Metasomatic Mineral Systems with IOA, IOCG, and Affiliated Critical and Precious Metal Deposits: A Review from a Field Geology Perspective
Abstract
1. Introduction
2. Case Studies
3. Definitions
3.1. Alteration, Alteration Facies, and Metasomatism
3.2. Definition of MIAC Systems
3.3. Classification for the Mineral Deposits in MIAC Systems

3.4. Review of Alteration Facies and Mineralization in MIAC Systems
4. Geo-Environment of MIAC Systems
4.1. Geological Context
4.2. Pre-MIAC Sedimentary Basins
4.3. Syn-MIAC Magmatic Flare-Up
4.4. Syn- to Post-MIAC Batholith Emplacement
4.5. Structural Context
5. Discussion
5.1. Metal and Fluid Sources: A Regional Field Geology Perspective
5.1.1. The Primary Sources of Regional Fluid Plumes
5.1.2. The Secondary Sources: Insights from Metasomatized Host Rocks and Geological Environments
5.2. Energy Driver of Fluid Flow
5.3. Structural–Stratigraphic Architecture and Porosity Creation That Enable Fluid Flow
5.4. Depositional Environment: Alteration Facies as Predictive Indicators of Mineralization
5.5. Perspectives on Knowledge and Data Gaps
5.5.1. The Need to Acquire Regional Geological Data on MIAC Systems
5.5.2. Beyond the Predictability of Metal Associations and Deposit Types at Each Facies
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Deposits | Total Resources (Unless Indicated Otherwise) 1 | ||
|---|---|---|---|
| Name | Class | Type | |
| Olympic Cu-Au Province (Volcano-Plutonic Environment with Additional Sedimentary Hosts) | |||
| Carrapateena | MI-Cu | IOCG | 900 Mt at 0.56% Cu, 0.24 g/t Au, 3 g/t Ag [217] |
| Oak Dam East | MI-Fe | IOA | 560 Mt at 41%–56% Fe, 0.2% Cu, 690 ppm U [132] |
| Oak Dam West | MI-Cu | IOCG | 1340 Mt at 0.66% Cu, 0.33 g/t Au [218] |
| Olympic Dam 2 | MI-Cu | IOCG | 9640 Mt at 0.58% Cu, 0.19 kg/t U3O8, 0.26 g/t Au, 1.0 g/t Ag+ 1740 Mt at 1.49% Cu, 0.44 kg/t U3O8, 0.58 g/t Au, 3 g/t Ag [217] |
| Prominent Hill | MI-Cu | IOCG | 162 Mt at 0.94% Cu, 0.81 g/t Au, 3.0 g/t Ag (UG sulfide) [217] |
| Hillside | MI-Cu | Fe-rich skarn-Cu | 337 Mt at 0.56% Cu, 0.14 g/t Au [219] |
| Cloncurry district and host Mount Isa Province (sedimentary basin with additional volcano-plutonic hosts) | |||
| Elaine 1 | MI-Cu | Skarn-hosted Cu-Au | 26.1 Mt at 0.56% Cu, 0.09 g/t Au [220] |
| Elaine-Dorothy | MAC-U | Skarn-hosted U-REE | 0.83 Mt at 280 ppm U3O8, 3200 ppm TREO [221] |
| Eloise | MI-Cu | ISCG | 4.8 Mt at 2.4% Cu, 0.6 g/t Au [222] |
| Ernest Henry 2 | MI-Cu | IOCG | 167 Mt at 1.1% Cu, 0.5 g/t Au (historic, pre-mining) [129] |
| Current: 101.5 Mt at 1.25% Cu, 0.73 g/t Au [223] | |||
| Jericho | MI-Cu | ISCG | 19.2 Mt at 2.0% Cu, 0.4 g/t Au [224] |
| Eva Cu 3 | MI-Cu | IOCG | 260.7 Mt at 0.42% Cu, 0.04 g/t Au [225] |
| Kalman | MI-Cu | ISCG | 39.2 Mt at 0.53% Cu, 0.27 g/t Au, 1.5 g/t Ag, 0.01% Mo, 2.1 g/t Re [226] |
| Mary Kathleen | MAC-U | Skarn-hosted U | 9.5 Mt at 1300 ppm U3O8 [227] |
| Merlin | MAC-Mo | MAC Mo | 6.4 Mt at 1.5% Mo, 26 g/t Re (reserves) [95] |
| Mt Elliot-Swan | MI-Cu | IOCG–ISCG | 353.7 Mt at 0.6% Cu, 0.35 g/t Au [228] |
| Mt Dore | MAC-Cu | Breccia-hosted Cu-Au | 110.4 Mt at 0.55% Cu, 0.10 g/t Au, 0.05% Pb, 0.30% Zn [228] |
| Rocklands | MI-Cu | IOCG | 56.7 Mt at 0.64% Cu, 294 ppm Co, 0.15 ppm Au, 5.3% magnetite; 178 Mt at 15% magnetite [229] |
| Tick Hill | MAC-Au | Albitite-hosted Au | 0.706 Mt at 22.52 g/t Au (mined) [131] |
| Valhalla | MI-U | Albitite-hosted U | 34.7 Mt at 830 ppm U3O8 [230] |
| Other representative deposits | |||
| Johnnies Reward | MI-Cu | IOCG (at granulite facies) | 2.19 Mt at 0.7 g/t Au, 0.4% Cu [231] |
| Peko (tailings) | MI-Cu | IOCG | 3.75 Mt at 1.14 g/t Au, 0.25% Cu, 0.11% Co, 80% magnetite [232] |
| Savage River | MI-Fe | IOA | 471.8 Mt at 68.3% Fe, 0.04% Ni, 0.71% TiO2, 1.43% MgO, 0.007% P, 0.35% V, 0.08% S [233] |
| Warrego | MI-Fe | IOCG | 6.95 Mt at 2.0% Cu, 6.6 g/t Au, 0.32% Bi [5] |
| Deposits | Total Resources (Unless Indicated Otherwise) 1 | ||
|---|---|---|---|
| Name | Class | Type | |
| United States | |||
| Ram/Sunshine/Sunshine East, Idaho | MI-Co | MI-Co | 5.77 Mt at 0.44% Co, 0.69% Cu, 0.53 g/t Au [123] |
| Iron Creek, Idaho | MI-Co | ISi-Co | 4.45 Mt at 0.19% Co, 0.73% Cu (indicated), 1.23 Mt at 0.08% Co, 1.34% Cu (inferred) [235] |
| Boss, SE Missouri | MI-Cu | IOCG | 40 Mt at 0.83% Cu, 18% Fe, 0.035% Co (historic) [236] |
| Pea Ridge, SE Missouri 2 | MI-Fe | IOA | 160.6 Mt at ~53%–55% Fe; 0.2 Mt at 12% TREE (historic) [100] |
| Pilot Knob, SE Missouri | MI-Fe | IOA | 20 Mt at 35 to 40% Fe (produced) [113] |
| Pumpkin Hollow, Yerrington district | MI-Cu | IOCG | 501.7 Mt at 0.452% Cu, 0.07 g/t Au, 1.85 g/t Ag (open pit, measured and indicated), 25.4 Mt at 0.358% Cu, 0.03 g/t Au, 1.37 g/t Ag (open pit, inferred); 49.1 Mt at 1.39% Cu, 0.17 g/t Au, 3.98 g/t Ag, 17.8% Fe (underground, measured and indicated), 26.5 Mt at 1.09% Cu, 0.10 g/t Au, 2.19 g/t Ag, 12.8% Fe (underground, inferred) [237] |
| Moonlight-Superior project, California | MI-Cu | IOCG | 402.83 Mt at 0.31% Cu, 1.85 g/t Ag, 0.012 g/t Au (measured and indicated); 64.59 Mt at 0.31% Cu, 0.77 g/t Ag, 0.005 g/t Au (inferred) [238] |
| Coles Hill, Virginia | MAC-U | Albitite-hosted U | 119 Mt at 0.056% U3O8 (indicated resources) [239] |
| Buena Vista | MI-Fe | IOA | 232 Mt at 18.6% Fe [240] |
| Canada 2 | |||
| NICO | MI-Co | IOx-Co | 33 Mt at 1.02 g/t Au, 0.12% Co, 0.14% Bi, 0.04% Cu [241] |
| Sue-Dianne 2 | MI-Cu | IOCG | 8.4 Mt at 0.80% Cu, 0.07 g/t Au, 3.2 g/t Ag [242] |
| Michelin | MI-U | Albitite-hosted U | 42.7 Mt at 0.098% U3O8 [243] |
| Upper C Moran Lake | MI-U | Albitite-hosted U | 6.9 Mt at 0.034% U3O8, 0.078% V2O5 (indicated, historic 3) + 5.3 Mt at 0.024% U3O8, 0.058% V2O5 (inferred, historic 3) [244] |
| Josette 2 | MI-REE | IOA-REE | 6.9 Mt at 2.7% REE2O3 (=1.83% LREE, 0.89% HREE) [245] |
| Fostung | MI-W | W skarn | 12.4 Mt at 0.2% WO3 (historic) [246] |
| Kiggavik | MAC-U | Albitite-hosted U | 10.4 Mt at 0.47 U3O8 [247] |
| Lac Cinquante | MAC-U | Albitite-hosted U | 2.8 Mt at 0.693% U3O8, 20.6 g/t Ag 0.167% Mo, 0.25% Cu (historic) [248] |
| Werner Lake | MI-Co | Isi-Co | 57.9kt at 0.51% Co, 0.25% Cu, 0.27% As, 0.22 g/t Au (indicated); 6.3kt at 0.48% Co, 0.14% Cu, 0.30% As, 0.24 g/t Au (inferred) [249] |
| Jaguar, Carajás, Brazil | MI-Ni | IOA-Ni | 58.6 Mt at 0.96% Ni [250] |
| Deposits | Total Resources (Unless Indicated Otherwise) 1 | ||
|---|---|---|---|
| Name | Class | Type | |
| Sweden | |||
| Per Geiger | MI-Fe | IOA-REE | 734 Mt at 47.3% Fe, 2.3% P, 0.18% TREO [153] |
| Kiruna (+Konsuln) | MI-Fe | IOA | 1437 Mt at 59.8% Fe, 0.33% P, 0.017% TREO [154] |
| Malmberget | MI-Fe | IOA | 1570 Mt at 53.4% Fe, 0.57% P, 0.022% TREO [154] |
| Svappavaara | MI-Fe | IOA | 785 Mt at 46.2% Fe, 0.47% P [154] |
| Grangesberg | MI-Fe | IOA | 148.3 Mt at 41.3% Fe, 0.81% P [251] |
| Nautanen North | MI-Cu | IOCG | 21 Mt at 1.46% Cu, 0.78 g/t Au, 6 g/t Ag, 99 g/t Mo [252] |
| Kiskamavaara | MI-Cu | IOCG | 7.67 Mt at 0.25% Cu, 0.04% Co [214] |
| Finland | |||
| Hannukainen | MI-Cu | IOCG | 221 Mt at 32% Fe, 0.17% Cu, 0.077 g/t Au, 135 ppm Co [253] |
| Sahavaara | MI-Fe | Fe skarn | 86.8 Mt at 39.82% Fe, 1.93% S (measured + indicated) [254] |
| Juomasuo | MAC-Au | Albitite-hosted Au-Co | 2.37 Mt at 0.13% Co, 4.6 g/t Au + 5.04 Mt at 0.12% Co [255] |
References
- Hofstra, A.; Lisitsin, V.; Corriveau, L.; Paradis, S.; Peter, J.; Lauzière, K.; Lawley, C.; Gadd, M.; Pilote, J.; Honsberger, I.; et al. Deposit Classification Scheme for the Critical Minerals Mapping Initiative Global Geochemical Database; Open-File Report 2021-1049; U.S. Geological Survey: Denver, CO, USA, 2021; 60 p.
- Williams, P.J.; Barton, M.D.; Johnson, D.A.; Fontboté, L.; de Haller, A.; Mark, G.; Oliver, N.H.S.; Marschik, R. Iron-oxide copper-gold deposits: Geology, space-time distribution, and possible modes of origin. Econ. Geol. 2005, 100, 371–405. [Google Scholar]
- Barton, M.D. Iron oxide(–Cu–Au–REE–P–Ag–U–Co) systems. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 13, pp. 515–541. [Google Scholar]
- Porter, T.M. Hydrothermal Iron Oxide Copper-Gold & Related Deposits: A Global Perspective, v. 4—Advances in the Understanding of IOCG Deposits; PGC Publishing: Adelaide, Australia, 2010; p. 294. [Google Scholar]
- Porter, T.M. Current understanding of iron oxide associated alkali-altered mineralised systems. Part 1—An overview. In Hydrothermal Iron Oxide Copper-Gold & Related Deposits: A Global Perspective, v. 3—Advances in the Understanding of IOCG Deposits; Porter, T.M., Ed.; PGC Publishing: Adelaide, Australia, 2010; pp. 5–32. [Google Scholar]
- Corriveau, L.; Montreuil, J.-F.; Blein, O.; Ehrig, K.; Potter, E.G.; Fabris, A.; Clark, J. Mineral systems with IOCG and affiliated deposits: Part 2—Geochemical footprints. In Mineral Systems with Iron Oxide Copper-Gold (IOCG) and Affiliated Deposits; Corriveau, L., Potter, E.G., Mumin, A.H., Eds.; Special Paper 52; Geological Association of Canada: St. John’s, NL, Canada, 2022; pp. 159–204. [Google Scholar]
- Corriveau, L.; Montreuil, J.-F.; Potter, E.G.; Blein, O.; De Toni, A.F. Mineral systems with IOCG and affiliated deposits: Part 3—Metal pathways and ore deposit model. In Mineral Systems with Iron Oxide Copper-Gold (IOCG) and Affiliated Deposits; Corriveau, L., Potter, E.G., Mumin, A.H., Eds.; Special Paper 52; Geological Association of Canada: St. John’s, NL, Canada, 2022; pp. 205–245. [Google Scholar]
- Hitzman, M.W.; Oreskes, N.; Einaudi, M.T. Geological characteristics and tectonic setting of Proterozoic iron oxide (Cu-U-Au-LREE) deposits. Precambrian Res. 1992, 58, 241–287. [Google Scholar]
- Groves, D.I.; Bierlein, F.P.; Meinert, L.D.; Hitzman, M.W. Iron oxide copper-gold (IOCG) deposits through Earth history. Implications for origin, lithospheric setting, and distinction from other epigenetic iron oxide deposits. Econ. Geol. 2010, 105, 641–654. [Google Scholar]
- Corriveau, L.; Montreuil, J.-F.; Potter, E.G.; Ehrig, K.; Clark, J.; Mumin, A.H.; Williams, P.J. Mineral systems with IOCG and affiliated deposits: Part 1—Metasomatic footprints of alteration facies. In Mineral Systems with Iron Oxide Copper-Gold (IOCG) and Affiliated Deposits; Corriveau, L., Potter, E.G., Mumin, A.H., Eds.; Special Paper 52; Geological Association of Canada: St. John’s, NL, Canada, 2022; pp. 113–158. [Google Scholar]
- Corriveau, L.; Montreuil, J.-F.; De Toni, A.F.; Potter, E.G.; Percival, J.B. Mapping mineral systems with IOCG and affiliated deposits: A facies approach. In Mineral Systems with Iron Oxide Copper-Gold (IOCG) and Affiliated Deposits; Corriveau, L., Potter, E.G., Mumin, A.H., Eds.; Special Paper 52; Geological Association of Canada: St. John’s, NL, Canada, 2022; pp. 69–111. [Google Scholar]
- Corriveau, L.; Mumin, A.H.; Setterfield, T. IOCG environments in Canada: Characteristics, geological vectors to ore and challenges. In Hydrothermal Iron Oxide Copper-Gold & Related Deposits: A Global Perspective, v. 3—Advances in the Understanding of IOCG Deposits; Porter, T.M., Ed.; PGC Publishing: Adelaide, Australia, 2010; pp. 311–344. [Google Scholar]
- Corriveau, L.; Potter, E.G.; Mumin, A.H. Mineral Systems with Iron Oxide Copper-Gold (IOCG) and Affiliated Deposits; Special Paper 52; Geological Association of Canada: St. John’s, NL, Canada, 2022; 424 p. [Google Scholar]
- Corriveau, L.; Williams, P.J.; Mumin, A.H. Alteration vectors to IOCG mineralization—From uncharted terranes to deposits. In Exploring for Iron Oxide Copper–Gold Deposits: Canada and Global Analogues; Corriveau, L., Mumin, A.H., Eds.; Short Course Notes, No. 20; Geological Association of Canada: St. John’s, NL, Canada, 2010; pp. 89–110. [Google Scholar]
- Corriveau, L.; Montreuil, J.-F.; Potter, E.G. Alteration facies linkages among IOCG, IOA and affiliated deposits in the Great Bear magmatic zone, Canada. Econ. Geol. 2016, 111, 2045–2072. [Google Scholar]
- Montreuil, J.-F.; Corriveau, L.; Davis, W. Tectonomagmatic evolution of the southern Great Bear magmatic zone (Northwest Territories, Canada)—Implications on the genesis of iron oxide alkali-altered hydrothermal systems. Econ. Geol. 2016, 111, 2111–2138. [Google Scholar]
- Montreuil, J.-F.; Corriveau, L.; Potter, E.G.; De Toni, A.F. On the relation between alteration facies and metal endowment of iron oxide–alkali-altered systems, southern Great Bear magmatic zone (Canada). Econ. Geol. 2016, 111, 2139–2168. [Google Scholar] [CrossRef]
- Potter, E.G.; Corriveau, L.; Kjarsgaard, B. Paleoproterozoic iron oxide apatite (IOA) and iron oxide-copper-gold (IOCG) mineralization in the East Arm Basin, Northwest Territories, Canada. Can. J. Earth Sci. 2020, 57, 167–183. [Google Scholar]
- Corriveau, L.; Blein, O.; Gervais, F.; Trapy, P.H.; De Souza, S.; Fafard, D. Iron-Oxide and Alkali-Calcic Alteration, Skarn and Epithermal Mineralizing Systems of the Grenville Province: The Bondy Gneiss Complex in the Central Metasedimentary Belt of Quebec as a Case Example—A Field Trip to the 14th Society for Geology Applied to Mineral Deposits (SGA) Biennial Meeting; Open File 8349; Geological Survey of Canada: Ottawa, ON, Canada, 2018; 136 p.
- Slack, J.; Corriveau, L.; Hitzman, M. Proterozoic Iron Oxide-Apatite (± REE) and Iron Oxide-Copper-Gold and Affiliated Deposits of Southeast Missouri, USA, and the Great Bear Magmatic Zone, Northwest Territories, Canada. Econ. Geol. 2016, 111, 1803–1814. [Google Scholar]
- Drejing-Carroll, D.; Hitzman, M.W.; Coller, D. Geology of the Nautanen North Cu-Au-Ag-(Mo) Deposit, Norrbotten, Sweden. Econ. Geol. 2023, 118, 1765–1794. [Google Scholar]
- Hamilton, M.; Montreuil, J.-F.; Adlakha, E.; Corriveau, L.; Bain, W. Base, Critical, and Precious Metals Mineralization in the Metasomatic Iron and Alkali-Calcic Systems of the Southern Province in the Sudbury Area: A Geological Guidebook; Open File Report 6391; Ontario Geological Survey: Sudbury, ON, Canada, 2023; 66p. [Google Scholar]
- Fabris, A.; Michaelsen, B. Reference Drillholes from IOCG and Associated Deposits in South Australia; Report Book 2024/00008; Department for Energy and Mining: Adelaide, Australia, 2024; 60p.
- Blein, O.; Corriveau, L.; Montreuil, J.-F.; Ehrig, K.; Fabris, A.; Reid, A.; Pal, D. Geochemical signatures of metasomatic ore systems hosting IOCG, IOA, albite-hosted uranium and affiliated deposits: A tool for process studies and mineral exploration. In Mineral Systems with Iron Oxide Copper-Gold (IOCG) and Affiliated Deposits; Corriveau, L., Potter, E.G., Mumin, A.H., Eds.; Special Paper 52; Geological Association of Canada: St. John’s, NL, Canada, 2022; pp. 263–298. [Google Scholar]
- Blein, O.; Harlaux, M.; Corriveau, L.; Lynch, E.P.; Niiranen, T.; Lisitsin, V.; Ehrig, K.; Montreuil, J.-F.; Gourcerol, B. Alteration footprints of metasomatic iron and alkali-calcic systems in the Northern Norrbotten, Sweden. In Proceedings of the 17th Biennial Meeting of the SGA, Zürich, Switzerland, 28 August–1 September 2023; 4p. [Google Scholar]
- Acosta-Góngora, P.; Potter, E.G.; Lawley, C.J.M.; Corriveau, L.; Sparkes, G. Geochemical characterization of the Central Mineral Belt U±Cu±Mo±V mineralization, Labrador, Canada: Evaluation of IOCG potential and application of unsupervised machine-learning. J. Geochem. Expl. 2022, 237, 106995. [Google Scholar]
- Montreuil, J.-F.; Potter, E.; Corriveau, L.; Davis, W.J. Element mobility patterns in magnetite-group IOCG systems: The Fab IOCG system, Northwest Territories, Canada. Ore Geol. Rev. 2016, 72, 562–584. [Google Scholar]
- Montreuil, J.-F.; Corriveau, L.; Potter, E. Formation of albitite-hosted uranium within IOCG systems: The Southern Breccia, Great Bear magmatic zone, Northwest Territories, Canada. Mineral. Depos. 2015, 50, 293–325. [Google Scholar]
- Corriveau, L.; Lauzière, K.; Montreuil, J.-F.; Potter, E.G.; Hanes, R.; Prémont, S. Dataset of Geochemical Data from Iron Oxide Alkali-Altered Mineralizing Systems of the Great Bear Magmatic Zone (NWT); Open File 7643; Geological Survey of Canada: Ottawa, ON, Canada, 2015; 19p.
- Enkin, R.J.; Corriveau, L.; Hayward, N. Metasomatic alteration control of petrophysical properties in the Great Bear magmatic zone (Northwest Territories, Canada). Econ. Geol. 2016, 111, 2073–2085. [Google Scholar]
- Zeng, L.-P.; Zhao, X.-F.; Spandler, C.; Mavrogenes, J.A.; Mernagh, T.P.; Liao, W.; Fan, Y.-Z.; Hu, Y.; Fu, B.; Li, J.-W. The role of iron-rich hydrosaline liquids in the formation of Kiruna-type iron oxide–apatite deposits. Sci. Adv. 2024, 10, eadk2174. [Google Scholar]
- Xu, X.; Steele-MacInnis, M.; Bain, W.; Tornos, F.; Hanchar, J.M.; Lamadrid, H.M.; Lehmann, B.; Xu, X.; Steadman, J.A.; Bottrill, R.S.; et al. Magnetite-apatite ores record widespread involvement of molten salts. Geology 2024, 52, 417–422. [Google Scholar]
- Skirrow, R.G. Iron oxide copper-gold (IOCG) deposits—A review (part 1): Settings, mineralogy, ore geochemistry and classification. Ore Geol. Rev. 2022, 140, 104569. [Google Scholar]
- Skirrow, R.G.; Murr, J.; Schofield, A.; Huston, D.L.; van der Wielen, S.E.; Czarnota, K.; Coghlan, R.; Highet, L.M.; Connolly, D.; Doublier, M.; et al. Mapping iron oxide Cu-Au (IOCG) mineral potential in Australia using a knowledge-driven mineral systems-based approach. Ore Geol. Rev. 2019, 113, 103011. [Google Scholar]
- Williams, P.J. Classifying IOCG deposits. In Exploring for Iron Oxide Copper–Gold Deposits: Canada and Global Analogues; Corriveau, L., Mumin, A.H., Eds.; Short Course Notes, No. 20; Geological Association of Canada: St. John’s, NL, Canada, 2010; pp. 13–21. [Google Scholar]
- Wyborn, L.A.I.; Heinrich, C.A.; Jaques, A.L. Australian Proterozoic mineral systems: Essential ingredients and mappable criteria. In Proceedings of the AusIMMM Annual Conference, Transactions, Melbourne, Australia, 5–9 August 1994; pp. 109–115. [Google Scholar]
- Corriveau, L.; Montreuil, J.-F.; Blein, O.; Potter, E.; Ansari, M.; Craven, J.; Enkin, R.; Fortin, R.; Harvey, B.; Hayward, N.; et al. Metasomatic iron and alkali calcic (MIAC) system frameworks: A TGI-6 task force to help de-risk exploration for IOCG, IOA and affiliated primary critical metal deposits. In Scientific Presentation 127; Geological Survey of Canada: Ottawa, ON, Canada, 2021; 105p. [Google Scholar]
- Corriveau, L.; Potter, E.G. Advancing exploration for iron oxide-copper-gold and affiliated deposits in Canada: Context, scientific overview, outcomes and impacts. In Canada’s Northern Shield: New Perspectives from the Geo-Mapping for Energy and Minerals Program; Pehrsson, S., Wodicka, N., Rogers, N., Percival, J., Eds.; Bulletin 612; Geological Survey of Canada: Ottawa, ON, Canada, 2024; pp. 55–98. [Google Scholar]
- Corriveau, L.; Mumin, A.H. Exploring for iron oxide copper–gold deposits: The need for case studies, classifications and exploration vectors. In Exploring for Iron Oxide Copper–Gold Deposits: Canada and Global Analogues; Corriveau, L., Mumin, A.H., Eds.; Short Course Notes, No. 20; Geological Association of Canada: St. John’s, NL, Canada, 2010; pp. 1–12. [Google Scholar]
- Sillitoe, R.H. Iron oxide-copper-gold deposits: An Andean view. Mineral. Depos. 2003, 38, 787–812. [Google Scholar]
- Slack, J. Descriptive and Geoenvironmental Model for Cobalt–Copper–Gold Deposits in Metasedimentary Rocks; Scientific Investigations Report 2010–5070–G; U.S. Geological Survey: Denver, CO, USA, 2013; 218 p.
- Reid, A. The Olympic Cu-Au Province, Gawler Craton: A review of the lithospheric architecture, geodynamic setting, alteration systems, cover successions and prospectivity. Minerals 2019, 9, 371. [Google Scholar] [CrossRef]
- Skirrow, R.G. Hematite-group IOCG ± U deposits: An update on their tectonic settings, hydrothermal characteristics, and Cu-Au-U mineralizing processes. In Mineral Systems with Iron Oxide Copper-Gold (IOCG) and Affiliated Deposits; Corriveau, L., Potter, E.G., Mumin, A.H., Eds.; Special Paper 52; Geological Association of Canada: St. John’s, NL, Canada, 2022; pp. 27–51. [Google Scholar]
- Schutesky, M.E.; Oliveira, C.; Hagemann, S.; Monteiro, L.V.S. A thematic issue dedicated to the 50 years of the discovery of the Carajás Mineral Province, Brazil. Ore Geol. Rev. 2021, 129, 103819. [Google Scholar]
- Hickin, A.S.; Ootes, L.; Orovan, E.A.; Brzozowski, M.J.; Northcote, B.K.; Rukhlov, A.S.; Bain, W.M. Critical Minerals and Mineral Systems in British Columbia; Paper 2024–01; British Columbia Geological Survey: Victoria, Canada, 2024; pp. 13–51.
- Rukhlov, A.S. Review of Metallic Mineralization in Alberta with Emphasis on Gold Potential; ERCB/AGS Open File Report 2011–01; Energy Resources Conservation Board: Edmonton, AB, Canada, 2011; 93 p.
- Avery, G.; Adlakha, E.; Powell, J.W.; Tschirhart, V. Petrography and Geochemistry of the Lac Cinquante Uranium Deposit, Nunavut; Canada; Geological Survey of Canada: Ottawa, ON, Canada, 2024.
- Burron, I.; Fayek, M.; Brown, J.; Quirt, D. Remnants of a 1.55 Ga hybrid between metasomatic iron alkali-calcic and unconformity-related uranium environments in the Kiggavik Region, Nunavut, Canada. Econ. Geol. 2024, 119, 1861–1888. [Google Scholar]
- Corriveau, L.; Nadeau, O.; Montreuil, J.-F.; Desrochers, J.-P. Report of Activities for the Core Zone: Strategic Geomapping and Geoscience to Assess the Mineral Potential of the Labrador Trough for Multiple Metals IOCG and Affiliated Deposits, Canada; Open File 7714; Geological Survey of Canada: Ottawa, ON, Canada, 2014; 12 p.
- Montreuil, J.-F.; Desrochers, J.-P.; Masters, J. Exploration Report (July and August 2013 program) on the Sagar Property, Romanet Horst, Labrador Trough, Québec, Canada; GM 68408; Ministère de l’Énergie et des Ressources naturelles: Québec, QC, Canada, 2014; 80 p. [Google Scholar]
- Clark, T.; Gobeil, A.; Chevé, S. Alterations in IOCG-type and related deposits in the Manitou Lake area, Eastern Grenville Province, Québec. In Exploring for Iron Oxide Copper–Gold Deposits: Canada and Global Analogues; Corriveau, L., Mumin, A.H., Eds.; Short Course Notes, No. 20; Geological Association of Canada: St John’s, NL, Canada, 2010; pp. 127–146. [Google Scholar]
- Hildebrand, R.S. Kiruna-type deposits: Their origin and relationship to intermediate subvolcanic plutons in the Great Bear Magmatic Zone, northwest Canada. Econ. Geol. 1986, 81, 640–659. [Google Scholar] [CrossRef]
- Hildebrand, R.S.; Hoffman, P.F.; Housh, T.; Bowring, S.A. The nature of volcano-plutonic relations and shapes of epizonal plutons of continental arcs as revealed in the Great Bear magmatic zone, northwestern Canada. Geosphere 2010, 6, 812–839. [Google Scholar] [CrossRef]
- Reardon, N.C. Altered Rocks and Magnetite—Apatite—Actinolite Deposits Associated with the Mystery Island Intrusive Suite, Echo Bay, District of Mackenzie; Open File 2507; Geological Survey of Canada: Ottawa, ON, Canada, 1992; 89 p.
- Mumin, A.H.; Corriveau, L.; Somarin, A.K.; Ootes, L. Iron oxide copper-gold-type polymetallic mineralisation in the Contact Lake Belt, Great Bear magmatic zone, Northwest Territories, Canada. Expl. Mining Geol. 2007, 16, 187–208. [Google Scholar]
- Mumin, A.H.; Somarin, A.K.; Jones, B.; Corriveau, L.; Ootes, L.; Camier, J. The IOCG-porphyry-epithermal continuum of deposit types in the Great Bear magmatic zone, Northwest Territories, Canada. In Exploring for Iron Oxide Copper–Gold Deposits: Canada and Global Analogues; Corriveau, L., Mumin, A.H., Eds.; Short Course Notes, No. 20; Geological Association of Canada: St. John’s, NL, Canada, 2010; pp. 59–78. [Google Scholar]
- Mumin, A.H. Echo Bay IOCG Thematic Map Series: Geology, Structure and Hydrothermal Alteration of a Stratovolcano Complex, Northwest Territories, Canada; Open File 7807; Geological Survey of Canada: Ottawa, ON, Canada, 2015; 19 p.
- Jackson, V.A.; Ootes, L.; Pierce, K.L.; Bennett, V.; Smar, L.; Mackay, D.; Sandeman, H.A. Geology of the South-Central Wopmay Orogen, Northwest Territories (Parts of NTS 86B, 86C, And 86D); Results from the South Wopmay Bedrock Mapping Project; Open File 2017-01; Northwest Territories Geological Survey: Yellowknife, NT, Canada, 2022; 103 p.
- Potter, E.G.; Acosta-Góngora, P.; Corriveau, L.; Montreuil, J.-F.; Yang, Z. Uranium enrichment processes in iron oxide and alkali-calcic alteration systems as revealed by trace element signatures of uraninite. In Mineral Systems with Iron Oxide Copper-Gold (IOCG) and Affiliated Deposits; Corriveau, L., Potter, E.G., Mumin, A.H., Eds.; Special Paper 52; Geological Association of Canada: St. John’s, NL, Canada, 2022; pp. 325–345. [Google Scholar]
- Somarin, A.K.; Zhou, L.; Zheng, G.; Ma, X. Hydrothermal mineralization and mineral chemistry of arsenides and sulfarsenides in the Fe-Co-Ni-As-S system and introduction of three unique minerals, Port Radium deposit, Canada. Minerals 2024, 14, 85. [Google Scholar] [CrossRef]
- De Toni, A.F. Les Paragénèses à Magnétite des Altérations Associées aux Systèmes à Oxydes de fer et Altérations en Éléments Alcalins, Zone Magmatique du Grand lac de l’Ours. Master’s Thesis, Institut national de la Recherche Scientifique, Québec, QU, Canada, 2016; 534 p. [Google Scholar]
- Ootes, L.; Snyder, D.; Davis, W.J.; Acosta-Góngora, P.; Corriveau, L.; Mumin, A.H.; Montreuil, J.-F.; Gleeson, S.A.; Samson, I.A.; Jackson, V.A. A Paleoproterozoic Andean-type iron oxide copper-gold environment, the Great Bear magmatic zone, Northwest Canada. Ore Geol. Rev. 2017, 81, 123–139. [Google Scholar]
- Putnis, A. Fluid–mineral interactions: Controlling coupled mechanisms of reaction, mass transfer and deformation. J. Petrol. 2021, 62, egab092. [Google Scholar]
- Zhao, X.-F.; Chen, H.; Zhao, L.; Zhou, M.-F. Linkages among IOA, skarn, and magnetite-group IOCG deposits in China: From deposit studies to mineral potential assessment. In Mineral Systems with Iron Oxide Copper-Gold (IOCG) and Affiliated Deposits; Corriveau, L., Potter, E.G., Mumin, A.H., Eds.; Special Paper 52; Geological Association of Canada: St. John’s, NL, Canada, 2022; pp. 83–407. [Google Scholar]
- Marschik, R.; Fontboté, L. The Candelaria-Punta del Cobre iron oxide Cu-Au (-Zn-Ag) deposits, Chile. Econ. Geol. 2001, 96, 1799–1826. [Google Scholar]
- Oliver, N.H.S.; Cleverley, J.S.; Mark, G.; Pollard, P.J.; Fu, B.; Marshall, L.C.; Rubenach, M.J.; Williams, P.J.; Baker, T. Modeling the role of sodic alteration in the genesis of iron oxide-copper-gold deposits: Eastern Mount Isa Block, Australia. Econ. Geol. 2004, 99, 1145–1176. [Google Scholar] [CrossRef]
- Oliver, N.H.S.; Butera, K.M.; Rubenach, M.J.; Marshall, L.J.; Cleverley, J.S.; Mark, G.; Tullemans, F.; Esser, D. The protracted hydrothermal evolution of the Mount Isa Eastern Succession: A review and tectonic implications. Precambrian Res. 2008, 163, 108–130. [Google Scholar]
- Engvik, A.K.; Corfu, F.; Solli, A.; Austrheim, H. Sequence and timing of mineral replacement reactions during albitisation in the high-grade Bamble lithotectonic domain, S-Norway. Precambrian Res. 2017, 291, 1–16. [Google Scholar] [CrossRef]
- Barton, M.D.; Dilles, J.H.; Girardi, J.D.; Haxel, G.B.; Johnson, D.A.; Kreiner, D.C.; Seedorff, E.; Zurcher, L. Jurassic igneous-related metallogeny of southwestern North America. In Great Basin Evolution and Metallogeny, Proceedings of the Geological Society of Nevada, Symposium, Reno Sparks, Reno, NV, USA, 14 May 2010; Steininger, R.C., Pennell, W.M., Eds.; Destech Publications Inc.: Lancaster, PA, USA, 2011; Volume 1, pp. 373–396. [Google Scholar]
- Mark, G.; Oliver, N.H.S.; Williams, P.J. Mineralogical and chemical evolution of the Ernest Henry Fe oxide-Cu-Au ore system, Cloncurry district, northwest Queensland, Australia. Mineral. Depos. 2006, 40, 769–801. [Google Scholar] [CrossRef]
- Warr, L.M. IMA–CNMNC approved mineral symbols. Min. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Ehrig, K.; McPhie, J.; Kamenetsky, V. Geology and mineralogical zonation of the Olympic Dam iron oxide Cu-U-Au-Ag deposit, South Australia. in Hedenquist, J.W., Harris, M.; Camus, F., eds., Geology and genesis of major copper deposits and districts of the world: A tribute to Richard, H. Sillitoe. Soc. Econ. Geol. Spec. Publ. 2012, 16, 237–267. [Google Scholar]
- Keyser, W.; Ciobanu, C.L.; Ehrig, K.; Dmitrijeva, M.; Wade, B.P.; Courtney-Davies, L.; Verdugo-Ihl, M.; Cook, N.J. Skarn-style alteration in Proterozoic metasedimentary protoliths hosting IOCG mineralization: The Island Dam Prospect, South Australia. Mineral. Depos. 2022, 57, 1227–1250. [Google Scholar] [CrossRef]
- Chen, H.; Clark, A.H.; Kyser, T.K.; Ullrich, T.D.; Baxter, R.; Chen, Y.; Moody, T.C. Evolution of the giant Marcona-Mina Justa iron oxide-copper-gold district, south-central Peru. Econ. Geol. 2010, 105, 155–185. [Google Scholar] [CrossRef]
- del Real, I.; Thompson, J.F.H.; Carriedo, J. Lithological and structural controls on the genesis of the Candelaria-Punta del Cobre iron oxide copper-gold district, Northern Chile. Ore Geol. Rev. 2018, 102, 106–153. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, L. Mineralization, alteration, and fluid compositions in selected Andean IOCG deposits. In Mineral Systems with Iron Oxide Copper-Gold (IOCG) and Affiliated Deposits; Corriveau, L., Potter, E.G., Mumin, A.H., Eds.; Special Paper 52; Geological Association of Canada: St. John’s, NL, Canada, 2022; pp. 365–381. [Google Scholar]
- Daliran, F.; Stosch, H.-G.; Williams, P.J.; Jamali, H.; Dorri, M.-B. Early Cambrian IOA-REE, U/Th and Cu(Au)-Bi-Co-Ni-Ag-As-sulphide-U/Th deposits of the Bafq district, East-Central Iran. In Mineral Systems with Iron Oxide Copper-Gold (IOCG) and Affiliated Deposits; Corriveau, L., Potter, E.G., Mumin, A.H., Eds.; Special Paper 52; Geological Association of Canada: St. John’s, NL, Canada, 2022; pp. 409–424. [Google Scholar]
- Harlov, D.E.; Meighan, C.J.; Kerr, I.D.; Samson, I.M. 2016, Mineralogy, chemistry, and fluid-aided evolution of the Pea Ridge Fe oxide- (Y + REE) deposit, southeast Missouri, USA. Econ. Geol. 2016, 111, 1963–1984. [Google Scholar] [CrossRef]
- Bonyadi, Z.; Davidson, G.J.; Mehrabi, B.; Meffre, S.; Ghazban, F. Significance of apatite REE depletion and monazite inclusions in the brecciated Se-Chahun iron oxide–apatite deposit, Bafq district, Iran: Insights from paragenesis and geochemistry. Chem. Geol. 2011, 281, 253–269. [Google Scholar] [CrossRef]
- Hitzman, M.W.; Valenta, R.K. Uranium in iron oxide-copper-gold (IOCG) systems. Econ. Geol. 2005, 100, 1657–1661. [Google Scholar] [CrossRef]
- Kirschbaum, M.J.; Hitzman, M.W. Guelb Moghrein: An unusual carbonate-hosted iron oxide copper-gold deposit in Mauritania, northwest Africa. Econ. Geol. 2016, 111, 763–770. [Google Scholar]
- Cave, B.; Healy, M.; Overall, D.; Lilly, R. Genesis of the Mount Colin Cu-Au deposit, NW Queensland: Evidence from geology, multi-mineral U–Pb geochronology, S-, O- and C-isotope constraints. J. Geochem. Explor. 2024, 256, 107350. [Google Scholar] [CrossRef]
- Zharikov, V.A.; Pertsev, F.N.; Rusinov, V.L.; Callegari, E.; Fettes, D.J. Metasomatism and Metasomatic rocks: Recommendations by the IUGS Subcommission on the Systematics of Metamorphic Rocks, Web version 01.02.07. Available online: www.bgs.ac.uk/scmr/home.html (accessed on 3 May 2012).
- Kreiner, D.; Barton, M.D. Sulfur-poor intense acid hydrothermal alteration: A distinctive hydrothermal environment. Ore Geol. Rev. 2017, 88, 174–187. [Google Scholar] [CrossRef]
- Ehrig, K.; Kamenetsky, V.S.; McPhie, J.; Macmillan, E.; Thompson, J.; Kamenetsky, M.; Maas, R. Staged formation of the supergiant Olympic Dam uranium deposit, Australia. Geology 2021, 49, 1312–1316. [Google Scholar] [CrossRef]
- Zhao, X.-F.; Zeng, L.-P.; Liao, W.; Fan, Y.-Z.; Hofstra, A.H.; Emsbo, P.; Hu, H.; Wen, G.; Li, J.W. Iron oxide–apatite deposits form from hydrosaline liquids exsolved from subvolcanic intrusions. Mineral. Depos. 2024, 59, 655–669. [Google Scholar]
- Potter, E.G.; Montreuil, J.-F.; Corriveau, L.; Davis, W. The Southern Breccia metasomatic uranium system of the Great Bear magmatic zone, Canada: Iron oxide-copper-gold (IOCG) and albitite-hosted uranium linkages. In Ore Deposits: Origin, Exploration, and Exploitation; Decrée, S., Robb, L., Eds.; Geophysical Monograph 242, American Geophysical Union; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 109–130. [Google Scholar]
- Seymour, N.M.; Singleton, J.S.; Gomila, R.; Arancibia, G.; Ridley, J.; Gevedon, M.L.; Stockli, D.F.; Seman, S.M. Sodic-calcic alteration and transpressional shear along the Atacama fault system during IOCG mineralization, Copiapó, Chile. Mineral. Depos. 2024, 59, 1295–1323. [Google Scholar] [CrossRef]
- Rubenach, M. Structural controls of metasomatism on a regional scale. In Metasomatism and the Chemical Transformation of Rock: The Role of Fluids in Terrestrial and Extraterrestrial Processes; Harlov, D.E., Austrheim, H., Eds.; Lecture Notes in Earth System Sciences; Springer: New York, NY, USA, 2012; pp. 93–140. [Google Scholar]
- Frietsch, R.; Tuisku, P.; Martinsson, O.; Perdahl, J.-A. Early Proterozoic Cu-(Au) and Fe ore deposits associated with regional Na-Cl metasomatism in northern Fennoscandia. Ore Geol. Rev. 1997, 12, 1–34. [Google Scholar] [CrossRef]
- Marshall, L.J.; Oliver, N.H.S. Constraints on hydrothermal fluid pathways within Mary Kathleen Group stratigraphy of the Cloncurry iron-oxide–copper–gold District, Australia. Precambrian Res. 2008, 163, 151–158. [Google Scholar] [CrossRef]
- Conor, C.; Raymond, O.; Baker, T.; Teale, G.; Say, P.; Lowe, G. Alteration and mineralisation in the Moonta-Wallaroo copper-gold mining field region, Olympic domain, South Australia. In Hydrothermal Iron Oxide Copper-Gold & Related Deposits: A Global Perspective, v. 3—Advances in the Understanding of IOCG Deposits; Porter, T.M., Ed.; PGC Publishing: Adelaide, Australia, 2010; pp. 147–170. [Google Scholar]
- Poulet, T.; Karrech, A.; Regenauer-Lieb, K.; Fisher, L.; Schaubs, P. Thermal–hydraulic–mechanical–chemical coupling with damage mechanics using ESCRIPTRT and ABAQUS. Tectonophysics 2012, 526–529, 124–132. [Google Scholar] [CrossRef]
- Austin, J.R.; Blenkinsop, T.G. Local to regional scale structural controls on mineralisation and the importance of a major lineament in the eastern Mount Isa Inlier, Australia: Review and analysis with autocorrelation and weights of evidence. Ore Geol. Rev. 2009, 35, 298–316. [Google Scholar]
- Babo, J.; Spandler, C.; Oliver, N.; Brown, M.; Rubenach, M.; Creaser, R.A. The high-grade Mo-Re Merlin deposit, Cloncurry District, Australia: Paragenesis and geochronology of hydrothermal alteration and ore formation. Econ. Geol. 2017, 112, 397–422. [Google Scholar]
- Geijer, P.; Ödman, O.H. The Emplacement of the Kiruna Iron Ores and Related Deposits; SER C NR 700; Sveriges Geologiska Undersökning: Uppsala, Sweden, 1974; 48 p.
- Meyer, W. Soda metasomatism. In 1986 Regional Resident Geologists Report on Activities; Kustra, C.R., Ed.; Miscellaneous Paper 134; Ontario Geological Survey: Sudbury, ON, Canada, 1987; pp. 256–257. [Google Scholar]
- Ray, S.K. The albitite line of northern Rajasthan—A fossil intracontinental rift zone. J. Geol. Soc. India 1990, 86, 413–423. [Google Scholar]
- Champion, D.C.; Huston, D.L.; Bastrakov, E.N.; Siegel, C.; Thorne, J.; Gibson, G.M.; Hauser, J. Alteration of mafic igneous rocks of the southern McArthur Basin: Comparison with the Mount Isa region and implications for basin-hosted base metal deposits. In Exploring for the Future: Extended Abstracts; Geoscience Australia: Canberra, Australia, 2020. [Google Scholar]
- Schandl, E.S.; Gorton, M.P.; Davis, D.W. Albitization at 1700 ± 2 Ma in the Sudbury—Wanapitei Lake area, Ontario: Implications for deep-seated alkalic magmatism in the Superior Province. Can. J. Earth Sci. 1994, 31, 597–607. [Google Scholar]
- Polito, P.A.; Kyser, T.K.; Stanley, C. The Proterozoic, albitite-hosted, Valhalla uranium deposit, Queensland, Australia: A description of the alteration assemblage associated with uranium mineralization in diamond drill hole V39. Mineral. Depos. 2009, 44, 11–40. [Google Scholar]
- Kreiner, D.C. Epithermal Style Iron Oxide(-Cu-Au) (=IOCG) Vein Systems and Related Alteration. Ph.D. Thesis, University of Arizona, Tuscon, AZ, USA, 2011; 659 p. [Google Scholar]
- Barton, M.D.; Johnson, D.A.; Kreiner, D.C.; Jensen, E.P. Vertical zoning and continuity in Fe oxide (-Cu-Au-Ag-Co-U-P-REE) (or IOCG) systems: Cordilleran insights. In Proceedings of the 12th SGA Biennial Meeting, Uppsala, Sweden, 12–15 August 2013; Volume 3, pp. 1348–1351. [Google Scholar]
- Wilde, A. Towards a model for albitite-type uranium. Minerals 2013, 3, 36–48. [Google Scholar] [CrossRef]
- Putnis, A. Transient porosity resulting from fluid–mineral interaction and its consequences. Rev. Mineral. Geochem. 2015, 80, 1–23. [Google Scholar]
- Yadav, G.S.; Muthamilselvan, A.; Shaji, T.J.; Nanda, L.K.; Rai, A.K. Recognition of a new albitite zone in northern Rajasthan: Its implications on uranium mineralization. Curr. Sci. 2015, 108, 1994–1998. [Google Scholar]
- Sparkes, G.W. Uranium Mineralization within the Central Mineral Belt of Labrador: A Summary of the Diverse Styles, Settings and Timing of Mineralization; Open File LAB/1684; Government of Newfoundland and Labrador, Department of Natural Resources; Geological Survey: St. John’s, NL, Canada, 2017; 198 p.
- Baidya, A.S.; Sen, A.; Pal, D.C. Textures and compositions of cobalt pentlandite and cobaltian mackinawite from the Madan-Kudan copper deposit, Khetri Copper Belt, Rajasthan, India. J. Earth Syst Sci 2018, 127, 56. [Google Scholar]
- Rasilainen, K.; Eilu, P.; Huovinen, I.; Konnunaho, J.; Niiranen, T.; Ojala, J.; Törmänen, T. Quantitative Assessment of Undiscovered Resources in Kuusamo-Type Co-Au Deposits in Finland; Bulletin 410; Geological Survey of Finland: Espoo, Finland, 2020; 32 p. [Google Scholar]
- Dwivedy, S.; Sahoo, P.R. Geology and trace element geochemistry of the albitite hosted iron ore mineralization around Khetri copper deposit, India: Implications for an IOA type deposit. Ore Geol. Rev. 2021, 138, 104343. [Google Scholar]
- Fabris, A.; Katona, L.; Gordon, G.; Reed, G.; Keeping, T.; Gouthas, G.; Swain, G. Characterising and Mapping Alteration in the Punt Hill Region: A Data Integration Project; Report Book, 2018/00010; Government of South Australia; Department of the Premier and Cabinet: Adelaide, Australia, 2018; 604 p.
- Magyarosi, Z.; Baker, J.; MacKenzie, J. Data Compilation and Interpretation Report, Fostung Project, Ontario, Canada. 2012. Available online: https://www.geologyontario.mines.gov.on.ca/persistent-linking?assessment=20000008530 (accessed on 27 June 2021).
- Day, W.C.; Slack, J.F.; Ayuso, R.A.; Seeger, C.M. Regional geologic and petrologic framework for iron oxide ± apatite ± rare earth element and iron oxide copper-gold deposits of the Mesoproterozoic St. François Mountains Terrane, Southeast Missouri, USA. Econ. Geol. 2016, 111, 1825–1858. [Google Scholar]
- Tornos, F.; Hanchar, J.M.; Steele-MacInnis, M.; Crespo, E.; Kamenetsky, V.S.; Casquet, C. Formation of magnetite-(apatite) systems by crystallizing ultrabasic iron-rich melts and slag separation. Mineral. Depos. 2024, 59, 189–225. [Google Scholar]
- Normandeau, P.X.; Harlov, D.E.; Corriveau, L.; Paquette, J.; McMartin, I. Characterization of fluorapatite within iron oxide alkali-calcic alteration systems of the Great Bear magmatic zone: A potential metasomatic process record. Can. Min. 2018, 56, 167–187. [Google Scholar]
- Sappin, A.-A.; Perreault, S. Drill Core Pictures and Description of Samples Collected from the REE Kwyjibo Deposit (SOQUEM Warehouse, Val d’Or, QC—October 2015); Open File 8794; Geological Survey of Canada: Ottawa, ON, Canada, 2021; 14 p.
- Garcia, V.B.; Schutesky, M.E.; Oliveira, C.G.; Whitehouse, M.J.; Huhn, S.R.B.; Augustin, C.T. The Neoarchean GT-34 Ni deposit, Carajás mineral Province, Brazil: An atypical IOCG-related Ni sulfide mineralization. Ore Geol. Rev. 2020, 127, 103773. [Google Scholar]
- Veloso, A.S.R.; Monteiro, L.V.S.; Juliani, C. The link between hydrothermal nickel mineralization and an iron oxide-copper-gold (IOCG) system: Constraints based on mineral chemistry in the Jatobá deposit, Carajás Province. Ore Geol. Rev. 2020, 121, 103555. [Google Scholar]
- Mansur, E.; Dare, S.; Ferreira Filho, C.; Miranda, A.C.R.; Monteiro, L.V.S. The distribution of trace elements in sulfides and magnetite from the Jaguar hydrothermal nickel deposit: Exploring the link with IOA and IOCG deposits within the Carajás Mineral Province, Brazil. Ore Geol. Rev. 2023, 152, 105256. [Google Scholar]
- Campo Rodriguez, Y.T.; Cook, N.J.; Ciobanu, C.L.; Schutesky, M.E.; King, S.A.; Gilbert, S.; Ehrig, K. Platinum group minerals associated with nickel-bearing sulfides from the Jatobá iron oxide-copper-gold deposit, Carajás domain, Brazil. Minerals 2024, 14, 757. [Google Scholar] [CrossRef]
- Acosta-Góngora, P.; Gleeson, S.; Samson, I.; Corriveau, L.; Ootes, L.; Jackson, S.E.; Taylor, B.E.; Girard, I. Origin of sulfur and crustal recycling of copper in polymetallic (Cu-Au-Co-Bi-U ± Ag) iron-oxide-dominated systems of the Great Bear magmatic zone, NWT, Canada. Mineral. Depos. 2018, 53, 353–376. [Google Scholar]
- Acosta-Góngora, P.; Gleeson, S.; Samson, I.; Corriveau, L.; Ootes, L.; Taylor, B.E.; Creaser, R.A.; Muehlenbachs, K. Genesis of the Paleoproterozoic NICO iron-oxide-cobalt-gold-bismuth deposit, Northwest Territories, Canada: Evidence from isotope geochemistry and fluid inclusions. Precambrian Res. 2015, 268, 168–193. [Google Scholar]
- Sletten, M.; Zelligan, S.; Frost, D.; Yugo, N.; Charbonneau, C.; Cameron, D.P. Idaho Cobalt Operations, Form 43-101F1 Technical Report, Feasibility Study Idaho, USA; M3 Engineering and Technology Corporation for Jervois Mining Limited: Salmon, ID, USA, 2020; 416 p. [Google Scholar]
- Goad, R.E.; Mumin, A.H.; Duke, N.A.; Neale, K.L.; Mulligan, D.L. Geology of the Proterozoic iron oxide-hosted NICO cobalt-gold-bismuth, and Sue-Dianne copper-silver deposits, southern Great Bear Magmatic Zone, Northwest Territories, Canada. In Hydrothermal Iron Oxide Copper-Gold & Related Deposits: A Global Perspective; Porter, T.M., Ed.; PGC Publishing: Adelaide, Australia, 2000; Volume 1, pp. 249–267. [Google Scholar]
- Acosta-Góngora, G.P.; Gleeson, S.A.; Samson, I.; Ootes, L.; Corriveau, L. Gold refining by bismuth melts in the iron oxide-dominated NICO Au-Co-Bi (±Cu±W) deposit, NWT, Canada. Econ. Geol. 2015, 110, 291–314. [Google Scholar]
- Rusk, B.; Oliver, N.; Blenkinsop, T.; Zhang, D.; Williams, P.; Cleverley, J.; Habermann, H. Physical and chemical characteristics of the Ernest Henry iron oxide copper gold deposit, Cloncurry, Queensland, Australia; Implications for IOCG genesis. In Hydrothermal Iron Oxide Copper-Gold & Related Deposits: A Global Perspective, v. 3—Advances in the Understanding of IOCG Deposits; Porter, T.M., Ed.; PGC Publishing: Adelaide, Australia, 2010; pp. 201–218. [Google Scholar]
- Zhao, X.-F.; Zhou, M.F.; Su, Z.K.; Li, X.C.; Chen, W.T.; Li, J.W. Geology, geochronology, and geochemistry of the Dahongshan Fe-Cu-(Au-Ag) deposit, Southwest China: Implications for the formation of iron oxide copper-gold deposits in intracratonic rift settings. Econ. Geol. 2017, 112, 603–628. [Google Scholar]
- Zeng, M.; Zhang, D.; Zhang, Z.; Li, T.; Li, C.; Wei, C. Structural controls on the Lala iron-copper deposit of the Kangdian metallogenic province, southwestern China: Tectonic and metallogenic implications. Ore Geol. Rev. 2018, 97, 35–54. [Google Scholar]
- Lilly, R.; Case, G.; Miller, B. Ernest Henry iron oxide copper-gold deposit. Publ. Australas. Inst. Min. Metall. 2017, 32, 501–506. [Google Scholar]
- Jébrak, M. Use of breccias in IOCG(U) exploration: An update review. In Mineral Systems with Iron Oxide Copper-Gold (IOCG) and Affiliated Deposits; Corriveau, L., Potter, E.G., Mumin, A.H., Eds.; Special Paper 52; Geological Association of Canada: St. John’s, NL, Canada, 2022; pp. 315–324. [Google Scholar]
- Le, T.X.; Dirks, P.H.G.M.; Sanislav, I.V.; Huizenga, J.M.; Cocker, H.; Manestar, G.N. Geological setting and mineralisation characteristics of the Tick Hill Gold Deposit, Mount Isa Inlier, Queensland, Australia. Ore Geol. Rev. 2021, 137, 104288. [Google Scholar]
- Davidson, G.J.; Paterson, H.; Meffre, S.; Berry, R.F. Characteristics and origin of the Oak Dam East breccia-hosted, iron oxide-Cu-U-(Au) deposit: Olympic Dam region, Gawler Craton, South Australia. Econ. Geol. 2007, 102, 1471–1498. [Google Scholar]
- Fox, N.; Valenta, R.; Gow, P. Chapter 7: Eloise Cu-Au deposit. NWMP Deposit Atlas; Geological Survey of Queensland: Brisbane, Australia, 2023; pp. 257–279.
- Somarin, A.K.; Mumin, A.H. P–T-composition and evolution of paleofluids in the Paleoproterozoic Mag Hill IOCG hydrothermal system, Contact Lake belt, Northwest Territories, Canada. Mineral. Depos. 2014, 49, 199–215. [Google Scholar]
- Lobo-Guerrero, S.A. Iron oxide-copper-gold mineralization in the Greater Lufilian Arc, Africa. In Exploring for Iron Oxide Copper-Gold Deposits: Canada and Global Analogues; Corriveau, L., Mumin, A.H., Eds.; Short Course Notes, No. 20; Geological Association of Canada: St. John’s, NL, Canada, 2010; pp. 161–175. [Google Scholar]
- Gandhi, S.S.; Potter, E.G.; Fayek, M. New constraints on genesis of the polymetallic veins at Port Radium, Great Bear Lake, Northwest Canadian Shield. Ore Geol. Rev. 2018, 96, 28–47. [Google Scholar]
- Cave, B.; Lilly, R.; Hand, M.; Varga, J.; Light, S.; Leslie, D.; North, B.; Park, J.; Klingberg, L. A temporal framework for the Carrapateena Iron Oxide Copper-Gold (IOCG) deposit, Eastern Gawler Craton, South Australia. Ore Geol. Rev. 2024, 169, 106092. [Google Scholar]
- Hoggard, M.J.; Czarnota, K.; Richards, F.D.; Huston, D.; Jaques, L.; Ghelichkhan, S. Global distribution of sediment-hosted metals controlled by craton edge stability. Nat. Geosci. 2020, 13, 504–510. [Google Scholar]
- Tiddy, C.J.; Giles, D. Suprasubduction zone model for metal endowment at 1.60–1.57 Ga in eastern Australia. Ore Geol. Rev. 2020, 122, 103483. [Google Scholar]
- Hayward, N.; Corriveau, L.; Craven, J.A.; Enkin, R.J. Geophysical signature of the NICO Au-Co-Bi-Cu deposit and its iron oxide-alkali alteration system, Northwest Territories, Canada. Econ. Geol. 2016, 111, 2087–2110. [Google Scholar]
- Tornos, F.; Hanchar, J.M.; Munizaga, R.; Velasco, F.; Galindo, C. The role of the subducting slab and melt crystallization in the formation of magnetite-(apatite) systems, Coastal Cordillera of Chile. Mineral. Depos. 2021, 56, 253–278. [Google Scholar]
- Porter, T.M. The Carrapateena iron oxide copper-gold deposit, Gawler Craton, South Australia: A review. In Hydrothermal Iron Oxide Copper-Gold & Related Deposits: A Global Perspective, v. 3—Advances in the Understanding of IOCG Deposits; Porter, T.M., Ed.; PGC Publishing: Adelaide, Australia, 2010; pp. 191–200. [Google Scholar]
- Jara, J.J.; Barra, F.; Reich, M.; Leisen, M.; Romero, R.; Morata, D. Episodic construction of the early Andean Cordillera unravelled by zircon petrochronology. Nat. Comm. 2021, 12, 4930. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liu, X.; Wang, S.; Xie, J.; Liu, J. Metallogenic type controlled by magma source and tectonic regime: Geochemical comparisons of Mesozoic magmatism between the Middle–Lower Yangtze River Belt and the Dabie Orogen, eastern China. Ore Geol. Rev. 2021, 133, 104095. [Google Scholar] [CrossRef]
- Bickford, M.E.; Van Schmus, W.R.; Karlstrom, K.E.; Mueller, P.A.; Kamenov, G.D. Mesoproterozoic-trans-Laurentian magmatism: A synthesis of continent-wide age distributions, new SIMS U–Pb ages, zircon saturation temperatures, and Hf and Nd isotopic compositions. Precambrian Res. 2015, 265, 286–312. [Google Scholar] [CrossRef]
- Chen, H.; Cooke, D.R.; Baker, M.J. Mesozoic iron oxide copper-gold mineralization in the central Andes and the Gondwana supercontinent breakup. Econ. Geol. 2013, 108, 37–44. [Google Scholar] [CrossRef]
- Verbaas, J.; Thorkelson, D.J.; Crowley, J.; Davis, W.J.; Foster, D.A.; Gibson, H.D.; Marshall, D.D.; Milidragovic, D. A sedimentary overlap assemblage links Australia to northwestern Laurentia at 1.6 Ga. Precambrian Res. 2018, 305, 19–39. [Google Scholar] [CrossRef]
- Bockmann, M.J.; Payne, J.L.; Hand, M.; Morrissey, L.J.; Belperio, A.P. Linking the Gawler Craton and Mount Isa Province through hydrothermal systems in the Peake and Denison Domain, northeastern Gawler Craton. Geosci. Front. 2023, 14, 101596. [Google Scholar] [CrossRef]
- Trunfull, E.F.; Hagemann, S.G.; Xavier, R.P.; Moreto, C.P.N. Critical assessment of geochronological data from the Carajás Mineral Province, Brazil: Implications for metallogeny and tectonic evolution. Ore Geol. Rev. 2020, 121, 103556. [Google Scholar] [CrossRef]
- Regan, S.P.; Walsh, G.J.; Williams, M.L.; Chiarenzelli, J.R.; Toft, M.; McAleer, R. Syn-collisional exhumation of hot middle crust in the Adirondack Mountains (New York, USA): Implications for extensional orogenesis in the southern Grenville Province. Geosphere 2019, 15, 1240–1261. [Google Scholar] [CrossRef]
- Xavier, R.P.; Monteiro, L.V.S.; de Souza Filho, C.R.; Torresi, I.; de Resende Carvalho, E.; Dreher, A.M.; Wiedenbeck, M.; Trumbull, R.B.; Pestilho, A.L.S.; Moreto, C.P.N. The iron oxide copper-gold deposits of the Carajás mineral province, Brazil: An updated and critical review. In Hydrothermal Iron Oxide Copper-Gold & Related Deposits: A Global Perspective, v. 3—Advances in the Understanding of IOCG Deposits; Porter, T.M., Ed.; PGC Publishing: Adelaide, Australia, 2010; pp. 285–306. [Google Scholar]
- Martinsson, O.; Billström, K.; Broman, C.; Weihed, P.; Wanhainen, C. Metallogeny of the Northern Norrbotten Ore Province, northern Fennoscandian Shield with emphasis on IOCG and apatite-iron ore deposits. Ore Geol. Rev. 2016, 78, 447–492. [Google Scholar] [CrossRef]
- LKAB. A Summary Technical Report on the Mineral Resources of LKAB, Sweden—Per Geijer Iron Ore Apatite Deposit, June 2023. Available online: https://lkab.mediaflowportal.com/documents/folder/446946/ (accessed on 3 August 2023).
- LKAB. LKAB 2022 Mineral Resources Summary Report, December 2022. Available online: https://lkab.mediaflowportal.com/documents/folder/446946/ (accessed on 3 August 2023).
- Rainbird, R.H.; Rooney, A.D.; Creaser, R.A.; Skulski, T. Shale and pyrite Re-Os ages from the Hornby Bay and Amundsen basins provide new chronological markers for Mesoproterozoic stratigraphic successions of northern Canada. Earth Planet. Sci. Lett. 2020, 548, 116492. [Google Scholar]
- Hussey, K.J.; Huston, D.L.; Claoué-Long, J.C. Geology and Origin of some Cu-Pb-Zn (Auag) Deposits in the Strangways Metamorphic Complex, Arunta Region, Northern Territory; Report 17; Northern Territory Geological Survey: Darwin, Australia, 2005; 96 p.
- Corriveau, L.; Spry, P. Metamorphosed hydrothermal ore deposits. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 13, pp. 175–194. [Google Scholar]
- Hildebrand, R.S. Precambrian Geology, Leith Peninsula-Rivière Grandin Area, Northwest Territories. Canadian Geoscience Map 153; Geological Survey of Canada: Ottawa, ON, Canada, 2017.
- Bretzlaff, R.; Kerswill, J.A. Mineral Occurrences of the Great Bear Magmatic Zone; Open File 7959; Geological Survey of Canada: Ottawa, ON, Canada, 2016; 7p.
- Hayward, N.; Corriveau, L. Fault reconstructions using aeromagnetic data in the Great Bear magmatic zone, Northwest Territories, Canada. Can. J. Earth Sci. 2014, 51, 927–942. [Google Scholar]
- Chen, H. External sulphur in IOCG mineralization: Implications on definition and classification of the IOCG clan. Ore Geol. Rev. 2013, 51, 74–78. [Google Scholar]
- Badham, J.P.N.; Stanworth, C.W. Evaporites from the lower Proterozoic of the East Arm, Great Slave Lake. Nature 1977, 268, 516–518. [Google Scholar]
- Curtis, S.; Wade, C.; Reid, A. Sedimentary basin formation associated with a silicic large igneous province: Stratigraphy and provenance of the Mesoproterozoic Roopena Basin, Gawler Range Volcanics. Aust. J. Earth Sci. 2018, 65, 447–463. [Google Scholar]
- Schlegel, T.U.; Wagner, T.; Wälle, M.; Heinrich, C.A. Hematite breccia-hosted iron oxide copper-gold deposits require magmatic fluid components exposed to atmospheric oxidation: Evidence from Prominent Hill, Gawler Craton, South Australia. Econ. Geol. 2018, 113, 597–644. [Google Scholar] [CrossRef]
- Hildebrand, R.S. Geology of the Rainy Lake-White Eagle Falls area district of Mackenzie: Early Proterozoic cauldrons, stratovolcanoes and subvolcanic plutons: Paper 83–20; Geological Survey of Canada: Ottawa, ON, Canada, 1984; 42 p.
- Kelly, C.J.; Davis, W.J.; Potter, E.G.; Corriveau, L. Geochemistry of hydrothermal tourmaline from IOCG occurrences in the Great Bear magmatic zone: Implications for fluid source(s) and fluid composition evolution. Ore Geol. Rev. 2020, 118, 103329. [Google Scholar]
- Hildebrand, R.S.; Bowring, S.A. Continental intra-arc depressions: A non-extensional model for their origin, with a Proterozoic example from Wopmay Orogen. Geology 1984, 12, 73–77. [Google Scholar]
- Huang, Q.; Kamenetsky, V.S.; Ehrig, K.; McPhie, J.; Kamenetsky, M.; Cross, K.; Meffre, S.; Agangi, A.; Chambefort, I.; Direen, N.G.; et al. Olivine-phyric basalt in the Mesoproterozoic Gawler silicic large igneous province, South Australia: Examples at the Olympic Dam iron oxide Cu–U–Au–Ag deposit and other localities. Precambrian Res. 2016, 281, 185–199. [Google Scholar] [CrossRef]
- Schlegel, T.U.; Heinrich, C.A. Lithology and hydrothermal alteration control the distribution of copper grade in the Prominent Hill iron oxide-copper-gold deposit (Gawler Craton, South Australia). Econ. Geol. 2015, 110, 1953–1994. [Google Scholar]
- Jagodzinski, E.A.; Reid, A.; Crowley, J.; Wade, C.; Curtis, S. Precise zircon U-Pb dating of the Mesoproterozoic Gawler large igneous province, South Australia. Results Geochem. 2023, 10, 100020. [Google Scholar]
- Cherry, A.; Ehrig, K.; Kamenetsky, V.S.; McPhie, J.; Crowley, J.; Kamenetsky, M. Precise geochronological constraints on the origin, setting and incorporation of ca. 1.59 Ga surficial facies into the Olympic Dam Breccia Complex, South Australia. Precambrian Res. 2018, 315, 162–168. [Google Scholar]
- Courtney-Davies, L.; Ciobanu, C.L.; Verdugo-Ihl, M.R.; Dmitrijeva, M.; Cook, N.J.; Ehrig, K.; Wade, B.P. Hematite geochemistry and geochronology resolve genetic and temporal links among iron-oxide copper gold systems, Olympic Dam district, South Australia. Precambrian Res. 2019, 335, 105480. [Google Scholar]
- Courtney-Davies, L.; Ciobanu, C.L.; Tapster, S.R.; Cook, N.J.; Ehrig, K.; Crowley, J.L.; Verdugo-Ihl, M.R.; Wade, B.P.; Condon, D.J. Opening the magmatic-hydrothermal window: High-precision U-Pb geochronology of the Mesoproterozoic Olympic Dam Cu-U-Au-Ag deposit, South Australia. Econ. Geol. 2020, 115, 1855–1870. [Google Scholar]
- Marschik, R.; Söllner, F. Early Cretaceous U-Pb zircon ages for the Copiapo plutonic complex and implications for the IOCG mineralization at Candelaria, Atacama Region, Chile. Mineral. Depos. 2006, 41, 785–801. [Google Scholar]
- Yu, J.; Chen, Y.; Mao, J.; Pirajno, F.; Duan, C. Review of geology, alteration and origin of iron oxide–apatite deposits in the Cretaceous Ningwu basin, Lower Yangtze River Valley, eastern China: Implications for ore genesis and geodynamic setting. Ore Geol. Rev. 2011, 43, 170–181. [Google Scholar]
- Wang, J.; Li, L.; Santosh, M.; Yan, G.-Y.; Shen, J.-F.; Yuan, M.-W.; Alam, M.; Li, S.-R. Multistage ore formation in the world’s largest REE-Nb-Fe deposit of Bayan Obo, North China Craton: New insights and implications. Ore Geol. Rev. 2024, 164, 105817. [Google Scholar]
- Davis, W.J.; Corriveau, L.; van Breemen, O.; Bleeker, W.; Montreuil, J.-F.; Potter, E.; Pelleter, E. Timing of IOCG mineralising and alteration events within the Great Bear magmatic zone [abs.]. In 39th Annual Yellowknife Geoscience Forum Abstracts; Fischer, B.J., Watson, D.M., Eds.; Northwest Territories Geological Survey: Yellowknife, NT, Canada, 2011; Abstracts Volume 2011, 97p. [Google Scholar]
- Verdugo-Ihl, M.R.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K.; Courtney-Davies, L. Defining early stages of IOCG systems: Evidence from iron-oxides in the outer shell of the Olympic Dam deposit, South Australia. Mineral. Depos. 2020, 55, 429–452. [Google Scholar]
- Rodriguez-Mustafa, M.A.; Simon, A.C.; Holder, R.M.; Stein, H.; Kylander-Clark, A.R.C.; Jicha, B.R.; Blakemore, D.; Machado, E.L.B. Integrated Re-Os, Ar/Ar, and U-Pb geochronology directly dates the timing of mineralization at the Mina Justa and Marcona deposits, Peru. GSA Bull. 2024, 136, 2861–2874. [Google Scholar]
- Chapman, N.D.; Ferguson, M.; Meffre, S.J.; Stepanov, A.; Maas, R.; Ehrig, K.J. Pb-isotopic constraints on the source of A-type suites: Insights from the Hiltaba Suite-Gawler Range Volcanics magmatic event, Gawler craton, South Australia. Lithos 2019, 346–347, 105156. [Google Scholar]
- Wade, C.E.; Payne, J.L.; Hill, J.; Curtis, S.; Barovich, K.; Reid, A.J. Temporal, geochemical and isotopic constraints on plume-driven felsic and mafic components in a Mesoproterozoic flood rhyolite province. Results Geochem. 2022, 9, 100019. [Google Scholar]
- Wade, C.E.; Payne, J.L.; Barovich, K.M.; Crowley, J.; Jagodzinski, E.; Gilbert, S.E.; Wade, B.P.; Reid, A.J. Zircon trace element geochemistry as an indicator of magma fertility in iron oxide-copper-gold provinces. Econ. Geol. 2022, 117, 703–718. [Google Scholar]
- Williams, M.R.; Holwell, D.A.; Lilly, R.M.; Case, G.N.D.; McDonald, I. Mineralogical and fluid characteristics of the fluorite-rich Monakoff and E1 Cu–Au deposits, Cloncurry region, Queensland, Australia: Implications for regional F–Ba-rich IOCG mineralisation. Ore Geol. Rev. 2015, 64, 103–127. [Google Scholar]
- Williams, P.J.; Kendrick, M.; Xavier, R.P. Sources of ore fluid components in IOCG deposits. In Hydrothermal Iron Oxide Copper-Gold & Related Deposits: A Global Perspective, v. 3—Advances in the Understanding of IOCG Deposits; Porter, T.M., Ed.; PGC Publishing: Adelaide, Australia, 2010; pp. 107–116. [Google Scholar]
- Bastrakov, E.N.; Skirrow, R.G.; Davidson, G.J. Fluid evolution and origins of iron oxide Cu-Au prospects in the Olympic Dam district, Gawler Craton, South Australia. Econ Geol. 2007, 102, 1415–1440. [Google Scholar]
- Baker, T.; Mustard, R.; Fu, B.; Williams, P.J.; Dong, G.; Fisher, L.; Mark, G.; Ryan, C.G. Mixed messages in iron oxide–copper–gold systems of the Cloncurry district, Australia: Insights from PIXE analysis of halogens and copper in fluid inclusions. Mineral. Depos. 2008, 43, 599–608. [Google Scholar]
- Williams, P.J. Iron mobility during synmetamorphic alteration in the Selwyn Range area, NW Queensland: Implications for the origin of ironstone-hosted Au-Cu deposits. Mineral. Depos. 1994, 29, 250–260. [Google Scholar]
- Pelleter, E.; Gasquet, D.; Cheilletz, A.; Mouttaqi, A. Alteration processes and impacts on regional-scale element mobility and geochronology, Tamlalt-Menhouhou deposit, Morocco. In Exploring for Iron Oxide Copper-Gold Deposits: Canada and Global Analogues; Corriveau, L., Mumin, A.H., Eds.; Short Course Notes, No. 20; Geological Association of Canada: St. John’s, NL, Canada, 2010; pp. 177–185. [Google Scholar]
- Li, W.; Audétat, A.; Zhang, J. The role of evaporites in the formation of magnetite–apatite deposits along the Middle and Lower Yangtze River, China: Evidence from LA-ICP-MS analysis of fluid inclusions. Ore Geol. Rev. 2015, 67, 264–278. [Google Scholar] [CrossRef]
- Bain, W.M.; Steele-MacInnis, M.; Li, K.; Li, L.; Mazdad, F.K.; Marsh, E.E. A fundamental role of carbonate–sulfate melts in the formation of iron oxide–apatite deposits. Nat. Geosci. 2020, 13, 751–757. [Google Scholar]
- Wise, T.; Thiel, S. Proterozoic tectonothermal processes imaged with magnetotellurics and seismic reflection in southern Australia. Geosci. Front. 2020, 11, 885–893. [Google Scholar]
- Blundy, J.; Afanasyev, A.; Tattitch, B.; Sparks, S.; Melnik, O.; Utkin, I.; Rust, A. The economic potential of metalliferous sub-volcanic brines. Royal Soc. Open Sci. 2021, 8, 202192. [Google Scholar]
- Pietruszka, D.K.; Hanchar, J.M.; Tornos, F.; Wirth, R.; Graham, N.A.; Severin, K.P.; Velasco, F.; Steele-MacInnis, M.; Bain, W.M. Magmatic immiscibility and the origin of magnetite-(apatite) iron deposits. Nat. Comm. 2023, 14, 8424. [Google Scholar] [CrossRef] [PubMed]
- Lowell, G.R. The Butler Hill Caldera: A mid-Proterozoic ignimbrite-granite complex. Precambrian Res. 1991, 51, 245–263. [Google Scholar] [CrossRef]
- Marshall, L.J.; Oliver, N.H.S.; Davidson, G.J. Carbon and oxygen isotope constraints on fluid sources and fluid-wallrock interaction in regional alteration and iron-oxide–copper–gold mineralisation, eastern Mt Isa Block, Australia. Mineral. Depos. 2006, 41, 429–452. [Google Scholar] [CrossRef]
- Chinova Resources. Mount Dore mineral resource update summary. In Technical Report 2016; Chinova Resources: Dajarra, QLD, Australia, 2016; 15p, Available online: https://www.chinovaresources.com/images/pdfs/Mt_Dore_Resource_Estimation_Update_summary.pdf (accessed on 3 August 2023).
- Reid, A.J.; Fabris, A. Influence of pre-existing low metamorphic grade sedimentary successions on the distribution of iron oxide copper-gold mineralisation in the Olympic Cu-Au Province, Gawler Craton. Econ. Geol. 2015, 110, 2147–2157. [Google Scholar] [CrossRef]
- Morrissey, L.J.; Tomkins, A.G. Evaporite-bearing orogenic belts produce ligand-rich and diverse metamorphic fluids. Geochim. Cosmochim. Acta 2020, 275, 163–187. [Google Scholar] [CrossRef]
- de Melo, G.H.C.; Monteiro, L.V.S.; Hunger, R.B.; Toledo, P.I.F.; Xavier, R.P.; Zhao, X.-F.; Su, Z.-K.; Moreto, C.P.N.; De Jesus, S.d.S.G.P. Magmatic-hydrothermal fluids leaching older seafloor exhalative rocks to form the IOCG deposits of the Carajás Province, Brazil: Evidence from boron isotopes. Precambrian Res 2021, 365, 106412. [Google Scholar] [CrossRef]
- Emproto, C.; Mathur, R.; Simon, A.; Bindeman, I.; Godfrey, L.; Dhnaram, C.; Lisitsin, V. Integrated O, Fe, and Ti isotopic analysis elucidates multiple metal and fluid sources for magnetite from the Ernest Henry iron oxide copper gold (IOCG) Deposit, Queensland, Australia. Ore Geol. Rev. 2022, 150, 105170. [Google Scholar] [CrossRef]
- Melfou, M.; Richard, A.; Tarantola, A.; Villeneuve, J.; Carr, P.; Peiffert, C.; Mercadier, J.; Dean, B.; Drejing-Carroll, D. Tracking the origin of metasomatic and ore-forming fluids in IOCG deposits through apatite geochemistry (Nautanen North deposit, Norrbotten, Sweden). Lithos 2023, 438–439, 106995. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.-L. External fluid incursion during Cu-mineralization stage of Mina Justa iron oxide copper-gold (IOCG) deposit: Evidence from triple sulfur isotope geochemistry of chalcopyrite. Ore Geol. Rev. 2022, 149, 105102. [Google Scholar] [CrossRef]
- Duan, G.; Ram, R.; Xing, Y.; Etschmann, B.; Brugger, J. Kinetically driven successive sodic and potassic alteration of feldspar. Nat. Commun. 2021, 12, 4435. [Google Scholar] [CrossRef]
- Nayebi, N.; Esmaeily, D.; D’Antonio, M.; Xia, X.-P.; Di Renzo, V.; Lehmann, B.; Shinjo, R.; Babazadeh, S.; Deevsalar, R.; Modabberi, S. Zircon U–Pb ages and Sr–Nd–Pb–Hf isotopic compositions constrain the tectono-magmatic evolution of the Anomaly 21-A iron ore region, Bafq metallogenic province, Central Iran. J. Asian Earth Sci. 2023, 250, 105646. [Google Scholar] [CrossRef]
- Bergman, S.; Kübler, L.; Martinsson, O. Description of Regional Geological and Geophysical Maps of Northern Norrbotten County (East of the Caledonian Orogen); BA-56; Sveriges Geologiska Undersökning: Uppsala, Sweden, 2001; 110 p.
- Logan, L.; Andersson, J.B.H.; Whitehouse, M.J.; Martinsson, O.; Bauer, T.E. Energy drive for the Kiruna mining district mineral system(s): Insights from U-Pb zircon geochronology. Minerals 2022, 12, 875. [Google Scholar] [CrossRef]
- Case, G.; Blenkinsop, T.; Chang, Z.; Huizenga, J.M.; Lilly, R.; McLellan, J. Delineating the structural controls on the genesis of iron oxide-Cu-Au deposits through implicit modelling: A case study from the E1 Group, Cloncurry District, Australia. In Characterization of Ore-forming Systems from Geological, Geochemical and Geophysical Studies; Gessner, K., Blenkinsop, T.G., Sorjonen-Ward, P., Eds.; Geological Society, London, Special Publications: London, UK, 2017; 453 p. [Google Scholar]
- Lu, Y.; Liu, L.; Xu, G. Constraints of deep crustal structures on large deposits in the Cloncurry district, Australia: Evidence from spatial analysis. Ore Geol. Rev. 2016, 79, 316–331. [Google Scholar]
- Bauer, T.E.; Andersson, J.B.H. Regional structural setting of late-orogenic IOCG mineralization along the northern Nautanen deformation zone, Norrbotten, Sweden. Ore Geol. Rev. 2023, 163, 105814. [Google Scholar]
- Gadd, M.G.; Lawley, C.J.; Corriveau, L.; Houlé, M.; Peter, J.M.; Plouffe, A.; Potter, E.G.; Sappin, A.-A.; Pilote, J.-L.; Marquis, G.; et al. Public geoscience solution for diversifying Canada’s critical mineral production. In Geological Society; Special Publications: London, UK, 2022; Volume 526, pp. 25–50. [Google Scholar]
- Cave, B.W.; Lilly, R.; Glorie, S.; Gillespie, J. Geology, apatite geochronology, and geochemistry of the Ernest Henry inter-lens: Implications for a re-examined deposit model. Minerals 2018, 8, 405. [Google Scholar] [CrossRef]
- Rojas, P.A.; Barra, F.; Reich, M.; Deditius, A.; Simon, A.; Uribe, F.; Romero, R.; Rojo, M. A genetic link between magnetite mineralization and diorite intrusion at the El Romeral iron oxide-apatite deposit, northern Chile. Mineral. Depos. 2018, 53, 947–966. [Google Scholar] [CrossRef]
- Martinsson, O. Kiskamavaara a shear zone hosted IOCG-style of Cu-Co-Au deposit in Northern Norrbotten, Sweden. In Proceedings of the 11th SGA Biennial Meeting, Antofagasta, Chile, 26–29 September 2011; Volume 2, pp. 470–472. [Google Scholar]
- Martinsson, O.; Wanhainen, C. Economic potential of battery metals and minerals in Sweden. In Proceedings of the Volume for the 16th SGA Biennial Meeting, Rotorua, New Zealand, 28–31 March 2022; pp. 227–230. [Google Scholar]
- Corriveau, L.; Bonnet, A.-L. Pinwarian (1.5 Ga) volcanism and hydrothermal activity at the eastern margin of the Wakeham Group, Grenville Province, Quebec. Can. J. Earth Sci. 2005, 42, 1749–1782. [Google Scholar]
- Corriveau, L.; Morin, D. Modelling 3D architecture of western Grenville from surface geology, xenoliths, styles of magma emplacement and Lithoprobe reflectors. Can. J. Earth Sci. 2000, 37, 235–251. [Google Scholar]
- BHP. Annual Report 2024. Available online: https://www.bhp.com/investors/annual-reporting/annual-report-2024 (accessed on 17 December 2024).
- BHP. Financial Results for the Year Ended 30 June 2024. Available online: https://www.bhp.com/-/media/documents/media/reports-and-presentations/2024/240827_bhpresultsfortheyearended30june2024.pdf (accessed on 13 January 2025).
- Rex Minerals Limited. Rex Minerals-Hillside Resources & Reserves. Available online: https://www.rexminerals.com.au/resourceshillside (accessed on 10 January 2025).
- Chinalco Yunnan Copper Resources. 26.1 Mt Inferred JORC Resource Estimate Elaine 1 Copper-Gold Deposit. Available online: https://www.aukingmining.com/site/pdf/efaa23c8-91c4-4c98-9019-7fc824701307/261Mt-Inferred-JORC-Resource-Elaine-1-Copper-Gold-Deposit.pdf (accessed on 29 June 2012).
- China Yunnan Copper Resources. Inferred Resource Estimate—Elaine-Dorothy Uranium—Rare Earth Element (REE). Available online: https://www.aukingmining.com/site/PDF/69270e44-d780-40f0-9aec-b1e695241a0e/CuCoREEUMineralisationatElaineDorothyProject (accessed on 27 January 2025).
- AIC Mines Limited. Eloise Copper Mine Almanac, April 2024. Available online: https://www.aicmines.com.au/downloads/eloise-almanac.pdf (accessed on 10 January 2025).
- Evolution Mining. Further Increase in Ernest-Henry Mineral Resource: ASX Announcement, 17 August 2023. Available online: www.evolutionmining.com.au (accessed on 15 May 2024).
- AIC Mines Limited. Significant Increase in Mineral Resource. 2025. Available online: https://www.listcorp.com/asx/a1m/aic-mines-limited/news/significant-increase-in-mineral-resources-3166632.html (accessed on 27 January 2025).
- Copper Mountain Mining Corporation. NI 43-101 Technical Report for the Eva Copper Project—Feasibility Study Update North West Queensland, Australia, 2020. Available online: https://www.sedarplus.ca/csa-party/records/document.html?id=d47610acaa2a771985e5f54edf16b9135b71722a01bb857a83b97a332d67db57 (accessed on 18 December 2024).
- Hammer Metal Corporation, 2023, Annual Report 2023. Available online: www.hammermetals.com.au (accessed on 23 January 2024).
- McKay, A.D.; Miezitis, Y. Australia’s uranium resources, geology and development of deposits. In Mineral Resources Report 1; AGSO–Geoscience Australia: Symonston, Australia, 2001. [Google Scholar]
- Chinova, R. Mount Elliott Swan Resource Estimation Update Summary. 2017. Available online: www.chinovaresources.com (accessed on 10 August 2017).
- Cudeco. 2017 Annual Report. Available online: www.cudeco.com.au (accessed on 17 May 2023).
- Paladin, E. Valhalla Uranium Deposit, Mineral Resources. 2015. Available online: http://www.paladinenergy.com.au/default.aspx?MenuID=35 (accessed on 1 February 2015).
- Collier, J. Johnnies Reward, Au-Cu, Mineral Resource Estimation, 24th January 2018, Prepared for Conarco Consulting (Conarco). Available online: https://geoscience.nt.gov.au (accessed on 31 January 2025).
- Peko, B. Peko Tailings Retreatment Project Information Memorandum. 2017. Available online: https://www.99mines.com/wp-content/uploads/2017/07/345_geodir_key_documents_Peko-IM-July-2017-Executive-Summary-1.pdf (accessed on 16 December 2024).
- Grange Resources Limited. Annual Resource & Reserve Statement—December 2023; Maiden underground Ore Reserve Declared for Savage River Mine: ASX Announcement, 28 February 2024. Available online: https://www.listcorp.com/ (accessed on 18 December 2024).
- Natural Resources Canada, Canada’s Critical Minerals. 2024. Available online: https://www.canada.ca/en/campaign/critical-minerals-in-canada/critical-minerals-an-opportunity-for-canada.html (accessed on 16 January 2025).
- Perron, P.M.; Beauvais, M.R.; Kinnan, E.; Roy, P. NI 43-101 Technical Report and Mineral Resource Estimate for the Iron Creek Cobalt-Copper Property, Lemhi County, Idaho, USA; Electra Battery Materials Corporation: Toronto, On, Canada, 2023; 196p, Available online: https://www.sedarplus.ca/csa-party/records/document.html?id=fa56487bc1f25e4e1a0bdaee8cbca3c95ffdbcbe99044a729e5a046abe01e4a4 (accessed on 9 January 2025).
- Hammarstrom, J.; Dicken, C.; Day, W.; Hofstra, A.; Drenth, B.; Shah, A.; McCafferty, A.; Woodruff, L.; Foley, N.; Ponce, D.; et al. Focus Areas for Data Acquisition for Potential Domestic Resources of 11 Critical Minerals in the Conterminous United States, Hawaii, and Puerto Rico—Aluminum, Cobalt, Graphite, Lithium, Niobium, Platinum-Group Elements, Rare Earth elements, tantalum, tin, titanium, and tungsten (ver. 1.1, July 2022), chap. B of U.S. Geological Survey, Focus Areas for Data Acquisition for Potential Domestic Sources of Critical Minerals; Open-File Report 2019–1023; U.S. Geological Survey: Denver, CO, USA, 2020; 67 p. [CrossRef]
- Nevada Copper Corporation. Pumpkin Hollow Project, Open Pit and Underground Mine Prefeasibility Study: NI 43-101 Technical Report; Nevada Copper Corporation: Yerington, NV, USA, 2019; 520p, Available online: https://www.sedarplus.ca/csa-party/records/document.html?id=abf27b5892bfb4c5a9d18dd96e8474e9a58b05136024c81a3ffd693b10ca629e (accessed on 9 January 2025).
- Samari, H.; Lane, T.; Harvey, T.; Breckenridge, L. Preliminary Economic Assessment NI 43-101 Technical Report on the Moonlight-Superior Project; US Copper Corp: Toronto, ON, Canada, 2024; Available online: https://www.sedarplus.ca/csa-party/records/document.html?id=0510c94aec7ae511be0f0aed8bb8b237d3b00860377b44353a278c9f527ff540 (accessed on 9 January 2025).
- Kyle, J.; Beahm, D. NI 43-101 Preliminary Economic Assessment Update, Coles Hill Uranium Property; Virginia Energy Resources: Pittsylvania County, VA, USA, 2012. Available online: https://www.sedarplus.ca/csa-party/records/document.html?id=721388e103dc592c58d59cda0a57eebd97be070851512380857d1d9ce053d63d (accessed on 10 January 2025).
- Magnum Mining and Exploration. Maiden JORC 2012 resource for Buena Vista magnetite project. Magnum Mining and Exploration: Subiaco, WA, USA. Available online: https://www.mmel.com.au/site/projects-and-products/buena-vista-magnetite-project (accessed on 27 January 2025).
- Burgess, H.; Gowans, R.M.; Hennessey, B.T.; Lattanzi, C.R.; Puritch, E. Technical Report on the Feasibility Study for the NICO Gold–Cobalt–Bismuth–Copper Deposit; NI 43-101 Technical Report No. 1335; Fortune Minerals Ltd.: London, ON, Canada, 2014; 385p, Available online: https://www.sedarplus.ca/csa-party/records/document.html?id=b7f689dd67e909fa62179555a672714fc561c8a7d5fcd21b5d1ee14a15edf946 (accessed on 15 September 2015).
- Hennessey, B.T.; Puritch, E. A Technical Report on a Mineral Resource Estimate for the Sue-Dianne Deposit, Mazenod Lake Area; NI 43-101 Technical Report; Fortune Minerals Limited: London, ON, Canada, 2008; 125p, Available online: https://www.sedarplus.ca/csa-party/records/document.html?id=c64ebb5d1c5a5e3c37a62392a9209df9a59529b1c4b9a0a3a1e7b6c08f9c1a8e (accessed on 21 December 2009).
- Paladin Energy. Michelin Project, Labrador Canada, 2019. Available online: http://www.paladinenergy.com.au/project/michelin-canada (accessed on 1 September 2019).
- Kruse, F. NI 43-101 technical report, Moran Lake project, Central Mineral Belt, Newfoundland and Labrador, Canada. NI 43-101 Technical Report. Consolidated-Uranium-Inc., 2021. Available online: https://www.sedarplus.ca/csfsprod/data446/filings/03325658/00000001/z%3A%5CPUBLIC%5CConsolidated-Uranium-Inc%5C2022%5CSMG%5CCUR-MoranLk-TechRpt.pdf (accessed on 28 January 2025).
- Gagnon, R.; Buro, Y.A.; Ibrango, S.; Gagnon, D.; Stapinsky, M.; Del Carpio, S.; Larochelle, E. Projet de Terres Rares Kwyjibo: Rapport Technique NI 43-101 Révisé; Dra Met-Chem: Montréal, QC, Canada, 2018; 292p, Available online: https://www.sedarplus.ca/csa-party/records/document.html?id=6fe7e177d4d03d91a694437e1ea496e30055103b092233ae7ba5039ea38c7265 (accessed on 15 December 2023).
- Chadwick, P.J.; Péloquin, A.S.; Suma-Momoh, J.; Daniels, C.M.; Hinz, S.L.K.; Kennedy, C.A.; Streit, L.; Todd, R.M. Report of Activities 2019, Resident Geologist Program, Kirkland Lake Regional Resident Geologist Report: Kirkland Lake and Sudbury Districts; Open File Report 6367; Ontario Geological Survey: Sudbury, ON, Canada, 2020; 143 p. [Google Scholar]
- Orano. Annual Activity Report 2023. Available online: https://cdn.orano.group/orano/docs/default-source/orano-doc/finance/publications-financieres-et-reglementees/2023/orano_annual-activity-report_2023_online.pdf (accessed on 27 January 2024).
- Dufresne, M.B.; Sim, R.; Davis, B. Technical Report and Resource Update for the Angilak Property; Kivalliq Energy Corporation: Kivalliq region, NU, Canada, 2013; 174p, Available online: https://www.sedarplus.ca/csa-party/records/document.html?id=c6d42de1edac935a02f2c333558a9aac71279d3bc086be3a8c59e25d6a5d3604 (accessed on 7 January 2025).
- AGP Mining Consultants Inc. NI 43-101 Resource Estimate for Werner Lake Cobalt Project; Global Energy Metals Corp: Werner Lake, ON, Canada, 2017; Available online: https://www.sedarplus.ca/csa-party/records/document.html?id=f086f72323b8bdc899fa7c7d9350404b84ccd3c00e5b25e57465b1a0b6d7255f (accessed on 7 January 2025).
- Centaurus Metals Limited. The Jaguar Nickel Sulphide Project Value-Add Scoping Study, Executive Summary, May 2021. Available online: www.centaurus.com.au (accessed on 28 June 2021).
- Anglesey Mining plc. Grängesberg PFS Highlights Post-Tax NPV8 of US$688m; 2022. Available online: https://www.angleseymining.co.uk/ (accessed on 7 January 2025).
- Dean, B.; McGimpsey, I.; Drejing-Carroll, D.; Årebäck, H. Boliden Summary Report, Mineral Resources and Mineral Reserves 2022, Nautanen. Available online: https://www.boliden.com/operations/exploration/mineral-reserves-and-mineral-resources (accessed on 3 August 2023).
- Baker, H.; MacDougall, C.; Pattinson, D. Technical Review of the Hannukainen Iron-Copper-Gold Project, Kolari District, Finland: SRK Consulting, National Instrument 43-101 Technical Report. 2014. Available online: https://www.sedarplus.ca/csa-party/records/document.html?id=92dc83b898c7088db2d0282a1309d34fd8bc36b97671d2a6c615fa08f2f22d21 (accessed on 28 July 2015).
- Baker, H.; Pattinson, D.; Reardon, C. Technical Review of the Kaunisvaara Iron Project, Sweden, June 2011. Report U4067 prepared for Northland Resources AB. SRK (UK) Limited. Available online: https://www.sedarplus.ca/csa-party/records/document.html?id=47ef8c7ab490c1266285f285485325e4cb62122df76830b7cb1c79e1bd6b3d56 (accessed on 28 January 2025).
- Geological Survey of Finland. Juomasuo, Paleoproterozoic Kuusamo belt. Available online: https://minsysfin.gtk.fi/index.php/juomasuo-paleoproterozoic-kuusamo-belt/#:~:text=The%20host%20rocks%20of%20the,by%20pyrite%20and%20lesser%20chalcopyrite (accessed on 28 January 2025).
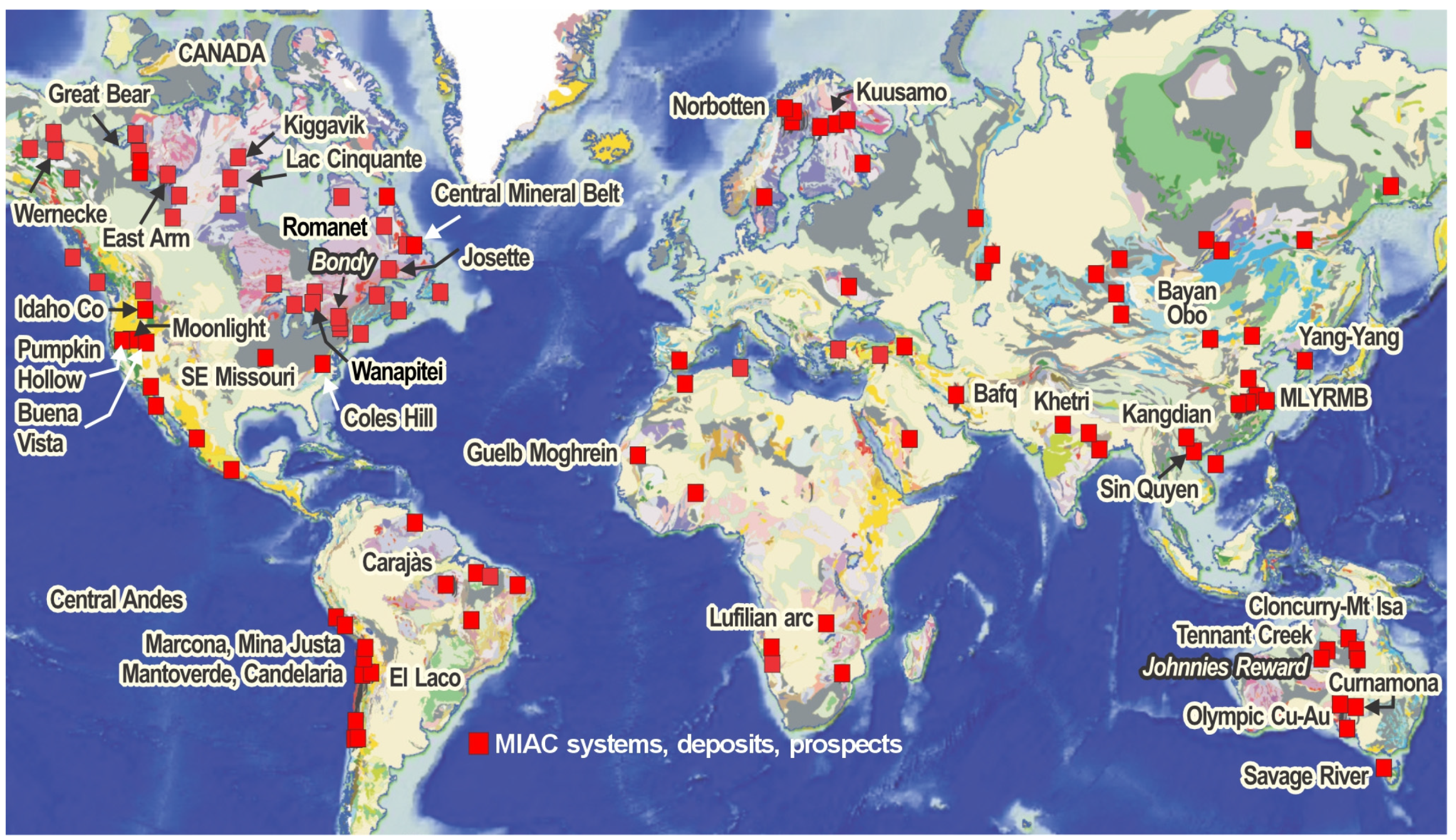
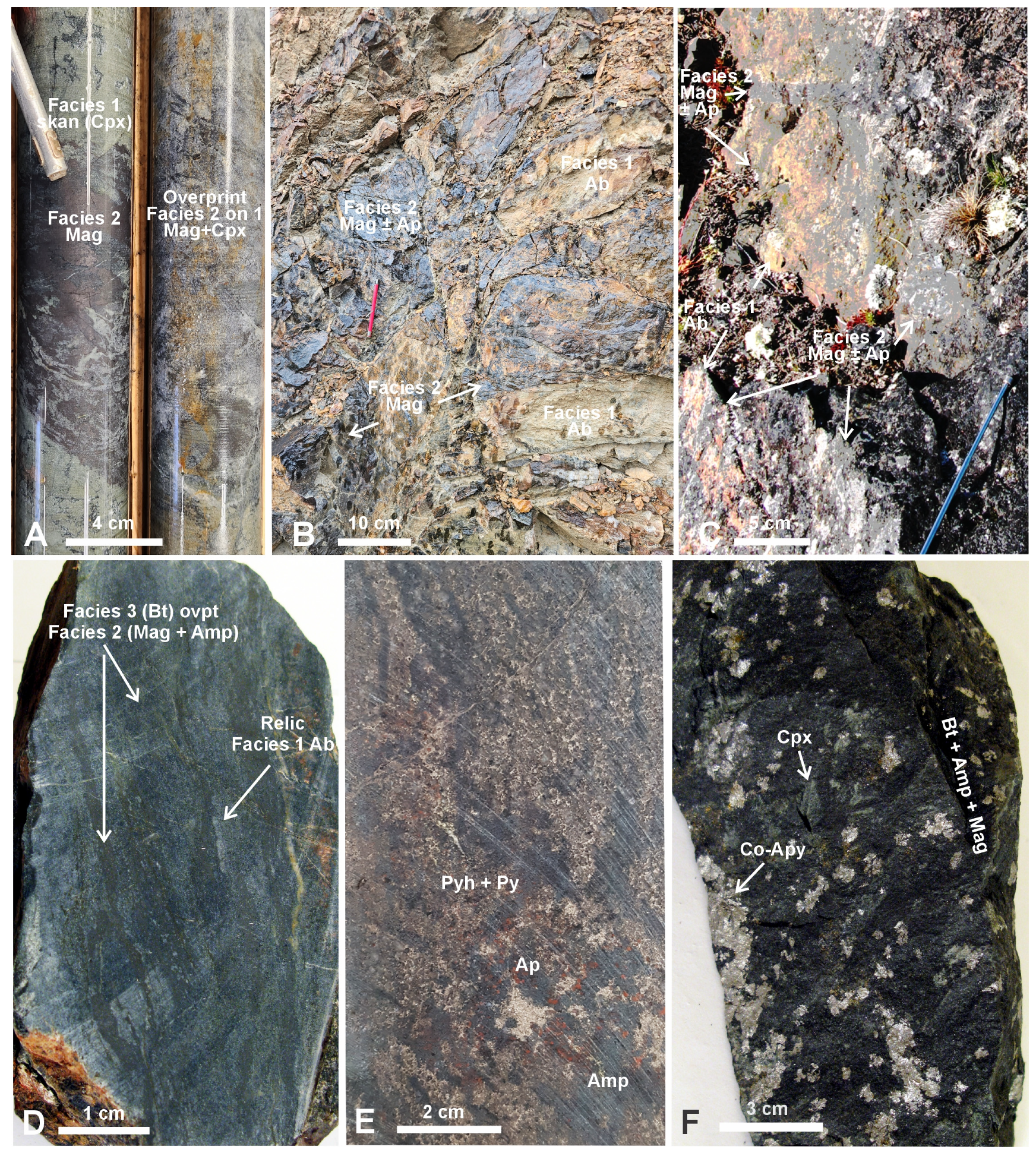
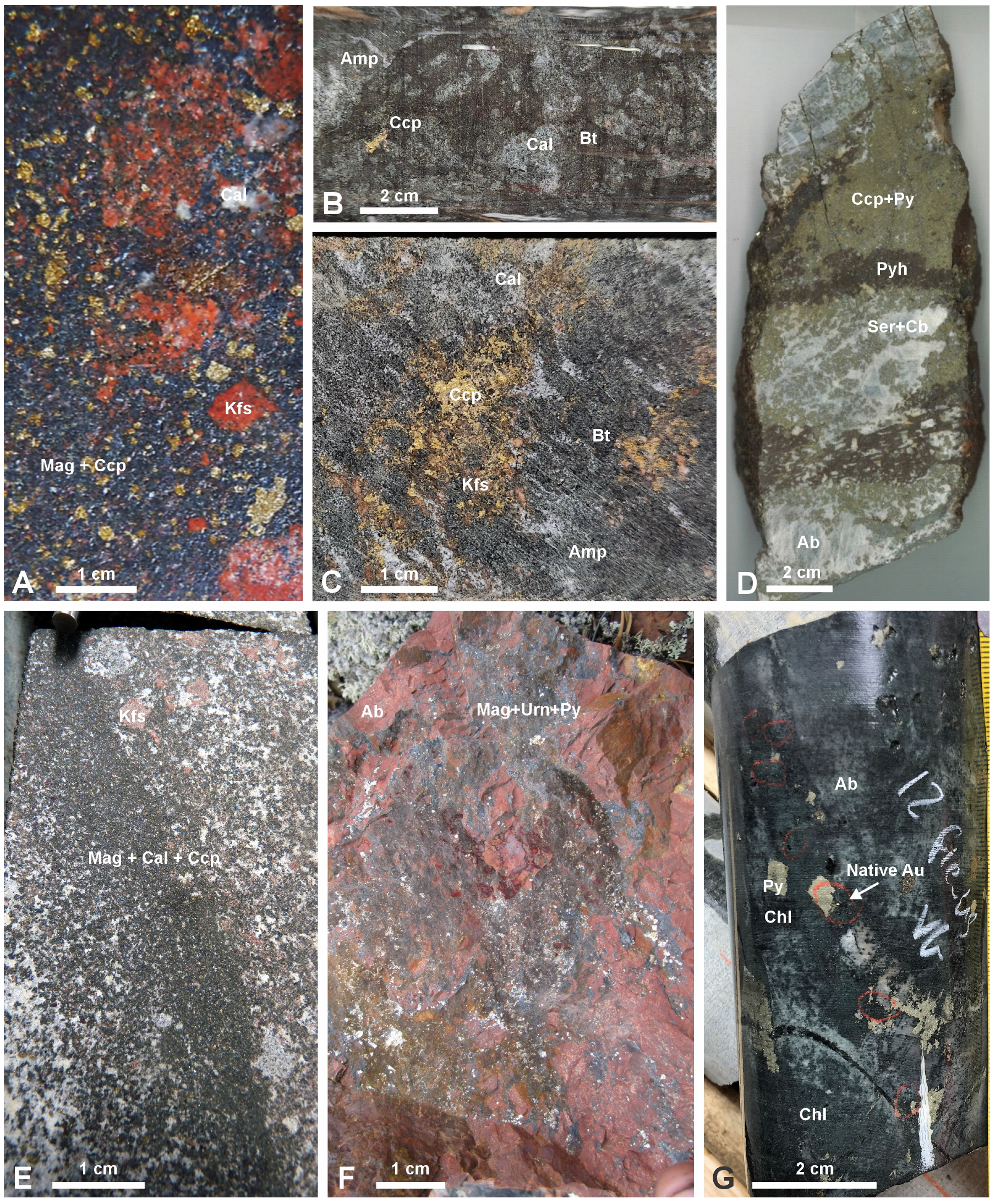
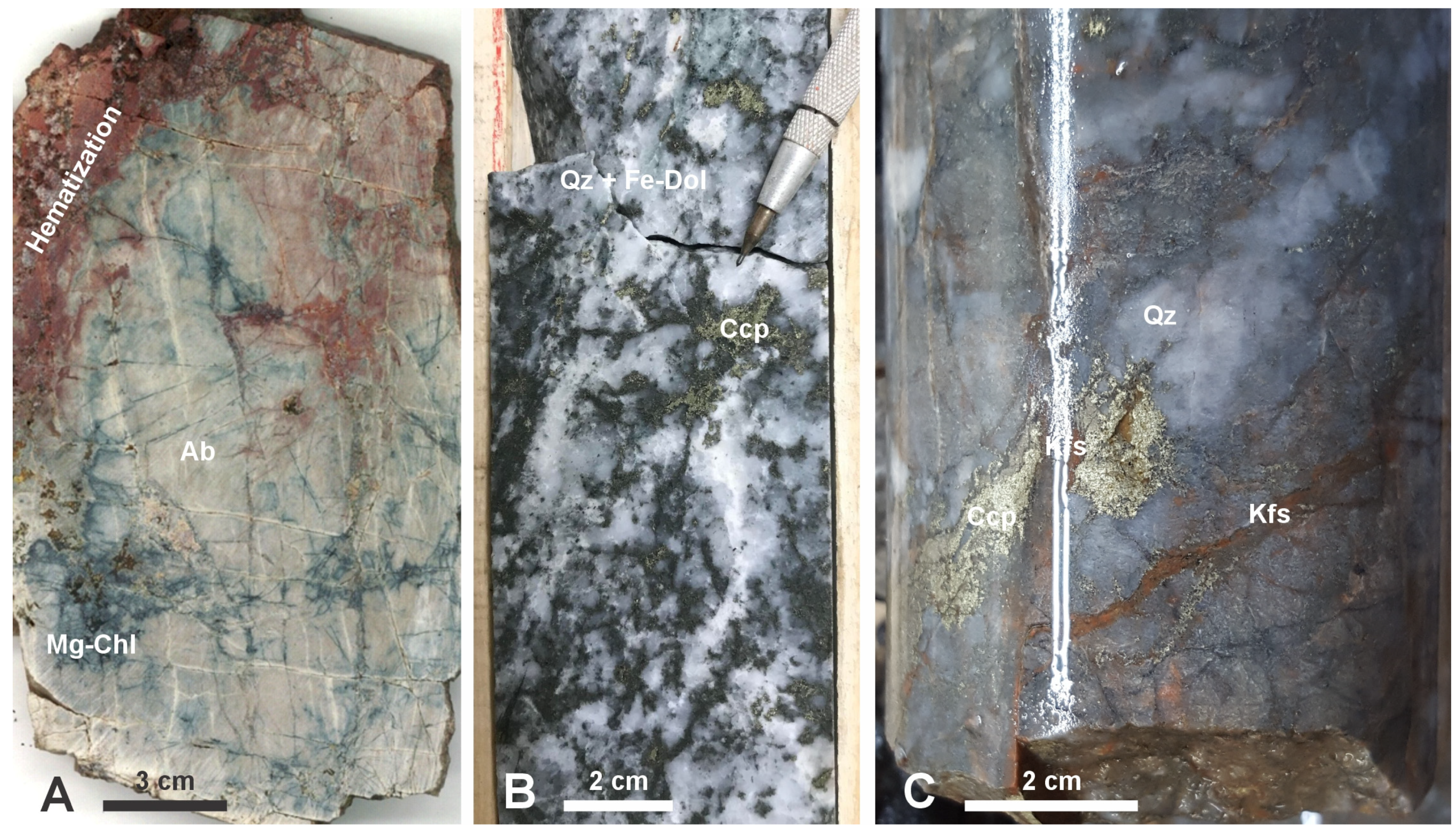
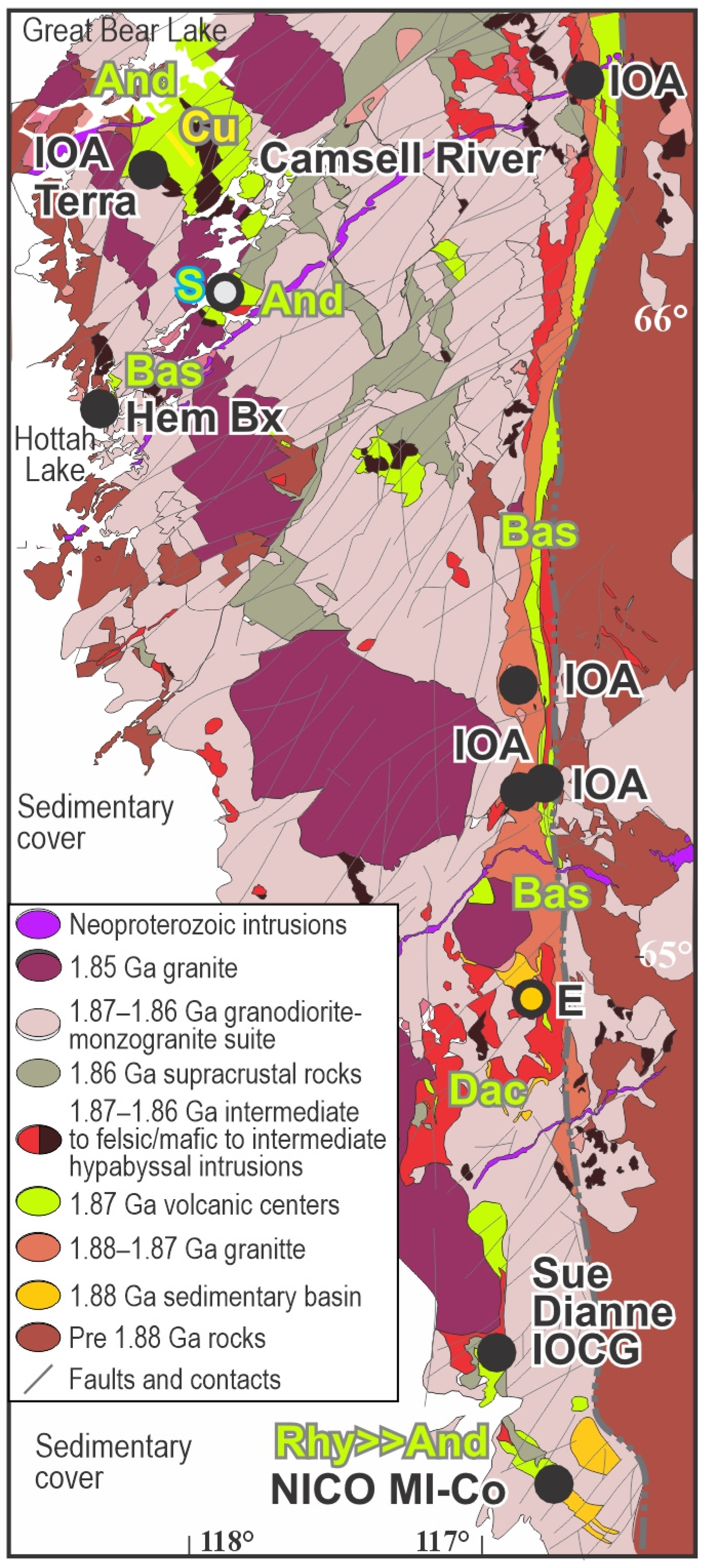
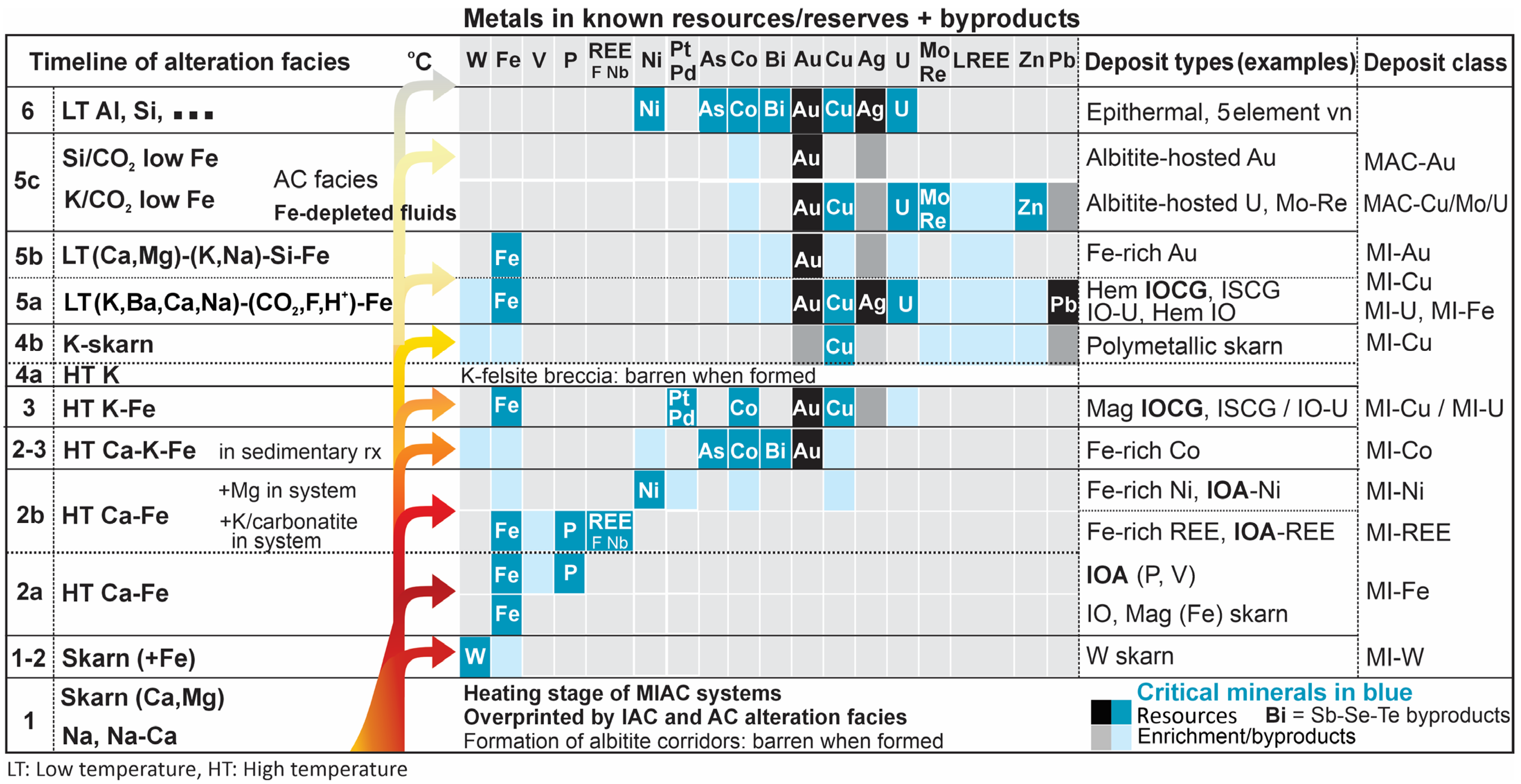
| Alteration Facies | Minerals 1 | General Characteristics | Association with Mineralization and Case Examples | ||
| Alteration | Mineralization | ||||
| Fe-Poor | Accessory | ||||
| Facies 1a Na | Ab–Olc; res. Qz | Mnz, Ru, Ttn, Zrn | Barren |
| Barren when formed, e.g., Southern Province, Great Bear, CAN; Kethri Cu Belt and its Albitite Line, IND; Central Andes, CHL-PER; Cloncurry district and Mt Isa Province, AUS; Norrbotten district, SWE; Kuusamo belt, FIN; and Middle–Lower Yangtze Metallogenic Belt, CHN. Host to subsequent albitite-hosted mineralization. Sources: [10,11,12,13,14,15,22,26,52,54,55,66,67,68,69,70,76,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110]. |
| Facies 1b Na-Ca | Ab–Olc, Anh, Scp; res. Qz | Amp, Cpx, Mnz, Ru, Ttn, Zrn | Barren | ||
| Facies 1c Skarn | Mg-Fe Amp (Tr), Mg-Fe Chl, Cpx, Grt, Phl, Tlc, res. Cb | Barren |
| Barren when formed, e.g., Great Bear, CAN; Punt Hill (Olympic Cu-Au Province), AUS; and Middle–Lower Yangtze Metallogenic Belt, CHN. Host to subsequent skarn-hosted mineralization Sources: [14,64,92,111]. | |
| Alteration Facies | Minerals | General Characteristics | Association with Mineralization and Case Examples | ||
| Alteration | Mineralization | ||||
| Fe-Rich Fe-Poor | Accessory | Main Accessory | |||
| Facies 1–2 HT Na-Ca-Fe | Amp (Act, Hbl), Cpx, Mag Ab–Olc | Ap, Mnz, Ru, Qz, Ttn, Zrn | Mag, Py |
| Proximal to iron oxide (IO) and IOA deposits. Incipient REE mineralization possible. Examples: Great Bear magmatic zone, CAN and Moonta-Wallaroo district (Olympic Cu-Au Province), AUS. Sources: [12,14,55,61,92]. |
| Facies 2–1 Fe-rich skarn Figure 3A | Fe-rich Cpx and Grt, Mag, Pyh res. Cb | Amp, Aln, Ap, Qz, Ttn | Mag, Sch |
| MI-W, e.g., Fostung (Wanapitei district), CAN and Punt Hill, AUS. Sources: [6,7,111,112]. |
| Facies 2a HT Ca-Fe (Fe, P, or REE endowed) Figure 3B–D | Fe-rich Amp (Act, Hbl), Ep, Mag or Pyh Ap, Qz | res. Cpx and Grt; Ttn | Ap, Mag, REE-min. (e.g., Aln, Adr, Bri, Mnz Flr) Ccp, Py, Thr, Urn; rem. Sch |
| MI-Fe (IO, IOA) deposits, e.g., El Laco, CHL; Kiruna, Per Geiger, Malberget (Norrbotten district), SWE; Pilot Knob, Pea Ridge (southeast Missouri district), USA; Middle–Lower Yangtze Metallogenic Belt, CHN. Magnetite-skarn as magnetite-dominant HT Ca-Fe overprint on skarn. REE remobilization from IOA apatite forms MI-REE deposits, e.g., Josette, CAN and Pea Ridge (southeast Missouri district), USA. Sources: [6,11,14,27,61,70,77,78,110,113,114,115,116]. |
| Facies 2b HT Ca-(Mg)-Fe (Ni-endowed) Figure 3E | Fe-rich Amp (Act, Hbl), Mag, Pyh, Py Ap, Qz | Bt res. Cpx and Grt | Mlr, Pn Ccp |
| MI-Ni deposits, e.g., Jaguar, Jatobá, GT-34 (Carajás), BRA Sources: [117,118,119,120]. |
| Facies 2–3 HT Ca-K-Fe to Bt-rich HT K-Fe (Bt > Kfs) Figure 3F | Fe-rich Amp (Act, Hbl), Bt, Mag, Pyh, Sid Ap, Kfs, Qz | res. Cpx and Grt, Flr, Ilm, Ttn | Apy, Co-As/AsS, Bin, Au in As-min. or native Overprint: Bin, Emp, Mdo, native Au/Bi Ccp, Mlr, Mol, Py, Sch |
| MI-Co deposits, e.g., Guelb Morghein, MRT; NICO, CAN; Sin Quyen, VNM; Blackbird, Ram, Sunshine, Sunshine East, Merle (Idaho Cobalt Belt), USA; and components of Ernest-Henry, AUS. Sources: [7,10,15,17,41,61,78,80,121,122,123,124,125,126]. |
| Facies 2–3 HT Ca-K-Fe (Kfs>Bt) | Fe-rich Amp (Act), Bt, Mag, Pyh, Kfs | Ap, Ttn, Zrn | Ccp, Py, Urn |
| Halo of MI-Cu deposits. Breccia infill and Kfs-altered ore breccia, e.g., Ernest Henry, AUS Sources: [7,10,15,17,70,126]. |
| Alteration Facies | Minerals | General Characteristics | Association with Mineralization and Case Examples | ||
| Alteration | Mineralization | ||||
| Fe-rich Fe-poor | Accessory | Main Accessory | |||
| Facies 3 HT K-Fe (Bt-rich) Figure 4B,C | Bt, Mag and/or Pyh Kfs, Qz | Ap, Amp, Grt | Brn, Ccp, Py, native Au, Urn |
| MI-Cu, including Mag-group IOCG and ISCG, e.g., components of Candelaria, CHL; Dahongshan (Kangdian district), CHN; and components of Ernest-Henry (Cloncurry district), AUS. ISCG, which are typical of geological sequences with graphitic units. Sources: [64,70,74,75,123,126,127,128,129]. |
| Facies 3 HT K-Fe (Kfs-rich) Figure 4A,F | Bt, Mag Kfs, Qz | Ap, Brt, Flr, Ilm, Mnz, Thr, Ttn, Zrn | Ccp, native Au, Py, Urn Apy, Bn, Mol, Pyh |
| MI-Cu, including Mag-group IOCG, e.g., components of Candelaria, CHL; Ernest Henry (Cloncurry district), AUS; and Sue-Dianne, CAN. MI-U, including albite-hosted U, e.g., Southern Breccia (Great Bear magmatic zone), CAN. Sources: [7,10,11,14,28,35,51,70,72,87,126,130]. |
| Alteration Facies | Minerals | General Characteristics | Association with Mineralization and Case Examples | ||
| Alteration | Mineralization | ||||
| Fe-rich Fe-poor | Accessory | Main Accessory | |||
| Facies 4a K-felsite | Kfs |
| Haloes to zones of mineralization, e.g., Ernest-Henry (Cloncurry district), AUS. Sources: [14,70]. | ||
| Facies 4b K-skarn | Ank, Mag Aln, Cpx (Adr-Grs), Ep, Kfs | Cal, Dol | Ccp, Gn, Mol, Py, Sp |
| Example: Mile Lake prospect (Great Bear magmatic zone), CAN. Source: [56]. |
| Alteration Facies | Minerals | General Characteristics | Association with Mineralization and Case Examples | ||
| Alteration | Mineralization | ||||
| Fe-rich Fe-poor | Accessory | Main Accessory | |||
| Facies 5a LT (K, Ba, Ca, Na)-(CO2, F, H+)-Fe Figure 4D,E | Chl, Ep, Hem, Sid Ab, Ank, Brt, Cal, Dol, Flr, Kfs, Msc, Qz | Anh, Ank, Ap, Dol, Tur | Brn, Ccp, Cct, LREE-min., native Au, Py, Urn Apy, Mol, Sch |
| MI-Cu, e.g., Hem-group IOCG Olympic Dam, AUS. MI-U, e.g., albitite-hosted U Michelin (Central Mineral Belt), CAN. Sources: [2,10,33,43,71,107,131,132]. |
| Aln, Amp (Act, Hst, Stp), Chl, Ep, Grt, Hem, Mag Ab, Ank, Brt, Cal, Dol, Flr, Kfs, Msc, Qz | Ap | Brn, Ccp, Cct, LREE-min., native Au, Py, Urn Gn, Mol, Sp |
| MI-Cu (e.g., Hem-group IOCG and K-skarn hosted; Sossego (Carajás), BRA; and Hillside, Punt Hill (Olympic Cu-Au Province), AUS. MI-U, e.g., Moran Lake (Central Mineral Belt), CAN. Sources: [6,7,56,92,107,111]. | |
| Act, Bt, Chl, Pyh, Py Msc, Qz | Ap, Cb, LREE-min., Tur | Brn, Ccp Gn, Mol, Sp |
| MI-Cu, e.g., Eloise, Jericho (Cloncurry district), AUS; Delhi Pacific (Romanet Horst), CAN. Sources: [50,133]. | |
| Facies 5b Si-rich LT (Ca, Mg)-(K, Na)-Si-Fe; Figure 4G | Amp, Chl, Ep, Hem, Mag, Pyh Ab, Ank, Brt, Dol, Flr, Qz | LREE-min., Kfs, Msc | Native Au, Py Ccp |
| MI-Au, e.g., Scadding (Wanapitei district), CAN. MI-Ag. Sources: [7,22,33,80]. |
| Alteration Facies | Minerals | General Characteristics | Association with Mineralization and Case Examples | ||
| Alteration | Mineralization | ||||
| Fe-rich Fe-poor | Accessory | Main Accessory | |||
| Facies 5c K/CO2 Figure 5B,C | Ank Chl, Cal, Dol (ferroan), Kfs, Msc, Qz, Tur | Ccp, Mol, Native Au, Pyh, Py Brn |
| MAC-Cu, e.g., Mount Dore (Cloncurry district), AUS; Alwyn (Wanapitei district), CAN; Pahtohavare (Norrbotten district), SWE. MAC-Mo, e.g., Merlin (Cloncurry district), AUS. Hanging wall of MI-REE, e.g., Per Geijer (Norrbotten district), SWE. Sources: [22,95,96]. | |
| Facies 5d Si/CO2 Figure 5A | Cal, Chl, Dol (ferroan), Qz | Ab, Kfs, Msc | Native Au, Pyh, Py, Urn Ccp, LREE-min. | MAC-Au, e.g., Tick Hill (Cloncurry district), AUS; Kish showing (Romanet Horst), CAN. Sources: [50,131]. | |
| Alteration Facies | Minerals | General Characteristics | Association with Mineralization and Case Examples | |
| Alteration | Mineralization | |||
| Fe-rich Fe-poor | Main | |||
| Facies 6 K-Si-Al ± Fe-Ba epithermal caps | Hem Phyllic, sericitic, silicic, advanced argillic, silicification | Py |
| Epithermal mineralization, e.g., Gossan Island, Echo Bay Gossan (Great Bear magmatic zone), CAN; Central Andes, CHL [10,55,56,64,84]. |
| Facies 6 LT Fe-(Si,CO2) ± Ba | Hem Brt, Cal, Dol, Qz | As–AsS, Ccp, Gn, Sp, Urn |
| Vein-type mineralization, five-element veins, e.g., Camsell River and Port Radium-Echo Bay districts (Great Bear magmatic zone), CAN; Olympic Cu-Au Province, AUS; Lufilian arc, Africa [21,56,60,85,134,135,136,137]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Portions of this work are reproduced with permission under crown copyright, held by his Majesty the King in Right of Canada, and are made available under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corriveau, L.; Montreuil, J.-F. Metasomatic Mineral Systems with IOA, IOCG, and Affiliated Critical and Precious Metal Deposits: A Review from a Field Geology Perspective. Minerals 2025, 15, 365. https://doi.org/10.3390/min15040365
Corriveau L, Montreuil J-F. Metasomatic Mineral Systems with IOA, IOCG, and Affiliated Critical and Precious Metal Deposits: A Review from a Field Geology Perspective. Minerals. 2025; 15(4):365. https://doi.org/10.3390/min15040365
Chicago/Turabian StyleCorriveau, Louise, and Jean-François Montreuil. 2025. "Metasomatic Mineral Systems with IOA, IOCG, and Affiliated Critical and Precious Metal Deposits: A Review from a Field Geology Perspective" Minerals 15, no. 4: 365. https://doi.org/10.3390/min15040365
APA StyleCorriveau, L., & Montreuil, J.-F. (2025). Metasomatic Mineral Systems with IOA, IOCG, and Affiliated Critical and Precious Metal Deposits: A Review from a Field Geology Perspective. Minerals, 15(4), 365. https://doi.org/10.3390/min15040365






