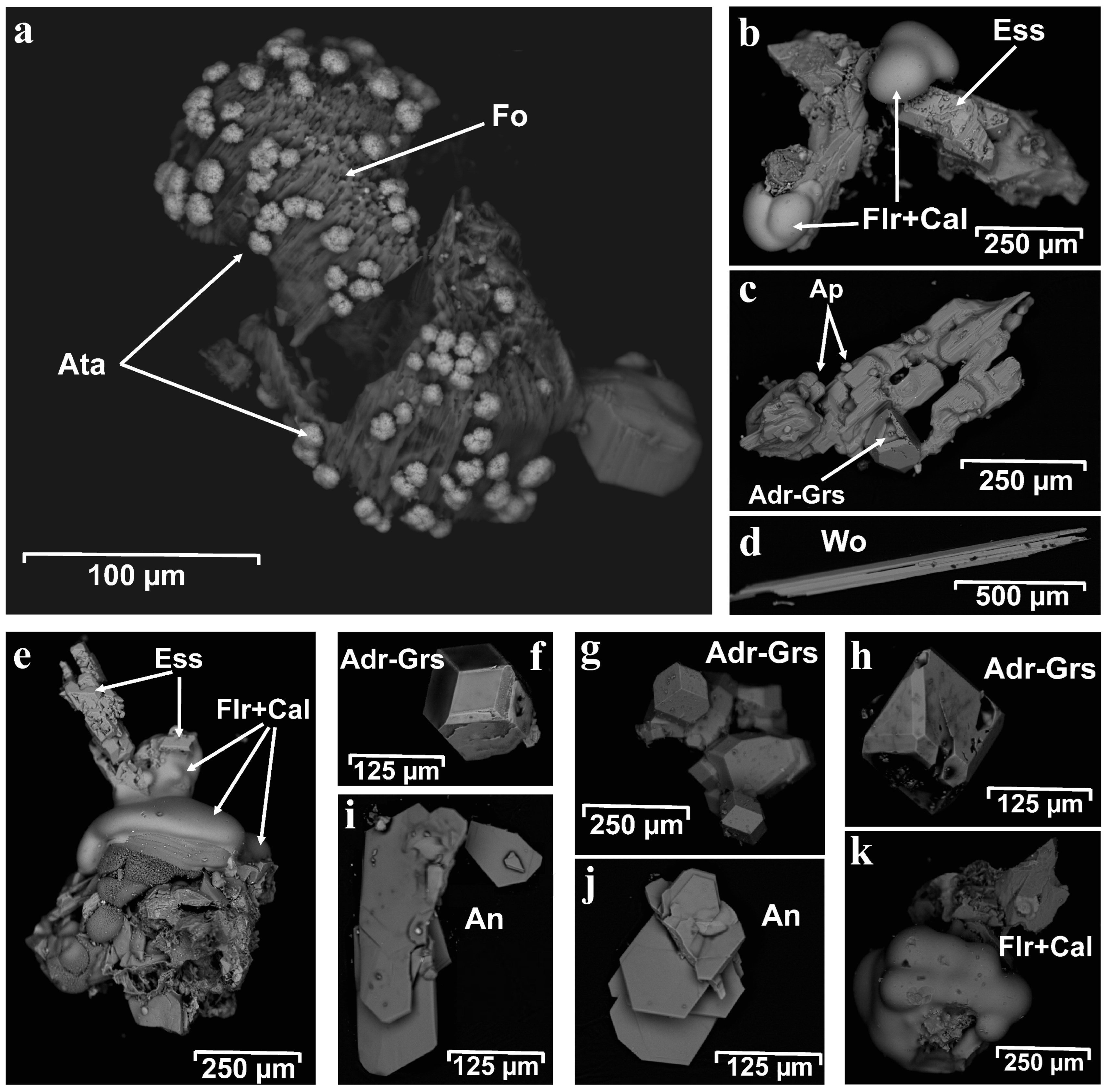
Figure 7.
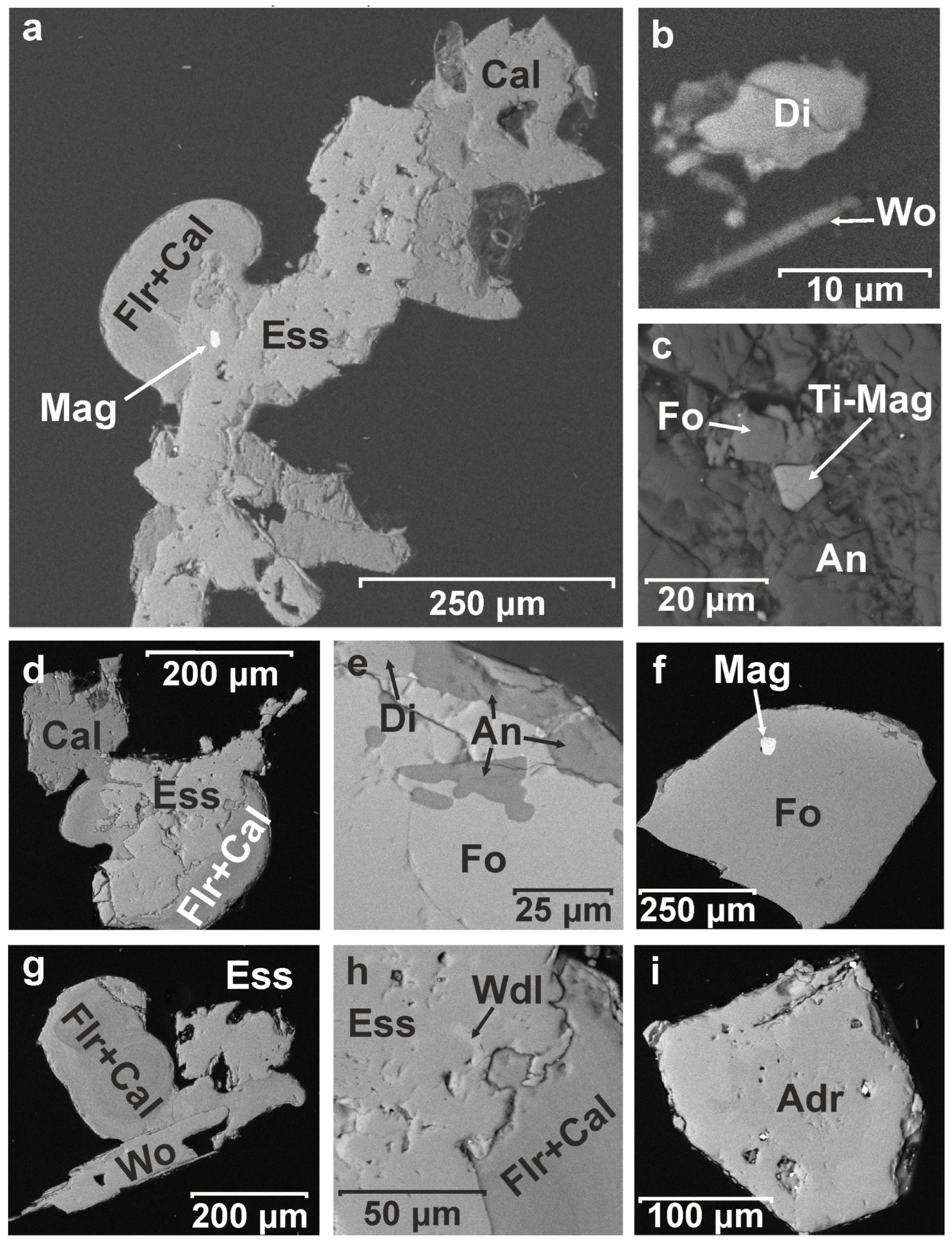
Back-scattered electron image (BSE) of unpolished mineral grains: (a) atacamite (Ata) covering forsterite (Fo); (b,e,k) fluorite–calcite aggregates (Flr + Cal); (c) apatite crystals (Apa) with garnet (Adr-Grs); (d) wollastonite (Wo); (f–h) well-developed isometric garnet crystals (Adr-Grs) and (i,j) anorthite (An).

Table 2.
The chemical composition 1 (in wt. %) of unpolished mineral samples.

Table 3.
The chemical composition (in wt. %) of polished mineral samples.
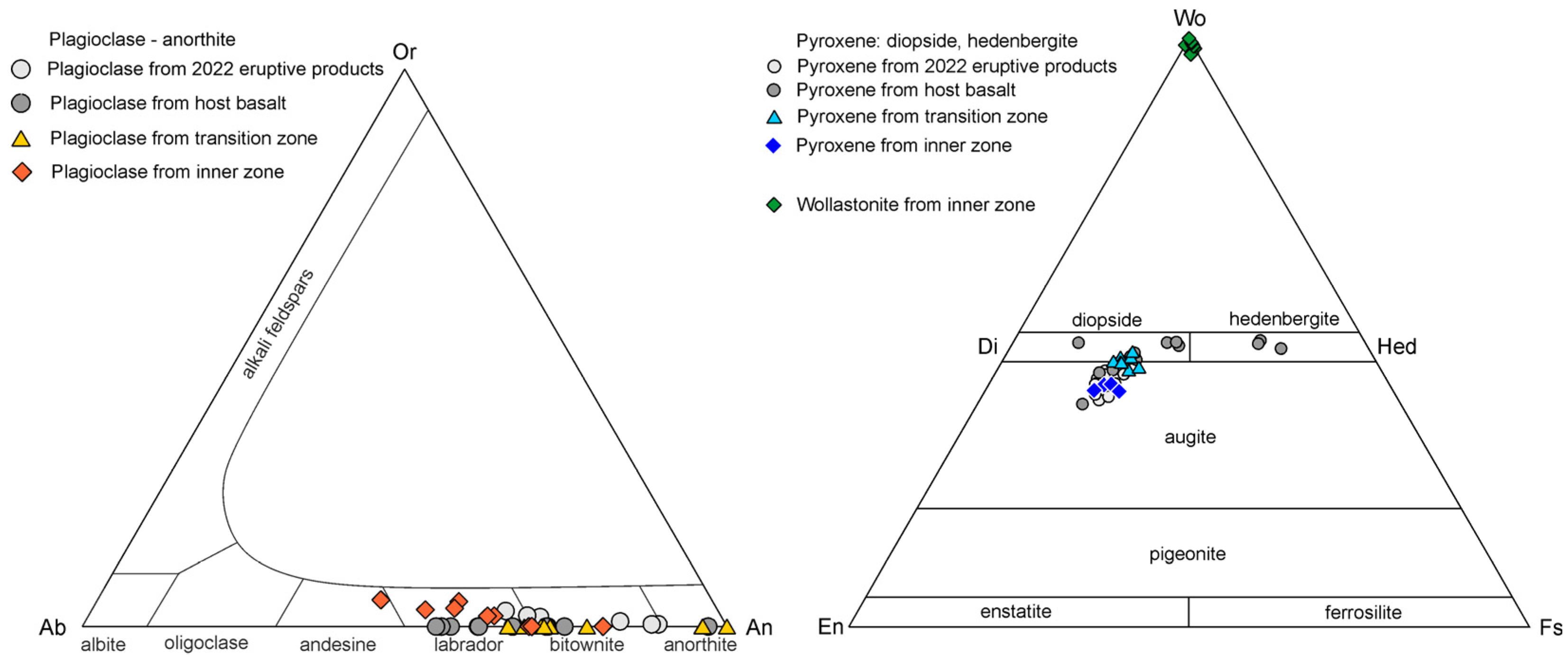
It can be seen from the diagrams in Figure 8 that anorthite and diopside from basalt and the transition zone have nearly identical chemical compositions. The minerals that are found in different zones also include magnetite, which forms a Ti-rich variety, Fe2+(Fe3+,Ti)2O4 (with Fe3+:Ti~1.50:0.50), in basalts (four analyses), and a Ti-free variety, (Fe2+,Mn,Mg)(Fe3+,Al)2O4, in the inner zone. Atacamite is found in both inner and transition zones; in the transition zone, it is abundant and forms shapeless manifestations (it possibly fills microcracks) up to 25 microns. The chemical compositions of olivine correspond to forsterite Fo60-75; this variety is also known as hyalosiderite and chrysolite.
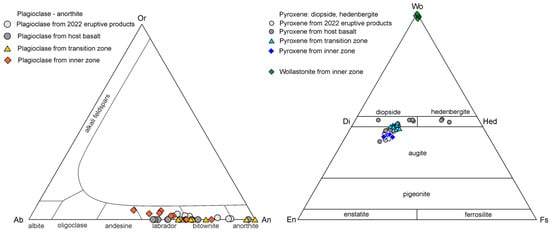
Figure 8.
The chemical composition of anorthite (left); pyroxene and wollastionite (right) from eruptive products 2022, host basalt, and transition and inner zones.
The identified minerals include wollastonite, anorthite, diopside, forsterite, andradite (intermediate between andradite and grossular), esseneite, wadalite, rhönite-like mineral, magnetite (Table 3), fluorapatite, calcite, atacamite and an intergrowth of fluorite, calcite, and amorphous silica. Most of the minerals were identified through X-ray diffraction and electro-microprobe analysis, while wadalite and rhönite-like minerals were determined exclusively through electron–microprobe analysis because they form submicron phases. The assignment of the latter minerals is less accurate, and, therefore, some additional information is presented on them in this section, which is intended to justify their classification as these mineral species based on the analytical capabilities of this work.
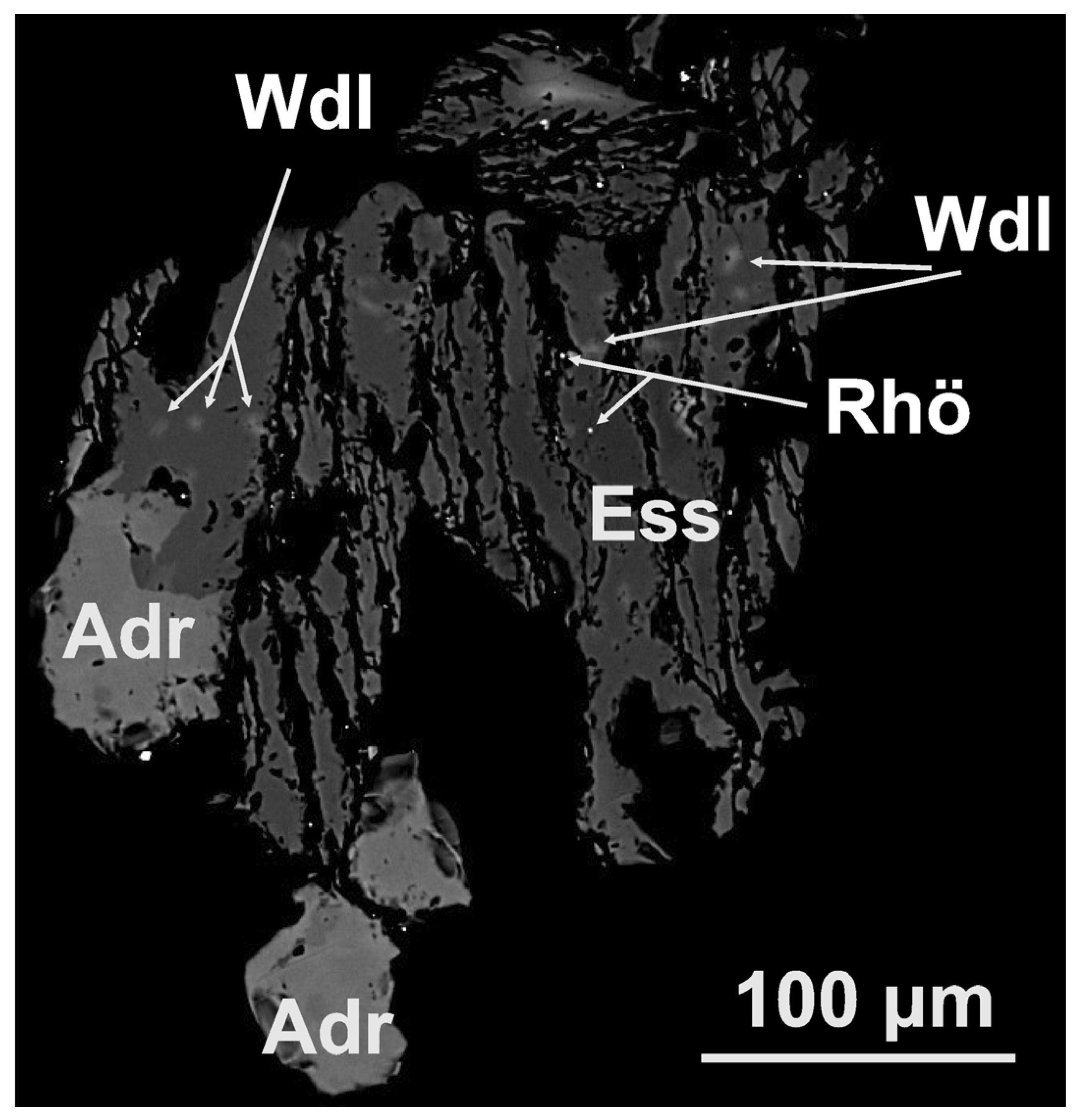
Submicron manifestations of wadalite are found in most of the esseneite grains (Figure 9 and Figure 10). Their composition is fairly well-maintained; the ranges (in wt. %) (compared to the averaged value) are Na2O 0.28–0.65 (0.39), MgO 1.56–3.21 (2.49), Al2O3 16.14–20.38 (17.55), SiO2 20.21–25.95 (23.86), CaO 35.95–40.45 (38.35), MnO 0–0.4 (0.23), FeOtot 4.09–8.54 (6.14), Cl 9.04–12.37 (10.87), and F 0–2.45 (0.11). From these values, it can be assumed that the main species-defining elements are Ca, Al, O, Cl, and possibly Fe and Mg. The search among known minerals shows two options: wadalite, (Ca,Mg)6(Al,Fe3+)4((Si,Al)O4)3O4Cl3, and its Mg-Si analogue adrianite, Ca12(Al4Mg3Si7)O32Cl6. The experimental formula obtained in this work can be given as (Ca5.60Na0.10)Σ5.70(Al2.82Fe2+0.70Mg0.51Mn0.02)Σ4.05Si3.25O16(Cl2.51OH0.45F0.05)Σ3.00, and it can be simplified as (Ca,Na)6(Al,Fe2+,Mg,Mn)4(SiO4)3O4(Cl,OH,F)3, which shows wadalite despite excess Si (0.25 apfu) and demonstrates that all Fe is divalent. It is worth noting that in the studied wadalite, the Si content (3.25 apfu for 13 cations and 16 O + 3Cl) is intermediate between wadalite (3.00 apfu) and adrianite (3.50 apfu), while the Mg content (0.51 apfu) is too low for adrianite (vs. 1.5 apfu). The excess charge produced by the extra 0.25 apfu of Si is compensated mainly by the divalent cation of Fe2+ and Mg (instead of trivalent once, as in the ideal chemical formula, i.e., Fe3+); however, their total content (Fe2+ + Mg = 1.21 apfu) is lower than that of the hypothetical Fe2+ analogue of adrianite because some charge is also compensated by Na substituting Ca. Thus, following the chemical composition data, the studied mineral can be identified as Si,Fe2+-rich wadalite.
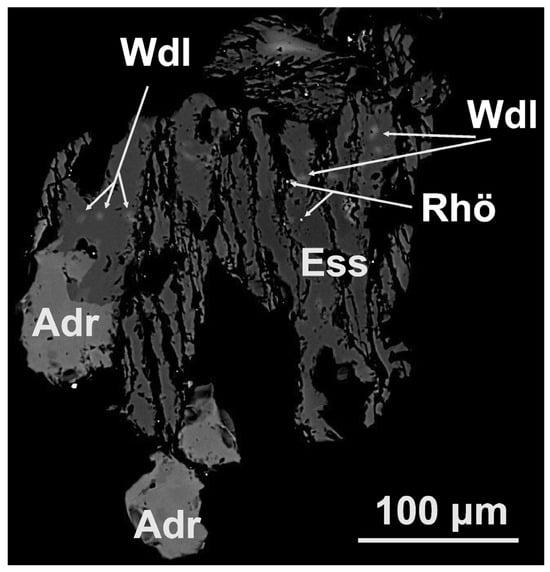
Figure 9.
BSE image of esseneite (Ess) grain that includes submicron wadalite (Wdl) and rhönite-like mineral (Rhö) in association with andradite (Adr).

Figure 10.
BSE image of polished mineral grains: fluorite (Flr) (a,d,g); esseneite (Ess) (a,d,g,h); magnetite (Mag) (a,f); calcite (Cal) (a,d); diopside (Di) (b); wollastonite (Wo) (b,g); forsterite (Fo) (c,e); Ti-rich magnetite (Ti-Mag) (c); anorthite (An) (c,e); wadalite (Wdl) (h); andradite (Adr) (i).
The chemical composition of the mineral identified as rhönite-like [44,45] is given in Table 3, from which it can be seen that the highest content is observed for such elements as Fe, Al, Mg, Ca, Si, and O. We have less statistics on this mineral in terms of the number of analyses, but it is found in different grains, and it is characteristic of this mineral association. At the same time, the tiny grain size (Figure 9) and the small number of analyses do not allow us to reliably discuss variations in cations. The search among known minerals shows sapphirine-group (dorrite/rhönite), tourmaline-group, amphibole-supergroup, and vesuvianite-group minerals. However, the calculation of the chemical formula using different bases shows a resemblance (among approved minerals) to dorrite (ideally, Ca4(Mg3Fe3+9)O4(Si3Al8Fe3+O36)), resulting in the empirical formula of rhönite-like mineral, which is Ca4(Fe2+3.36Mg1.50Ca0.49Mn0.37)Fe3+6.28O4[Si5.54Al4.52Fe3+1.77Ti0.17]O36, with Fe2+ exceeding mineral-defining Mg, high Si, and low Al contents (or the excess of Si, Ca, and Fe2+ and the lack of Al, Mg, and Fe3+). A more detailed analysis shows the closest (but still incomplete) match with the unnamed Ti-free Fe2+ analogue of rhönite, Ca4Fe82+Fe43+O4[Si8Al4O36], [46], which, however, has considerably higher Fe2+ and Al content and lower Fe3+. Because Fe2+ > Mg, the phase has been identified as rhönite-like; the obtained composition may correspond to a new unnamed mineral variety.
Summing up the results given above, the mineral assignment follows. The well-developed brown isometric crystals (Figure 4 and Figure 7f–h) are andradite (garnet) (Table 3). The elongated white or colorless crystals (Figure 4, Figure 7d and Figure 11a) belong to wollastonite, having a nearly ideal chemical composition (Table 3). The colorless and transparent elongated laths are of anorthite (Figure 4, Figure 7i,j and Figure 11d). Black prismatic crystals (Figure 4 and Figure 11d) are rare clinopyroxene–esseneite (Table 3), which has a light brown color, and the black color and blue tints are given by a thin film on the surface. Wadalite forms micron-sized zones (~10 µm) in esseneite; however, it is abundant and found in all esseneite grains (Table 3). The rhönite-like mineral is found in a couple of nanograins up to 5 µm within the esseneite matrix; a more detailed characterization of this phase is problematic due to its size. The fluorite–calcite–amorphous silica dripstone aggregates (Figure 11b) are the latest phase in the mineral sequence; they cover wollastonite and esseneite. The chemical analyses obtained from them represent a mixture, and they were not recalculated as the chemical formulas are very simple: CaF2 and CaCO3.
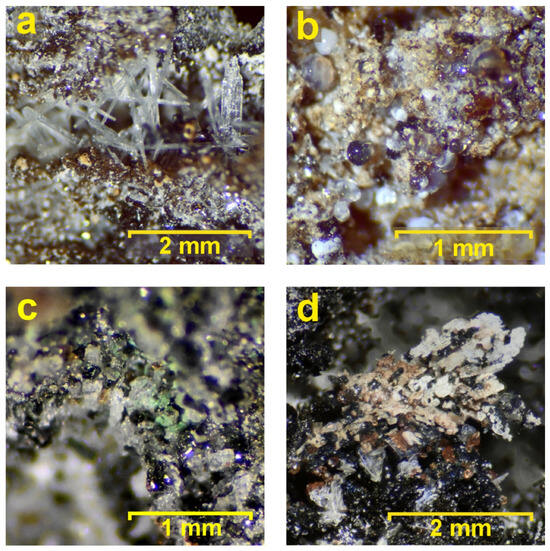
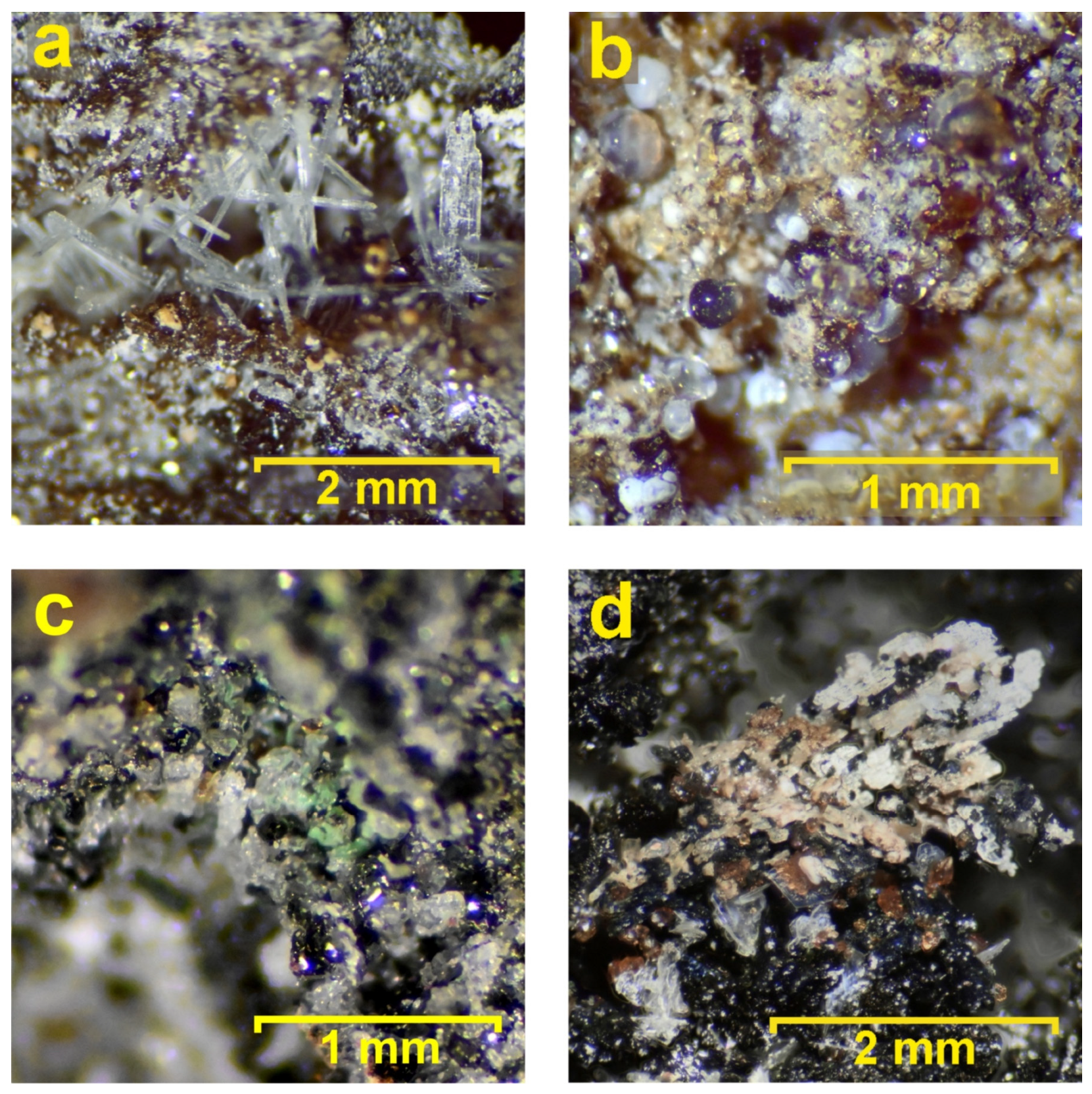
Figure 11.
Minerals from the inner zone: (a) elongated transparent crystals of wollastonite-1A; (b) colorless to white spherules of calcite–fluorite intergrowth with amorphous silica; (c) green coating (atacamite) covering black clinopyroxene crystals (esseneite and diopside); and (d) association of crystals of black esseneite, colorless anorthite leisty, brown andradite, beige wollastonite, and white apatite crystals covering the top.
4.3. Crystal Structures
The crystal structures of esseneite, andradite, and wollastonite have been refined (Table 4 and Table S5). Atom coordinates, equivalent isotropic displacement parameters, site occupancies, selected bond distances, and anisotropic displacement parameters are provided in Tables S6–S8 for andradite, Tables S9–S11 for wollastonite, and Tables S12–S14 for esseneite. For esseneite, the site occupancy suggests 19 electrons for the position incorporating Fe, Al, and Mg, which agrees well with the experimentally obtained chemical formula (Table 3). The structure refinement of andradite gives Fe/Al as 50/50, which completely agrees with the electron microprobe examination (Table 3). The structure refinement of wollastonite identified that it crystallizes as a 1A (one-layer triclinic) polytype; the site occupancies agree with the ideal chemical formula of the mineral.

Table 4.
The unit cell parameters of esseneite, andradite, and wollastonite-1A.
5. Discussion
This mineral association simultaneously bears the characteristics of skarn, pyrometamorphic (or combustion metamorphic), and fumarolic (or, in our case, more accurately, formed with the participation of magmatic gas) rocks.
The association of the calcium silicates wollastonite, andradite, diopside, and anorthite corresponds to the typical skarn. The findings of these minerals are vastly interpreted as a skarnification process of crustal xenoliths due to interaction with magma [2,13,47,48,49] (Table 5). The process of xenolith skarnification is widespread for volcanoes with carbonate basements like Somma–Vesuvius, Colli Albani, and Merapi [48]. The described mineral paragenesis as a whole indeed shares a lot with skarn xenoliths (Table 5). To the best of our knowledge, such xenoliths have not been described for the Kuril Islands, and carbonates have not been described in the basement of Alaid. Following the xenolith conception, the untypical xenolith should have been fully reworked with no traces left, and we do not observe any relics of the original carbonates or structures/textures of sedimentary rocks. The most probable origination of such a xenolith is the walls of a magmatic system. It is worth noting that the skarn-like mineral associations have been recorded on volcanoes without underlying carbonate rocks, which have recently been interpreted as multiple carbonatitic and alkaline silicate metasomatism of pristine basalt to picrobasalt [49]. A similar skarn-like mineral association has been documented in the xenoliths of the Bezymianny volcano in Kamchatka, where carbonate rocks are also absent. These xenoliths contain Fe-rich wollastonite, anorthite, diopside, hedenbergite, and micron-sized garnet with andradite–grossular composition. They formed due to the pyrometamorphic recrystallization of basalts that had previously undergone hydrothermal alteration within the magmatic system walls, influenced by a CO2-rich fluid [50].

Table 5.
The comparison of mineral paragenesis from the Alaid volcano to volcanic fumaroles of the Kuril-Kamchatka Island arc and skarn association due to carbonate xenolith interaction with silicate magmas.
Under volcanic conditions, calcium silicates can also form in fumaroles (Table 5), mainly in their high-temperature parts, or, more generally, in environments of high-temperature gas transport and metasomatism (not necessarily with the formation of classical fumaroles but along cracks and weakened zones). For Tolbachik, fumarolic anorthite, diopside, andradite, fluorapatite, esseneite, and other silicates were reported [51,52]. For high-temperature fumaroles (>850 °C) of the Kudryavy volcano (South Kuril), wollastonite, anorthite, diopside, and andradite were documented [53]. In our case, the fumarolic scenario could have explained mineral crystallization in the form of a geode, i.e., on the walls of the small cavity. The CO2 can be of volcanic origin. The participation of the gas component in the process of the formation of the mineral association described in this work is reflected in the formation of halogen-containing minerals (Cl, F) and later Cu-mineral–atacamite, which is a ubiquitous mineral on volcanoes, where it is formed from hot magmatic gas in fumaroles and crater zones.
Finally, the majority of the reported minerals are found in pyrometamorphic (or combustion metamorphic) rocks of natural and/or anthropogenic origin, like the Hatrurim complex (Israel), the Durham Ranch paralava occurrence (USA), and worldwide burning coal dumps.
The submicron globules of fluorite and calcite are an interesting petrological association that could have formed during the high-temperature decomposition of CaCO3–CaF2 melt (T > 800 °C, p < 1 kbar). Evidence of the partially melted state is the dripstone shape of aggregates and their strong association with the latest mineral zone. The stability of carbonate melts under magmatic temperature is ensured by the high F activity in the system [54,55,56,57].
Among the described minerals, esseneite, wadalite, and rhönite are quite rare and deserve some attention.
Esseneite, CaFe3+[AlSiO6], is a member of the clinopyroxene group. It forms a solid solution series with diopside, CaMgSi2O6, and kushiroite, CaAl[AlSiO6]. In our sample, esseneite makes up a significant portion of the sample. Esseneite was originally described in paralava created by incomplete burning of coal near Durham (Wyoming, USA). Since then, it (and its technogenic analogue) has been confirmed in similar settings, like Hatrurim [57,58], the Lapanouse-de-Sévérac slag locality (Aveyron, France) [59], and the Chelyabinsk coal basin (Russia) [60]. Apart from that, esseneite has been reported in a couple of volcanic environments in (a) Pliocene lavas of the Western Taurids, Türkiye; (b) xenoliths of ultramafic rocks in dacitic lavas of the Ten`-01 volcano within the Lena–Vilyui watershed (Yakutia, Russia) [61]; and (c) fumarolic sublimates and lava tube incrustations of the Tolbachik volcano (Kamchatka, Russia) [51,52]. Esseneite has also been reported in meteorites and comets [62]. Previously, a close genetic connection was shown between esseneite and rhönite (both identified therein) in pyromethamorphic rocks. Both minerals are formed in high-temperature, low-pressure conditions and oxidizing environments [63]. Esseneite also forms during alteration of magmatic system walls by high-temperature (~850 °C) CO2-rich fluid [64]. Also, the same Al-rich clinopyroxene is typical for high-temperature skarns and calcareous rocks affected by high-grade contact metamorphism of metasedimentary rocks, including pyrometamorphic changes of Ca-rich xenoliths [64,65]. For example, in Saint Vincent, Lesser Antilles, a nodule containing Al-rich pyroxene associated with wollastonite, anorthite, and grossular was found [66]. The studied esseneite has a complex composition and contains 32% esseneite, 26% diopside, 20% hedenbergite, and about 18% kushiroite end-members. It should be noted that the presence of impurities is also characteristic of the previously described findings of esseneite: (a) the sample from type locality (Gillette, Wyoming, USA) contains 72% esseneite, 16% diopside, and <5% hedenbergite and kushiroite [67] and (b) the sample from the Lena–Vilyui watershed (Yakutiya, Russia) contains 48% esseneite, 33% diopside, and 14% kushiroite [61]. The mineral is determined through a combination of chemical composition with powder and single-crystal X-ray diffraction data. The unit cell parameters of esseneite determined through two methods fell within the range previously published for that mineral and are distinct from diopside and kushiroite (Table 6). The refinement of site occupancy (Table S12) also agrees with esseneite’s chemical composition, as the refined and calculated number of electrons in that site is 19.

Table 6.
Comparison of the unit cell parameters of esseneite, diopside, and kushiroite.
Rhönite-group minerals in terrestrial conditions are known in the pyrometamorphic (or combustion metamorphic) rocks [70] and in high-Al, Si-undersaturated systems [71,72]. In high-Al basaltic systems, it is stable under temperatures between 840 and 1200 °C and pressures below 600 bars [73].
Another rather rare mineral found in our sample is wadalite, (Ca,Mg)6(Al,Fe3+)4((Si,Al)O4)3O4Cl3. Initially, the mineral was found in calcsilicate xenolith in two-pyroxene andesite from Koriyama City, Fukushima Prefecture, Japan, but it was scarcely described (mainly, the crystal structure of the mineral from that locality is published) [74]. The majority of current wadalite findings refer to meteorites [75], coal fires [76], and skarn zones with carbonate xenoliths embayed in volcanic rocks [13,47]. Wadalite occurs in skarn xenoliths with wollastonite, grossular, andradite, and gehlenite in Tadano, Japan [77]. The abundance of halogen-rich mineral phases in the skarn xenoliths traditionally testifies to their infiltration by magmatic brine [64,78].
It can be assumed that the described mineral paragenesis crystallized at a high temperature (over 800 °C) and, in addition to the temperature (the pyrometamorphic effect), crystallization occurred with the participation of volcanic gases (enriched with CO2, Cl, F). We have not found clear traces of (reworked) xenolith; however, we do not deny the possibility of the xenolithic nature of this neoformation. The presence of well-developed, faceted crystals of different minerals (Figure 11) indirectly indicates the duration of their crystallization, that is, prolonged exposure to high temperatures.
6. Conclusions
We have provided the first description and detailed mineralogical study of a neoformation composed of paragenesis of calcium minerals, including silicates, phosphates, fluorides, and carbonates, from the Northern Kuril Islands. The samples were studied structurally and chemically. Chemically, we show that plagioclase (anorthite) and clinopyroxene (diopside) have persistent chemical compositions in different zones of the neoformation and in eruptive (2022) products of the Alaid volcano. The paragenesis is formed mainly by such elements as Si, Al, Ca, Fe, Mg, and O, among which the mineral-forming specificity lies in the high content of calcium with the almost complete absence of sodium and potassium. Structurally, it was shown that wollastonite crystallizes in the 1A polytype, garnet is an intermediate andradite–grossular member, and esseneite can be distinguished from the closest related minerals (diopside and kushiroite) by the unit cell parameters. The paragenesis described here is atypical for the Kuril–Kamchatka arc, although its individual minerals have been described sporadically for this territory, mainly as fumarole formations. The rarest of those studied are esseneite, wadalite, and rhönite. At present, there is no clear evidence of how this neoformation was formed. Among the most probable scenarios, we see both a xenolith and the processing (or deposition from) hot volcanic gas or rather a combination of these processes. Based on the literature data on the formation conditions of the described minerals, mineral genesis should have occurred at a temperature above 800 °C and a pressure of less than 1 kbar, and the size and shape of the crystals indirectly indicate the long duration of heating. Alaid is a volcano without a carbonate base for which skarnified sedimentary fragments are not characteristic, which actualizes the question of the genesis of such neoformations. A detailed mineralogical study of such formations is one of the important steps in understanding the functioning of magmatic systems, the circulation and transformation of natural matter, and mineral-forming processes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/min15030237/s1, Table S1: The powder X-ray diffraction of plagioclase (anorthite); Table S2: The powder X-ray diffraction of garnet (andradite); Table S3: The powder X-ray diffraction of clinopyroxene (esseneite); Table S4: The powder X-ray diffraction of fluorite and calcite and fluorite; Table S5: Crystal data and structure refinement of andradite, wollastonite-1A, and esseneite; Table S6: Anisotropic displacement parameters (Å2) and site occupancies for andradite; Table S7: Selected bond distances (Å) for andradite; Table S8: Anisotropic displacement parameters (Å2) for andradite; Table S9: Atom coordinates, equivalent isotropic displacement parameters (Å2), and site occupancies for wollastonite-1A; Table S10: Selected bond distances for (Å) for wollastonite-1A; Table S11: Anisotropic displacement parameters (Å2) for wollastonite-1A; Table S12: Atom coordinates, equivalent isotropic displacement parameters (Å2), and site occupancies for esseneite; Table S13: Selected bond distances (Å) for esseneite; Table S14: Anisotropic displacement parameters (Å2) for esseneite; Table S15: The powder X-ray diffraction obtained for atacamite.
Author Contributions
Conceptualization, E.S.Z. and A.A.N.; methodology, E.S.Z., A.A.N., R.M.S., P.S.Z., A.V.K. and V.O.D.; software, E.S.Z., R.M.S., M.A.K. and N.S.V.; validation, E.S.Z., A.A.N., R.M.S. and P.S.Z.; formal analysis, E.S.Z., R.M.S. and N.S.V.; investigation, E.S.Z., A.A.N., R.M.S., R.A.K., P.S.Z., A.V.K. and V.O.D.; resources, A.A.N., R.A.K. and A.V.K.; data curation, E.S.Z., A.A.N., P.S.Z., A.V.K. and V.O.D.; writing—original draft preparation, E.S.Z., A.A.N., R.M.S., R.A.K., P.S.Z., A.V.K. and V.O.D.; writing—review and editing, E.S.Z., A.A.N., R.M.S., R.A.K., P.S.Z., M.A.K., A.V.K., V.O.D. and N.S.V.; visualization, E.S.Z., R.A.K., P.S.Z. and M.A.K.; supervision, E.S.Z.; project administration, A.A.N.; funding acquisition, A.A.N. All authors have read and agreed to the published version of the manuscript.
Funding
The work was carried out according to the state assignment of the IVS FEB RAS within the framework of the research topic “Geochemistry of products of modern volcanic and post-volcanic activity” (FWME-2024-0013), approved by the Ministry of Education and Science of Russia (topic No. 124080600037-9). The analyses carried out in the X-ray Diffraction and Geomodel Resource Centers are undertaken as part of projects No. 118201839 (ID Pure) and No. 124032000029-9, respectively.
Data Availability Statement
The crystal structure data for esseneite, andradite, and wollastonite-1A are available as CIF files from the CCDC/FIZ Karlsruhe database as CSD # 2395350 and 2395349, respectively.
Acknowledgments
We would like to thank the colleagues taking part in the expedition (L.P. Anikin, I.E. Bolshakov and K.A. Kudryashova). The X-ray diffraction studies were performed in the X-ray Diffraction Resource Centre of St. Petersburg State University. Scanning electron microscopy was performed in the “Geomodel” Resource Centre of St. Petersburg State University. We express our gratitude to Konstantin A. Gribushin, who took part in the early rounds of chemical characterization of the sample. We thank the reviewers for their constructive comments that improved the manuscript’s quality.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Markhinin, E.K. Volcanism as an agent of formation of the Earth’s crust. Crust Up. Mantle Pac. Area 2012, 12, 413–422. [Google Scholar]
- Martin, A.P.; Cooper, A.F.; Price, R.C.; Doherty, C.L.; Gamble, J.A. A review of mantle xenoliths in volcanic rocks from southern Victoria Land, Antarctica. Geochem. Geophys. Antarct. Mantle 2023, 56, 33–55. [Google Scholar] [CrossRef]
- Rodríguez, A.; van Bergen, M.J. Superficial alteration mineralogy in active volcanic systems: An example of Poás volcano, Costa Rica. J. Volcanol. Geoth. Res. 2017, 346, 54–80. [Google Scholar] [CrossRef]
- Sillitoe, R.H.; Bonham, H.F. Volcanic landforms and ore deposits. Econ. Geol. 1984, 79, 1286–1298. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Lowenstern, J.B. The role of magmas in the formation of hydrothermal ore deposits. Nature 1994, 370, 519–527. [Google Scholar] [CrossRef]
- Campeny, M.; Menéndez, I.; Ibáñez-Insa, J.; Rivera-Martínez, J.; Yepes, J.; Álvarez-Pousa, S.; Méndez-Ramos, J.; Mangas, J. The ephemeral fumarolic mineralization of the 2021 Tajogaite volcanic eruption (La Palma, Canary Islands, Spain). Sci. Rep. 2023, 13, 6336. [Google Scholar] [CrossRef] [PubMed]
- Shchipalkina, N.V.; Pekov, I.V.; Koshlyakova, N.N.; Belakovskiy, D.I.; Zubkova, N.V.; Agakhanov, A.A.; Britvin, S.N.; Nazarova, M.A. A new mineral cuprodobrovolskyite Na4Cu(SO4)3 from the Tolbachik volcano (Kamchatka, Russia) and relationships in the family of natural anhydrous Na–Cu sulfates. Mineral. Mag. 2024, 88, 49–60. [Google Scholar] [CrossRef]
- Borisov, A.S.; Siidra, O.I.; Vlasenko, N.S.; Platonova, N.V.; Schuldt, T.; Neuman, M.; Strauss, H.; Holzheid, A. The Yadovitaya fumarole, Tolbachik volcano: A comprehensive mineralogical and geochemical study and driving factors for mineral diversity. Geochemistry 2024, 84, 126179. [Google Scholar] [CrossRef]
- Balić-Žunić, T.; Nestola, F.; Pamato, M.G.; Rasmussen, M.B. Kristjánite, KNa2H(SO4)2, a new fumarolic mineral from Iceland containing the [SO4–H–SO4]3– anion in the crystal structure. Mineral. Mag. 2024, 88, 211–217. [Google Scholar] [CrossRef]
- Renggli, C.J.; Klemme, S. Experimental constraints on metal transport in fumarolic gases. J. Volcanol. Geoth. Res. 2020, 400, 106929. [Google Scholar] [CrossRef]
- Vander Auwera, J.; Montalbano, S.; Namur, O.; Bechon, T.; Schiano, P.; Devidal, J.L.; Bolle, O. The petrology of a hazardous volcano: Calbuco (Central Southern Volcanic Zone, Chile). Contrib Mineral Petrol. 2021, 176, 46. [Google Scholar] [CrossRef]
- Grapes, R. Pyrometamorphism, 2nd ed.; Springer-Verlag Berlin: Heidelberg, Germany, 2011; p. 365. [Google Scholar]
- Galuskina, I.O.; Krüger, B.; Galuskin, E.V.; Armbruster, T.; Gazeev, V.M.; Włodyka, R.; Dulski, M.; Dzierżanowski, P. Fluorchegemite, Ca7(SiO4)3F2, a new mineral from the edgrewite-bearing endoskarn zone of an altered xenolith in ignimbrites from upper Chegem caldera, northern Caucasus, Kabardino-Balkaria, Russia: Occurrence, crystal structure, and new data on the mineral assemblages. Canad Miner. 2015, 53, 325–344. [Google Scholar]
- Zubkova, N.V.; Chukanov, N.V.; Pekov, I.V.; Ternes, B.; Schüller, W.; Pushcharovskii, D.Y. Ta-Free Nb-Dominant Ixiolite Analogue from the Eifel Paleovolcanic Region, Germany, and Its Crystal Structure. On the Problem of “Ashanite”. Geol. Ore Depos. 2021, 63, 805–811. [Google Scholar] [CrossRef]
- Pekov, I.V.; Agakhanov, A.A.; Zubkova, N.V.; Koshlyakova, N.N.; Shchipalkina, N.V.; Sandalov, F.D.; Yapaskurt, V.O.; Turchkova, A.G.; Sidorov, E.G. Oxidizing-type fumaroles of the Tolbachik Volcano, a mineralogical and geochemical unique. Russ. Geol. Geophys. 2020, 61, 675–688. [Google Scholar] [CrossRef]
- Garavelli, A.; Pinto, D.; Mitolo, D.; Kolitsch, U. Thermessaite-(NH4), (NH4)2AlF3 (SO4), a new fumarole mineral from La Fossa crater at Vulcano, Aeolian Islands, Italy. Mineral. Mag. 2021, 85, 665–672. [Google Scholar] [CrossRef]
- Balassone, G.; Panikorovskii, T.L.; Pellino, A.; Bazai, A.V.; Bocharov, V.N.; Goychuk, O.F.; Avdontseva, E.Y.; Yakovenchuk, V.N.; Krivovichev, S.V.; Petti, C.; et al. Enricofrancoite, KNaCaSi4O10, a new Ca–K–Na silicate from Somma–Vesuvius volcano, southern Italy. Mineral. Mag. 2024, 88, 277–287. [Google Scholar] [CrossRef]
- Campostrini, I.; Castellano, C.; Demartin, F.; Rocchetti, I.; Russo, M.; Vignola, P. Paradimorphite, β-As4S3, a vintage new mineral from Solfatara di Pozzuoli and Vesuvius, Napoli, Italy. Mineral. Mag. 2022, 86, 500–506. [Google Scholar] [CrossRef]
- Kasatkin, A.V.; Siidra, O.I.; Nestola, F.; Pekov, I.V.; Agakhanov, A.A.; Koshlyakova, N.N.; Chukanov, N.V.; Nazarchuk, E.V.; Molinari, S.; Rossi, M. Napoliite, Pb2OFCl, a new mineral from Vesuvius volcano, and its relationship with dimorphous rumseyite. Mineral. Mag. 2023, 87, 711–718. [Google Scholar] [CrossRef]
- Balić-Žunić, T.; Garavelli, A.; Jakobsson, S.P.; Jonasson, K.; Katerinopoulos, A.; Kyriakopoulos, K.; Acquafredda, P. Fumarolic minerals: An overview of active European volcanoes. In Updates in Volcanology-From Volcano Modelling to Volcano Geology; Nemeth, K., Ed.; IntechOpen Limited: London, UK, 2016; pp. 267–322. [Google Scholar]
- Gorshkov, G.S. Volcanism of the Kuril Island Arc; Nauka: Moscow, Russia, 1967; 288p. (In Russian) [Google Scholar]
- Martynov, Y.A.; Martynov, A.Y.; Chashchin, A.A.; Rybin, A.V. Basalts of Tyatya volcano: Petrology and genesis (Kunashir Island, Kuril Island Arc). Russ. J. Pac. Geol. 2005, 24, 22–31. [Google Scholar]
- Blokh, Y.I.; Bondarenko, V.I.; Rashidov, V.A.; Trusov, A.A. The Alaid Volcanic Massif (Kuril Island-Arc System). In Proceedings of the International Symposium “Problems of Modern Volcanism”; Institute of volcanology and seismology FEB RAS: etropavlovsk-Kamchatsky, Russian, 2006; pp. 135–143. (In Russian) [Google Scholar]
- Bolshakov, I.E.; Nuzhdaev, A.A.; Kuznetsov, R.A.; Anikin, L.P.; Kudryashova, K.A.; Zhitova, E.S. Expedition to Alaid and Ebeko Volcanoes (Kuril Islands) in Summer 2023. Bull. Krasec. Earth Sci. 2023, 4, 105–113. (In Russian) [Google Scholar] [CrossRef]
- Degterev, A.V.; Chibisova, M.V.; Romanyuk, F.A. An Explosive–Effusive Eruption of Alaid Volcano in 2022 (Atlasova Island, Northern Kuril Islands). Russ. J. Pac. Geol. 2023, 17 (Suppl. S2), S249–S258. [Google Scholar] [CrossRef]
- Didenko, A.H.; Rashidov, V.A.; Markov, G.P.; Trusenko, M.S.; Petrova, V.V.; Anikin, L.P. Petromagnetic and geochemical characterization of volcanics from the 2015-2016 eruption of Alaid volcano, Kuril Island Arc. J. Volcanol. Seismol. 2021, 1, 3–21. (In Russian) [Google Scholar]
- Fedotov, S.A.; Ivanov, B.V.; Avdeiko, G.P.; Flerov, G.P.; Andreyev, V.N.; Dvigalo, V.N.; Dubik, Y.M.; Chirkov, A.M. Eruption of Alaid Volcano in 1981. J. Volcanol. Seismol. 1981, 5, 82–87. (In Russian) [Google Scholar]
- Avdeiko, G.P.; Khrenov, A.P.; Flerov, G.B.; Tokarev, P.I.; Shirokov, V.A.; Meniaylov, I.A.; Chirkov, A.M.; Volinets, O.N.; Dubik, Y.M.; Vergasova, L.P.; et al. Eruption of Alaid volcano in 1972. Bull. Volconological Stn. 1974, 50, 64–80. (In Russian) [Google Scholar]
- Abdurakhmanov, B.N.; Piskunov, I.G.; Smirnov, I.G.; Fedorchenko, V.I. Alaid volcano (Kuril Islands). In East Asian Island Systems (Tectonics and Volcanism); Tuezov, I.K., Ed.; SakhKNII: Yuzhno-Sakhalinsk, Russia, 1978; pp. 85–107. (In Russian) [Google Scholar]
- Bergal-Kuvikas, O.V. Peculiarities of the spatial variations from Paramushir volcanic Group, Kurile Islands. Arc. Bull. Krasec. Earth Sci. 2012, 20, 194–207. (In Russian) [Google Scholar]
- Geodekyan, A.A.; Udintsev, G.B.; Baranov, B.V.; Beresnev, A.F.; Burk, C.; Bogatikov, O.A.; Gabov, V.V.; Gnibidenko, G.S.; Dmitriyev, Y.I.; Zonenshayn, L.P.; et al. Solid rocks of the floor of the central part of the Sea of Okhotsk. Int. Geol. Rev. 1977, 19, 817–834. [Google Scholar] [CrossRef]
- Vasiliev, B.; Putintsev, V.K.; Markovsky, V.; Svyatogorova, N.; Selivanov, V.; Udintsev, G. Results of dragging the bottom of the Sea of Okhotsk. Sov. Geol. 1984, 12, 100–107. [Google Scholar]
- Zhitova, E.S.; Anikin, L.P.; Sergeeva, A.V.; Ismagilova, R.M.; Rashidov, V.A.; Chubarov, V.M.; Kupchinenko, A.N. Volborthite Occurrence at the Alaid Volcano (Atlasov Island, Kuril Islands, Russia). Geol. Ore Depos. 2021, 63, 735–748. [Google Scholar] [CrossRef]
- Britvin, S.N.; Dolivo-Dobrovolsky, D.V.; Krzhizhanovskaya, M.G. Software for processing of X-ray powder diffraction data obtained from the curved image plate detector of Rigaku RAXIS Rapid II diffractometer. Zapiski RMO 2017, 146, 104–107. (In Russian) [Google Scholar]
- Bruker-AXS Topas. General Profile and Structure Analysis Software for Powder Diffraction Data, Version 4.2; Bruker-AXS: Karlsruhe, Germany, 2009.
- CrysAlisPro Software System, Version 1.171.39.44; Rigaku Oxford Diffraction: Oxford, UK, 2015.
- Sheldrick, G.M. Crystal Structure Refinement with It SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. It OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Cameron, M.; Sueno, S.; Prewitt, C.T.; Papike, J.J. High-Temperature Crystal Chemistry of Acmite, Diopside, Hedenbergite Jadeite, Spodumene and Ureyite. Am. Mineral. 1973, 58, 594–618. [Google Scholar]
- Quartieri, S.; Oberti, R.; Boiocchi, M.; Dalconi, M.C.; Boscherini, F.; Safonova, O.; Woodland, A.B. Site preference and local geometry of Sc in garnets: Part II. The crystal-chemistry of octahedral Sc in the andradite–Ca3Sc2Si3O12 join. Am. Mineral. 2006, 91, 1240–1248. [Google Scholar] [CrossRef]
- Klein, S.; Korekawa, M. Die gemittelte Struktur des Labradorits. Neues Jahrb. Fur Mineral. Monatshefte 1976, 1976, 66–69. [Google Scholar]
- Speziale, S.; Duffy, T.S. Single-crystal elastic constants of fluorite (CaF2) to 9.3 GPa. Phys. Chem. Miner. 2002, 29, 465–472. [Google Scholar] [CrossRef]
- Graf, D.L. Crystallographic tables for the rhombohedral carbonates. Am. Mineral. 1961, 46, 1283–1316. [Google Scholar]
- Grew, E.S.; Hålenius, U.; Pasero, M.; Barbier, J. Recommended nomenclature for the sapphirine and surinamite groups (sapphirine supergroup). Mineral. Mag. 2008, 72, 839–876. [Google Scholar] [CrossRef]
- Zolotarev, A.A.; Krivovichev, S.V.; Avdontceva, M.S.; Zhitova, E.S.; Shchipalkina, N.V.; Pekov, I.V. Crystal Chemistry of “Malakhovite”, an Anthropogenic Analog of Khesinite from Burnt Dumps of the Chelyabinsk Coal Basin (South Urals). Crystallogr. Rep. 2021, 66, 66–75. [Google Scholar] [CrossRef]
- Peretyazhko, I.S.; Savina, E.A.; Khromova, E.A. Minerals of the rhönite-kuratite series in paralavas from a new combustion metamorphic complex in the Choir–Nyalga basin (Central Mongolia): Composition, mineral assemblages and formation conditions. Mineral. Mag. 2017, 81, 949–974. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Krüger, B.; Krüger, H.; Blass, G.; Widmer, R.; Galuskina, I.O. Wernerkrauseite, CaFe3+2Mn4+O6: The first nonstoichiometric post-spinel mineral, from Bellerberg volcano, Eifel, Germany. EJM 2016, 28, 485–493. [Google Scholar] [CrossRef]
- Knuever, M.; Mele, D.; Sulpizio, R. Mineralization and Skarn Formation Associated with Alkaline Magma Chambers Emplaced in a Limestone Basement: A Review. Minerals 2023, 13, 1184. [Google Scholar] [CrossRef]
- Reato, L.; Huraiová, M.; Ackerman, L.; Ďurišová, J.; Horschinegg, M.; Konečný, P.; Meicel, T.C.; Hurai, V. Forearc magmatism in the Pannonian basin recorded by metasomatised skarnoid xenoliths in Pliocene basalt (Novohrad-Gemer Volcanic Field, Slovakia). Lithos 2024, 482, 107741. [Google Scholar] [CrossRef]
- Davydova, V.O.; Shcherbakov, V.D.; Nekrylov, N.A.; Plechov, P.Y.; Yapaskurt, V.O. Sulfide Mineralization in Pyrometamorphosed Upper Crustal Xenoliths, Bezymianny Volcano, Kamchatka. Petrology 2023, 31, 358–382. [Google Scholar] [CrossRef]
- Shchipalkina, N.V.; Pekov, I.V.; Koshlyakova, N.N.; Britvin, S.N.; Zubkova, N.V.; Varlamov, D.A.; Sidorov, E.G. Unusual silicate mineralization in fumarolic sublimates of the Tolbachik volcano, Kamchatka, Russia–Part 1: Neso-, cyclo-, ino-and phyllosilicates. EJM 2020, 32, 101–119. [Google Scholar]
- Shchipalkina, N.V.; Pekov, I.V.; Koshlyakova, N.N.; Britvin, S.N.; Zubkova, N.V.; Varlamov, D.A.; Sidorov, E.G. Unusual silicate mineralization in fumarolic sublimates of the Tolbachik volcano, Kamchatka, Russia–Part 2: Tectosilicates. EJM 2020, 32, 121–136. [Google Scholar]
- Ganino, C.; Libourel, G.; Bernard, A. Fumarolic incrustations at Kudryavy volcano (Kamchatka) as a guideline for high-temperature (>850 C) extinct hydrothermal systems. J. Volcanol. Geoth. Res. 2019, 376, 75–85. [Google Scholar] [CrossRef]
- Chebotarev, D.A.; Veksler, I.V.; Wohlgemuth-Ueberwasser, C.; Doroshkevich, A.G.; Koch-Müller, M. Experimental study of trace element distribution between calcite, fluorite and carbonatitic melt in the system CaCO3 + CaF2 + Na2CO3 ± Ca3(PO4)2 at 100 MPa. Contrib. Mineral. Petrol. 2018, 174, 4. [Google Scholar] [CrossRef]
- Gozzi, F.; Gaeta, M.; Freda, C.; Mollo, S.; Di Rocco, T.; Marra, F.; Dallai, L.; Pack, A. Primary magmatic calcite reveals origin from crustal carbonate. Lithos 2014, 190–191, 191–203. [Google Scholar] [CrossRef]
- Veksler, I.V.; Dorfman, A.M.; Dulski, P.; Kamenetsky, V.S.; Danyushevsky, L.V.; Jeffries, T.; Dingwell, D.B. Partitioning of elements between silicate melt and immiscible fluoride, chloride, carbonate, phosphate and sulfate melts, with implications to the origin of natrocarbonatite. Geochim. Cosmochim. Acta 2012, 79, 20–40. [Google Scholar] [CrossRef]
- Vapnik, Y.; Sokol, E.; Murashko, M.; Sharygin, V. The enigma of Hatrurim. Mineral. Alm. 2006, 10, 69–77. [Google Scholar]
- Kruszewski, Ł.; Palchik, V.; Vapnik, Y.; Nowak, K.; Banasik, K.; Galuskina, I. Mineralogical, Geochemical, and Rock Mechanic Characteristics of Zeolite-Bearing Rocks of the Hatrurim Basin, Israel. Minerals 2021, 11, 1062. [Google Scholar] [CrossRef]
- Stracher, G.B. (Ed.) Coal and Peat Fires: A Global Perspective: Volume 5: Case Studies–Advances in Field and Laboratory Research; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Cesnokov, B.; Kotrly, M.; Nisanbajev, T. Brennende Abraumhalden und Aufschlüsse im Tscheljabinsker Kohlenbecken–eine reiche Mineralienküche [Burning tailings and outcrops in the Chelyabinsk coal basin–a rich mineral kitchen]. Miner. Welt 1998, 9, 54–63. [Google Scholar]
- Yakubovich, O.V.; Zayakina, N.V.; Oleinikov, O.B.; Kostin, A.V. Esseneite from xenoliths in dacite lavas: Crystal structure and genesis. Geol. Ore Depos. 2019, 61, 689–695. [Google Scholar] [CrossRef]
- Ciesielczuk, J.; Kruszewski, Ł.; Majka, J. Comparative mineralogical study of thermally-altered coal-dump waste, natural rocks and the products of laboratory heating experiments. Int. J. Coal Geol. 2015, 139, 114–141. [Google Scholar] [CrossRef]
- Cosca, M.A.; Rouse, R.R.; Essene, E.J. Dorrite [Ca2(Mg2Fe3+)4(Al4Si2)O20], a new member of the aenigmatite group from a pyrometamorphic melt-rock. Am. Min. 1988, 73, 1440–1448. [Google Scholar]
- Pascal, M.; Katona, I.; Fonteilles, M.; Verkaeren, J. Relics of high-temperature clinopyroxene on the join Di-CaTs with up to 72 mol.% Ca (Al, Fe3+) AlSiO6 in the skarns of Ciclova and Magureaua Vatei, Carpathians, Romania. Can. Miner. 2005, 43, 857. [Google Scholar] [CrossRef]
- Havette Ledebt, A.; Clocchiatti, R.; Nativel, P.; Montaggioni, L. Une paragenèse inhabituelle à fassaite, mélilite et rhönite dans un basalte alcalin contaminé au contact d’un récif corallien (Saint-Leu, Ile de la Réunion). Bull. De Minéralogie 1982, 105, 364–375. [Google Scholar] [CrossRef]
- Arculus, R.J.; Wills, K.J. The petrology of plutonic blocks and inclusions from the Lesser Antilles island arc. J. Petrol. 1980, 21, 743–799. [Google Scholar] [CrossRef]
- Cosca, M.A.; Peacor, D.R. Chemistry and structure of esseneite (CaFe3+AlSiO6); a new pyroxene produced by pyrometamorphism. Am. Min. 1987, 72, 148–156. [Google Scholar]
- Clark, J.A.; Appleman, D.E.; Papike, J.J. Crystal-chemical characterization of clinopyroxenes based on eight new structure refinements. Mineral. Soc. Amer. Spec. 1969, 2, 31–50. [Google Scholar]
- Kimura, M.; Mikouchi, T.; Suzuki, A.; Miyahara, M.; Ohtani, E.; El Goresy, A. Kushiroite, CaAlAlSiO6: A new mineral of the pyroxene group from the ALH 85085 CH chondrite, and its genetic significance in refractory inclusions. Am. Min. 2009, 94, 1479–1482. [Google Scholar] [CrossRef]
- Shchipalkina, N.V.; Pekov, I.V.; Chukanov, N.V.; Koshlyakova, N.N.; Ternes, B.; Schüller, W. Crystal chemistry of dorrite from the Eifel volcanic region, Germany, and chemical variations in the khesinite-dorrite-rhönite-kuratite solid-solution system. Mineral. Petrol. 2019, 113, 249–259. [Google Scholar] [CrossRef]
- Kong, F.-M.; Schertl, H.-P.; Zhao, L.-Q.; Li, X.-P.; Liu, X.-H. Rhönite in Cenozoic alkali basalt from Changle, Shandong Province, China, and its significance. EJM 2020, 32, 325–346. [Google Scholar] [CrossRef]
- Nizametdinov, I.R.; Smirnov, S.Z.; Shevko, A.Y.; Kuzmin, D.V.; Kotov, A.A.; Sekisova, V.S.; Timina, T.Y. High-Alumina Daughter Phases in Olivine-Hosted Melt Inclusions from Kudryavy and Menshiy Brat Volcanoesv (Medvezhia Caldera, Iturup Island). Russ. J. Pac. Geol. 2024, 18, 410–435. [Google Scholar] [CrossRef]
- Kunzmann, T. The aenigmatite-rhonite mineral group. EJM 1999, 11, 743–756. [Google Scholar] [CrossRef]
- Tsukimura, K.; Kanazawa, Y.; Aoki, M.; Bunno, M. Structure of wadalite Ca6Al5Si2O16Cl3. Acta Crystallogr. C 1993, 49, 205–207. [Google Scholar] [CrossRef]
- Ma, C.; Krot, A.N.; Nagashima, K.; Dunn, T. Louisfuchsite, Ca2(Mg4Ti2)(Al4Si2)O20, a new rhönite-type mineral from the NWA 4964 CK meteorite: A refractory phase from the solar nebula. Am. Min. 2024, 109, 2006–2012. [Google Scholar] [CrossRef]
- Sharygin, V.V. Mayenite-supergroup minerals from burned dump of the Chelyabinsk Coal Basin. Russ. Geol. Geophys. 2015, 56, 1603–1621. [Google Scholar] [CrossRef]
- Banno, Y.; Bunno, M.; Tsukimura, K. A reinvestigation of holotype wadalite from Tadano, Fukushima Prefecture, Japan. Mineral. Mag. 2018, 82, 1023–1031. [Google Scholar] [CrossRef]
- Whitley, S.; Halama, R.; Gertisser, R.; Preece, K.; Deegan, F.M.; Troll, V.R. Magmatic and Metasomatic Effects of Magma–Carbonate Interaction Recorded in Calc-silicate Xenoliths from Merapi Volcano (Indonesia). J. Petrol. 2020, 61, eagga048. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).