Interactions of Mineral Surfaces with Water Vapor: A Method for Analyzing Surface Condensation on Halite Crystals
Abstract
:1. Introduction
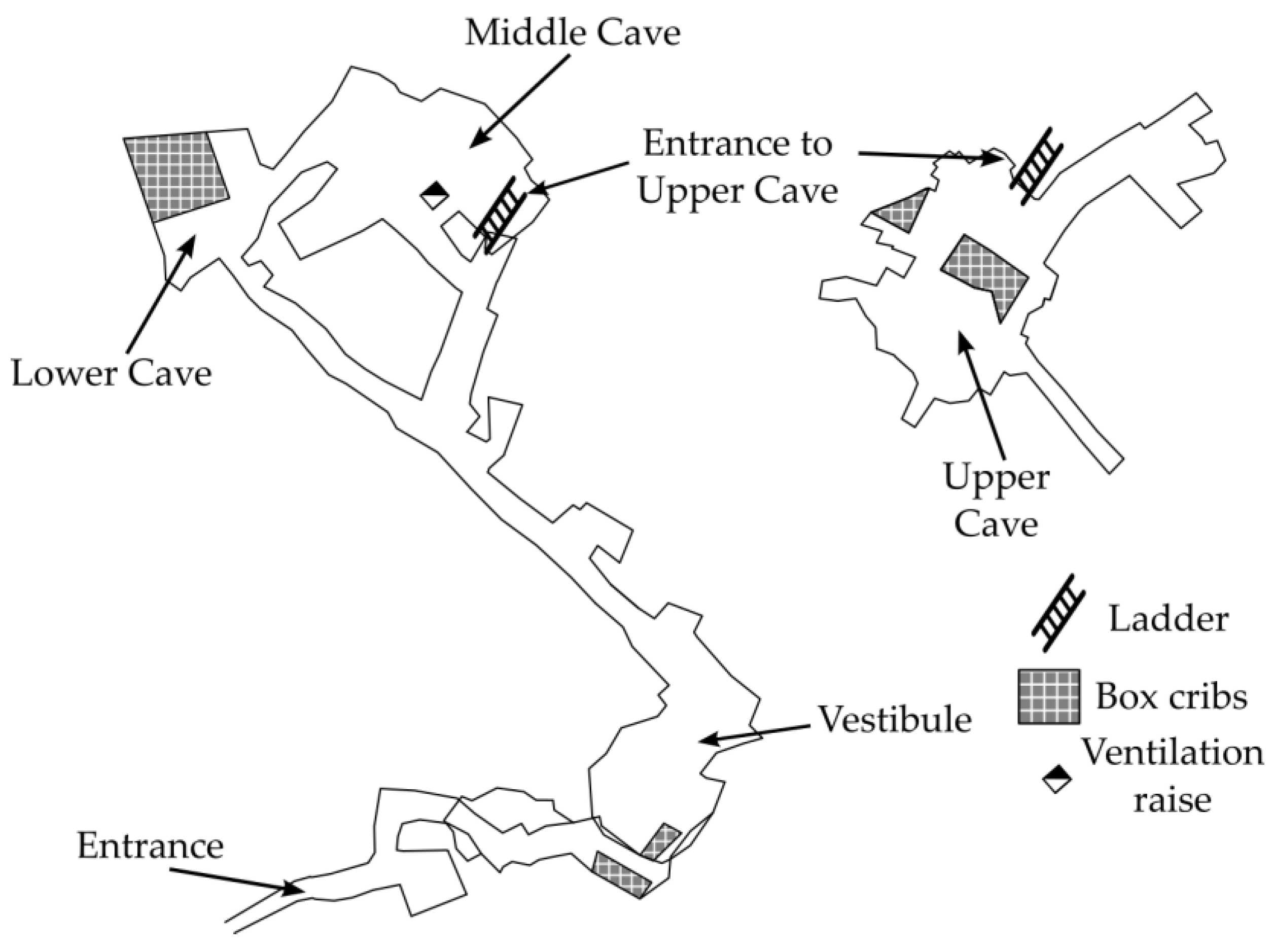
1.1. Microclimatic Conditions in the Crystal Caves
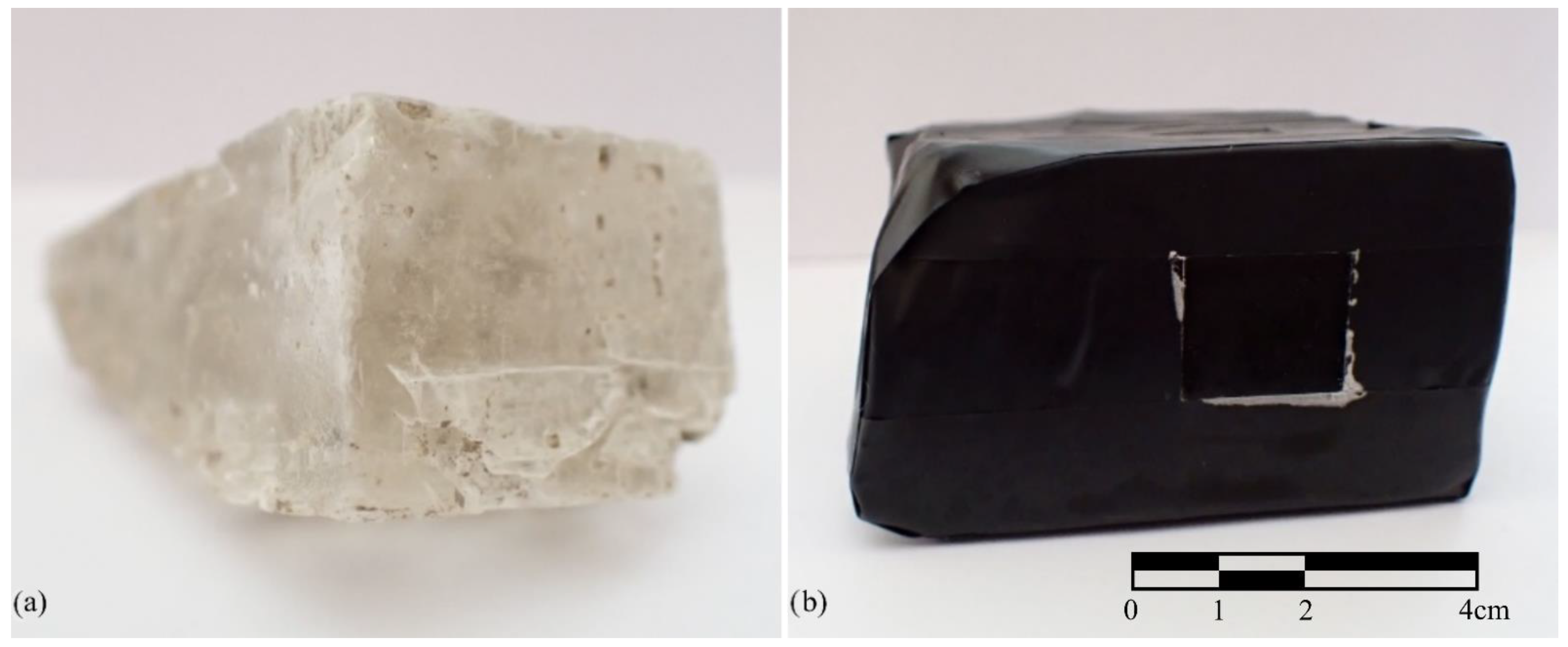
1.2. Halite Crystal Wetting Process
- Development of an optical method to detect significant changes on the surface of halite, caused by the formation of an aqueous film on this surface.
- Determination of the specific value of CRH for crystals derived directly from Crystal Caves so that the specific properties of the salts due to their unique composition can be taken into account.
- To evaluate the feasibility of using the proposed method for continuous monitoring in the underground environment. It is hypothesized that the proposed method will make it possible to detect optical changes in the halite surface in the visible spectrum caused by surface condensation and to correlate these changes with the relative humidity around the crystal, which may allow future qualitative classification of the state of the surface moisture and, as a result, an effective assessment of the risk of degradation.
- Refraction—The change in the direction of light propagation caused by the presence of a water film on the surface.
- Scattering—the appearance of water droplets or a heterogeneous water film in certain areas of the sample.
- Reflection—A wetted, rough surface or the presence of H2O molecules in cracks, fissures, and among salt dust/contaminant particles changes the amount of light reflected and transmitted through the sample.
- Interference and Polarization—The interaction of the waves at the boundary of media.
2. Concept and Methods
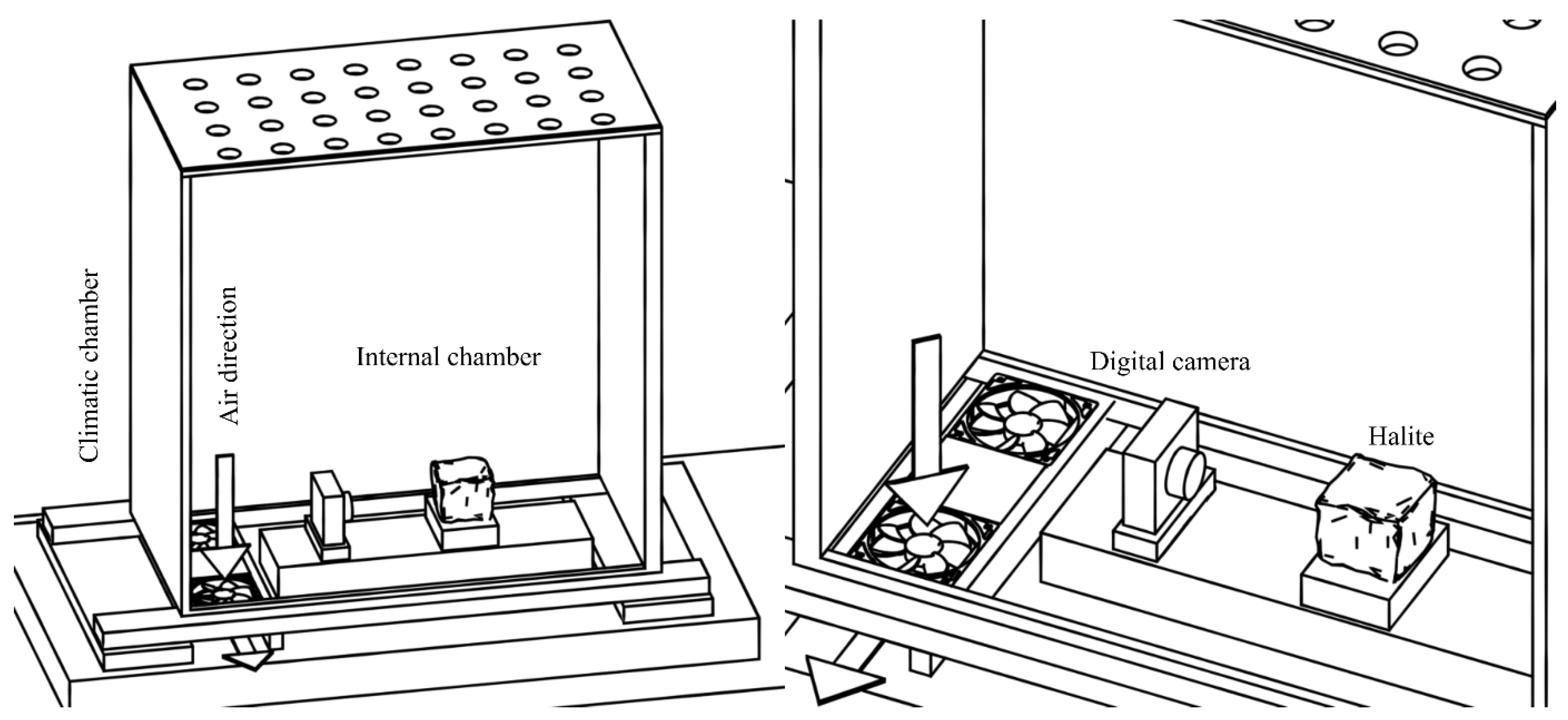
2.1. Measurement Setup
- BINDER MKF 720 climate chamber;
- OptiDew 501 thermohygrometer;
- OM System TG-7 digital camera with a resolution of 4000 × 3000 px.
2.2. Measurement Procedure
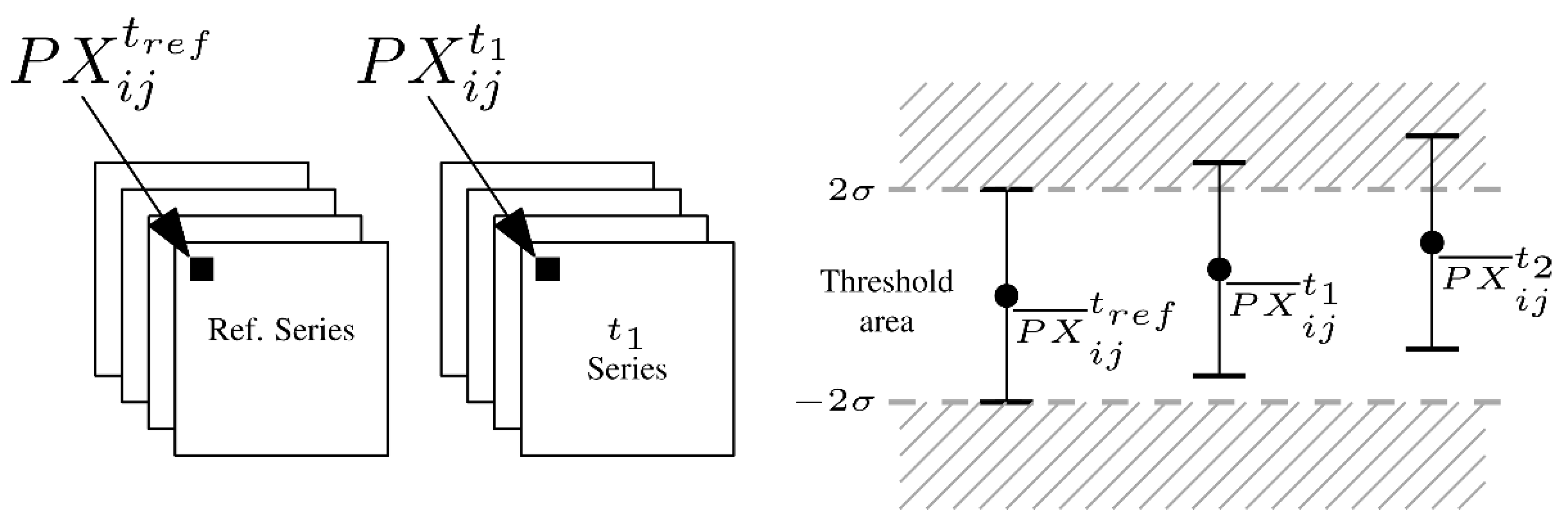
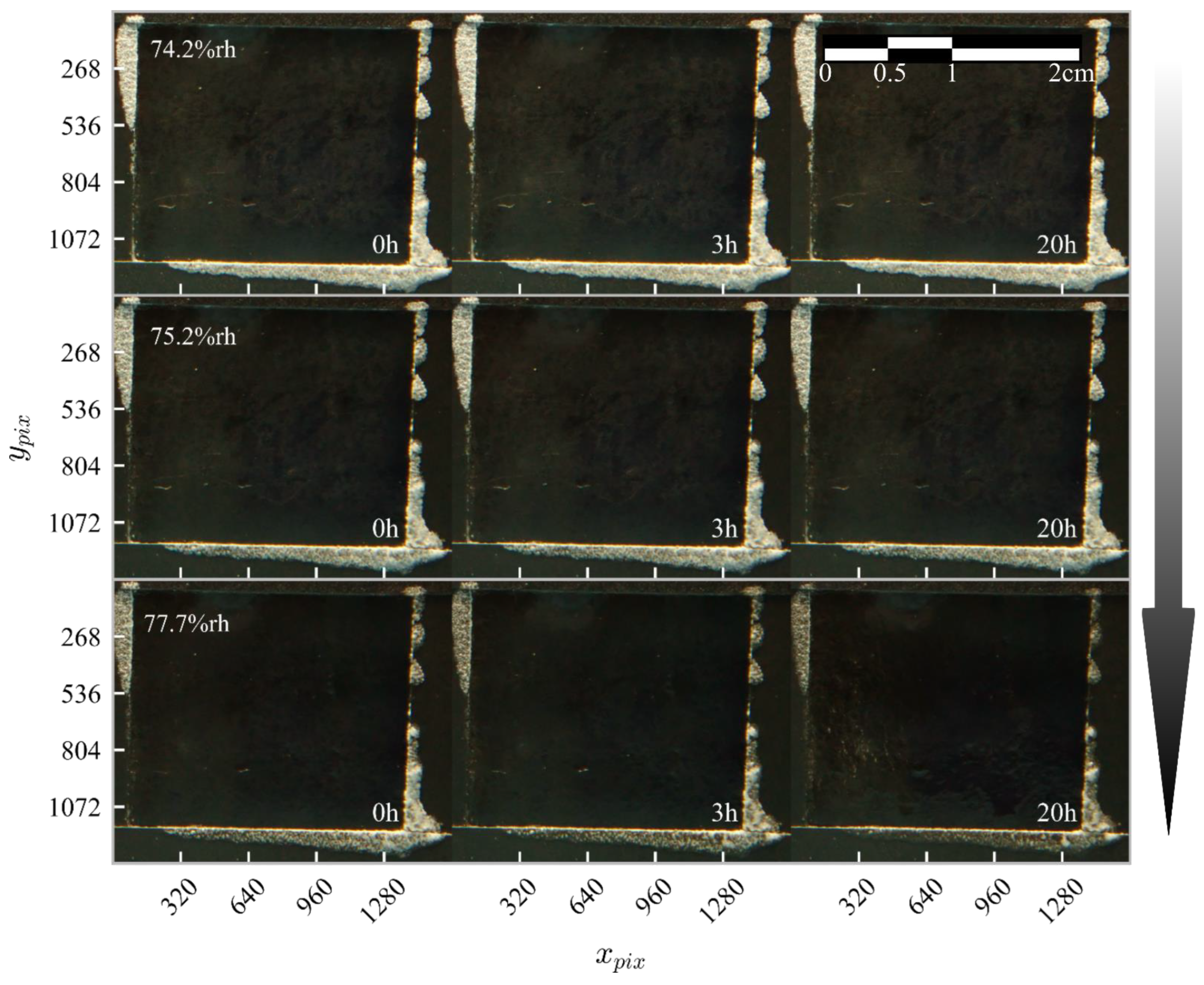
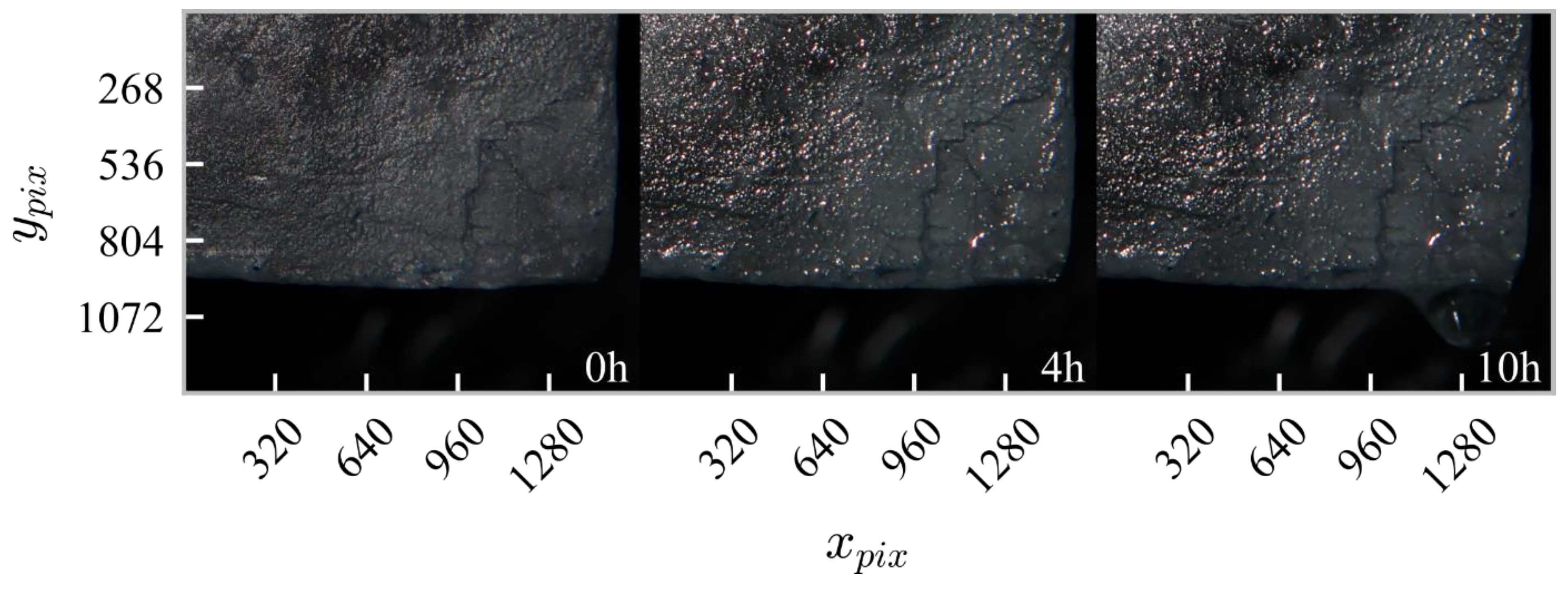
2.3. Image Analysis
- —acquired pixel brightness;
- —intensity of light reflected by the crystal surface element;
- —sum of noise from the sensor and vibrations of the climate chamber.
3. Experimental Results
3.1. Stage I—Crystal Testing in the Relative Humidity Range of 40%–77%
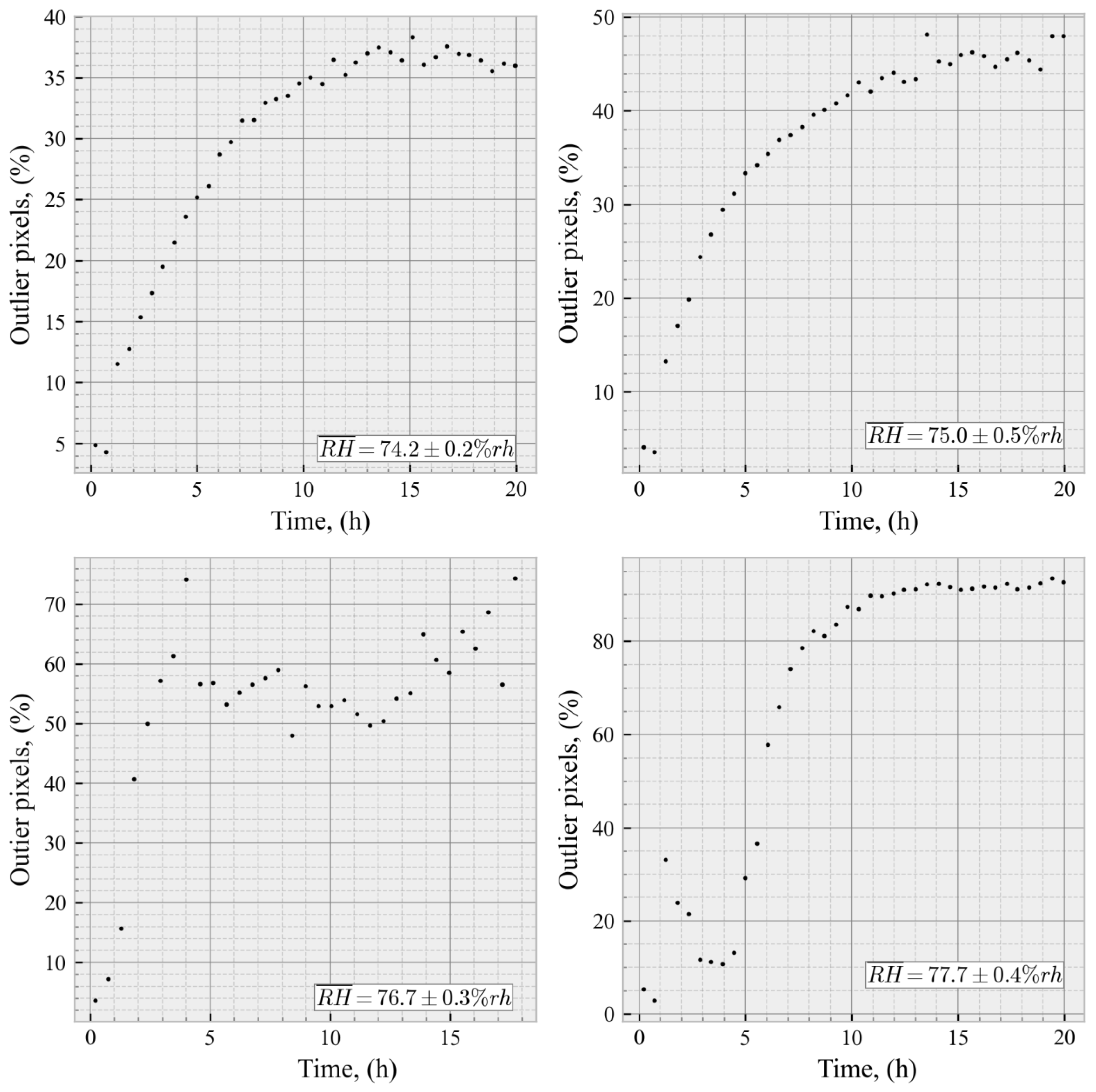
3.2. Stage II—Crystal Testing in the Relative Humidity Range of 74.2%–77.7%
- Dry and Early Adsorption State < 65.0% [54];
- Quasi-Static Condensation State >= 65.0% and <75.5%;
- Unstable State >= 75.5% < 77.0%rh;
- Dynamic Water Condensation State >= 77.0%—Crystal surface degradation.
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beysens, D. The Physics of Dew, Breath Figures and Dropwise Condensation; Lecture Notes in Physics; Springer International Publishing: Cham, Switzerland, 2022; Volume 994, ISBN 978-3-030-90441-8. [Google Scholar]
- Liu, P.; Song, M.; Zhao, T.; Gunthe, S.S.; Ham, S.; He, Y.; Qin, Y.M.; Gong, Z.; Amorim, J.C.; Bertram, A.K.; et al. Resolving the Mechanisms of Hygroscopic Growth and Cloud Condensation Nuclei Activity for Organic Particulate Matter. Nat. Commun. 2018, 9, 4076. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Zhang, C.; Dong, Z. Survival in Desert: Extreme Water Adaptations and Bioinspired Structural Designs. iScience 2023, 26, 105819. [Google Scholar] [CrossRef]
- Hauber, F.; Konrad, W.; Roth-Nebelsick, A. Aerial Roots of Orchids: The Velamen Radicum as a Porous Material for Efficient Imbibition of Water. Appl. Phys. A 2020, 126, 885. [Google Scholar] [CrossRef]
- Wierzchos, J.; Ascaso, C.; McKay, C.P. Endolithic Cyanobacteria in Halite Rocks from the Hyperarid Core of the Atacama Desert. Astrobiology 2006, 6, 415–422. [Google Scholar] [CrossRef]
- Robinson, C.K.; Wierzchos, J.; Black, C.; Crits-Christoph, A.; Ma, B.; Ravel, J.; Ascaso, C.; Artieda, O.; Valea, S.; Roldán, M.; et al. Microbial Diversity and the Presence of Algae in Halite Endolithic Communities Are Correlated to Atmospheric Moisture in the Hyper-arid Zone of the A Tacama D Esert. Environ. Microbiol. 2015, 17, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Vítek, P.; Edwards, H.G.M.; Jehlička, J.; Ascaso, C.; De Los Ríos, A.; Valea, S.; Jorge-Villar, S.E.; Davila, A.F.; Wierzchos, J. Microbial Colonization of Halite from the Hyper-Arid Atacama Desert Studied by Raman Spectroscopy. Phil. Trans. R. Soc. A 2010, 368, 3205–3221. [Google Scholar] [CrossRef]
- Ren, H.; Ma, Z.; Liu, J.; Gong, X.; Li, W. A Review of Heat and Mass Transfer Improvement Techniques for Dehumidifiers and Regenerators of Liquid Desiccant Cooling Systems. Appl. Therm. Eng. 2019, 162, 114271. [Google Scholar] [CrossRef]
- Salehi, A.A.; Ghannadi-Maragheh, M.; Torab-Mostaedi, M.; Torkaman, R.; Asadollahzadeh, M. A Review on the Water-Energy Nexus for Drinking Water Production from Humid Air. Renew. Sustain. Energy Rev. 2020, 120, 109627. [Google Scholar] [CrossRef]
- Barala, P.; Hooda, V. Hydrophilic Polymers in Agriculture and the Use of Nanoadditives to Improve Their Capabilities. J. Soil Sci. Plant Nutr. 2022, 22, 2992–3011. [Google Scholar] [CrossRef]
- Al-Rashed, R.; Al-Jabari, M. Concrete Protection by Combined Hygroscopic and Hydrophilic Crystallization Waterproofing Applied to Fresh Concrete. Case Stud. Constr. Mater. 2021, 15, e00635. [Google Scholar] [CrossRef]
- Esfahani, M.B.; Eshaghi, A.; Bakhshi, S.R. Transparent Hydrophobic, Self-Cleaning, Anti-Icing and Anti-Dust Nano-Structured Silica Based Thin Film on Cover Glass Solar Cell. J. Non-Cryst. Solids 2022, 583, 121479. [Google Scholar] [CrossRef]
- Muñóz, A.J.; Reca, J.; Martínez, J. Application of a Hydrophobic Coating to a Pressurized Pipe and Its Effect on Energy Losses and Fluid Flow Profile. Sci. Rep. 2024, 14, 8236. [Google Scholar] [CrossRef] [PubMed]
- d’Ambrosio Alfano, F.R.; Palella, B.I.; Riccio, G. Moisture in Historical Buildings from Causes to the Application of Specific Diagnostic Methodologies. J. Cult. Herit. 2023, 61, 150–159. [Google Scholar] [CrossRef]
- Vazquez, P.; Lux, J. Salt Crystallization on Crazannes Limestone in a Long-Term Storage Environment. Minerals 2023, 13, 1282. [Google Scholar] [CrossRef]
- Shi, W.; Liu, P.; Sun, B.; Wang, Q.; Xie, X.; Liang, Y.; Li, C. Water Vapor Condensation Prevention and Risk Rating Evaluation Based on Yang Can’s Tomb. Herit. Sci. 2024, 12, 178. [Google Scholar] [CrossRef]
- Li, F.; Yang, H.; Ayyamperumal, R.; Liu, Y. Evaluation of Hydrophilicity and Moisture Adsorption Characteristics of Inorganic Mineral Pigments to Sustain Ancient Paintings. J. King Saud Univ. Sci. 2022, 34, 102204. [Google Scholar] [CrossRef]
- Liñán, C.; Benavente, J.; Del Rosal, Y.; Vadillo, I.; Ojeda, L.; Carrasco, F. Condensation Water in Heritage Touristic Caves: Isotopic and Hydrochemical Data and a New Approach for Its Quantification through Image Analysis. Hydrol. Process. 2021, 35, e14083. [Google Scholar] [CrossRef]
- Columbu, A.; Audra, P.; Gázquez, F.; D’Angeli, I.M.; Bigot, J.-Y.; Koltai, G.; Chiesa, R.; Yu, T.-L.; Hu, H.-M.; Shen, C.-C.; et al. Hypogenic Speleogenesis, Late Stage Epigenic Overprinting and Condensation-Corrosion in a Complex Cave System in Relation to Landscape Evolution (Toirano, Liguria, Italy). Geomorphology 2021, 376, 107561. [Google Scholar] [CrossRef]
- Jamróz, P.; Socha, K. Monitoring of the Rock Mass Moisture in the Crystal Caves Nature Reserve. Arch. Min. Sci. 2023, 67, 729–742. [Google Scholar] [CrossRef]
- Alexandrowicz, Z. Groty Kryształowe w Kopalni Soli Wieliczka; Studia Naturae, Instytut Ochrony Przyrody PAN: Kraków, Poland, 2000; Volume 46. [Google Scholar]
- Alexandrowicz, Z.; Urban, J.; Andreychouk, V. Crystal Caves in the ‘Wieliczka’ Salt Mine—Unique Cave Site. Z. Für Geomorphol. Suppl. Issues 2021, 62, 235–254. [Google Scholar] [CrossRef]
- Walker, J.P.; Willgoose, G.R.; Kalma, J.D. In Situ Measurement of Soil Moisture: A Comparison of Techniques. J. Hydrol. 2004, 293, 85–99. [Google Scholar] [CrossRef]
- Kim, M.-I.; Jeong, G.-C.; Chung, I.-M.; Kim, N.-W. Experimental Assessment of the Infiltration Properties of a Coarse Soil Medium in a Dielectric Infiltration Test. Environ. Geol. 2009, 57, 591–600. [Google Scholar] [CrossRef]
- Placidi, P.; Gasperini, L.; Grassi, A.; Cecconi, M.; Scorzoni, A. Characterization of Low-Cost Capacitive Soil Moisture Sensors for IoT Networks. Sensors 2020, 20, 3585. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Hu, J.; Salmeron, M. Adsorption of Water on NaCl (100) Surfaces: Role of Atomic Steps. J. Phys. Chem. B 1997, 101, 1994–1998. [Google Scholar] [CrossRef]
- Foster, M.C.; Ewing, G.E. Adsorption of Water on the NaCl(001) Surface. II. An Infrared Study at Ambient Temperatures. J. Chem. Phys. 2000, 112, 6817–6826. [Google Scholar] [CrossRef]
- Dai, D.J.; Peters, S.J.; Ewing, G.E. Water Adsorption and Dissociation on NaCl Surfaces. J. Phys. Chem. 1995, 99, 10299–10304. [Google Scholar] [CrossRef]
- Ślązak, N.; Obracaj, D.; Borowski, M. Warunki Mikroklimatu W Podziemnych Obiektach Zabytkowych Kopalni Soli. Górnictwo I Geoinżynieria 2006, 30, 121–130. [Google Scholar]
- Adams, J.R.; Merz, A.R. Hygroscopicity of Fertilizer Materials and Mixtures. Ind. Eng. Chem. 1929, 21, 305–307. [Google Scholar] [CrossRef]
- Alexandrowicz, Z.; Urban, J. The conservation plan of the “Crystal Caves” Nature Reserve in the “Wieliczka” Salt Mine and its implementation in 2011–2015. Chrońmy Przyr. Ojczystą 2018, 74, 299–307. [Google Scholar]
- Kozłowski, R.; Hosker, R. Zabezpieczenie Przed Zniszczeniem Kopalni Soli w Wieliczce. Sprawozdanie z Realizacji Polsko—Amerykańskiego Programu Badawczego Za Okres 1992–1993; Instytut Katalizy i Fizykochemi Powierzchni PAN: Kraków, Poland, 1993. [Google Scholar]
- Salmon, L.G.; Cass, G.R.; Kozlowski, R.; Hejda, A.; Spiker, E.C.; Bates, A.L. Air Pollutant Intrusion into the Wieliczka Salt Mine. Environ. Sci. Technol. 1996, 30, 872–880. [Google Scholar] [CrossRef]
- Steiger, M.; Charola, A.E.; Sterflinger, K. Weathering and Deterioration. In Stone in Architecture; Siegesmund, S., Snethlage, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 227–316. ISBN 978-3-642-14474-5. [Google Scholar]
- Martínez-Martínez, J.; Arizzi, A.; Benavente, D. The Role of Calcite Dissolution and Halite Thermal Expansion as Secondary Salt Weathering Mechanisms of Calcite-Bearing Rocks in Marine Environments. Minerals 2021, 11, 911. [Google Scholar] [CrossRef]
- Bryan, C.R.; Knight, A.W.; Katona, R.M.; Sanchez, A.C.; Schindelholz, E.J.; Schaller, R.F. Physical and Chemical Properties of Sea Salt Deliquescent Brines as a Function of Temperature and Relative Humidity. Sci. Total Environ. 2022, 824, 154462. [Google Scholar] [CrossRef]
- Yalcin, S.E.; Legg, B.A.; Yeşilbaş, M.; Malvankar, N.S.; Boily, J.-F. Direct Observation of Anisotropic Growth of Water Films on Minerals Driven by Defects and Surface Tension. Sci. Adv. 2020, 6, eaaz9708. [Google Scholar] [CrossRef]
- Winardhi, C.W.; Godinho, J.R.D.A.; Gutzmer, J. The Effect of Macroscopic Particle Features on Mineral Dissolution. Minerals 2023, 13, 253. [Google Scholar] [CrossRef]
- Boily, J.-F.; Yeşilbaş, M.; Musleh Uddin, M.M.; Baiqing, L.; Trushkina, Y.; Salazar-Alvarez, G. Thin Water Films at Multifaceted Hematite Particle Surfaces. Langmuir 2015, 31, 13127–13137. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.; Ho, T.A.; Cole, D.R.; Striolo, A. Molecular Structure and Dynamics in Thin Water Films at Metal Oxide Surfaces: Magnesium, Aluminum, and Silicon Oxide Surfaces. J. Phys. Chem. C 2012, 116, 15962–15973. [Google Scholar] [CrossRef]
- Yeşilbaş, M.; Boily, J.-F. Particle Size Controls on Water Adsorption and Condensation Regimes at Mineral Surfaces. Sci. Rep. 2016, 6, 32136. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Chen, X.; Hou, D. Modeling of Monolayer Water Adsorption on Surface of Cement Minerals by Molecule Simulation and Adsorption Isotherm. Mater. Today Commun. 2023, 36, 106646. [Google Scholar] [CrossRef]
- Wu, S.; Yu, Q. Experimental Investigations on Water Condensation in the Nanopores of Shales. Energy Sources Part A Recovery Util. Environ. Eff. 2024, 46, 16378–16396. [Google Scholar] [CrossRef]
- Sheng, Q.; Sun, J.; Wang, Q.; Wang, W.; Wang, H.S. On the Onset of Surface Condensation: Formation and Transition Mechanisms of Condensation Mode. Sci. Rep. 2016, 6, 30764. [Google Scholar] [CrossRef]
- Liu, J.; Aizawa, H.; Yoshino, H. CFD Prediction of Surface Condensation on Walls and Its Experimental Validation. Build. Environ. 2004, 39, 905–911. [Google Scholar] [CrossRef]
- Staszczuk, P.; Biliński, B. Water Film Properties on Mineral Surfaces in Flotation Processes. Colloids Surf. A Physicochem. Eng. Asp. 1993, 79, 97–104. [Google Scholar] [CrossRef]
- Künzel, H.; Tanaka, E.; Zirkelbach, D. Tipping Point for Condensation Water Drainage on Surfaces and Interfaces of Insulated Wall Assemblies-Experimental Method to Define Water Content Limits for Hygrothermal Simulation Models. In Proceedings of the Architectural Science Association (ASA International Conference), New Orleans, LA, USA, 1–3 October 2023. [Google Scholar]
- Lad, R.A. Adsorption of Water on Sodium Chloride: The Effect of Prior Exposure to Hydrogen Chloride, Carbon Dioxide and Water Vapor. Surf. Sci. 1968, 12, 37–45. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Manaf, N.D.A.; Fukuda, K.; Subhi, Z.A.; Mohd Radzi, M.F. Influences of Surface Roughness on the Water Adsorption on Austenitic Stainless Steel. Tribol. Int. 2019, 136, 75–81. [Google Scholar] [CrossRef]
- Thi Bao Le, T.; Divine-Ayela, C.; Striolo, A.; Cole, D.R. Effects of Surface Contamination on the Interfacial Properties of CO2/Water/Calcite Systems. Phys. Chem. Chem. Phys. 2021, 23, 18885–18892. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Zhao, J.; Tan, J. Optical Properties of Sodium Chloride Solution within the Spectral Range from 300 to 2500 Nm at Room Temperature. Appl. Spectrosc. 2015, 69, 635–640. [Google Scholar] [CrossRef]
- Zhang, Z.; Ewing, G.E. Attenuated Partial Internal Reflection Infrared Spectroscopy. Anal. Chem. 2002, 74, 2578–2583. [Google Scholar] [CrossRef]
- Yang, L.; He, J.; Shen, Y.; Li, X.; Sun, J.; Czajkowsky, D.M.; Shao, Z. Nanoscopic Characterization of the Water Vapor-Salt Interfacial Layer Reveals a Unique Biphasic Adsorption Process. Sci. Rep. 2016, 6, 31688. [Google Scholar] [CrossRef]
- Ewing, G.E. Thin Film Water. J. Phys. Chem. B 2004, 108, 15953–15961. [Google Scholar] [CrossRef]
- Heavens, O.S. Optical Properties of Thin Films. Rep. Prog. Phys. 1960, 23, 1–65. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piga, T.; Jamróz, P.; Buksa, D. Interactions of Mineral Surfaces with Water Vapor: A Method for Analyzing Surface Condensation on Halite Crystals. Minerals 2025, 15, 435. https://doi.org/10.3390/min15050435
Piga T, Jamróz P, Buksa D. Interactions of Mineral Surfaces with Water Vapor: A Method for Analyzing Surface Condensation on Halite Crystals. Minerals. 2025; 15(5):435. https://doi.org/10.3390/min15050435
Chicago/Turabian StylePiga, Tymoteusz, Paweł Jamróz, and Dominik Buksa. 2025. "Interactions of Mineral Surfaces with Water Vapor: A Method for Analyzing Surface Condensation on Halite Crystals" Minerals 15, no. 5: 435. https://doi.org/10.3390/min15050435
APA StylePiga, T., Jamróz, P., & Buksa, D. (2025). Interactions of Mineral Surfaces with Water Vapor: A Method for Analyzing Surface Condensation on Halite Crystals. Minerals, 15(5), 435. https://doi.org/10.3390/min15050435







