Mineralogical and Micro-Computer Tomographic (μCT) Texture Investigations of Egyptian Blue Spheres (Aguntum, East Tyrol; Retznei and Wagna, Flavia Solva, South Styria)
Abstract
1. Introduction
1.1. Use and Significance of Egyptian Blue in Antiquity
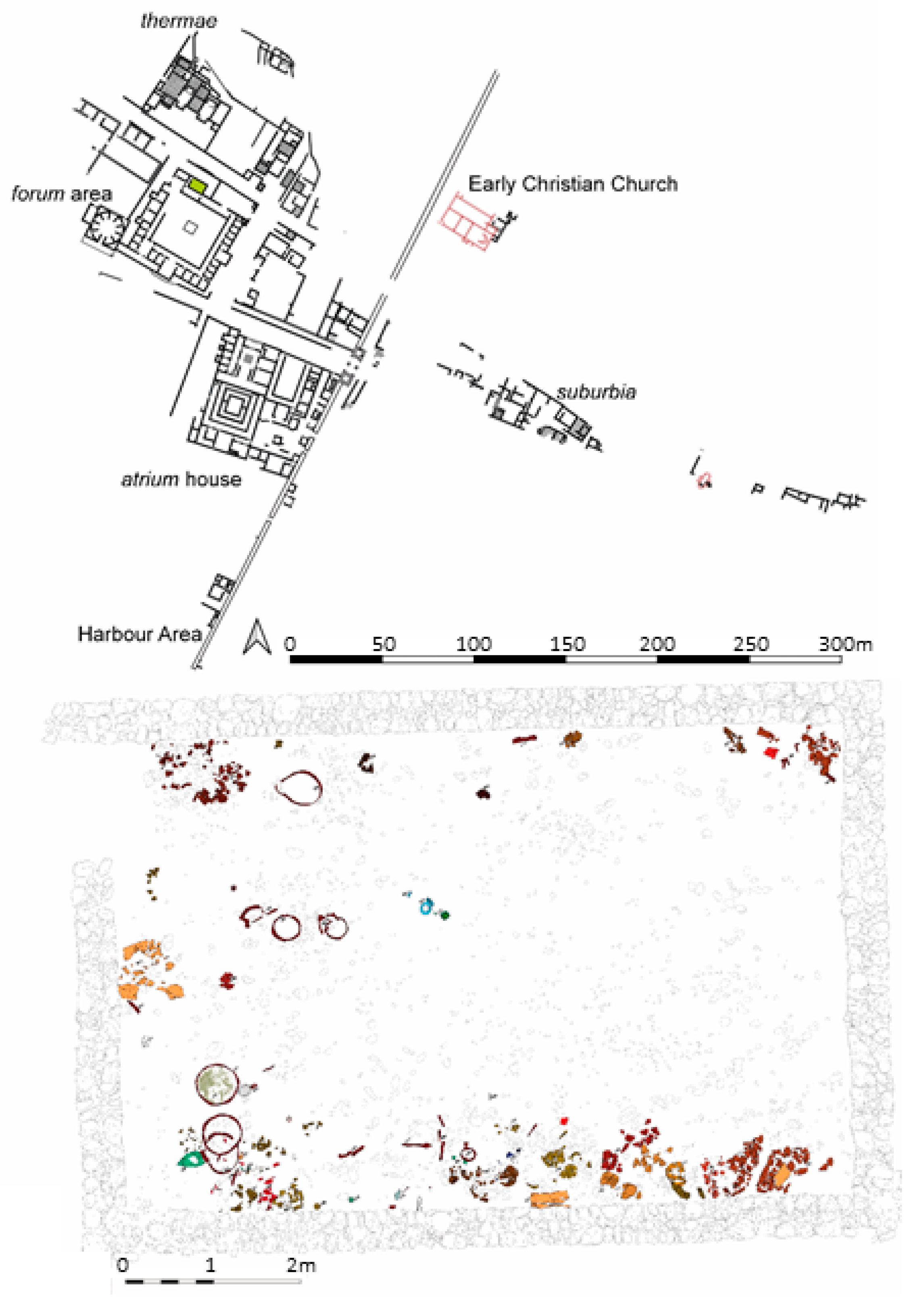
1.2. Geographical Location of the Roman Municipium Claudium Aguntum (Lienz)
1.3. Retznei and Flavia Solva (Wagna)
2. Materials and Methods
2.1. Scanning Electron Microscope
2.2. Micro X-Ray Fluorescence Analysis
2.3. Micro-Computed Tomography and Image Post Processing
3. Results
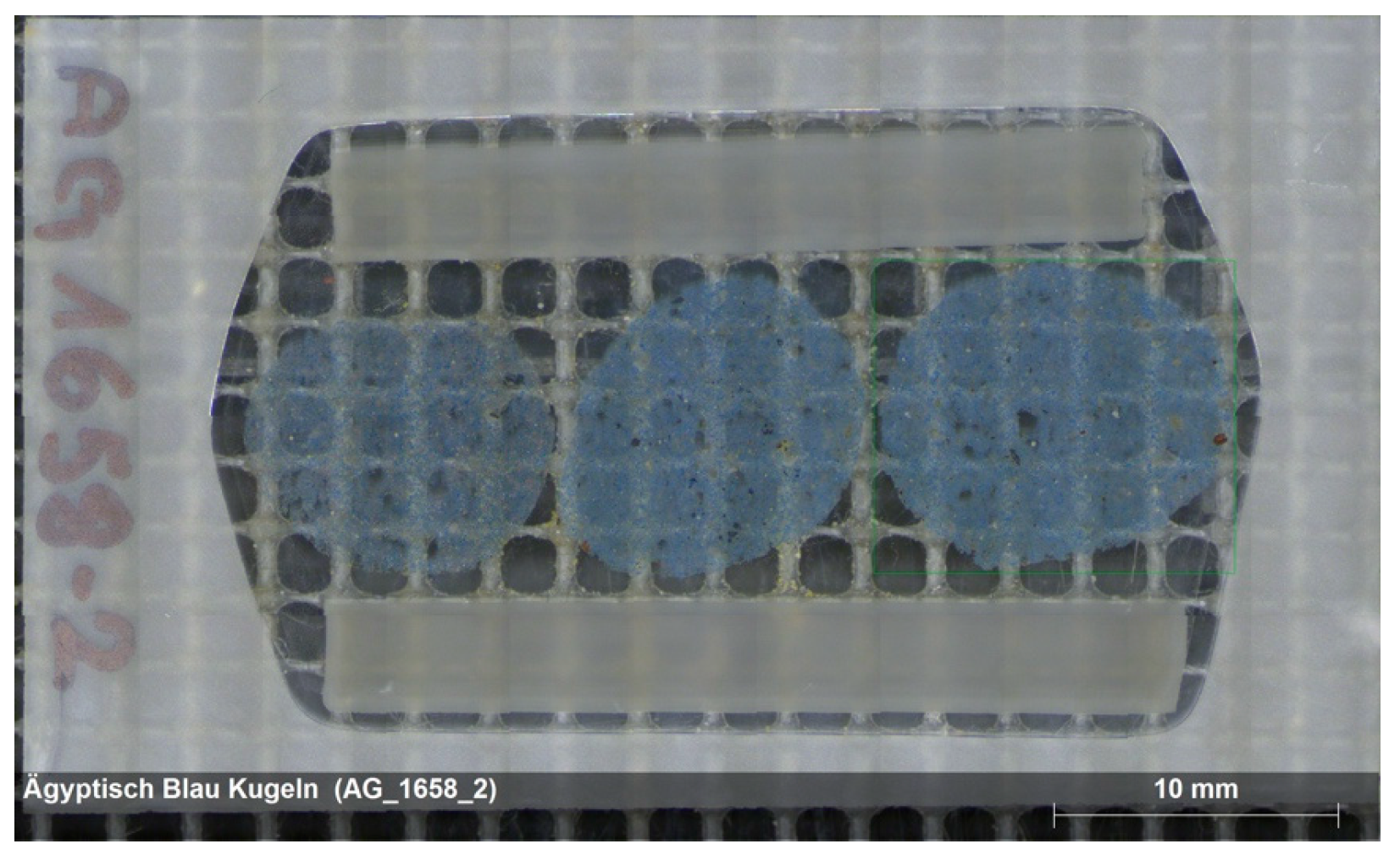
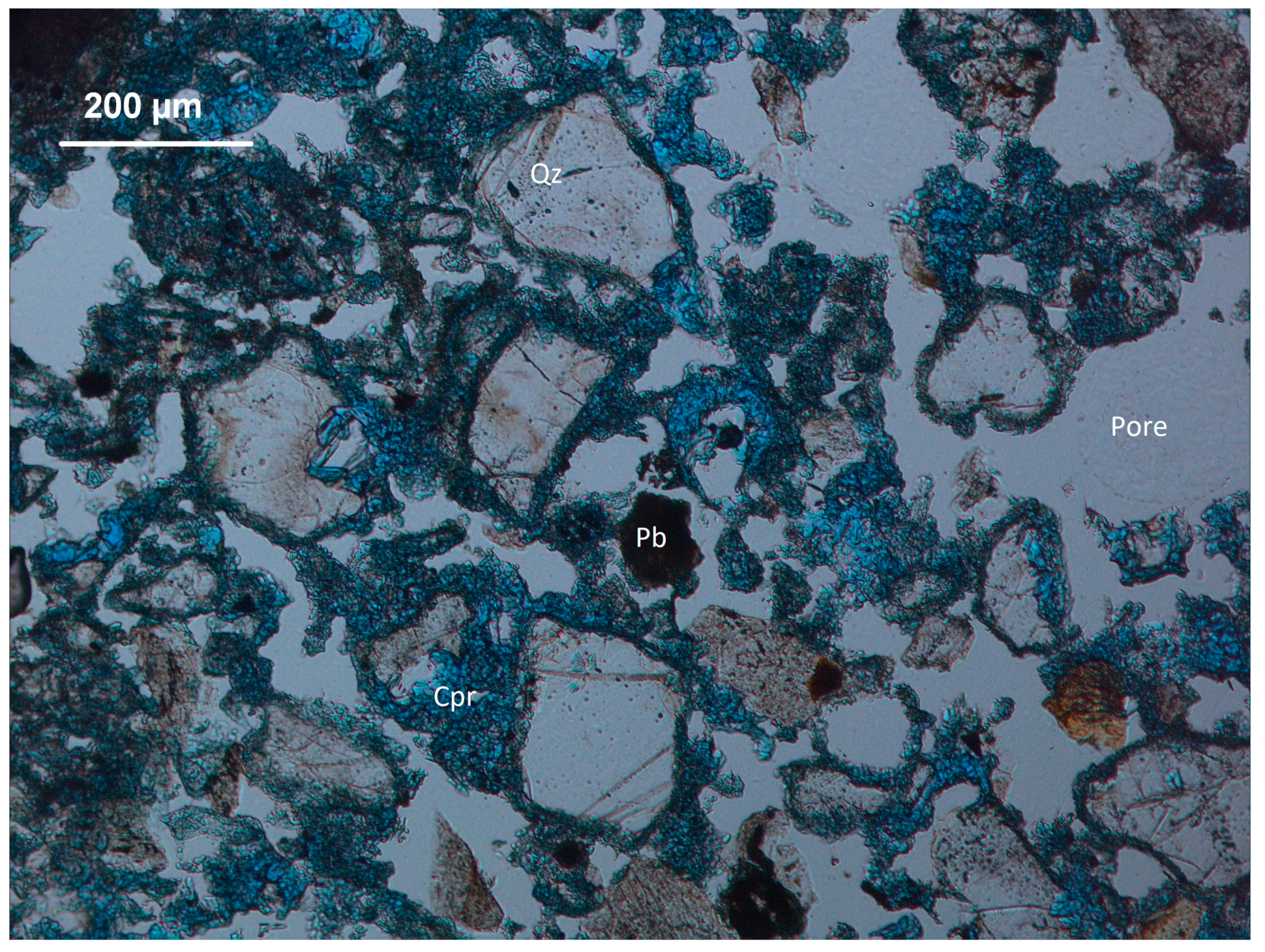
3.1. Thin Section Petrography
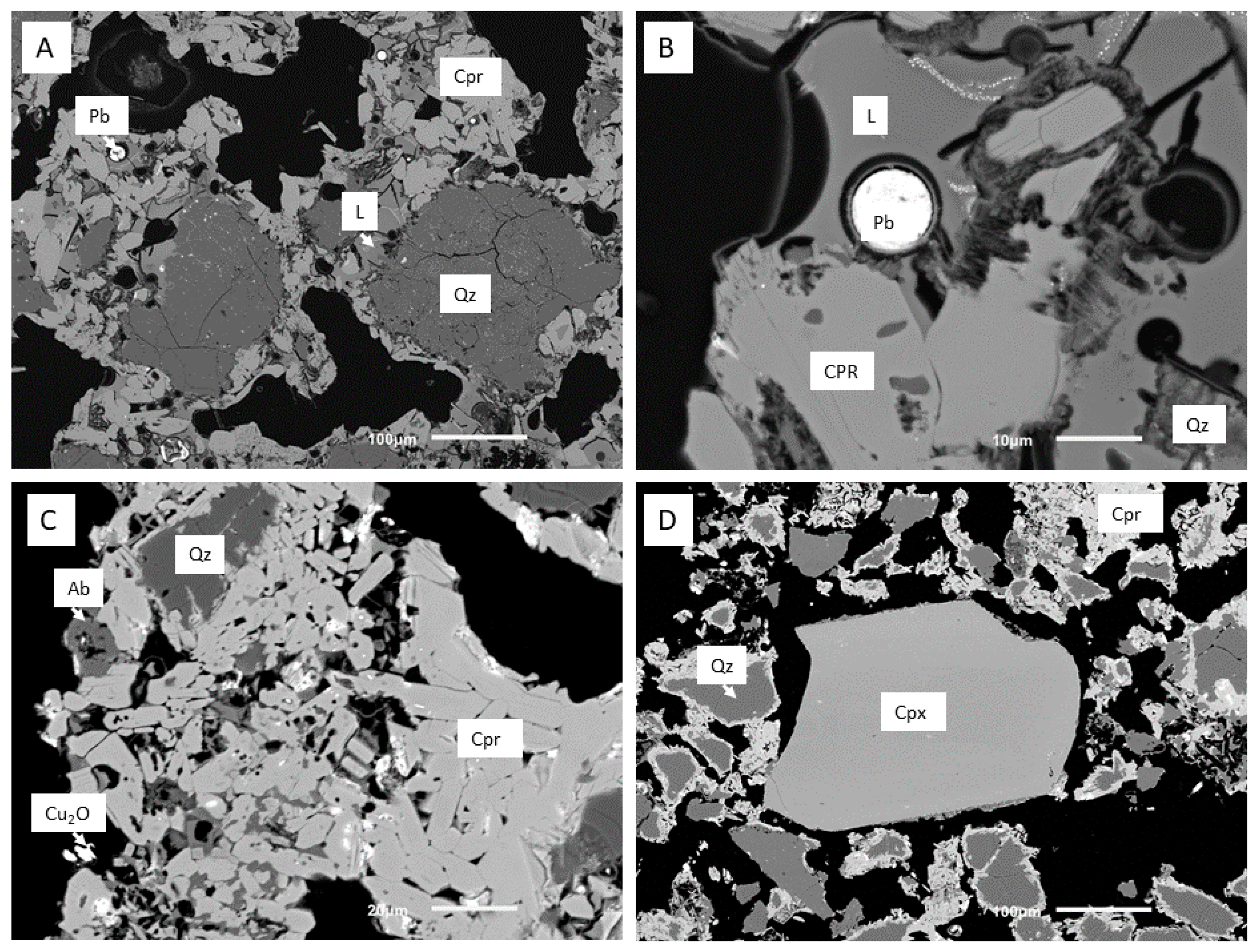
3.2. Scanning Electron Microscope, Energy Dispersive X-Ray Spectroscopy (SEM-EDX)
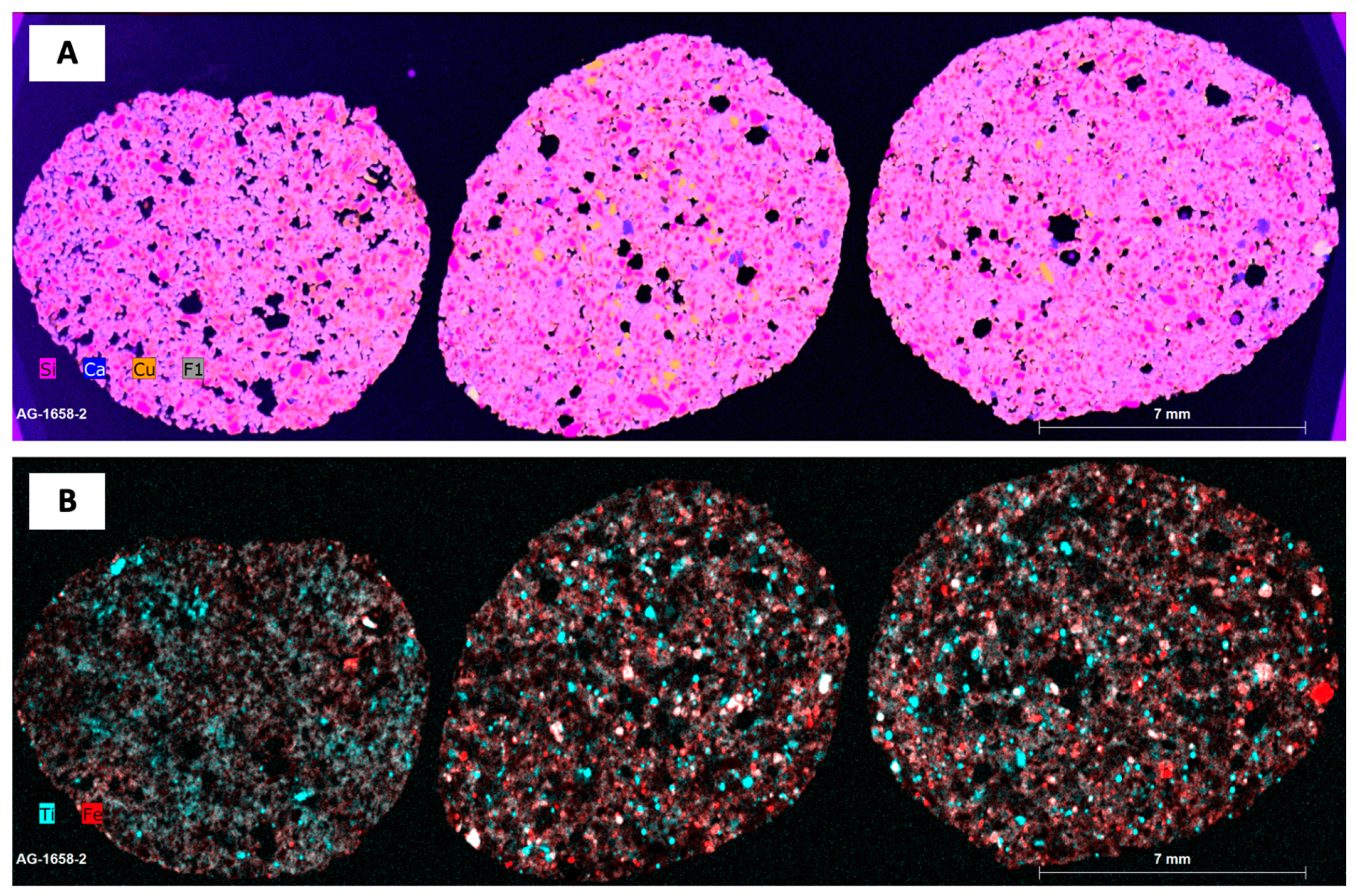
3.3. Micro X-Ray Fluorescence Analysis (μ-XRF)
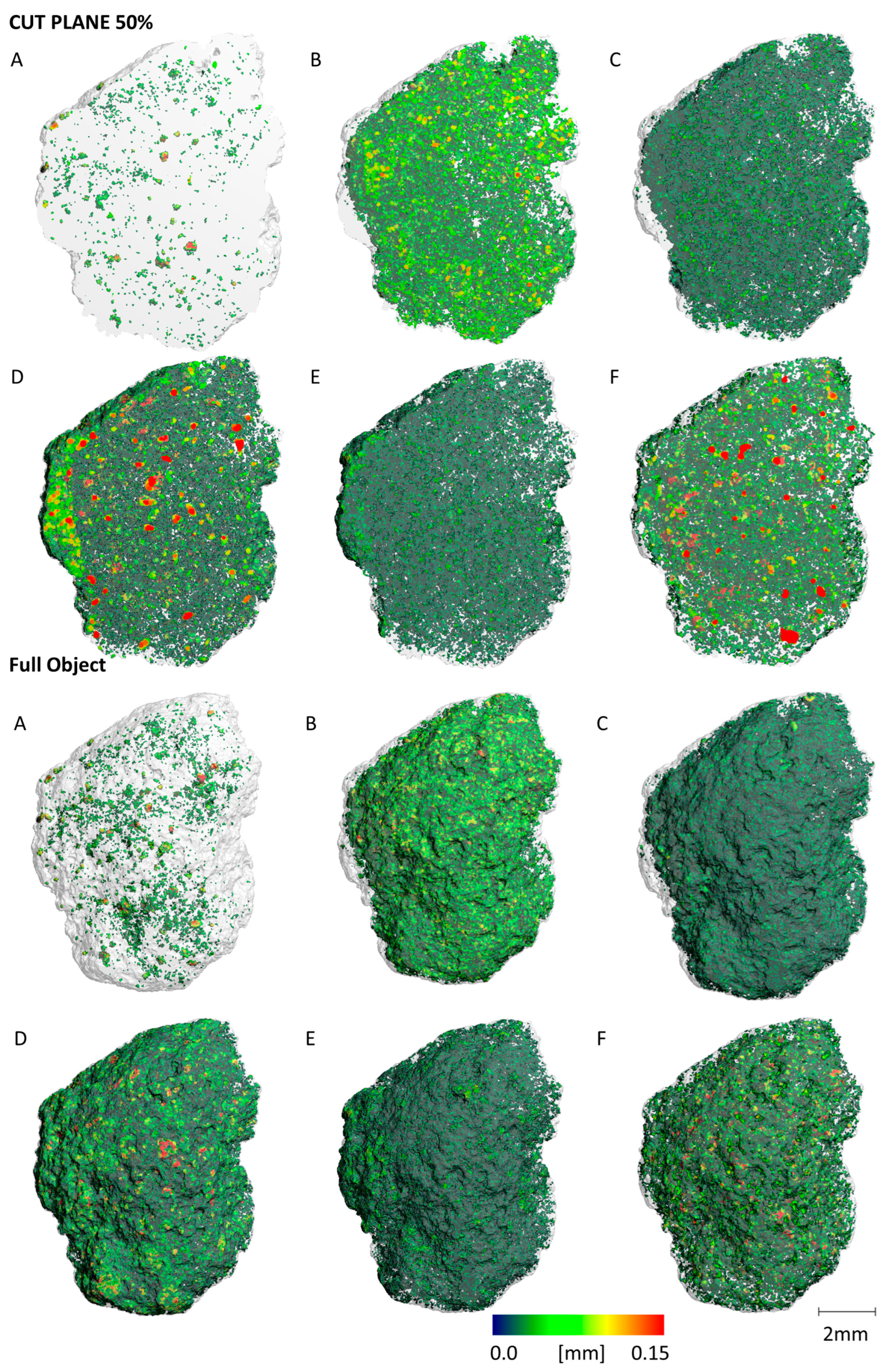
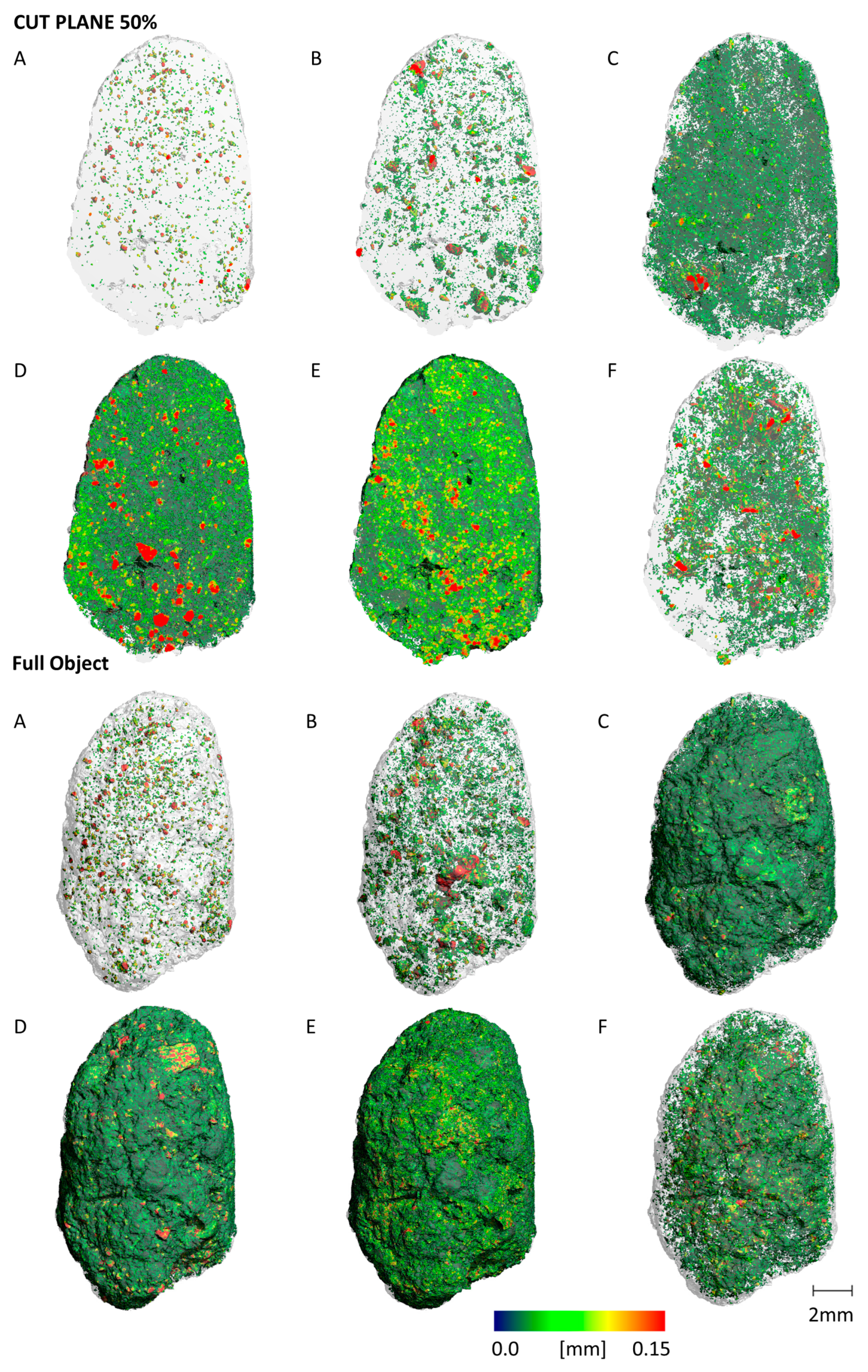
3.4. Micro Computer Tomography (µCT)
4. Discussion and Interpretation
Hypotheses on the Production Process of the Egyptian Blue Pellets from Aguntum
- 1.
- CaCO3 + 4 SiO2 + CuO = CaCuSi4O10 + CO2 (copper source: tenorite);
- 2.
- 2 CaCO3 + 8 SiO2 + Cu2O + 0.5 O2 = 2 CaCuSi4O10 + 2 CO2 (copper source: cuprite);
- 2.
- 2 CaCO3 + 8 SiO2 + Cu2S + O2 = 2 CaCuSi4O10 + 2 CO2 + 0.5 S2 (copper source: chalcocite);
- 3.
- CaCO3 + 4 SiO2 + Cu + 0.5 O2 = CaCuSi4O10 + CO2 (copper source: Cu metal).
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delamare, F. Egyptian blue, the blue pigment of Mediterranean antiquity: From Egyptian hsbd iryt to Roman caeruleum. In Blue Pigments: 5000 Years of Art and Industry; Archetype Publications: London, UK, 2013; pp. 1–36. [Google Scholar]
- Panagiotaki, M.; Tite, M.; Maniatis, Y. Egyptian blue in Egypt and beyond: The Aegean and the Near East. In Proceedings of the Tenth International Congress of Egyptologists, Rhodes, Greece, 22–29 May 2008; pp. 1769–1789. [Google Scholar]
- Minguzzi, C. Cuprorivaite: Un nuovo minerale. Period. Mineral. 1938, 9, 333–345. [Google Scholar]
- Rowland, I.D.; Howe, T.N. (Eds.) . Vitruvius: ‘Ten Books on Architecture’; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Mirti, P.; Appolonia, L.; Casoli, A.; Ferrari, R.P.; Laurenti, E.; Canesi, A.A.; Chiari, G. Spectrochemical and structural studies on a Roman sample of Egyptian blue. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1995, 51, 437–446. [Google Scholar] [CrossRef]
- Skovmøller, A.; Brøns, C.; Sargent, M.L. Egyptian blue: Modern myths, ancient realities. J. Rom. Archaeol. 2016, 29, 371–387. [Google Scholar] [CrossRef]
- Dariz, P.; Schmid, T. Trace compounds in Early Medieval Egyptian blue carry information on provenance, manufacture, application, and ageing. Sci. Rep. 2021, 11, 11296. [Google Scholar] [CrossRef] [PubMed]
- Laurie, A.P.; Mclintock, W.F.P.; Miles, F.D. Egyptian blue. Proc. R. Soc. London. Ser. A Contain. Pap. Math. Phys. Character 1914, 89, 418–429. [Google Scholar]
- Cavassa, L. La production du bleu égyptien durant l’époque hellénistique et l’Empire romain (IIIe av. J.-C.-Ier s. apr. J.-C.). Bull. Corresp. Hellénique-Supplément 2018, 56, 13–34. [Google Scholar]
- Zerobin, B.; Goldenberg, G.; Auer, M.; Töchterle, U.; Angerer, T.; Hejny, C.; Tropper, P. Ägyptisch Blau: Pigmentfunde aus Raum 289 des römischen Municipium Claudium Aguntum. In Alpine Landschaftsnutzung im Ager Aguntinus; Nearchos; Harrassowitz: Wiesbaden, Germany, 2021; pp. 183–192. [Google Scholar]
- Rodler-Rørbo, A.; Baragona, A.J.; Auer, M.; Zerobin, B.; Tropper, P.; Schrettle, B. Egyptian blue pellets from Noricum: On pigment production and trade. In Proceedings of the 11th International Round Table on Polychromy in Ancient Sculpture and Architecture, Rome, Italy, 9–12 November 2022. [Google Scholar]
- Mommsen, E.T. CIL III, Pars II: Inscriptionum Illyrici partes VI. VII. Res gestae divi Augusti. Edictum Diocletiani de pretiis rerum. Privilegia militum veteranorumque. Instrumenta Dacica. apud G. Reimerum: Berlin, Germany, 1873; ISBN 3-11-003190-6. [Google Scholar]
- Auer, M. Municipium Claudium Aguntum. Excavations in the city centre (2006–2015). In New Discoveries Between the Alps and the Black Sea. Results from Roman Sites in the Period Between 2005 and 2015; Janežič, M., Nadbath, B., Mulh, T., Žižek, I., Eds.; 2018; pp. 93–113. [Google Scholar]
- Schrettle, B.; Tsironi, S. Retznei: Villa, Vicus, Heiligtum? Ergebnisse der Grabungen der Jahre 2004 bis 2012, mit einem Beitrag von Gabriele Wrolli. Graz-Wien 2021, 84, 174. [Google Scholar]
- Heinemann, J. Mineralogische und Computertomographische Untersuchungen an Ägyptisch-Blau Kugeln (Aguntum, Osttirol; Retznei und Wagna, Flavia Solva, Südsteiermark). Bachelor‘s Thesis, University of Innsbruck, Innsbruck, Austria, 2022. Unpublished. [Google Scholar]
- Cornard, P.H.; Degenhart, G.; Tropper, P.; Moernaut, J.; Strasser, M. Application of micro-CT to resolve textural properties and assess primary sedimentary structures of deep-marine sandstones. Depos. Rec. 2024, 10, 559–580. [Google Scholar] [CrossRef]
- Guntoro, P.I.; Ghorbani, Y.; Koch, P.H.; Rosenkranz, J. X-ray microcomputed tomography (µCT) for mineral characterization: A review of data analysis methods. Minerals 2019, 9, 183. [Google Scholar] [CrossRef]
- Wang, Y.; Miller, J.D. Current developments and applications of micro-CT for the 3D analysis of multiphase mineral systems in geometallurgy. Earth-Sci. Rev. 2020, 211, 103406. [Google Scholar] [CrossRef]
- Cnudde, V.; Boone, M.N. High-resolution X-ray computed tomography in geosciences: A review of the current technology and applications. Earth-Sci. Rev. 2013, 123, 1–17. [Google Scholar] [CrossRef]
- Buyse, F.; Dewaele, S.; Boone, M.N.; Cnudde, V. Contribution of 3D automated mineralogy in unraveling the formation history of Nb-Ta-Sn mineralized LCT pegmatites in the Karagwe-Ankole belt (Central Africa). Geol. Belg. 2024, 27, 1–14. [Google Scholar] [CrossRef]
- Hildebrand, T.; Rüegsegger, P. A new method for the model-independent assessment of thickness in three-dimensional images. J. Microsc. 1997, 185, 67–75. [Google Scholar] [CrossRef]
- Napporn, T.W.; Canaff, C.; Bere, E.; Hacker, V. Characterization Methods for Components and Materials. In Fuel Cells and Hydrogen; Elsevier: Amsterdam, The Netherlands, 2018; pp. 155–173. [Google Scholar]
- Papadopoulou, D.; Sakalis, A.; Merousis, N.; Tsirliganis, N.C. Study of decorated archeological ceramics by micro X-ray fluorescence spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2007, 580, 743–746. [Google Scholar] [CrossRef]
- Pradell, T.; Salvado, N.; Hatton, G.D.; Tite, M.S. Physical processes involved in production of the ancient pigment, Egyptian blue. J. Am. Ceram. Soc. 2006, 89, 1426–1431. [Google Scholar] [CrossRef]
- El Goresy, A. Polychromatic Wall Painting Decorations in Monuments of Pharaonic Egypt: Compositions, chronology and painting techniques. In Proceedings of the First International Symposium the Wall Paintings of Thera: Proceedings of the First International Symposium Petros M. Nomikos Conference Centre (ed. S. Sherratt), (Thera, Hellas, 1997); 2000; Volume 1, pp. 49–70. [Google Scholar]
- Tite, M.S.; Bimson, M.; Cowell, M.R. Technological examination of Egyptian blue. Am. Chem. Soc. 1984, 205, 215–242. [Google Scholar]
- Lazzarini, L.; Verità, M. First evidence for 1st century AD production of Egyptian blue frit in Roman Italy. J. Archaeol. Sci. 2015, 53, 578–585. [Google Scholar] [CrossRef]
- Hatton, G.D.; Shortland, A.J.; Tite, M.S. The production technology of Egyptian blue and green frits from second millennium BC Egypt and Mesopotamia. J. Archaeol. Sci. 2008, 35, 1591–1604. [Google Scholar] [CrossRef]
- Kostomitsopoulou Marketou, A.; Andriulo, F.; Steindal, C.; Handberg, S. Egyptian Blue pellets from the first century BCE workshop of Kos (Greece): Microanalytical investigation by optical microscopy, scanning electron microscopy-X-ray energy dispersive spectroscopy and micro-Raman spectroscopy. Minerals 2020, 10, 1063. [Google Scholar] [CrossRef]
- Delamare, F. Sur les processus physiques intervenant lors de la synthèse du bleu égyptien. ArchéoSciences Rev. d’Archéométrie 1997, 21, 103–119. [Google Scholar] [CrossRef]




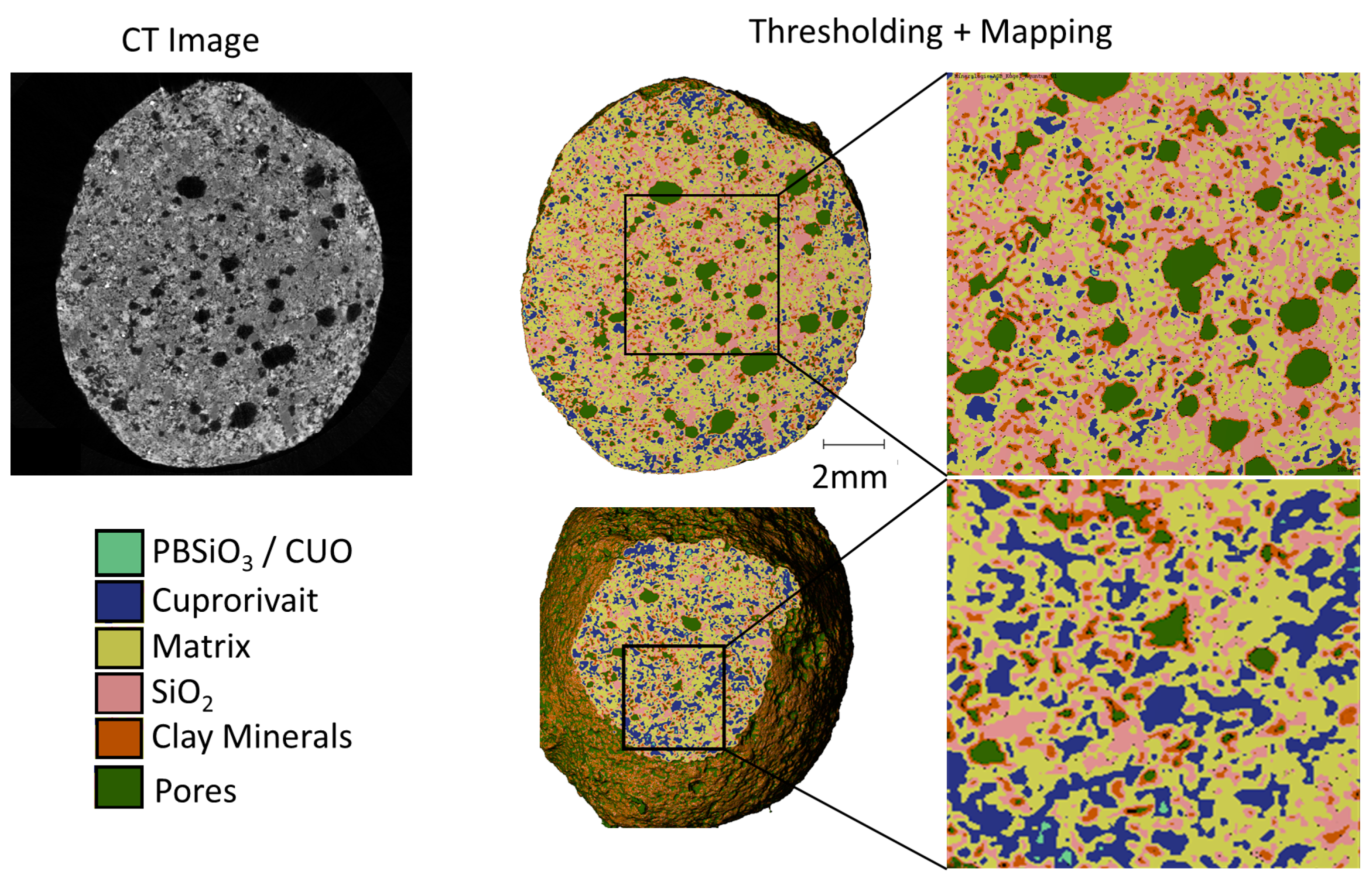
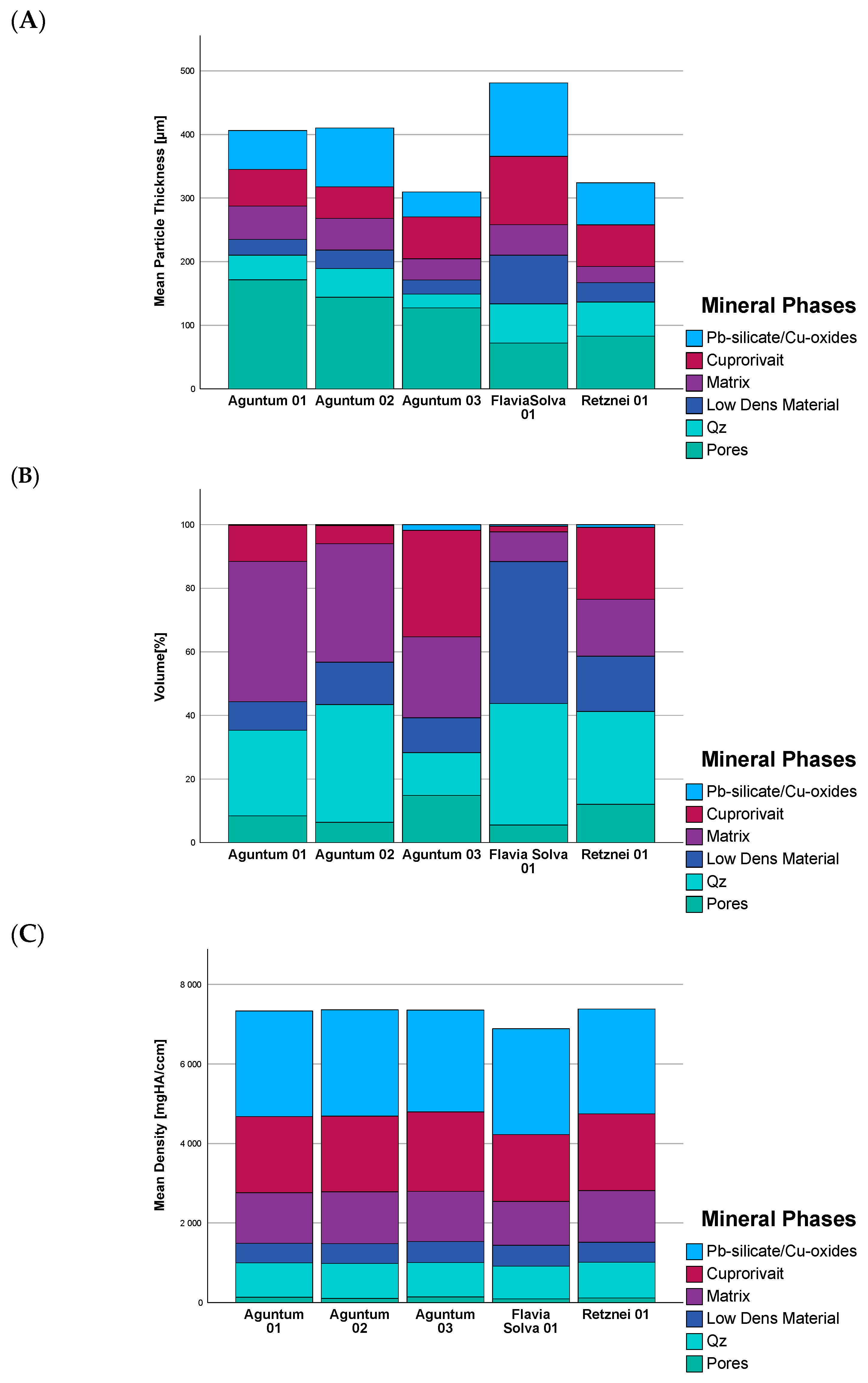

| Mineral Groups | Density |
|---|---|
| CuO, PbSiO3 (copper oxide, lead silicate) | 2301–3000 [mgHA/mm3] |
| CaCuSi4O10 (cuprorivaite) | 1521–2300 [mgHA/mm3] |
| Silicate matrix (clinopyroxene, plagioclase, albite, glass) | 1041–1500 [mgHA/mm3] |
| SiO2 (quartz, cristobalite) | 736–1040 [mgHA/mm3] |
| Undefined low-density mineral clays (alteration phases) | 456–735 [mgHA/mm3] |
| Pores | 0–455 [mgHA/mm3] |
| Analysis | SiO2 | Al2O3 | FeO | MgO | Cu2O | PbO | CaO | Na2O | K2O | Cl | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinopyroxene | 49.41 | 2.81 | 7.36 | 11.63 | n.d. | n.d. | 28.79 | n.d. | n.d. | n.d. | 100.00 |
| Pb-silicate | 32.35 | 2.43 | n.d. | 1.61 | n.d. | 63.62 | n.d. | n.d. | n.d. | n.d. | 100.00 |
| Pb-silicate | 29.96 | 1.24 | n.d. | 1.00 | n.d. | 67.79 | n.d. | n.d. | n.d. | n.d. | 100.00 |
| Albite | 66.83 | 17.37 | 3.31 | n.d. | n.d. | n.d. | n.d. | 10.48 | 2.01 | n.d. | 100.00 |
| Glass | 72.10 | 7.27 | 2.26 | 1.00 | 4.27 | n.d. | 1.69 | 8.70 | 2.31 | 0.41 | 100.00 |
| Glass | 68.70 | 7.75 | 3.13 | n.d. | 6.39 | n.d. | 2.77 | 6.22 | 2.34 | 2.70 | 100.00 |
| Glass | 71.18 | 6.15 | 1.61 | n.d. | 6.49 | n.d. | 2.57 | 10.02 | 1.60 | 0.38 | 100.00 |
| Cuprorivaite | 62.76 | n.d. | n.d. | 16.54 | 20.70 | n.d. | n.d. | n.d. | n.d. | n.d. | 100.00 |
| Cuprorivaite | 61.38 | n.d. | n.d. | 17.58 | 21.04 | n.d. | n.d. | n.d. | n.d. | n.d. | 100.00 |
| Cuprorivaite | 63.11 | n.d. | n.d. | 16.82 | 20.08 | n.d. | n.d. | n.d. | n.d. | n.d. | 100.00 |
| Cuprorivaite | 60.28 | n.d. | n.d. | 17.75 | 21.98 | n.d. | n.d. | n.d. | n.d. | n.d. | 100.00 |
| Cuprorivaite | 61.82 | n.d. | n.d. | 17.41 | 20.77 | n.d. | n.d. | n.d. | n.d. | n.d. | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degenhart, G.; Heinemann, J.; Tropper, P.; Rodler-Rørbo, A.; Zerobin, B.; Auer, M.; Goldenberg, G. Mineralogical and Micro-Computer Tomographic (μCT) Texture Investigations of Egyptian Blue Spheres (Aguntum, East Tyrol; Retznei and Wagna, Flavia Solva, South Styria). Minerals 2025, 15, 302. https://doi.org/10.3390/min15030302
Degenhart G, Heinemann J, Tropper P, Rodler-Rørbo A, Zerobin B, Auer M, Goldenberg G. Mineralogical and Micro-Computer Tomographic (μCT) Texture Investigations of Egyptian Blue Spheres (Aguntum, East Tyrol; Retznei and Wagna, Flavia Solva, South Styria). Minerals. 2025; 15(3):302. https://doi.org/10.3390/min15030302
Chicago/Turabian StyleDegenhart, Gerald, Julius Heinemann, Peter Tropper, Alexandra Rodler-Rørbo, Bianca Zerobin, Martin Auer, and Gert Goldenberg. 2025. "Mineralogical and Micro-Computer Tomographic (μCT) Texture Investigations of Egyptian Blue Spheres (Aguntum, East Tyrol; Retznei and Wagna, Flavia Solva, South Styria)" Minerals 15, no. 3: 302. https://doi.org/10.3390/min15030302
APA StyleDegenhart, G., Heinemann, J., Tropper, P., Rodler-Rørbo, A., Zerobin, B., Auer, M., & Goldenberg, G. (2025). Mineralogical and Micro-Computer Tomographic (μCT) Texture Investigations of Egyptian Blue Spheres (Aguntum, East Tyrol; Retznei and Wagna, Flavia Solva, South Styria). Minerals, 15(3), 302. https://doi.org/10.3390/min15030302






