Abstract
Ilmenite, the most widely distributed titanium ore resource globally, often coexists with titanaugite, one of its primary gangue minerals, which shares similar surface properties. This similarity significantly hampers the selective flotation separation efficiency of ilmenite. This study investigated the influence of grinding media shape—specifically steel balls, steel rods, and steel cylpebs—on the grinding characteristics of ilmenite and titanaugite through single-mineral micro-flotation experiments and related characterizations, and explored the potential of media shape to enhance the selective flotation separation of ilmenite. Experimental results demonstrate that in a weakly alkaline sodium oleate (NaOL) system at pH ≈ 8, the floatability of ilmenite milled with cylpebs is approximately 1.7% higher than that with balls and slightly lower than that with rods. In contrast, the floatability of titanaugite milled with cylpebs is similar to that with balls but almost 4% lower than that with rods. Compared to balls and rods, the difference in floatability between ilmenite and titanaugite increases from 29.96% and 29.04% to 32.71% with cylpeb milling. The primary reason is that cylpebs increase the exposure of the (104) face of ilmenite by approximately 2%, enhancing its interaction with NaOL, while minimizing the (−221) faces in titanaugite, thereby reducing its adverse impact on ilmenite flotation. Therefore, the use of cylpebs may facilitate the selective flotation separation of ilmenite.
1. Introduction
Titanium, a transition metal, is characterized by its high strength, low density, excellent corrosion resistance, and biocompatibility. These properties make titanium and its alloys highly valuable in various industries, including medical devices, energy, chemical engineering, shipbuilding, and aerospace [1,2,3,4]. The production of titanium products primarily relies on the smelting of titanium ores. Among the more than 140 titanium minerals discovered, only ilmenite (FeTiO3) and rutile (TiO2) are widely used in industrial production [5]. Notably, ilmenite is the most extensively distributed titanium resource globally, accounting for approximately 92% of the world’s titanium ore production [6].
China possesses abundant ilmenite resources. However, these resources are characterized by a low grade and are predominantly associated with metallic minerals. In the ideal crystal lattice of FeTiO3, a small proportion of metal ions are substituted by Mg, Mn, V, or Cr ions [7,8,9]. The primary gangue mineral is titanaugite, a silicate containing Ti, Fe, Mg, and Ca, among other elements. In the study [10] on the chemical composition, phase structure, and roasting transformation characteristics of various titanium-rich products (including hemo-ilmenite ore, ore concentrate, and various titania-rich slag), researchers noted that Fe2+ in the ilmenite lattice is easily replaced by metal ions with similar radii, forming solid solutions of MTiO3 (where M = Fe2+, Mg2+, and Mn2+). Additionally, ilmenite particles typically coexist with silicates, metal oxides, sulfides, and sulfates. Due to the similar surface properties between titanaugite and ilmenite, the separation process is challenging, leading to low comprehensive utilization efficiency of the resources [11]. Consequently, the beneficiation of ilmenite and the comprehensive utilization of its resources remain significant challenges [11,12].
In the beneficiation of ilmenite, grinding is an indispensable step to ensure the adequate liberation of valuable minerals and to provide feed material with suitable particle size for subsequent separation processes. Grinding is a complex process in which the comminution effect is achieved through the contact and interaction between grinding media and mineral particles. Consequently, the properties of grinding media significantly influence the characteristics of the milled product. Research [13] has demonstrated through mathematical models that the size of grinding media affects the breakage rate of ore. Experimental studies have further confirmed that smaller-sized balls generally exhibit a higher breakage rate compared to larger-sized balls [14,15], which is also influenced to some extent by the feed particle size [16]. In the review of the application of ball media in mineral processing, Matsanga et al. emphasized that the properties of grinding media are an integral part of the entire grinding process [17]. They noted that materials used for manufacturing grinding media should possess high hardness, fracture toughness, wear resistance, and corrosion resistance. In some cases, specific chemical reactions between the abrasive and the media must also be considered. Currently, the primary materials for grinding media include cast iron, steel, chromium alloys, and certain ceramics. The influence of media size distribution is primarily reflected in the different breakage mechanisms of balls of varying sizes: larger balls break particles through impact, effectively grinding coarser particles, while smaller balls break particles through abrasion, being more effective for finer particles. Additionally, they highlighted that the impact of media shape on the grinding process is mainly reflected in power and energy consumption, and further research is needed to understand how the shape characteristics of media affect the properties of mineral particles. In fact, a variety of grinding media with diverse shapes (e.g., short, bullet-shaped, cubes, concave-convex balls, hexagonal prisms, etc.) have been developed both domestically and internationally [18,19,20,21,22] to investigate their effects on grinding performance.
Balls and rods are traditional grinding media widely used in large- and medium-sized equipment in mineral processing plants. Studies [23,24,25] have found that grinding media of different shapes can have varying effects on the surface properties of scheelite and fluorite. Specifically, rod milling produces particles with a higher elongation ratio compared to ball milling, which exposes more (101) crystal faces in scheelite and more (110) and (310) crystal faces in fluorite, enhancing the flotation performance of these minerals. Therefore, using grinding media of different shapes should improve the floatability of minerals.
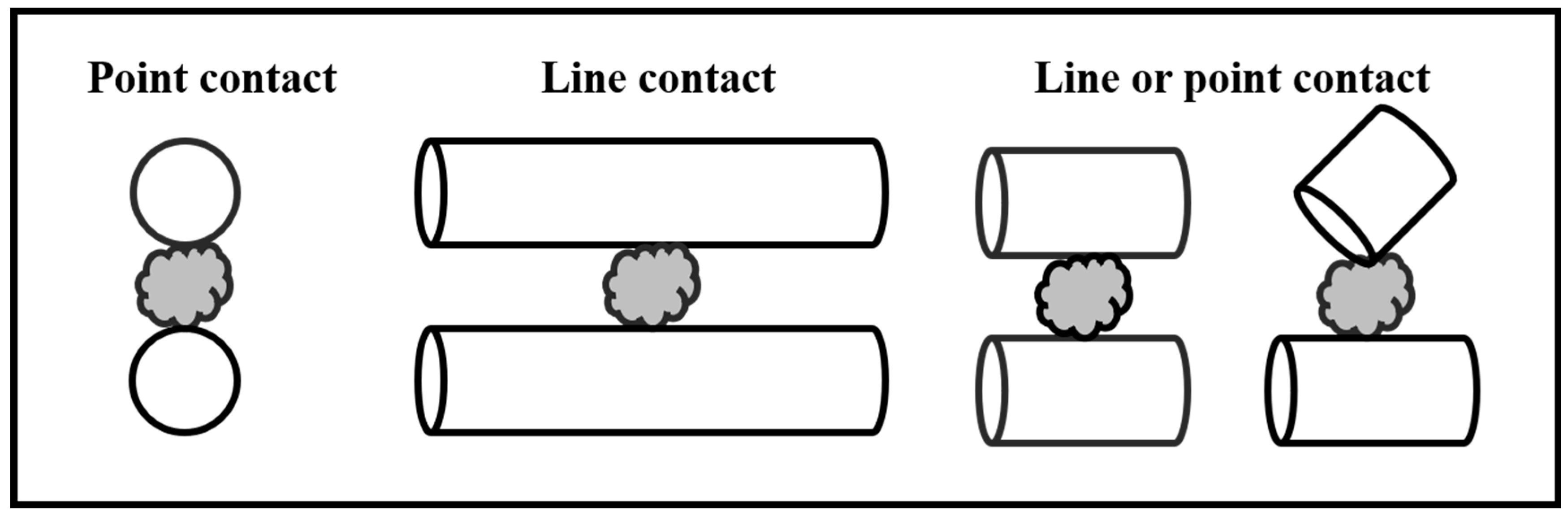
As shown in Figure 1, the difference in contact mechanisms between rod media (line contact) and ball media (point contact) with mineral particles may explain this phenomenon [26]. Cylpebs exhibit characteristics of both line contact (like rods) and point contact (like balls) when interacting with minerals, making them a media shape of growing interest. Some studies have shown that cylpebs achieve a higher breakage rate than balls (particularly more pronounced with larger feed particle sizes) [18] and are conducive to selective grinding, avoiding over-grinding [27,28]. When investigating the grinding efficiency of ball milling and cylpeb milling for +74 μm material, Jiahong et al. found [29] that cylpeb milling was more efficient for grinding times shorter than 142 s, while ball milling became more efficient for grinding times exceeding 142 s. At a grinding time of 180 s, the −74 μm fraction in ball-milled samples reached 74.23%, which was 12.08% higher than that of cylpeb-milled samples. They proposed that balls exhibit an equal probability of collision with particles across all size fractions, whereas cylpebs, due to their combined characteristics of rod-like line contact and ball-like point contact (with line contact being dominant), have a significantly higher collision probability with coarse particles compared to fine particles. Consequently, as grinding time increases and particle size decreases, cylpebs primarily break down coarse particles while providing a protective effect on fine particles, thereby preventing over-grinding.
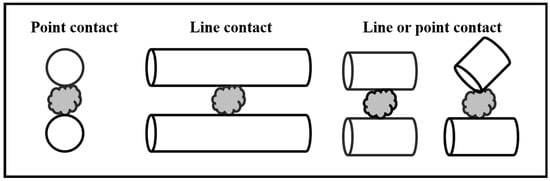
Figure 1.
Contact model between mineral particles and media.
Although researchers have recognized the influence of media shape on mineral liberation, studies focusing on the grinding of ilmenite remain scarce. This paper investigates the effects of three types of grinding media—steel balls, steel rods, and steel cylpebs—on the grinding performance and floatability of ilmenite and titanaugite through single-mineral grinding and flotation experiments. Utilizing analytical techniques such as scanning electron microscopy (SEM), contact angle measurement, and X-ray diffraction (XRD), complemented by surface simulation calculations of mineral crystals using Materials Studio 2019 software, this study examines the differences in surface properties of ilmenite and titanaugite milled products resulting from the use of different grinding media shapes.
2. Materials and Methods
2.1. Experimental Materials
The mineral samples were obtained from Panzhihua City, Sichuan Province, China. The deposit is located within the inner zone of the Emeishan Large Igneous Province, where the distribution of rock masses is predominantly composed of basic–ultrabasic rock. The process mineralogy analysis is presented in Table 1 and Table 2. The ilmenite and titanaugite used in the experiments were separated from the raw ore through a seven-stage shaking table process (with the ilmenite sample containing 48.51% TiO2 and exhibiting a purity of 92.17%). The −150 + 74 μm fraction was sieved and prepared for use. Sodium oleate (NaOL) at a concentration of 2×10ࢤ4 mol/L was employed as the flotation collector. The pH of the flotation process was adjusted using dilute NaOH and H2SO4 solutions. Deionized water was used throughout the experiments.

Table 1.
Main chemical components of the raw ore.

Table 2.
Main minerals and their contents in the raw ore.
2.2. Experimental Methods
2.2.1. Grinding Test
Grinding experiments were conducted using a stainless-steel container with a diameter (D) of 10 cm and a length (L) of 20 cm, along with stainless-steel balls and rod, and cylpeb grinding media. The diameter (D) of the grinding media was 12 mm, with the lengths of the rods and cylpebs media being = 200 mm and = 20 mm, respectively. The experimental parameters were set as follows: media filling rate of 45%, feed mass of 35 g, pulp concentration of 65%, mill rotation speed of 300 rpm, and grinding time of 3 min. After grinding, the products were washed and dried, and the dried samples were used for subsequent micro-flotation experiments and analyses.
2.2.2. Micro-Flotation Test
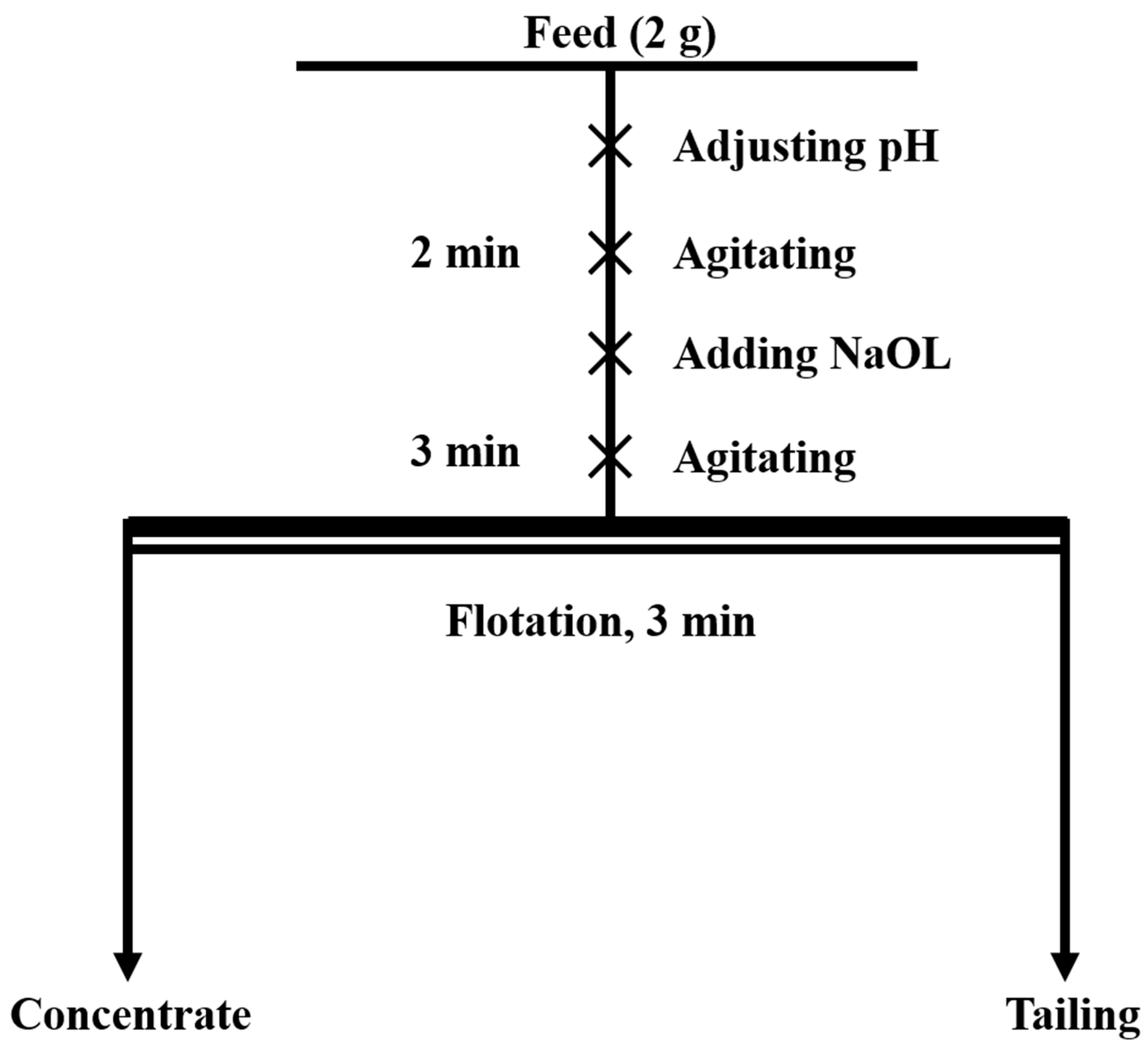
Single-mineral micro-flotation experiments were conducted using an XFG-type flotation machine by Jilin Province Prospecting Machinery Factory, China. The impeller speed of the flotation machine was set to 1700 rpm. In total, a 2 g sample of the milled single mineral and 35 mL of deionized water were placed in a 40 mL flotation cell. The pulp pH was adjusted using 5% NaOH and H2SO4 solutions. After agitating for 2 min, the collector ( = 2 × 10−4 mol/L) was added, and agitation was continued for an additional 3 min. Flotation was then initiated and maintained for 3 min. After flotation, the concentrate was filtered, dried, and weighed to calculate the recovery rate. The overall procedure of micro-flotation is illustrated in Figure 2. Each experiment was repeated three times, and the average value was taken as the final result.
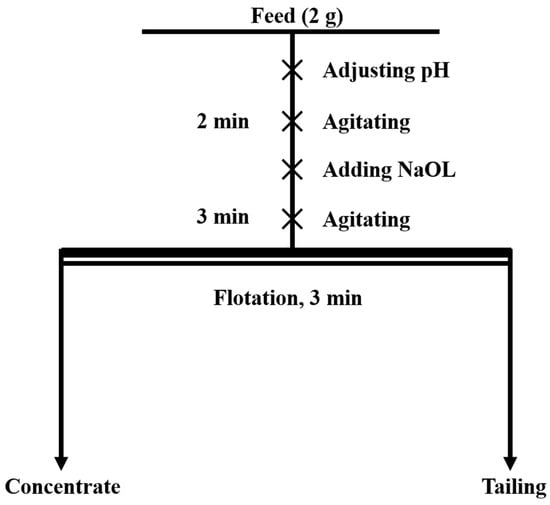
Figure 2.
The concise flow diagram of micro-flotation.
2.2.3. Scanning Electron Microscopy (SEM) Analysis
Conductive adhesive was applied to the surface of the sample holder, and the dried sample was evenly spread onto the adhesive. The sample was then sputter-coated with gold. A JSM-7900F scanning electron microscope (SEM), manufactured by JEOL, Tokyo Japan, was used to obtain SEM images of the milled products. The SEM images were processed using Image-Pro Plus 6.0 software. For each image, 50 particles were randomly selected ensuring they did not exceed the boundaries and had as clear and complete contours as possible. The perimeter (P) and area (A) of all particles were automatically measured by the software. Additionally, the length (L) and width (W) of each particle were measured three times, and the final results were averaged. The roundness () and elongation (E) were then calculated according to Equation (1) and Equation (2), respectively.
2.2.4. Particle Size Analysis
The particle size distribution of the milled products was analyzed using a Malvern Mastersizer 2000 laser particle size analyzer by Malvern, Britain. The particle size distribution curves were plotted, and the distribution of each sample across different particle size ranges was analyzed. During the experiment, a Hydro 2000MU (A) sample dispersion unit by Malvern, Britain, was used. The instrument has a measurable particle size range of 0.01 to 2000 μm.
2.2.5. Contact Angle Test
The contact angles of bulk ilmenite and titanaugite samples were measured before and after interaction with the collector using a JY-82C contact angle goniometer via the pendant drop method. The milled sample was placed in a solution with pH = 8 and stirred for 2 min, followed by filtration and natural air-drying at room temperature. Another portion of the milled sample was similarly placed in a pH = 8 solution and stirred for 2 min. Subsequently, a collector ( = 2 × 10−4 mol/L) was added, and the mixture was further stirred for 3 min before filtration and natural air-drying at room temperature. The air-dried samples were then pressed into tablets, and a micro-syringe was used to place water droplets at three different locations on the surface of each tablet. Images were captured using a camera, and the contact angles were measured using Image-Pro plus 6.0 software. The final results were obtained by averaging the measurements from the three points.
2.2.6. X-Ray Diffraction (XRD) Analysis
The crystal exposure of the milled products was analyzed using a D/MAX-rA X-ray diffractometer (XRD) manufactured by Shimadzu, Kyoto, Japan. The scanning speed was set to 5 °/min, with a scanning range of 5° to 80°. The obtained XRD patterns were processed using Jade 6.0 software, and the relative intensity changes of the diffraction peaks were statistically analyzed.
2.2.7. Crystal Surface Simulation
The Materials Studio 2019 software was used to construct the ideal primitive unit cell models of ilmenite and titanaugite. The Surface Builder module was employed to cleave the primitive unit cells of ilmenite and titanaugite, obtaining surface structure information for the main crystal planes of ilmenite ((104), (110), (116), (012), and (113)) and titanaugite ((−221), (220), and (330)). The exposure of active sites on each crystal plane was analyzed, and the density of active sites and broken bonds on the exposed surfaces was calculated.
3. Results and Discussion
3.1. Flotation Recovery
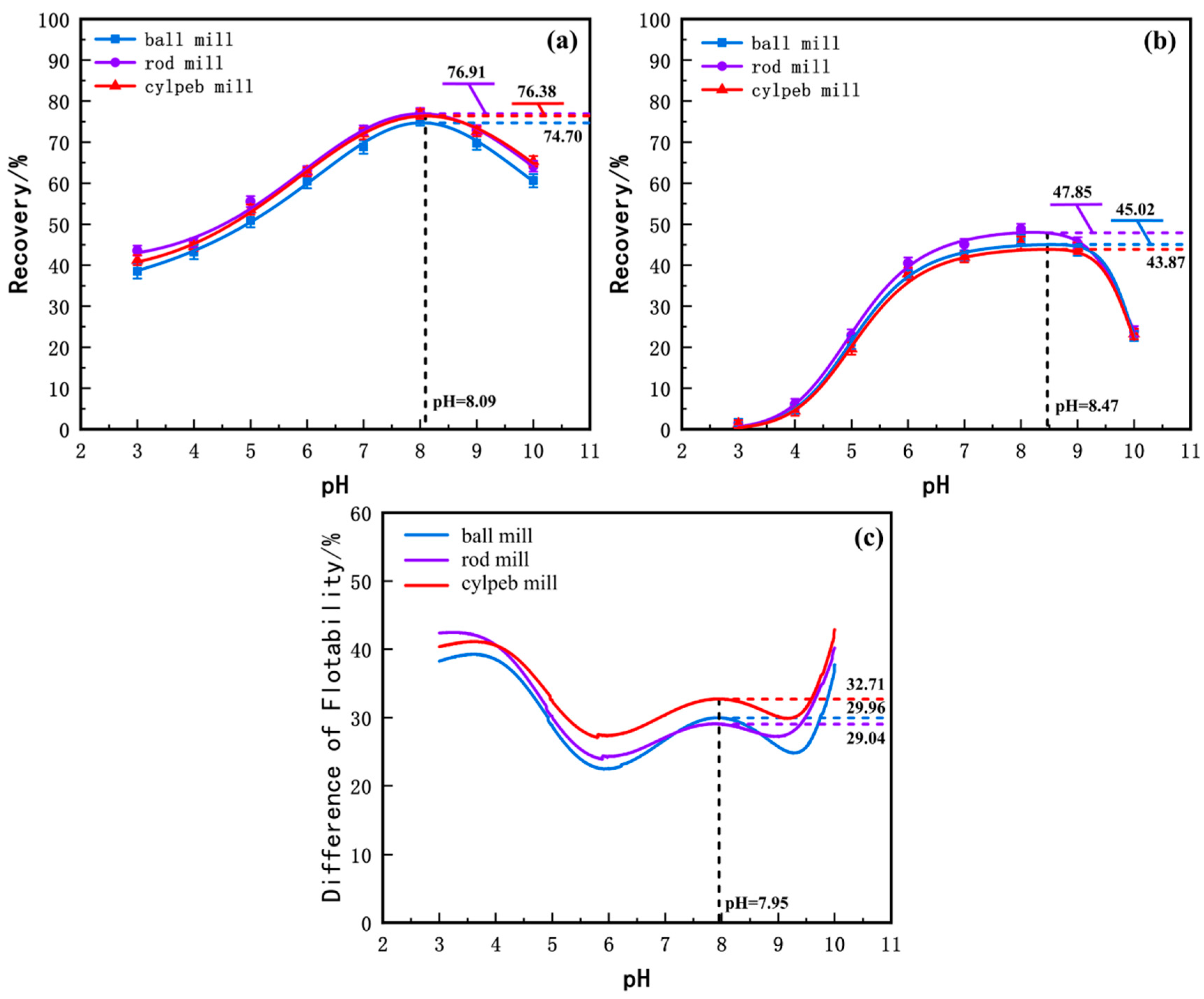
To investigate the effects of different grinding media shapes on the grinding characteristics of ilmenite and titanaugite, micro-flotation experiments were conducted on pure mineral samples of ilmenite and its primary gangue mineral, titanaugite, at various pH levels. The experimental results were fitted to curves, as shown in Figure 3. From Figure 3a, it can be observed that the flotation recovery of ilmenite generally increases and then decreases with increasing pulp pH. Specifically, starting from pH = 3, the recovery of ilmenite increases with pH, reaching a maximum at approximately pH ≈ 8, after which the recovery gradually decreases with further increases in pH. Comparing the recovery rates of products milled with different media shapes under the same pH conditions, the recovery of cylpeb-milled products is generally higher than that of ball-milled products but slightly lower than that of rod-milled products. The maximum recovery of cylpeb-milled products reaches 76.38% at pH = 8.09, while the recoveries of rod-milled and ball-milled products are 76.91% and 74.70%, respectively. Figure 3b shows the recovery rates of titanaugite milled with different media at various pH levels. Similar to ilmenite, the flotation recovery of titanaugite initially increases and then decreases with increasing pH. The recovery of cylpeb-milled titanaugite reaches a maximum of 43.87% at pH = 8.47, while the recoveries of rod-milled and ball-milled products are 47.85% and 45.02%, respectively. By calculating the difference in recovery rates between ilmenite and titanaugite, the floatability difference curves for products milled with different media were obtained, as shown in Figure 3c. The floatability difference curves for ilmenite and titanaugite exhibit a “W” shape, with the overall trend being cylpebs > rods > balls. The floatability difference for cylpeb-milled products reaches a maximum of 32.71% at pH = 7.95, while the differences for rod-milled and ball-milled products are 29.04% and 29.96%, respectively. Therefore, considering both the recovery of ilmenite and the floatability difference between ilmenite and titanaugite, a weakly alkaline environment at pH = 8 is most suitable for the flotation separation of ilmenite in the ilmenite/titanaugite/NaOL system. Additionally, using steel cylpebs as grinding media enhances the floatability difference between ilmenite and titanaugite, improving it by 3.67% and 2.75% compared to rod grinding and ball grinding, respectively.
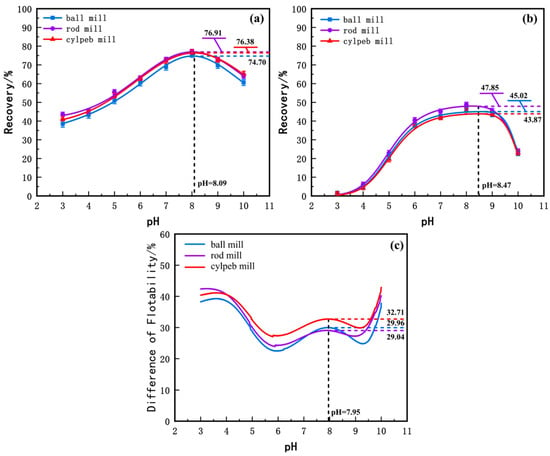
Figure 3.
Flotation recovery rate of milled (a) ilmenite, (b) titanaugite and (c) their D-value by different media.
3.2. SEM Microstructural Morphology
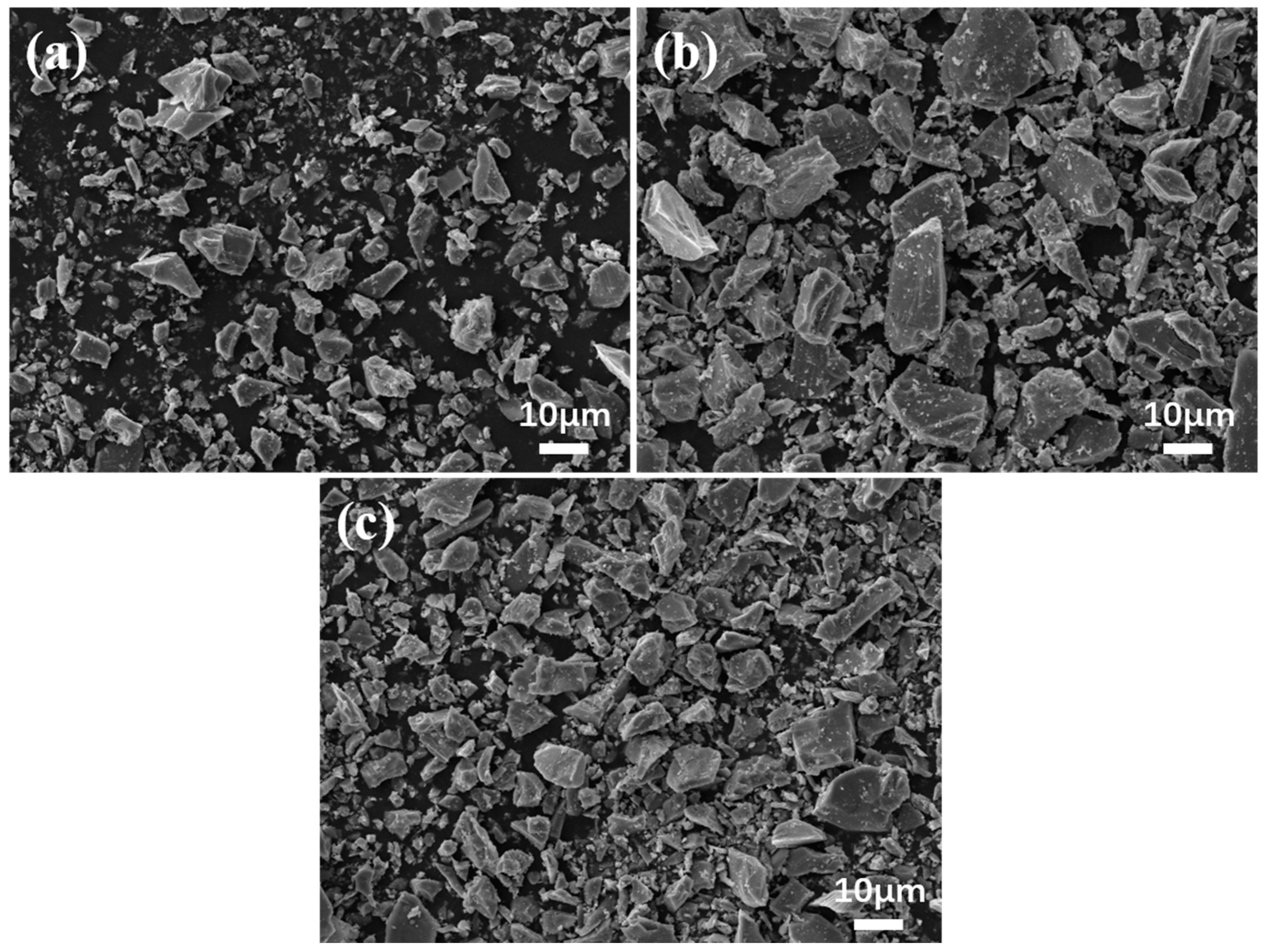
The SEM images of the three milled samples of ilmenite are shown in Figure 4. Morphologically, the rod-milled samples exhibit the coarsest overall particle size, with particles predominantly elongated in shape. The ball-milled samples have a finer overall particle size, with mostly irregular polygon-sized particles. The cylpeb-milled samples showed an intermediate particle size, with particle morphology combining characteristics of both ball-milled and rod-milled samples. The images were processed using Image-Pro Plus 6.0 software to calculate and E of each sample, and the results are presented in Table 3. The data are generally consistent with the morphological characteristics directly observed from the SEM images.
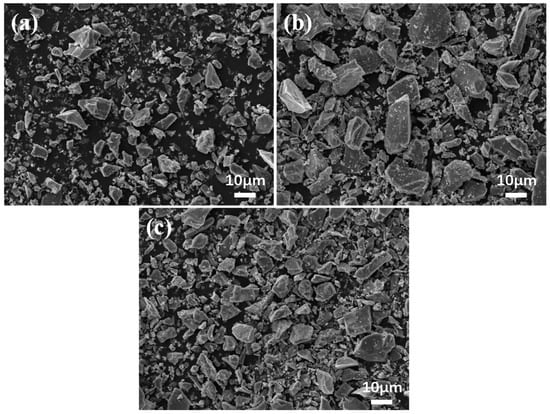
Figure 4.
SEM images of ilmenite for (a) ball milling, (b) rod milling and (c) cylpeb milling.

Table 3.
Average values of the shape factors of ilmenite particles for ball, rod and cylpeb milling.
3.3. Particle Size
Particle size significantly influences flotation performance. Minerals must be milled to a specific size to ensure adequate liberation of valuable minerals and to provide suitable feed material for subsequent separation processes. Overly large mineral particles may not be fully liberated as single particles; moreover, their higher specific gravity makes it more difficult for air bubbles to carry them to the surface. Extremely fine particles, on the other hand, reduce the likelihood of collision between mineral particles and air bubbles [30], which is also detrimental to flotation.
Studies [31,32] have shown that in the NaOL system, ilmenite exhibits the best flotation performance in the +20–38 μm size fraction. The flotation performance of the +38–74 μm and +74 μm fractions is slightly inferior to that of the +20–38 μm fraction, but the difference is not particularly significant. However, as the particle size decreases further, the floatability of ilmenite declines rapidly, and particles smaller than 20 μm are generally considered difficult to float.
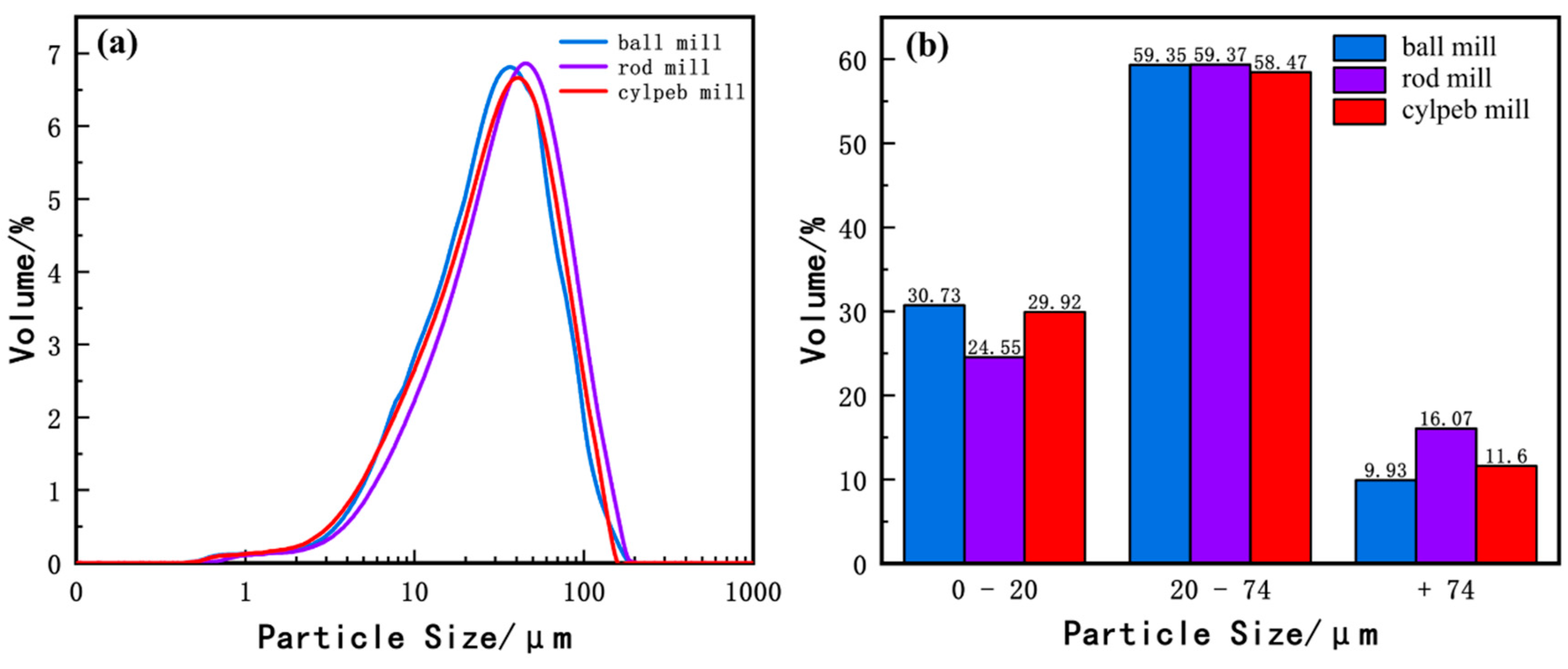
Figure 5 illustrates the particle size distribution of ilmenite. Figure 5a shows that the overall particle sizes of the three samples are similar, with the cylpeb-milled sample being slightly finer than the rod-milled sample but slightly coarser than the ball-milled sample. In Figure 5b, the contents of the most floatable +20–74 μm fraction in the ball-milled, rod-milled, and cylpeb-milled samples are 59.35%, 59.37%, and 58.47%, respectively. The contents of −20 μm particles are 30.73%, 24.55%, and 29.92%, respectively, while the +74 μm fraction is highest in the rod-milled sample at 16.07%. Among them, the intermediate size fraction content of the three samples is similar, with the cylpeb-milled sample being slightly lower; the rod-milled sample exhibits significantly less fine particle content and more coarse particle content. Considering the characteristics of ilmenite flotation recovery (where the rod-milled and cylpeb-milled samples are close to each other and notably higher than the ball-milled sample), the particle size distribution is not the primary factor contributing to the differences in recovery rates.
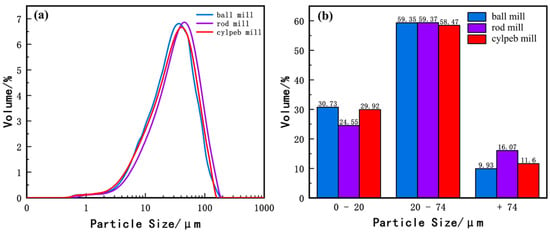
Figure 5.
(a) Particle-size distribution curve and (b) percentage of different particle sizes of ilmenite.
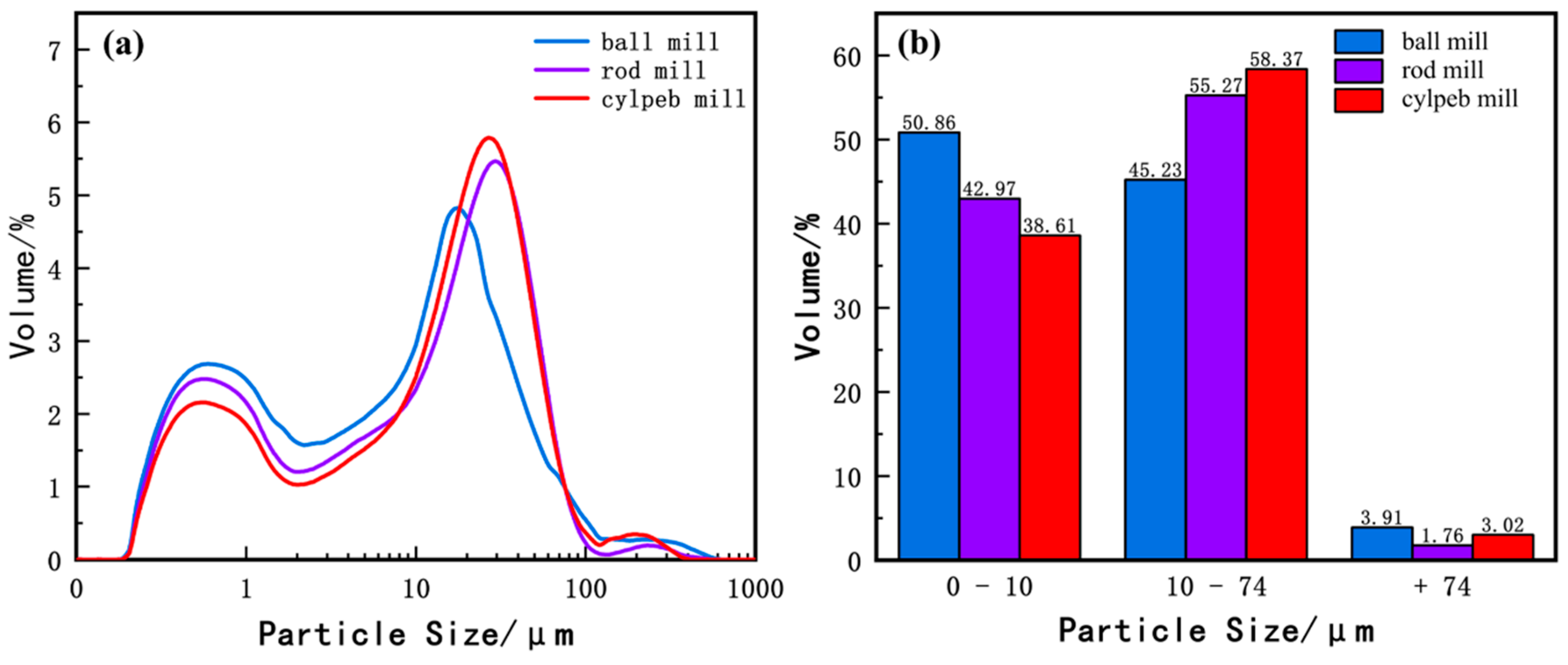
Figure 6 presents the particle size distribution of titanaugite. Figure 6a indicates that the overall particle size of titanaugite is smaller than that of ilmenite. Additionally, the ball-milled sample is significantly finer than the rod-milled and cylpeb-milled samples. Research [33,34] has shown that when the content of titanium particles smaller than 10 μm exceeds 40%, the recovery of ilmenite decreases significantly. In contrast, coarser titanaugite particles have a lesser impact on ilmenite recovery. As shown in Figure 6b, the contents of −10 μm particles in the ball-milled, rod-milled, and cylpeb-milled titanaugite samples are 50.88%, 42.97%, and 38.61%, respectively. The high content of fine particles in the ball-milled and rod-milled samples, exceeding 40%, is unfavorable for ilmenite recovery. Therefore, selecting steel cylpebs as the grinding medium is more conducive to avoiding the generation of fine titanaugite particles.
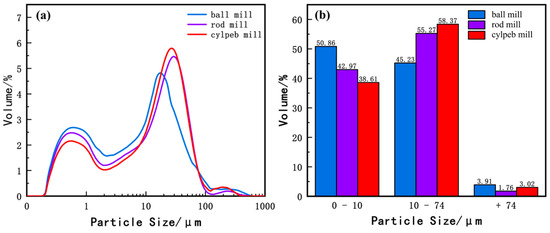
Figure 6.
(a) Particle size distribution curve and (b) percentage of different particle sizes of titanaugite.
3.4. Contact Angle
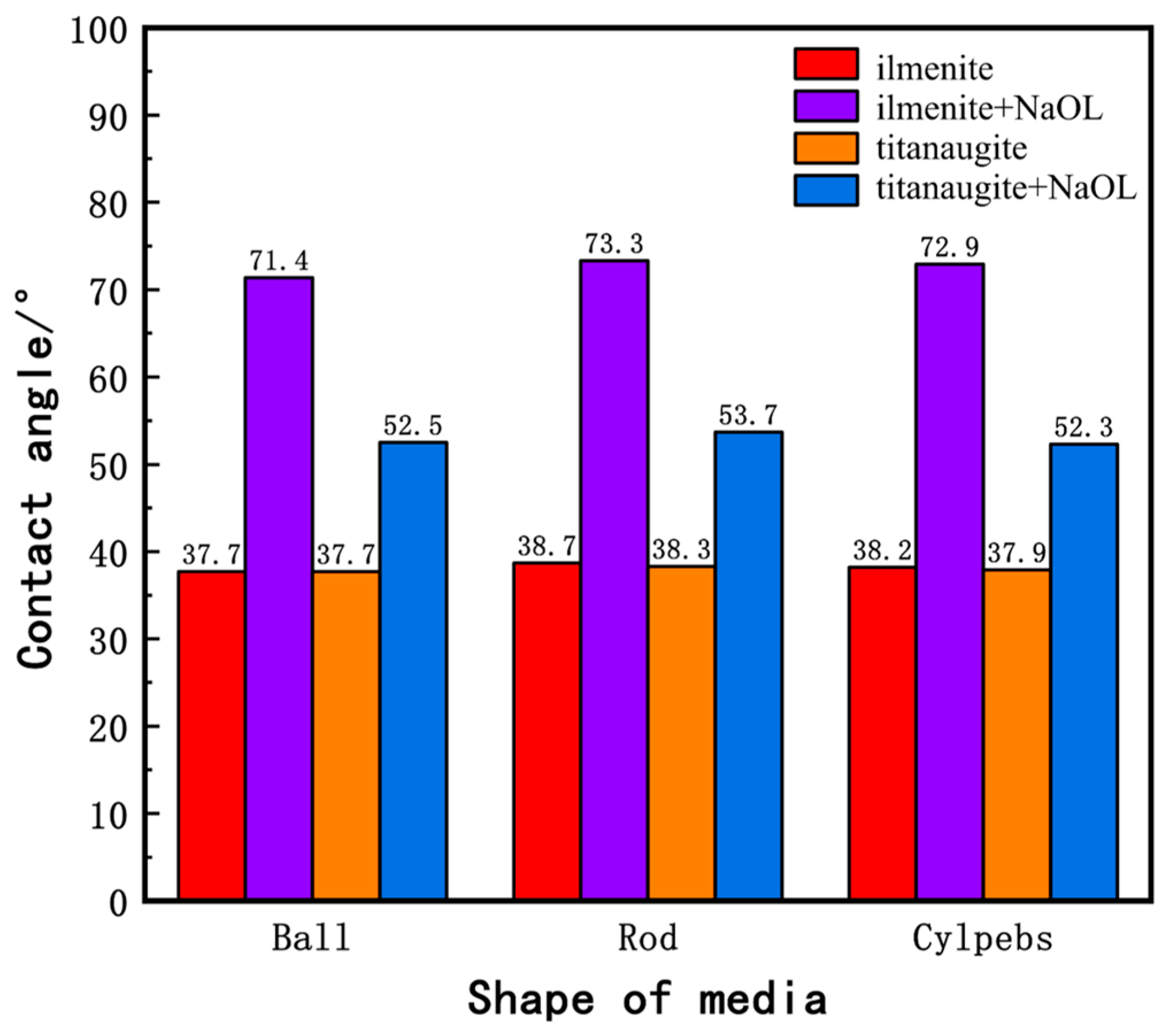
The contact angle is a measure of surface hydrophobicity, and its magnitude directly reflects the hydrophilicity or hydrophobicity of a mineral. Figure 7 presents the contact angle measurements of water on the milled products of ilmenite and titanaugite before and after interaction with the collector NaOL. The bar chart shows that, prior to interaction with NaOL, the contact angles of all milled products of ilmenite and titanaugite are relatively small, around 38°, indicating strong hydrophilicity. Although the contact angles of products milled with different media shapes vary slightly, these differences have almost no impact on their hydrophilicity. After interaction with NaOL, the contact angles of both ilmenite and titanaugite increase significantly. Specifically, the contact angles of ball-milled, rod-milled, and cylpeb-milled ilmenite increase to 71.4°, 73.3°, and 72.9°, respectively, while those of ball-milled, rod-milled, and cylpeb-milled titanaugite increase to 52.5°, 53.7°, and 52.3°, respectively. Clearly, the contact angle of ilmenite increases more significantly, indicating that NaOL has collecting capabilities for both ilmenite and titanaugite. The differences in contact angles between ball-milled, rod-milled, and cylpeb-milled products of ilmenite and titanaugite increase from 0°, 0.4°, and 0.3° to 18.9°, 19.6°, and 20.6°, respectively. This demonstrates that NaOL exhibits higher selectivity for ilmenite, enhancing the floatability difference between ilmenite and titanaugite, which is consistent with the single-mineral micro-flotation results of ilmenite presented in Section 3.1.
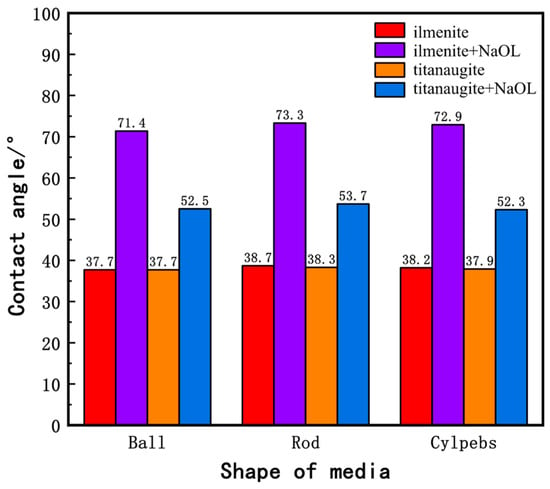
Figure 7.
Column diagram of contact angle between ilmenite and titanaugite.
3.5. Surface Structure Simulation
During the grinding process, the reduction in mineral particle size leads to the fracture of the crystal structure. This process is accompanied by the breaking of chemical bonds between surface atoms, generating new surfaces with unsaturated bonds. The fundamental principle of flotation relies on these unsaturated bonds as active sites on the mineral surface. By selectively adsorbing surfactants, the surface hydrophobicity of the mineral can be altered, facilitating the separation of valuable minerals from gangue minerals.
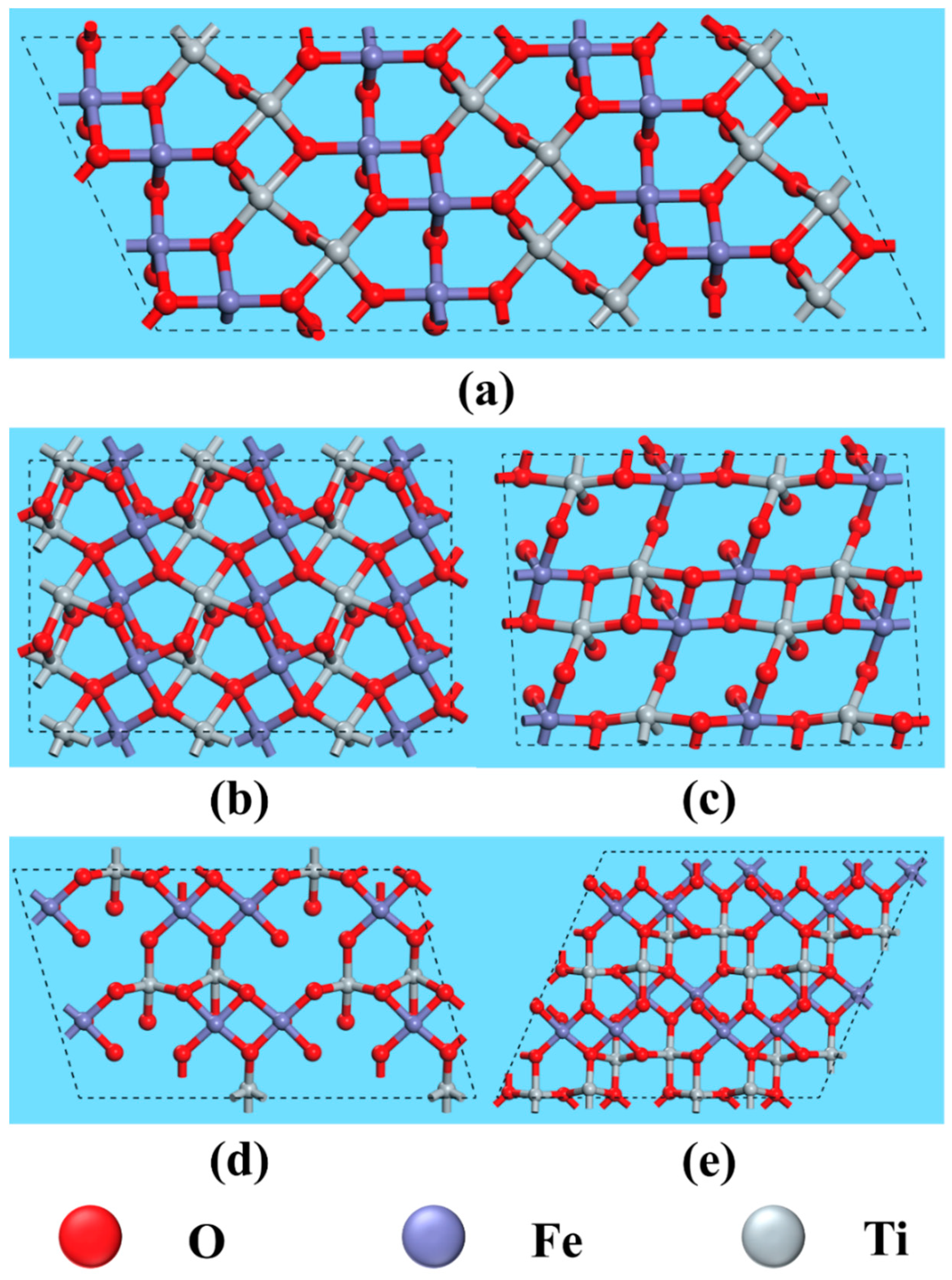
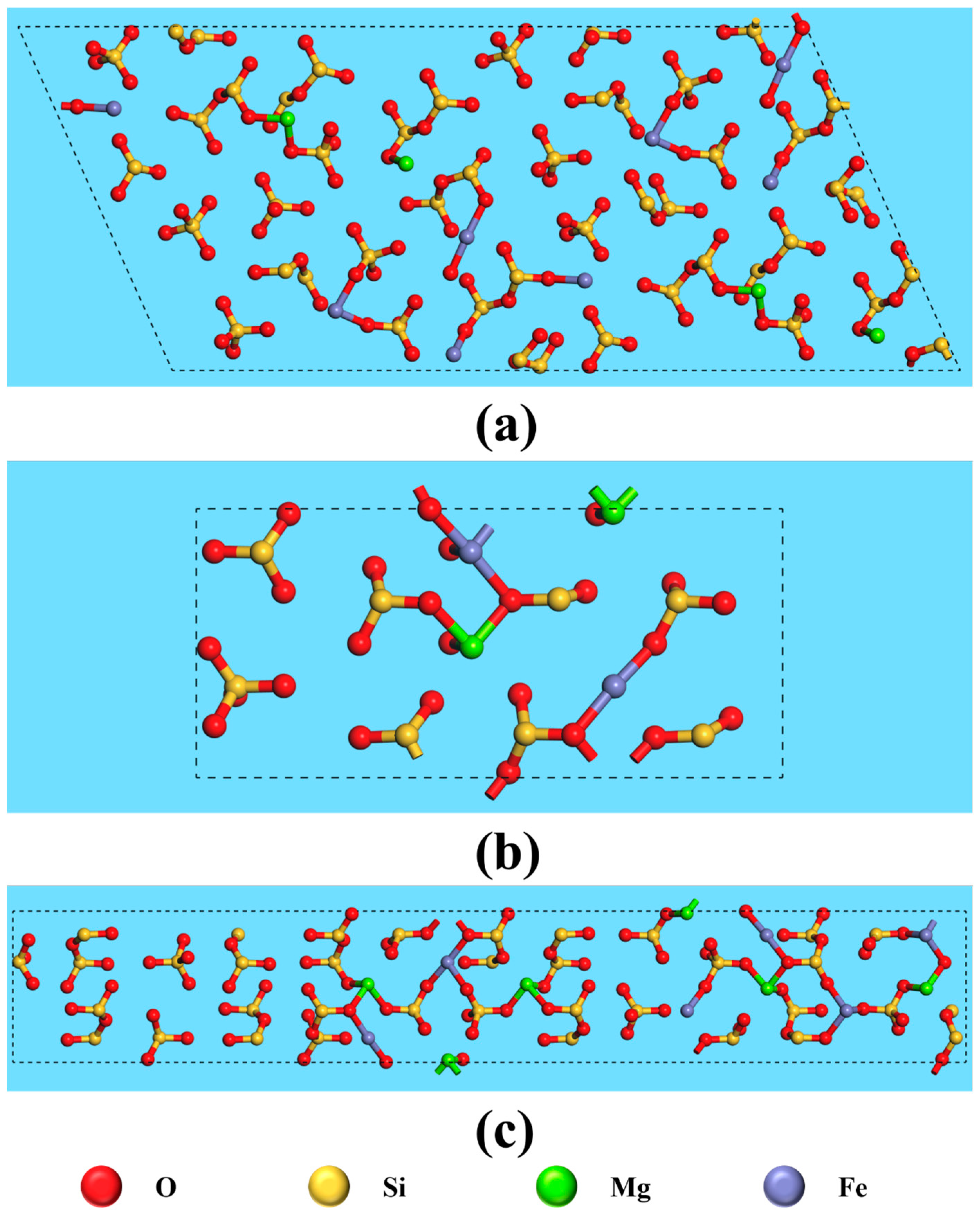
The anisotropy of crystals results in differences in the properties of various crystal planes, primarily due to variations in atomic arrangement and bond strength. Consequently, the interaction between exposed crystal planes and reagent molecules differs, ultimately affecting flotation outcomes. Figure 8 and Figure 9 illustrate the atomic arrangements of different cleavage planes of ilmenite and titanaugite, respectively, with appropriate unit cell expansions to better represent the crystal periodicity. The crystal structure information depicted in these figures is summarized in Table 4 and Table 5.
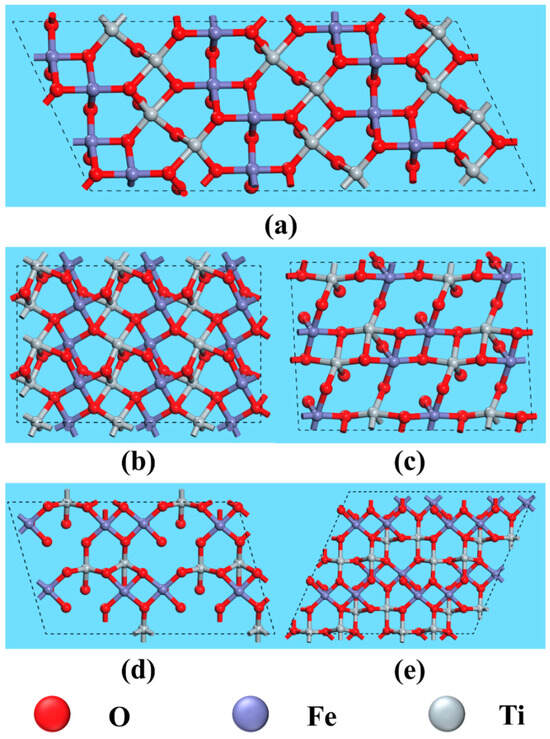
Figure 8.
Top view of fracture face of ilmenite on (a) (104), (b) (012), (c) (110), (d) (113) and (e) (116).
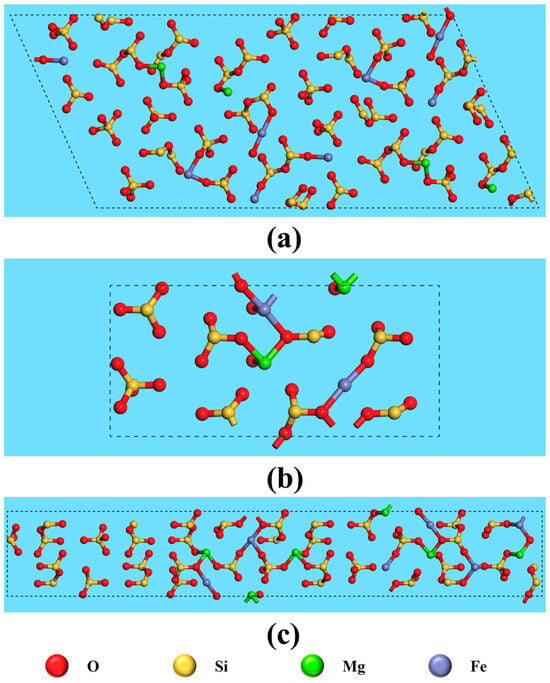
Figure 9.
Top view of fracture face of titanaugite on (a) (−221), (b) (220) and (c) (310).

Table 4.
Exposed Fe atoms and broken bonds on the surface of ilmenite.

Table 5.
Exposed metal atoms and broken bonds on the surface of titanaugite.
In existing studies on ilmenite flotation in the NaOL system [35,36,37], Fe is widely recognized as the primary active site for collector adsorption. Table 4 lists the structural information data for the main crystal planes of ilmenite, including (104), (110), (116), (012), and (113). The data indicate that the (104) plane has the highest density of broken bonds among Fe atoms. This high density increases the probability of reagent molecules binding to the (104) plane. Therefore, greater exposure of the (104) plane may favor ilmenite flotation. Du et al. [8] suggested that Fe and Mg sites on the surface of titanaugite are prone to interact with NaOL. As shown in Table 5, the overall broken bond densities of Fe and Mg on the (−221) plane of titanaugite are higher than those on the (220) and (310) planes, making it more likely to interact with the collector. This interaction could interfere with the adsorption of the collector on the ilmenite surface, thereby affecting ilmenite recovery.
3.6. XRD Test
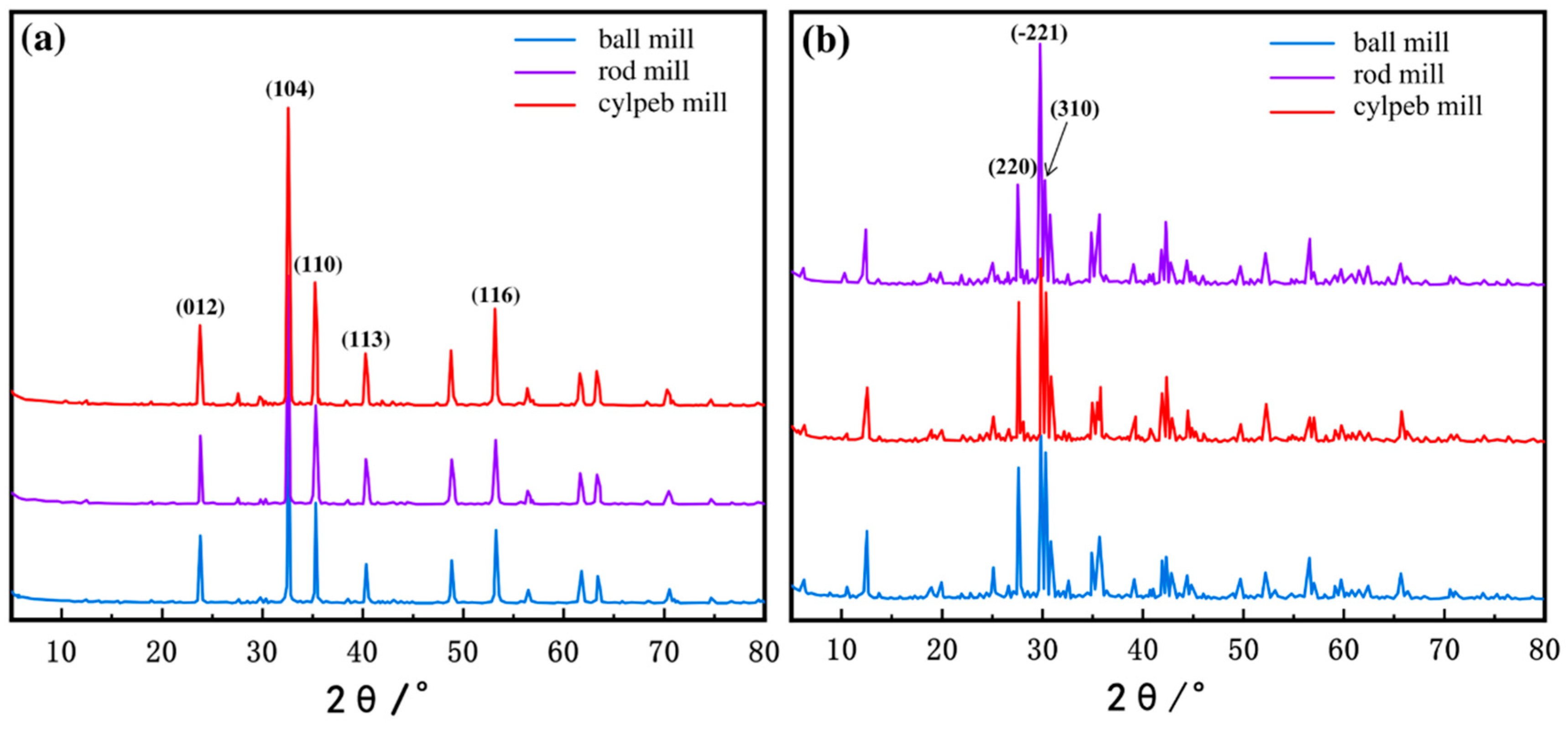
To analyze the exposure of different crystal planes in the milled ilmenite and titanaugite samples, X-ray diffraction (XRD) tests were conducted, and the diffraction patterns are shown in Figure 10. The intensity of the diffraction peaks corresponding to a specific crystal plane in the XRD pattern effectively reflects its exposure proportion in the mineral particles. By comparing the intensities of the diffraction peaks in the XRD patterns, the relationship between the exposure proportion of each crystal plane and the shape of the grinding media can be determined. Normalization of the XRD patterns was performed by defining the sum of the characteristic peak intensities of each major crystal plane as 100%, and the percentage of diffraction intensity was used to represent the relative proportion of a specific crystal plane. The results are presented in Table 6. The data indicate that, regardless of the grinding media shape, the (104) plane is the most exposed in ilmenite. Among the samples, the cylpeb-milled sample exhibits the highest proportion of the (104) plane at 45.78%, while the proportions in the ball-milled and rod-milled samples are 43.39% and 45.12%, respectively. In contrast, the (−221) plane is the most exposed in titanaugite, with the rod-milled sample showing the highest proportion of the (−221) plane at 53.86%, while the proportions in the ball-milled and cylpeb-milled samples are 37.76% and 38.71%, respectively. This suggests that using cylpebs as grinding media is more likely to generate a higher proportion of the (104) plane in ilmenite while avoiding excessive exposure of the (−221) plane in titanaugite.
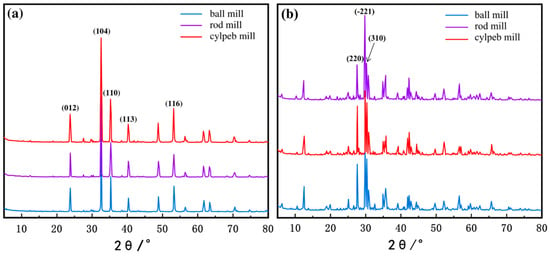
Figure 10.
XRD pattern of (a) ilmenite and (b) titanaugite.

Table 6.
Percentage of exposed crystal surfaces and recovery of milled ilmenite and titanaugite in media of different shapes.
3.7. Discussion
Previous studies have obtained the surface energies of common crystal planes of ilmenite through density functional theory (DFT) calculations [36,37,38]. In this study, the same method was employed to model and calculate the surface energies of titanaugite using Materials Studio 2019 software. The calculations were based on Equation (3).
In this equation, is the energy of the unit cell, is the energy of the monolayer atoms of the corresponding crystal plane, n is the ratio of the number of monolayer atoms to the number of unit cell atoms, and A is the cross-sectional area of the surface. The calculation results are presented in Table 7.

Table 7.
Surface energy of the lattice surface.
The data indicate that the (104) plane of ilmenite and the (−221) plane of titanaugite have the lowest surface energies, at 23.74 and 18.79 kcal·Å−2, respectively. Consequently, these two planes are the most likely to be exposed, which aligns with the XRD results showing that the diffraction peak intensities of these planes are the highest regardless of the grinding media used. Furthermore, combining the flotation and contact angle test results, the floatability and hydrophobicity of ilmenite and titanaugite are closely related to the exposure of the (104) and (−221) planes, respectively. However, rod media favor the exposure of the (−221) plane in titanaugite, while both rod and cylpeb media enhance the exposure of the (104) plane in ilmenite. This suggests that, in addition to the influence of particle size, the shape of the grinding media may directionally regulate the exposure of active mineral surfaces. In contrast, cylpeb grinding promotes the exposure of active surfaces in ilmenite while suppressing their exposure in titanaugite, which may explain the increased floatability difference between the two minerals. Gao and Xu et al. [24,25,39,40]. have mentioned that grinding media of different shapes can indeed influence the exposed crystal planes of mineral particles, providing an effective approach for the selective separation of minerals. However, the mechanism by which media shape promotes the exposure of active mineral surfaces remains unclear and represents a direction for future research.
4. Conclusions
By comparing the flotation recovery rates of products milled with the three types of media and analyzing the samples’ microscopic morphology, particle size distribution, contact angles, XRD patterns, and crystal structure simulations, it was found that using cylpebs as the grinding medium can enhance the floatability difference between ilmenite and titanaugite in a weakly alkaline NaOL system at pH ≈ 8. This facilitates the selective separation of ilmenite from titanaugite, the possible reasons for which are delineated below.
- (1)
- Compared to ball grinding, rod grinding and cylpeb grinding produce more elongated particles in ilmenite, which have lower roundness. This facilitates the exposure of more (104) planes in ilmenite, promoting the interaction between ilmenite particles and NaOL and thereby improving flotation recovery.
- (2)
- Compared to rod grinding, cylpeb grinding helps suppress the exposure of the (−221) plane in titanaugite, reducing the probability of interaction between titanaugite and NaOL. This weakens the interfering effect of titanaugite, favoring the selective separation of the two minerals.
Author Contributions
Validation, Analysis, software, Writing—original draft, Writing—review & editing, J.W.; Methodology, validation, Data curation C.D.; Funding acquisition, Project administration, Supervision P.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the support of the National Key Research and Development Program of China (2023YFC2908305), the National Natural Science Foundation of China (52474296, 52074357) China, the Sichuan Natural Science Foundation, China (24NSFSC1323) and the Hunan Natural Science Foundation, China (2022JJ30713). The authors also acknowledge the anonymous reviewers of the journal for providing many insightful suggestions for revisions.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviation
The following abbreviation is used in this manuscript:
| NaOL | Sodium Oleate |
References
- Boyer, R.R. Titanium for aerospace: Rationale and applications. Adv. Perform. Mater. 1995, 2, 349–368. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, Y.; Zhao, H.; Guo, D.; Wu, J.; Su, H.; Zhao, B. Research and Application Status of Titanium Alloys for Warships in China. Rare Met. Mater. Eng. 2011, 40, 538–544. [Google Scholar] [CrossRef]
- Yu, C.Y. Selection and application of titanium equipment in petrochemical industry. Rare Met. Mater. Eng. 1998, 27, 63–68. [Google Scholar]
- Balazic, M.; Kopac, J.; Jackson, M.J.; Ahmed, W. Review: Titanium and titanium alloy applications in medicine. Int. J. Nano Biomater. 2007, 1, 3–34. [Google Scholar] [CrossRef]
- Xiaowei, Z.; Wanyi, Z.; Ying, T.; Jiangcheng, O.; Minglei, S. Current Situation and Utilization Trend of Global Titanium Resources. Conserv. Util. Miner. Resour. 2019, 39, 68–75. [Google Scholar] [CrossRef]
- El Khalloufi, M.; Drevelle, O.; Soucy, G. Titanium: An Overview of Resources and Production Methods. Minerals 2021, 11, 1425. [Google Scholar] [CrossRef]
- Mehdilo, A.; Irannajad, M.; Rezai, B. Effect of chemical composition and crystal chemistry on the zeta potential of ilmenite. Colloids Surf. Physicochem. Eng. Asp. 2013, 428, 111–119. [Google Scholar] [CrossRef]
- Du, Y.; Meng, Q.; Yuan, Z.; Ma, L.; Zhao, X.; Xu, Y. Study on the flotation behavior and mechanism of ilmenite and titanaugite with sodium oleate. Miner. Eng. 2020, 152, 106366. [Google Scholar] [CrossRef]
- Didenko, P.; Efremov, A. study of self-consisted fractionation of iron and titanium isotopes in ilmenites. Appl. Surf. Sci. 2004, 231–232, 903–906. [Google Scholar] [CrossRef]
- Guéguin, M.; Cardarelli, F. Chemistry and mineralogy of titania-rich slags: Part 1—Hemo-ilmenite, sulphate, and upgraded titania slags. Miner. Process. Extr. Metall. Rev. 2007, 28, 1–58. [Google Scholar] [CrossRef]
- Cao, Y.C.; Huang, G.Y. Liu Study on High-efficiency Recovery of Ultrafine Ilmenite(−38 pm). Min. Metall. Eng. 2012, 32, 48–50. [Google Scholar]
- Ran, J.C.; Liu, Q.J.; Zhang, Z.G. Separation Test of Ilmenite in Yunnan. In Proceedings of the International Conference on Mechatronics Engineering and Computing Technology (ICMECT), Shanghai, China, 9–10 April 2014. [Google Scholar]
- Austin, L.; Shoji, K.; Luckie, P. The effect of rodrod size on mill performance. Powder Technol. 1976, 14, 71–79. [Google Scholar] [CrossRef]
- Yu, J.; Qin, Y.; Gao, P.; Han, Y.; Li, Y. An innovative approach for determining the grinding media system of ball mill based on grinding kinetics and linear superposition principle. Powder Technol. 2021, 378, 172–181. [Google Scholar] [CrossRef]
- Deniz, V. The effects of ball filling and ball diameter on kinetic breakage parameters of barite powder. Adv. Powder Technol. 2012, 23, 640–646. [Google Scholar] [CrossRef]
- Petrakis, E.; Karmali, V.; Komnitsas, K. Factors affecting nickel upgrade during selective grinding of low-grade limonitic laterites. Miner. Process. Extr. Metall. 2021, 130, 192–201. [Google Scholar] [CrossRef]
- Matsanga, N.; Nheta, W.; Chimwani, N. A Review of the Grinding Media in Ball Mills for Mineral Processing. Minerals 2023, 13, 1373. [Google Scholar] [CrossRef]
- Ipek, H. The effects of grinding media shape on breakage rate. Miner. Eng. 2006, 19, 91–93. [Google Scholar] [CrossRef]
- Lameck, N.S.; Kiangi, K.K.; Moys, M.H. Effects of grinding media shapes on load behaviour and mill power in a dry ball mill. Miner. Eng. 2006, 19, 1357–1361. [Google Scholar] [CrossRef]
- Caibin, W.U.; Yichao, Z.; Changmin, C.; Ruquan, Z.; Guiming, S.; Linfeng, Y.; Chen, W.J. Analysis of Different Contact Ways of Grinding Media in Grinding Kinetics in Tungsten. Nonferrous Met. Eng. 2016, 6, 58–62. [Google Scholar] [CrossRef]
- Lameck, N.S.; Moys, M.H. Effects of media shape on milling kinetics. Miner. Eng. 2006, 19, 1377–1379. [Google Scholar] [CrossRef]
- Simba, K.P.; Moys, M.H. Effects of mixtures of grinding media of different shapes on milling kinetics. Miner. Eng. 2014, 61, 40–46. [Google Scholar] [CrossRef]
- Huang, Z.-J.; Sun, W.; Gao, Z.-Y. Effects of grinding on mineral surface properties and flotation behaviors. Chin. J. Nonferr. Met. 2019, 29, 2671–2680. [Google Scholar] [CrossRef]
- Li, C.; Gao, Z. Effect of grinding media on the surface property and flotation behavior of scheelite particles. Powder Technol. 2017, 322, 386–392. [Google Scholar] [CrossRef]
- Li, C.; Gao, Z. Tune surface physicochemical property of fluorite particles by regulating the exposure degree of crystal surfaces. Miner. Eng. 2018, 128, 123–132. [Google Scholar] [CrossRef]
- Wang, C.; Deng, J.; Xiao, Q.; Sun, W.; Xu, S.; Gao, Z. Effect of grinding media shape on flotation separation of chalcopyrite and pyrite. Chin. J. Nonferr. Met. 2024, 34, 573–585. [Google Scholar] [CrossRef]
- Zhang, G.-F.; Feng, Q.-M.; Chen, Q.-Y.; Zhang, P.-M. Study on grinding media of selective grinding of bauxite. J. Cent. South Univ. Technol. Sci. Technol. 2004, 35, 552–556. [Google Scholar]
- Jin, Z.; Yong-Jie, G.; Ling-Pan, D.U.; Ci-Yun, C.; Tao, W.; Shu-Hong, Z.; Guo-Xian XJ, I.M. Processing Research on optimization test of grinding medium for ball mill. Ind. Miner. Process. 2016, 5, 28–30. [Google Scholar] [CrossRef]
- Jiahong, H.; Shujuan, D.; Zuojin, Z.; Jiahui, L. Study on grinding effects while steel forging as grinding medium. Min. Process. Equip. 2017, 45, 38–41. [Google Scholar] [CrossRef]
- Yoon, R.H.; Luttrell, G.H. The Effect of Bubble Size on Fine Particle Flotation. Miner. Process. Extr. Metall. Rev. 1989, 5, 101–122. [Google Scholar] [CrossRef]
- Yang, Y.H.; Xu, L.H.; Liu, S.J.; Deng, J. Influence of particle size on flotation separation of ilmenite, olivine, and pyroxene. Physicochem. Probl. Miner. Process. 2021, 57, 106–117. [Google Scholar] [CrossRef]
- Yong-Ming, G.; Huai-Fa, W.J. Micro-ilmenite Flotation Behavior of Study. Min. Eng. 2012, 10, 31–33. [Google Scholar]
- Zhang, G.-F.; Wang, L.; Feng, Q.-M.; Ou, L.-M.; Lu, Y.-P. Influence factors for interparticle interaction between titanaugite and ilmenite. Chin. J. Nonferr. Met. 2010, 20, 339–345. [Google Scholar] [CrossRef]
- Zhang, G.-F.; Wang, L.; Feng, Q.-M.; Lu, Y.-P.; Ou, L.-M. Effect of titanaugite on flotation behavior of ilmenite. Chin. J. Nonferr. Met. 2009, 19, 1124–1129. [Google Scholar] [CrossRef]
- Yang, S.Y.; Xu, Y.L.; Liu, C.; Soraya, D.A.D.; Li, C.; Li, H.Q. Investigations on the synergistic effect of combined NaOl/SPA collector in ilmenite flotation. Colloids Surf. Physicochem. Eng. Asp. 2021, 628, 127267. [Google Scholar] [CrossRef]
- Meng, Q.; Xu, Y.; Yuan, Z.; Zhao, X.; Du, Y. Separation mechanism of ilmenite from titanaugite with mixed BHA/NaOL collector. Miner. Eng. 2022, 176, 107363. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Yuan, Z.; Liu, Z.; Li, C. Selectivity of Benzyl Hydroxamic Acid in the Flotation of Ilmenite. Front. Chem. 2019, 7, 886. [Google Scholar] [CrossRef]
- Chettab, M.; Simon, Q.; Zaghrioui, M.; Autret-Lambert, C.; Laffez, P. Influence of sputtering conditions and annealing parameters on structure and morphology of NiTiO3 ilmenite thin films. Thin Solid Film. 2020, 714, 138384. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Wu, H.; Tian, J.; Liu, J.; Gao, Z.; Wang, L. Surface crystal chemistry of spodumene with different size fractions and implications for flotation. Sep. Purif. Technol. 2016, 169, 33–42. [Google Scholar] [CrossRef]
- Xu, L.; Tian, J.; Wu, H.; Deng, W.; Yang, Y.; Sun, W.; Gao, Z.; Hu, Y. New insights into the oleate flotation response of feldspar particles of different sizes: Anisotropic adsorption model. J. Colloid Interface Sci. 2017, 505, 500–508. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).