Green Process for the Preparation of MnCO3 and Recovery of By-Product Mg-Containing (NH4)2SO4 Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
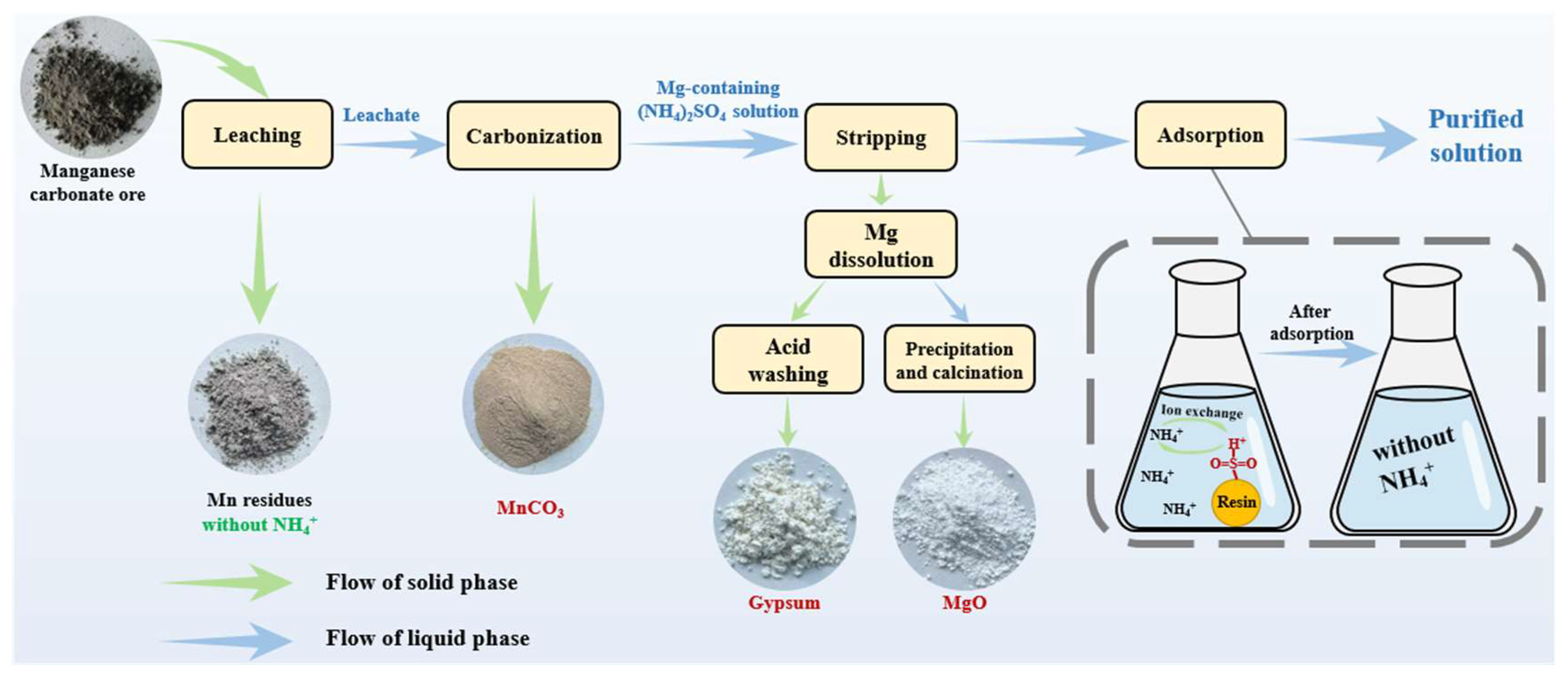
2.2. Green Process for Manganese Carbonate Ore Resource Utilization
2.2.1. Leaching Experiments
2.2.2. Carbonization Experiments
2.3. Stripping and Adsorption Tests
2.3.1. Stripping Test
2.3.2. Adsorption Test
2.4. Analytical Techniques
3. Results and Discussion
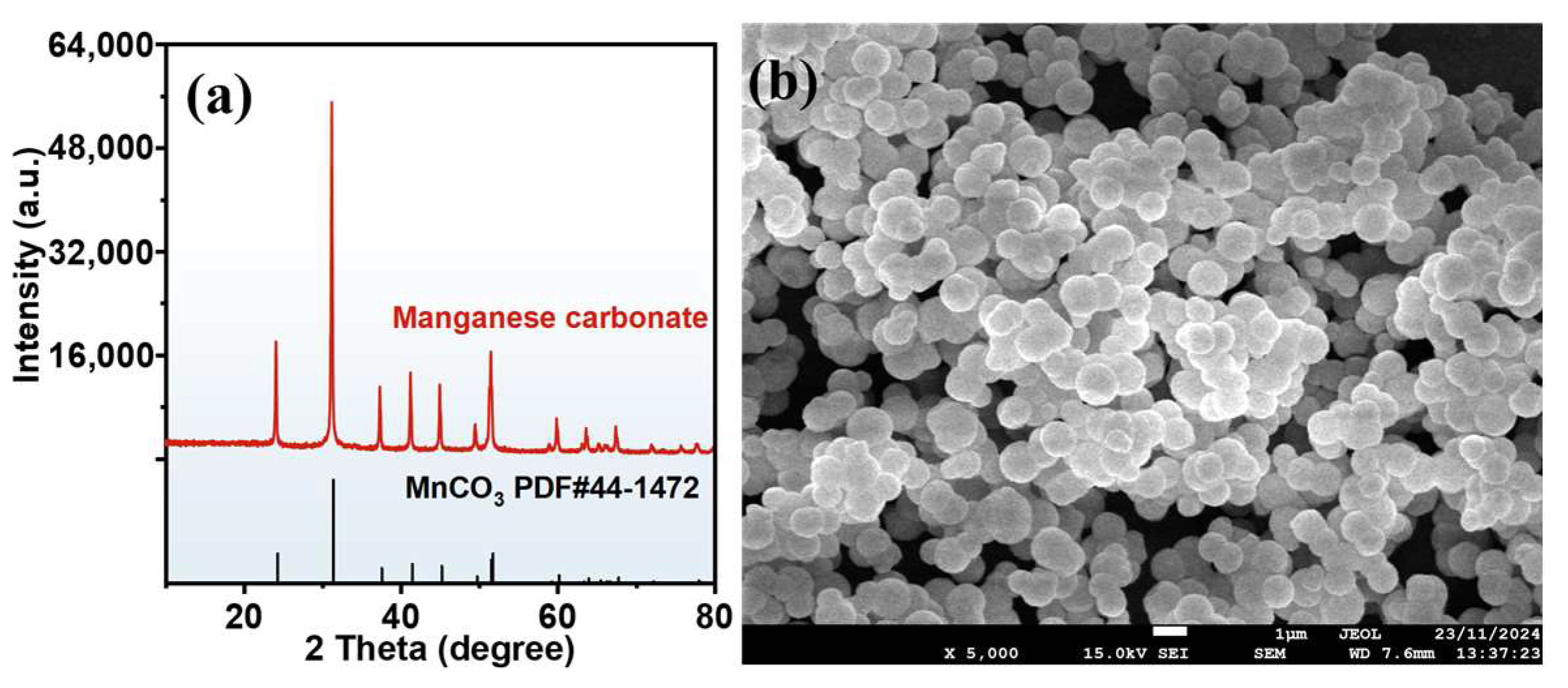
3.1. Preparation of Manganese Carbonate
3.2. Treatment of Mg-Containing Ammonium Sulfate Solution
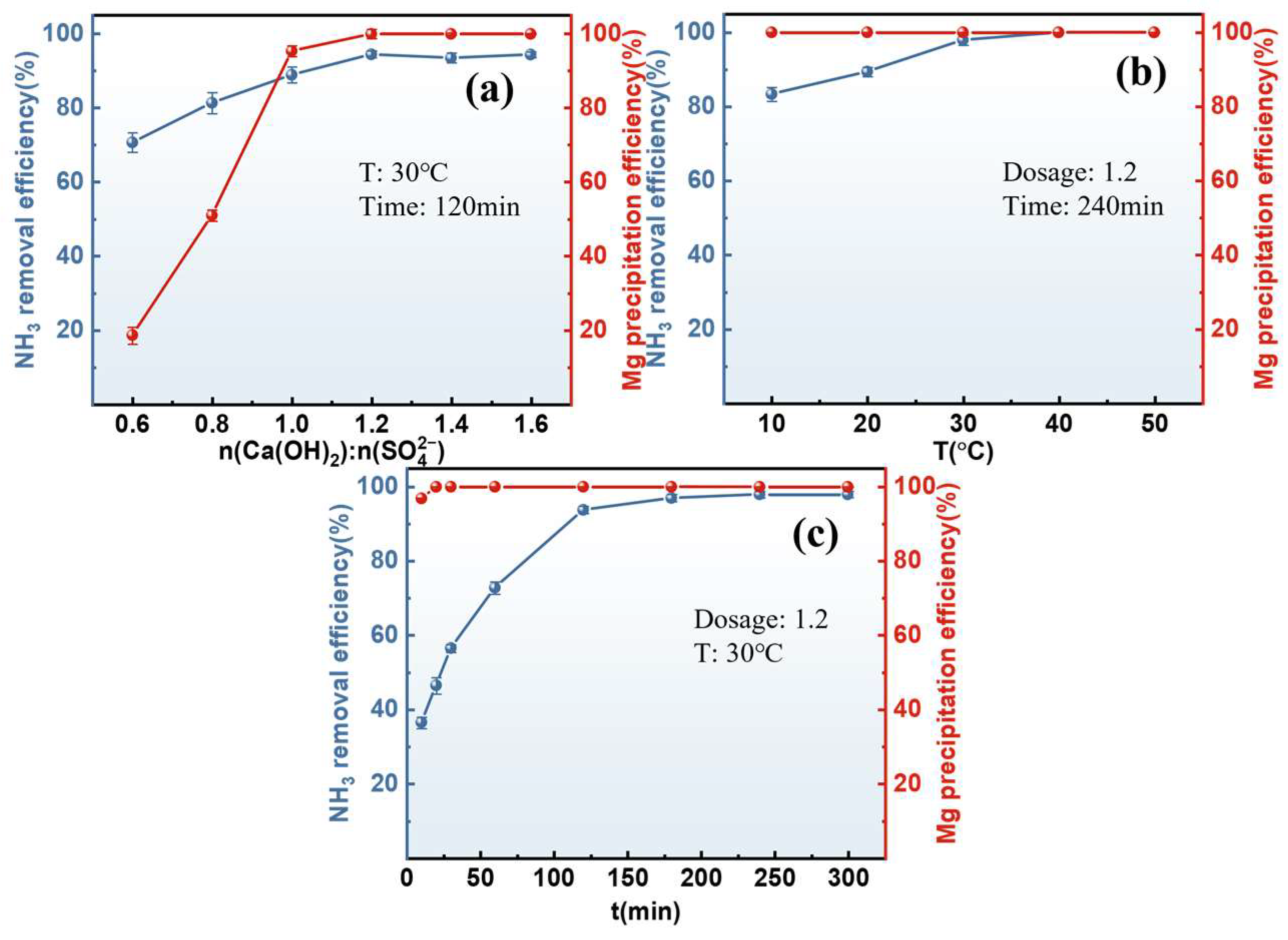
3.2.1. Stripping of Mg-Containing Ammonium Sulfate Solution
3.2.2. Resource Utilization of Magnesium
3.3. Adsorption Experiments
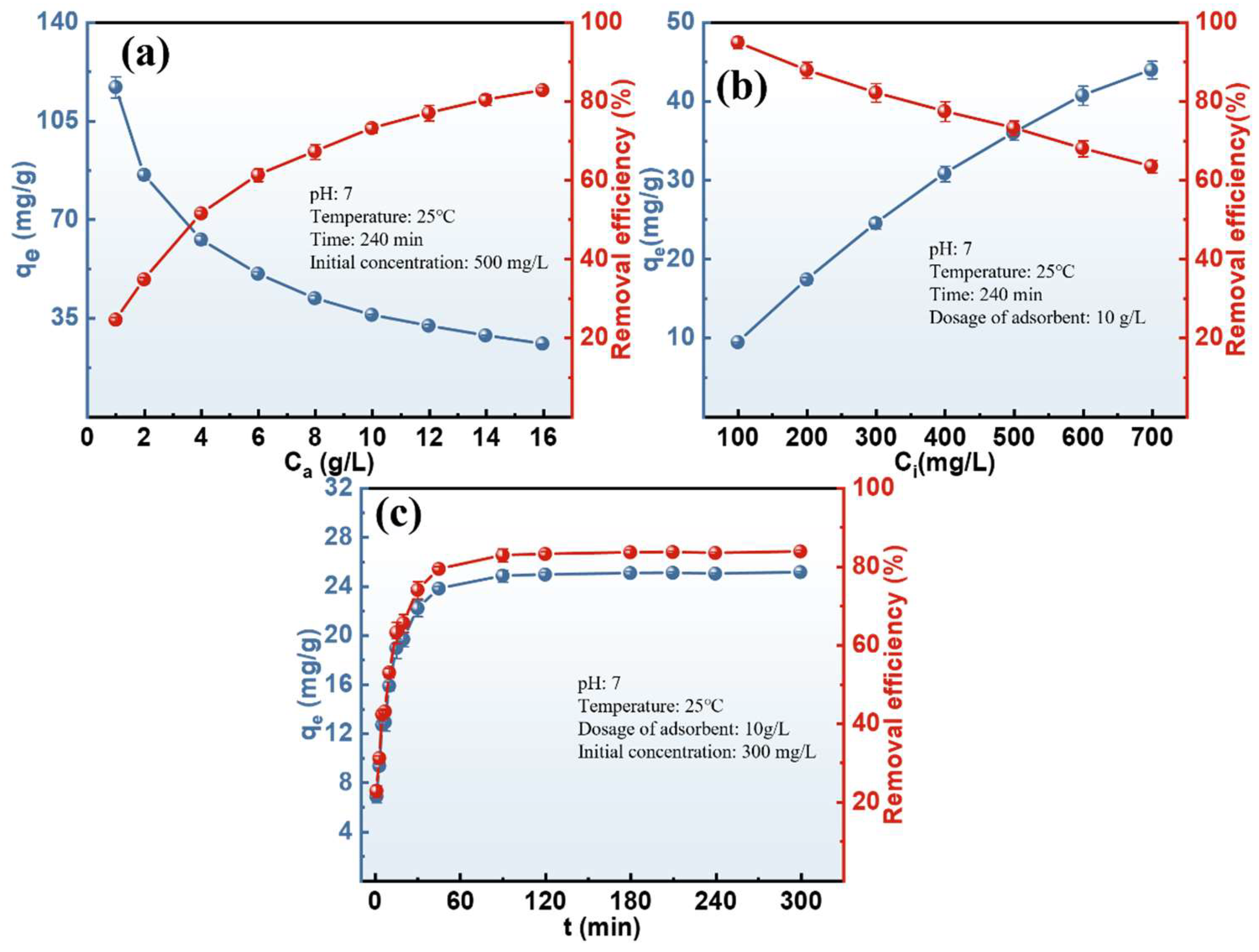
3.3.1. Batch Adsorption Experiment
3.3.2. Adsorption Kinetic and Isotherm Study
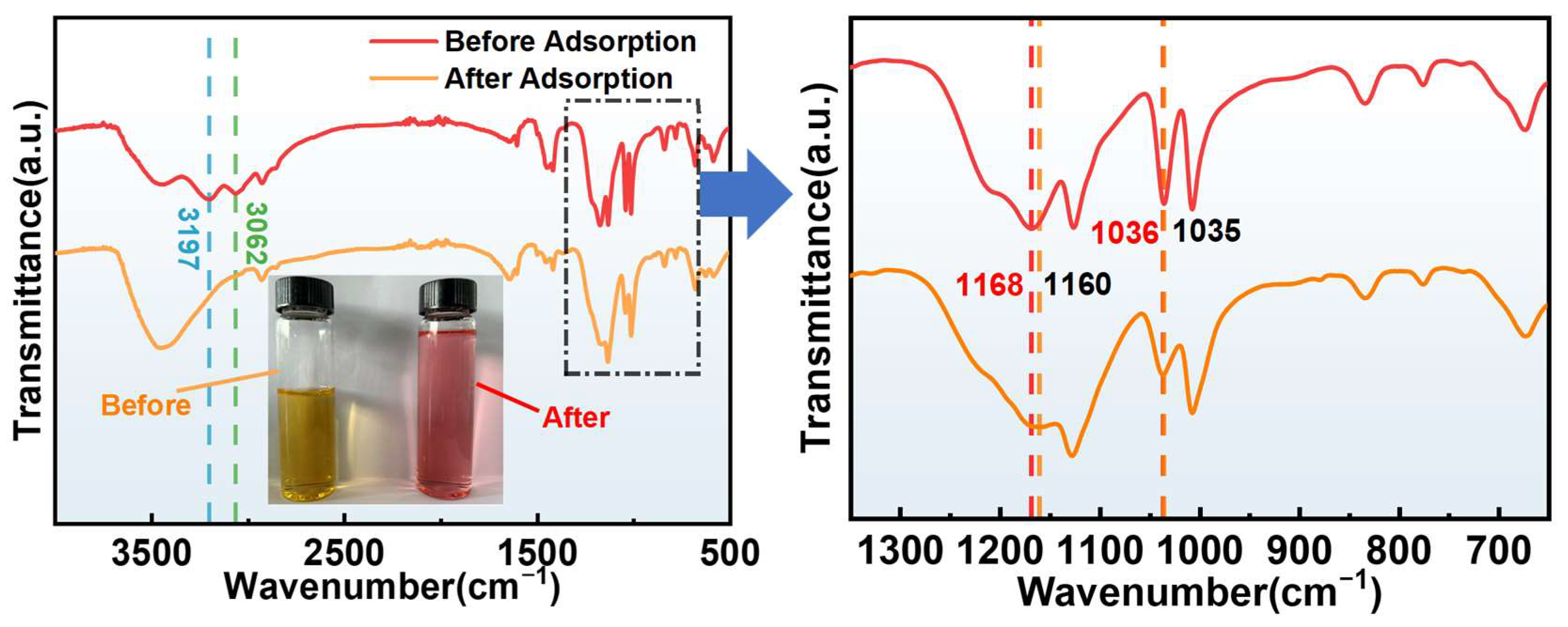
3.3.3. Adsorption Mechanism
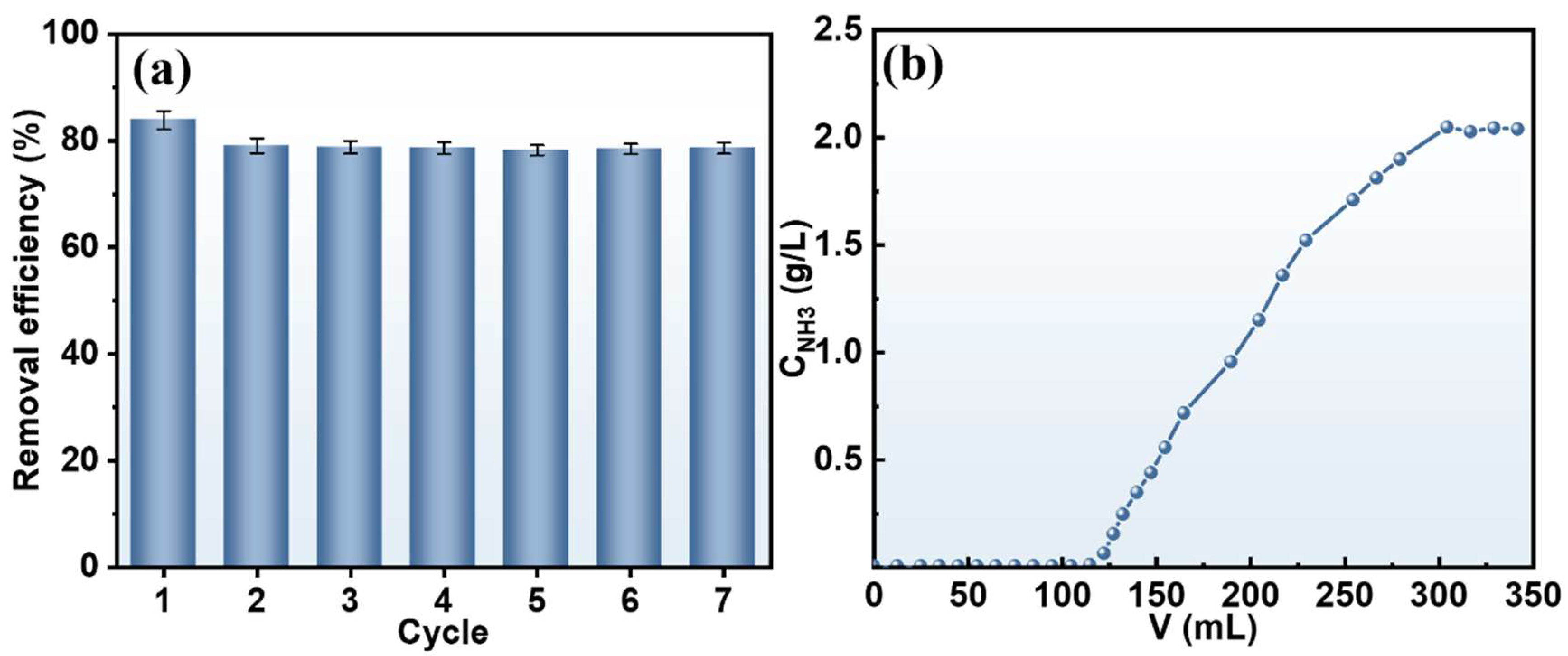
3.3.4. Regeneration
3.4. Adsorption Experiment of an Actual Ammonia Solution
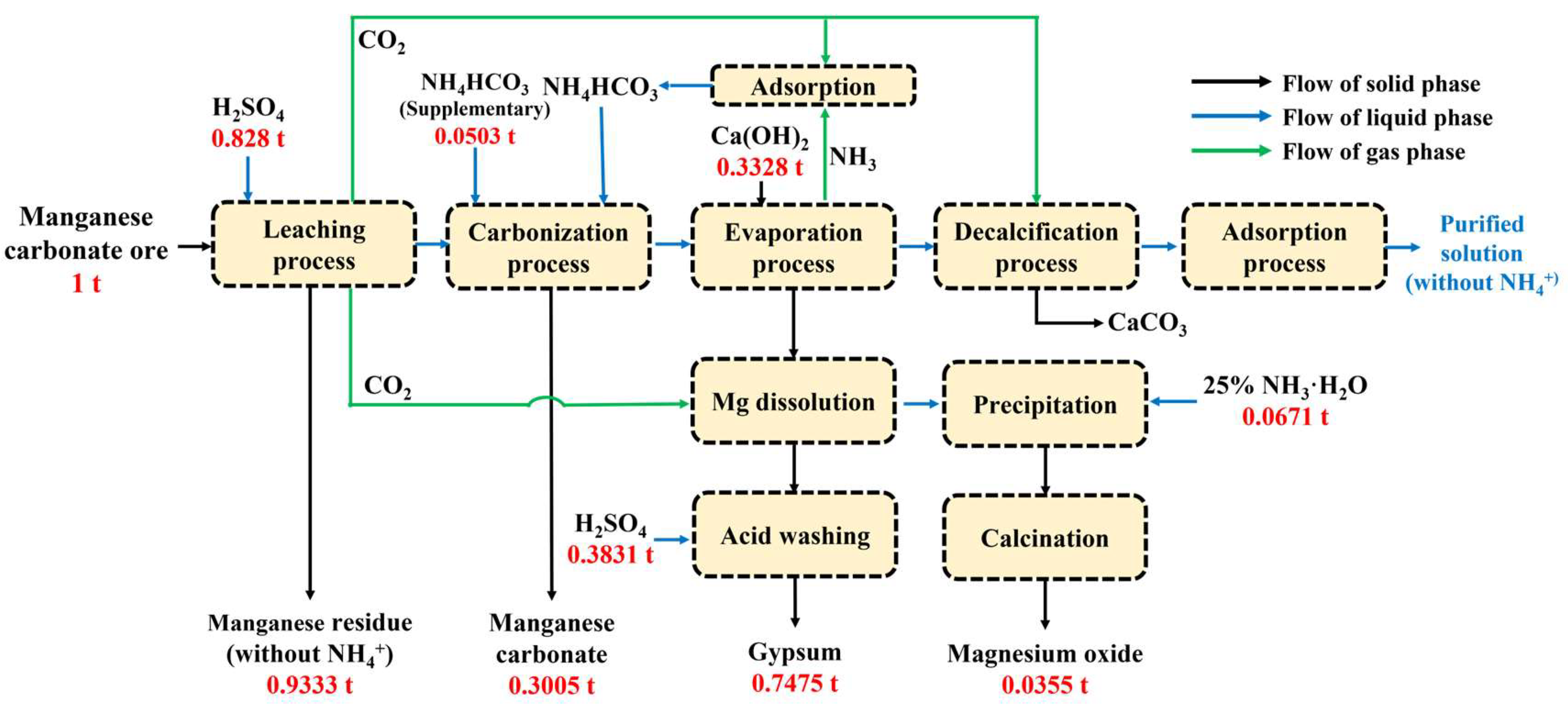
3.5. Preliminary Material Balance and Economic Evaluation
4. Conclusions
- (1)
- In the manganese carbonate preparation process, Mn and Mg in the ore were efficiently extracted via a two-stage leaching process. Compared to the direct leaching process, the leaching efficiencies of Mn and Mg increased from 94.11% to 97.49% and from 85.89% to 91.05%, respectively. The sulfuric acid from the leaching process was fully utilized, and the pH of the leachate was 5.60. The resulting manganese carbonate (Mn: 44.45%) obtained from the carbonization process met the first-class product indicators of HG/T 4203-2011 (Chinese manganese carbonate for industrial use).
- (2)
- A stripping–adsorption process for a Mg-containing ammonium sulfate solution was proposed. Under the optimized stripping conditions, a total of 88.20% of the ammonia nitrogen in the solution was stripped, and the stripped NH3 was recovered to prepare ammonium bicarbonate. A total of 99.99% of the Mg was precipitated and converted into magnesium oxide, while the calcium hydroxide used in the stripping process was transformed into gypsum products.
- (3)
- The 001×7 resin demonstrated excellent ammonia adsorption performance, with a maximum capacity of 51.14 mg/g. The dynamic adsorption experiment with the actual ammonia-containing solution after stripping indicates that the remaining ammonia nitrogen in the solution was completely removed by adsorption.
- (4)
- An economic analysis shows that this process is economically feasible. This process offers environmental and economic advantages and serves as a reference for process innovation in the manganese industry.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Lin, Q.; Li, L.; Fu, J.; Zhu, Z.; Wang, C.; Qian, D. Study on Hydrometallurgical Process and Kinetics of Manganese Extraction from Low-Grade Manganese Carbonate Ores. Int. J. Min. Sci. Technol. 2014, 24, 567–571. [Google Scholar] [CrossRef]
- Ren, G.; Liao, C.; Liu, Z.; Xiao, S. Lithium and Manganese Extraction from Manganese-Rich Slag Originated from Pyrometallurgy of Spent Lithium-Ion Battery. Trans. Nonferrous Met. Soc. China 2022, 32, 2746–2756. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.; Ma, X.; Cao, Z.; Di, J.; Yang, J.; Zhong, H. A Green Cyclic Leaching Process for Low-Grade Pyrolusite via a Recyclable Fe(II) Reductant. Minerals 2023, 13, 1191. [Google Scholar] [CrossRef]
- Sun, Y.; Fu, G.; Jiang, L. Kinetic Study of the Leaching of Low-Grade Manganese Ores by Using Pretreated Sawdust as Reductant. Minerals 2017, 7, 83. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Q.; Zou, Z.; Zhu, Q.; Li, H.; Li, H. CFD Simulation for Reduction of Pyrolusite in Fluidized Beds. Particuology 2023, 79, 109–120. [Google Scholar] [CrossRef]
- Elshkaki, A.; Graedel, T.E.; Ciacci, L.; Reck, B.K. Resource Demand Scenarios for the Major Metals. Environ. Sci. Technol. 2018, 52, 2491–2497. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, Y.; Li, K.; Shi, J.; Jin, X.; Xu, L.; Bai, X.; Shi, X.; Jin, P.; Wang, Q.; et al. Nucleation Crystallization Pelleting Process for Highly Efficient Manganese Ion Recovery in Electrolytic Manganese Wastewater. Chem. Eng. J. 2023, 475, 146271. [Google Scholar] [CrossRef]
- Palem, V.V. Ultrasonic-Assisted, Rapid Preparation of Mesoporous MnCO3 for Electrochemical Supercapacitor Applications: A Novel Approach. J. Electroanal. Chem. 2023, 942, 117571. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, H.; Liu, L.; Yao, Q.; Yang, X.; Hu, Z.; Yuan, Q.; Yao, J.; Liu, J. Regulation Role of Calcium Lignosulfonate on the Entrainment Behavior of Serpentine during Brucite Recycling: A New Perspective. Powder Technol. 2023, 428, 118764. [Google Scholar] [CrossRef]
- Wu, T.; Ma, B.; An, Y.; Chen, Y.; Wang, C. Improvement of Manganese Electrolytic Process and Secondary Resources Recovery of Manganese: A Review. Process Saf. Environ. Prot. 2024, 186, 895–909. [Google Scholar] [CrossRef]
- Yang, J.; Yang, F.; Shi, C.; Tan, C.; Chen, C.; Li, J.; Zhong, X. A Novel Electrochemical Deintercalation for Magnesium Ions Separation and Purification in Manganese Metallurgy. Sep. Purif. Technol. 2024, 351, 128031. [Google Scholar] [CrossRef]
- Wen, Q.; Liu, B.; Zhang, J.; Shen, H.; Lu, X.; Wang, S.; Zhang, S. Efficient Leaching of Manganese from Electrolytic Manganese Residue and Recovery of the Leaching Solution: Reuse and Purification. Sep. Purif. Technol. 2024, 332, 125648. [Google Scholar] [CrossRef]
- Ali, S.; Iqbal, Y.; Shah, K.H.; Fahad, M. Synthesis and Kinetic Modeling of Manganese Carbonate Precipitated from Manganese Sulfate Solution. Chem. Eng. Commun. 2022, 209, 96–107. [Google Scholar] [CrossRef]
- Zhang, H.; Song, D.; Zhang, Q.; Huang, X. Evaporation-Cooling Coupling Method to Remove the Calcium and Magnesium Impurities in Leaching Solution of Manganese Ore. IOP Conf. Ser. Earth Environ. Sci. 2021, 651, 042055. [Google Scholar] [CrossRef]
- Chen, C.; Peng, X.; Li, X.; Wei, C.; Deng, Z.; Li, M.; Fan, G. Magnesium Salt Removal from Manganese Electrolyte via Low-Temperature Crystallisation. Can. Metall. Q. 2024, 63, 194–202. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, L.; Zhang, G.; Guan, W.; Sun, Z.; Zhang, D.; Qing, J. A Novel Process on Separation of Manganese from Calcium and Magnesium Using Synergistic Solvent Extraction System. Hydrometallurgy 2019, 185, 55–60. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.; Jiang, G.; Tan, Z.; Pan, D.; Li, C. Research on the Purification Mechanism and High-Value Utilization of Manganese Leaching Solution from Electrolytic Manganese Anode Slime. Sep. Purif. Technol. 2025, 360, 130865. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, B.; Li, X.; Wang, C.; Chen, Y. Simultaneous Precipitation Removal of Fluoride Ion and Chloride Ion from Smelting Waste Acid Using Renewable Remover. J. Environ. Chem. Eng. 2024, 12, 114273. [Google Scholar] [CrossRef]
- Ma, R.; Feng, Y.; Li, H.; Ju, J.; Jiang, S.; Wang, H.; Li, Y.; Xu, C.; Xue, Z. An Environmentally Friendly Approach for the Extraction and Recovery of Mn from Pyrolusite by Ammonium Sulfate Roasting-Water Leaching and Ammonium Carbonate Precipitation. Asia-Pac. J. Chem. Eng. 2024, 19, e2999. [Google Scholar] [CrossRef]
- Pakarinen, J.; Paatero, E. Recovery of Manganese from Iron Containing Sulfate Solutions by Precipitation. Miner. Eng. 2011, 24, 1421–1429. [Google Scholar] [CrossRef]
- Tian, Y.; Shu, J.; Chen, M.; Wang, J.; Wang, Y.; Luo, Z.; Wang, R.; Yang, F.; Xiu, F.; Sun, Z. Manganese and Ammonia Nitrogen Recovery from Electrolytic Manganese Residue by Electric Field Enhanced Leaching. J. Clean. Prod. 2019, 236, 117708. [Google Scholar] [CrossRef]
- Lin, Q.; Gu, G.; Wang, H.; Wang, C.; Liu, Y.; Zhu, R.; Fu, J. Separation of Manganese from Calcium and Magnesium in Sulfate Solutions via Carbonate Precipitation. Trans. Nonferrous Met. Soc. China 2016, 26, 1118–1125. [Google Scholar] [CrossRef]
- Ju, J.; Ma, R.; Li, Y.; Feng, Y.; Li, H.; Wang, H.; Jiang, S. An Efficient and Clean Method for the Selective Extraction and Recovery of Manganese from Pyrolusite Using Ammonium Sulfate Roasting-Water Leaching and Carbonate Precipitation. Miner. Eng. 2023, 203, 108356. [Google Scholar] [CrossRef]
- Peng, J.; Yang, Z.; Lian, P. Process Optimization and Crystallization Kinetics of Ammonium Sulfate from Wet Ammonia-Based Desulfurization. Environ. Prog. Sustain. Energy 2024, 43, e14310. [Google Scholar] [CrossRef]
- Xu, H.; Fu, G.; Phan, D.; Xiang, L.; Le, T.; Zhang, L. Evaporating Crystallization Effect of Ammonium Sulfate at Atmospheric Pressure under the Action of Ultrasound. Ultrason. Sonochem. 2024, 106, 106896. [Google Scholar] [CrossRef]
- Park, J.; Lee, W.; Choe, J.K.; Choi, Y. Non-Evaporative Solid Phase Ammonium Sulfate Separation from Ammonia-Stripped Sulfuric Acid Solution by Solvent-Driven Fractional Crystallization. Sep. Purif. Technol. 2023, 318, 123869. [Google Scholar] [CrossRef]
- Jim, K.M.; Kim, K.J.; Jang, Y.N. Effect of Supersaturation on the Particle Size of Ammonium Sulfate in Semibatch Evaporative Crystallization. Ind. Eng. Chem. Res. 2013, 52, 11151–11158. [Google Scholar] [CrossRef]
- Zi, G.; Huang, B.; Dong, L.; Shi, Z.; Yang, L.; Luo, L. Effect of Evaporation Temperature and Mg2+ Concentration on the Crystallization of Ammonium Sulfate. Cryst. Res. Technol. 2024, 59, 2300312. [Google Scholar] [CrossRef]
- GB/T 1506-2016; Manganese Ores—Determination of Manganese Content—Potentiometric Method and Ammonium Iron(ll) Sulphate Titrimetric Method. Standardization Administration of China: Beijing, China, 2016.
- HJ 535-2009; Water quality—Determination of ammonia nitrogen Nessler’s reagent spectro photometry. Chinese Ministry of Environmental Protection: Beijing, China, 2010.
- GB/T 6730.10-2014; Iron Ores―Determination of Silicon Content―Gravimetric Methods. Standardization Administration of China: Beijing, China, 2014.
- GB/T 6730.73-2024; Iron Ores—Determination of Total Iron Content—EDTA Photometric Titration Method. Standardization Administration of China: Beijing, China, 2024.
- HJ-2009; Water Quality―Determination of Ammonia Nitrogen―Nessler’s Reagent Spectrophotometry. Chinese Ministry of Environmental Protection: Beijing, China, 2010.
- Lin, Q.; Gu, G.; Wang, H.; Zhu, R.; Liu, Y.; Fu, J. Preparation of Manganese Sulfate from Low-Grade Manganese Carbonate Ores by Sulfuric Acid Leaching. Int. J. Miner. Metall. Mater. 2016, 23, 491–500. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Q.; He, X.; Hu, H.; Chen, M. A Cleaner and Efficient Extraction of Mn from Low-Grade Mn Carbonate Ores by Ball Milling-Enhanced Fe2(SO4)3 Leaching: Acid Consumption Reduction. Clean. Eng. Technol. 2021, 4, 100220. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.; Ma, X.; Cao, Z.; Zhang, C.; Zhong, H. A Green Production Process of Electrolytic Manganese Metal Based on Solvent Extraction. Colloids Surf. Physicochem. Eng. Asp. 2023, 670, 131517. [Google Scholar] [CrossRef]
- Song, Y.C.; Woo, J.H.; Oh, G.G.; Kim, D.H.; Lee, C.Y.; Kim, H.W. External Electric Field Promotes Ammonia Stripping from Wastewater. Water Res. 2021, 203, 117518. [Google Scholar] [CrossRef] [PubMed]
- Farghali, M.; Chen, Z.; Osman, A.I.; Ali, I.M.; Hassan, D.; Ihara, I.; Rooney, D.W.; Yap, P.S. Strategies for Ammonia Recovery from Wastewater: A Review. Environ. Chem. Lett. 2024, 22, 2699–2751. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, H.; Lee, E. Influence of Ammonia Stripping Parameters on the Efficiency and Mass Transfer Rate of Ammonia Removal. Appl. Sci. 2021, 11, 441. [Google Scholar] [CrossRef]
- Shu, J.; Zhao, J.; Li, B.; Luo, D.; Zeng, X.; Chen, M.; Liu, Z. Cooperative Removal of Mn2+, NH4+−N, PO43−−P and F− from Electrolytic Manganese Residue Leachate and Phosphogypsum Leachate. J. Cent. South Univ. 2022, 29, 3656–3669. [Google Scholar] [CrossRef]
- Folino, A.; Calabrò, P.S.; Zema, D.A. Effects of Ammonia Stripping and Other Physico-Chemical Pretreatments on Anaerobic Digestion of Swine Wastewater. Energies 2020, 13, 3413. [Google Scholar] [CrossRef]
- Yoon, H.; Lim, J.H.; Chung, H.K. Ammonia Removal Model Based on the Equilibrium and Mass Transfer Principles. Bull. Korean Chem. Soc. 2008, 29, 555–561. [Google Scholar] [CrossRef]
- Dotto, G.L.; McKay, G. Current Scenario and Challenges in Adsorption for Water Treatment. J. Environ. Chem. Eng. 2020, 8, 103988. [Google Scholar] [CrossRef]
- Wei, Z.; Shao, Q.; Yuan, Y.; Jin, H.; Cao, J.; Liu, W.; Zhao, G.; Luo, J. Efficient Removal of Ammonia Nitrogen Using Biochar Derived from the Co-Fermentation Residue of Waste Activated and Orange Peel Waste: Linking Structure Properties and Reaction Kinetics. Process Saf. Environ. Prot. 2024, 189, 146–153. [Google Scholar] [CrossRef]
- Abu, N.F.; Yahya, N.Y.; Mansur, F.Z.; Muhammad, N. Removal of Ammonia from Rubber Wastewater Using Rubber-Sludge-Based Biochar to Enhance Biogas Production. Process Saf. Environ. Prot. 2024, 192, 378–385. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, N.; An, S.; Cai, C.; Peng, J.; Xie, M.; Peng, J.; Song, X. Synthesis of Novel Hierarchical Porous Zeolitization Ceramsite from Industrial Waste as Efficient Adsorbent for Separation of Ammonia Nitrogen. Sep. Purif. Technol. 2022, 297, 121418. [Google Scholar] [CrossRef]
- Cheng, H.; Zhu, Q.; Xing, Z. Adsorption of Ammonia Nitrogen in Low Temperature Domestic Wastewater by Modification Bentonite. J. Clean. Prod. 2019, 233, 720–730. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Qiao, H.; Liu, F.; Fu, Z. Synthesis of Manganese Oxides for Adsorptive Removal of Ammonia Nitrogen from Aqueous Solutions. J. Clean. Prod. 2020, 272, 123055. [Google Scholar] [CrossRef]
- Wang, X.S.; Tang, Y.P.; Tao, S.R. Kinetics, Equilibrium and Thermodynamic Study on Removal of Cr (VI) from Aqueous Solutions Using Low-Cost Adsorbent Alligator Weed. Chem. Eng. J. 2009, 148, 217–225. [Google Scholar] [CrossRef]
- Tran, H.N. Adsorption Technology for Water and Wastewater Treatments. Water 2023, 15, 2857. [Google Scholar] [CrossRef]
- Karadag, D.; Koc, Y.; Turan, M.; Armagan, B. Removal of Ammonium Ion from Aqueous Solution Using Natural Turkish Clinoptilolite. J. Hazard. Mater. 2006, 136, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Cao, Z.; Zhong, H.; Ma, X.; Wang, S. Highly Efficient Poly(6-Acryloylamino-N-Hydroxyhexanamide) Resin for Adsorption of Heavy Metal Ions. J. Environ. Manage. 2022, 308, 114631. [Google Scholar] [CrossRef]
- Laptash, N.M.; Maslennikova, I.G.; Kaidalova, T.A. Ammonium Oxofluorotitanates. J. Fluor. Chem. 1999, 99, 133–137. [Google Scholar] [CrossRef]
- Hu, Q.; Meng, Y.; Sun, T.; Mahmood, Q.; Wu, D.; Zhu, J.; Lu, G. Kinetics and Equilibrium Adsorption Studies of Dimethylamine (DMA) onto Ion-Exchange Resin. J. Hazard. Mater. 2011, 185, 677–681. [Google Scholar] [CrossRef]
- Ma, J.; Ji, J.; Yaseen, M.; Chen, X.; Liao, D.; Tong, Z. A Promising Strategy for the Large-Scale Preparation of Spherical Calcium Carbonate by Efficiently Using Carbon Dioxide. J. CO2 Util. 2022, 63, 102136. [Google Scholar] [CrossRef]


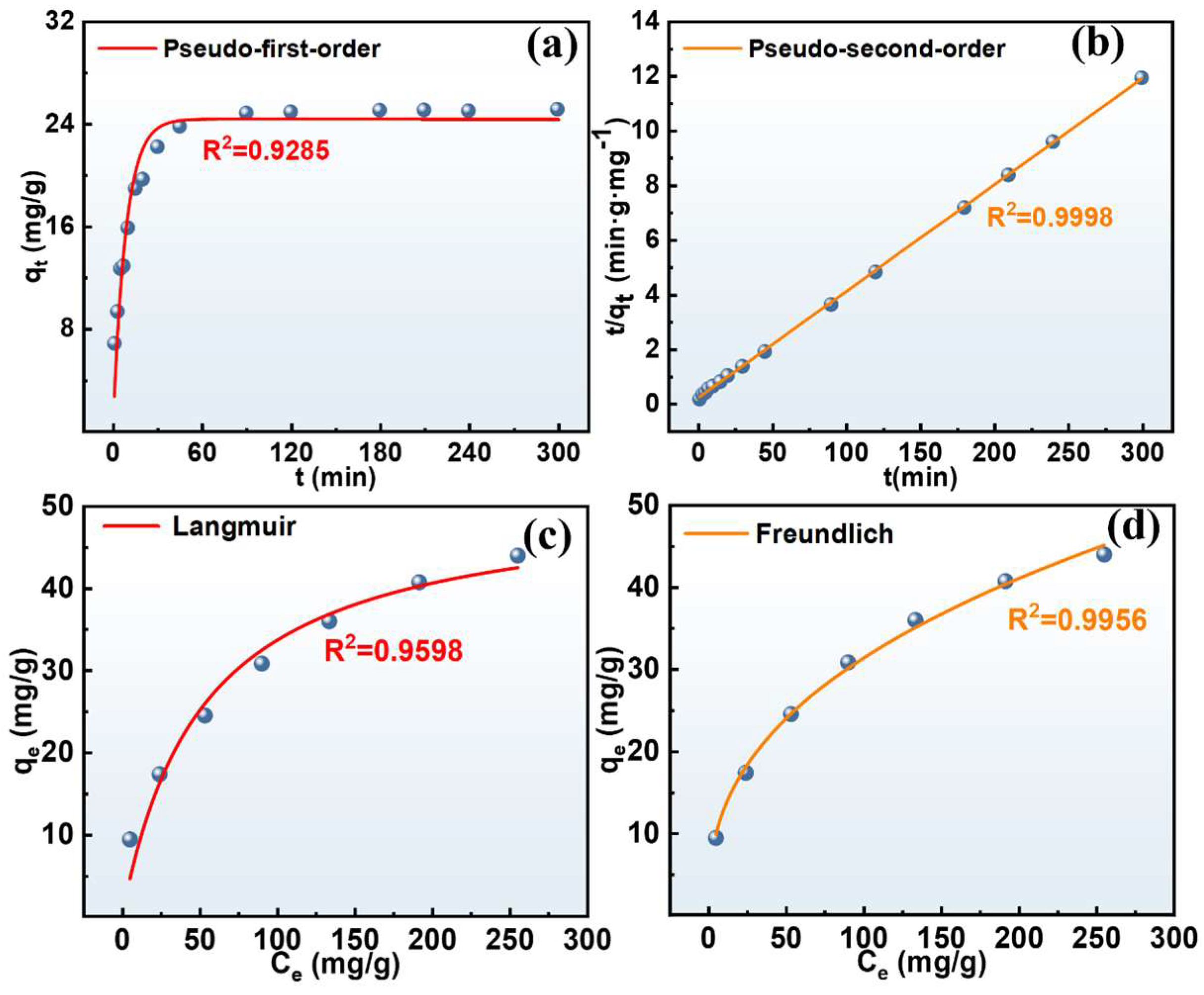
| Model | Parameter | Value | Model | Parameter | Value |
|---|---|---|---|---|---|
| Pseudo-first-order | qe | 24.38 | Langmuir | qm (mg/g) | 51.14 |
| k1 | 0.6147 | KL (L/mg) | 0.0192 | ||
| R2 | 0.9285 | R2 | 0.9598 | ||
| Pseudo-second-order | qe | 25.63 | Freundlich | KF (L/g) | 5.1930 |
| k2 | 0.0081 | 1/n | 0.3899 | ||
| R2 | 0.9998 | R2 | 0.9956 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Cheng, X.; Cao, Z.; Zhong, H.; Cai, H.; Xiao, G.; Ma, X.; Wang, S. Green Process for the Preparation of MnCO3 and Recovery of By-Product Mg-Containing (NH4)2SO4 Solution. Minerals 2025, 15, 304. https://doi.org/10.3390/min15030304
Ding X, Cheng X, Cao Z, Zhong H, Cai H, Xiao G, Ma X, Wang S. Green Process for the Preparation of MnCO3 and Recovery of By-Product Mg-Containing (NH4)2SO4 Solution. Minerals. 2025; 15(3):304. https://doi.org/10.3390/min15030304
Chicago/Turabian StyleDing, Xuran, Xunlong Cheng, Zhanfang Cao, Hong Zhong, Hongyan Cai, Gangxiang Xiao, Xin Ma, and Shuai Wang. 2025. "Green Process for the Preparation of MnCO3 and Recovery of By-Product Mg-Containing (NH4)2SO4 Solution" Minerals 15, no. 3: 304. https://doi.org/10.3390/min15030304
APA StyleDing, X., Cheng, X., Cao, Z., Zhong, H., Cai, H., Xiao, G., Ma, X., & Wang, S. (2025). Green Process for the Preparation of MnCO3 and Recovery of By-Product Mg-Containing (NH4)2SO4 Solution. Minerals, 15(3), 304. https://doi.org/10.3390/min15030304









