Abstract
Water pollution continues to pose a critical global challenge, largely due to the unregulated discharge of industrial, agricultural, and municipal effluents. Among emerging solutions, enzymatic bioremediation stands out as a sustainable and environmentally friendly approach, offering high specificity and efficiency under mild conditions. Nonetheless, the practical application of free enzymes is hindered by their inherent instability, poor reusability, and susceptibility to denaturation. To address these limitations, the immobilization of enzymes onto solid supports, particularly clay minerals, has garnered increasing attention. This review presents a detailed analysis of clay minerals as promising carriers for enzyme immobilization in wastewater treatment. It explores their classification, structural characteristics, and physicochemical properties, highlighting key advantages such as a large surface area, cation exchange capacity, and thermal stability. Functionalization techniques, including acid/base activation, intercalation, grafting, and pillaring, are discussed in terms of improving enzyme compatibility and catalytic performance. Various immobilization methods such as physical adsorption, covalent bonding, entrapment, crosslinking, and intercalation are critically evaluated with regard to enhancing enzyme activity, stability, and recyclability. Recent case studies demonstrate the effective removal of pollutants such as dyes, pharmaceuticals, and heavy metals using enzyme–clay composites. Despite these advances, challenges such as enzyme leaching, mass transfer resistance, and variability in clay composition persist. This review concludes by outlining future prospects, including the development of hybrid and magnetic clay-based systems and their integration into advanced water treatment technologies. Overall, enzyme immobilization on clay minerals represents a promising and scalable approach for the next generation of wastewater bioremediation strategies.
1. Introduction
Water pollution has become an escalating environmental concern in recent years due to the increasing discharge of industrial, agricultural, and municipal effluents. It is estimated that approximately 80% of industrial wastewater is released into natural water bodies without adequate treatment, often violating environmental discharge standards [1,2]. Consequently, various pollutants including dyes, heavy metals, pharmaceuticals, polychlorinated biphenyls (PCBs), and per- and polyfluoroalkyl substances (PFAS) are constantly released in watercourses, thus threatening the environment and human health [3]. These pollutants pose significant risks to human health and biodiversity, promoting phytotoxicity, alga proliferation, and aquatic biota devastation [4,5]. Furthermore, the stability of such emerging pollutants may lead to their seepage into groundwater, disrupting trophic chains and threatening ecological stability [6]. Accordingly, more than fifty waterborne diseases (cholera, hepatitis, cancer, etc.) ensue from water pollution, hence jeopardizing human life [7]. Considering all these deleterious environmental and health concerns associated with wastewater pollution, different techniques have been developed such as oxidation [8], adsorption [9], coagulation–flocculation [10], membrane filtration [11], batch reactors [12], and enzymatic bioremediation [13,14]. The choice of treatment method depends on the pollutant characteristics, treatment cost, reaction kinetics, and infrastructure availability. While physicochemical methods are efficient, they often involve the use of hazardous chemicals and may generate secondary pollutants. Despite the advantages of enzymatic bioremediation, its real-world adoption remains limited due to scalability challenges, regulatory constraints, and insufficient cost–benefit analyses compared to conventional methods. Bridging this gap requires interdisciplinary efforts to optimize immobilized enzyme systems for industrial wastewater treatment [15,16]. Furthermore, conventional techniques may be ineffective in removing low concentrations of emerging pollutants such as endocrine-disrupting chemicals (e.g., bisphenol A, phthalates) and certain heavy metals (e.g., mercury, arsenic) [17].
Enzymatic treatment has gained traction as a promising alternative due to its high substrate specificity, operation under mild conditions, and minimal secondary pollution [14,18]. Enzymes can selectively target pollutants without the need for toxic reagents, making them environmentally safe and efficient [14]. However, the practical application of free enzymes in wastewater treatment faces challenges such as low stability under extreme pH or temperature, susceptibility to denaturation, and difficulty in recovery and reuse [19,20].
To address these limitations, various strategies have been explored to improve enzyme stability, notably chemical modification and immobilization. Chemical modifications, such as surface functionalization with amino (-NH2) or thiol (-SH) groups, can enhance enzymatic performance; however, they often require expensive and potentially hazardous reagents [21]. Moreover, these approaches depend heavily on synthetic reactants (e.g., organic solvents, surfactants), which may generate byproducts that interfere with the enzyme’s native activity and reduce its reusability. Concerns regarding the safety, environmental impact, and overall cost of these methods further limit their practical application [22]. In contrast, enzyme immobilization, where enzymes are either attached to or entrapped within solid supports, represents a more sustainable and economically viable alternative. This technique not only improves enzyme stability under a wide range of operational conditions but also enables easy recovery and repeated use, often resulting in enhanced catalytic efficiency [23,24].
The choice of support material is critical. Various materials, such as organic polymers [25], carbon nanotubes [26], mesoporous silica [27], zeolites [28], and clay minerals [25], have been explored. Among these, clay minerals stand out due to their affordability, natural abundance, high surface area, and chemical stability. Unlike synthetic polymers, clay minerals are non-toxic and thermally stable, making them ideal candidates for enzyme immobilization in wastewater treatment applications [25].
Although enzyme immobilization and the properties of clay minerals have been independently reviewed [29,30], there is limited literature that comprehensively explores the synergistic use of clay minerals as enzymatic carriers specifically for wastewater treatment [3,6,23,31,32]. This review aims to fill that gap by presenting an in-depth analysis of clay minerals for enzyme immobilization. We begin with a discussion on the classification, structure, and physicochemical properties of clay minerals, followed by a detailed overview of their functionalization methods. Subsequently, we explore various enzyme immobilization techniques and their application in pollutant degradation. This review concludes by highlighting the current challenges and prospects of enzyme–clay systems for sustainable wastewater treatment. To ensure transparency and reproducibility, a structured methodology was applied for the selection of the studies included in this review. Relevant publications were retrieved from major scientific databases, including Scopus, Web of Science, and Science Direct. The literature search covered the period 2010 to 2025, with a particular focus on recent advances from 2020 onward. The inclusion criteria comprised peer-reviewed articles and reviews reporting experimental data or critical evaluations of enzyme immobilization on clay minerals for pollutant removal. Studies addressing related hybrid clay biocatalyst systems were also considered. The exclusion criteria included publications lacking experimental data, conference abstracts without peer review, and studies related to wastewater treatment. Following this process, a total of 97 studies were considered and analyzed in this review. This systematic approach ensures a comprehensive and balanced overview of the state of knowledge and recent progress in enzyme–clay composites for wastewater treatment applications
2. Clay Minerals: Classification, Structure, Properties, and Functionalization
2.1. Classification of Clay Minerals
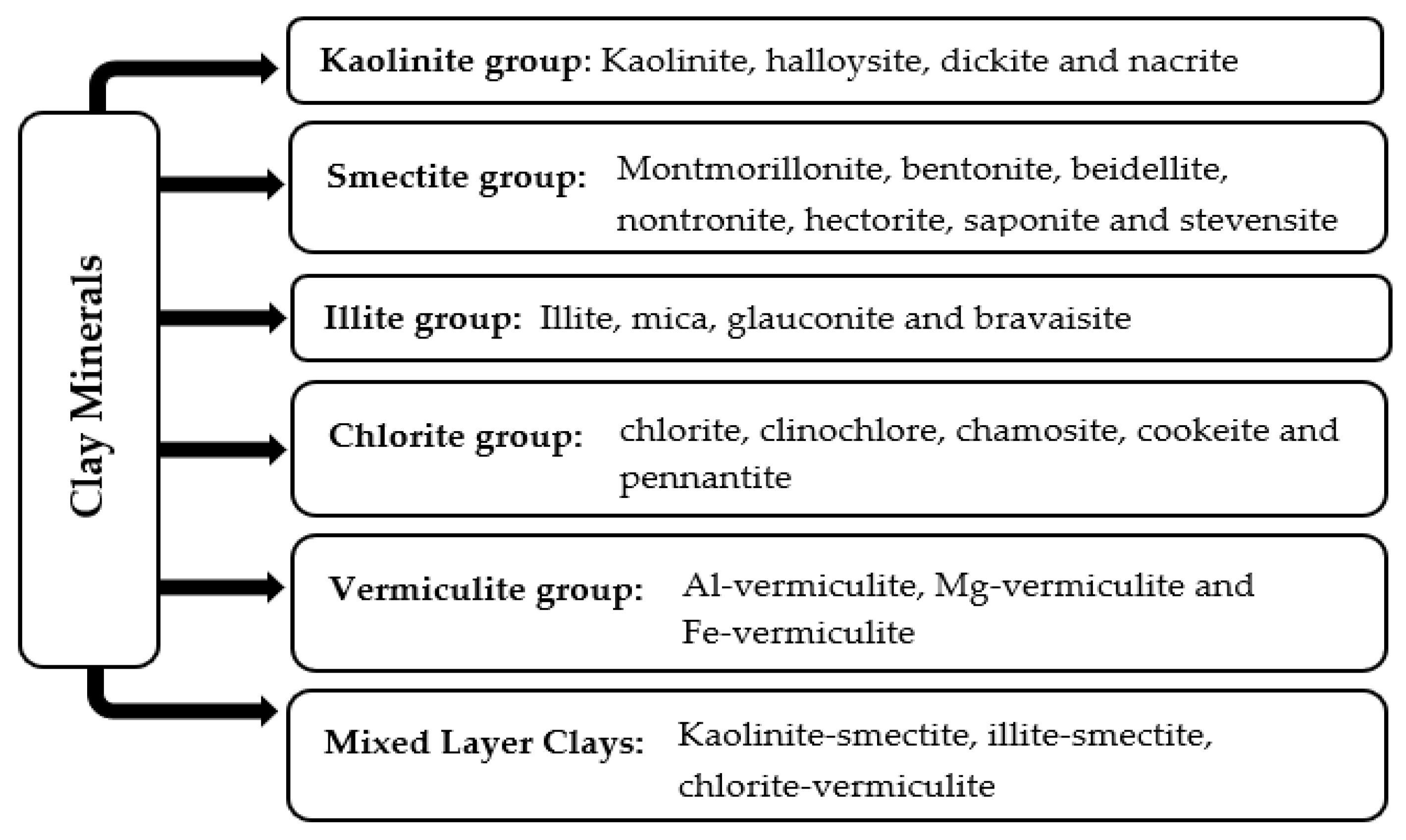
Clay minerals are naturally occurring materials, generally issued from weathering and diagenesis processes. Adopting the physicochemical features of their parent rocks, they are classified into five main groups: kaolinite, smectite, illite, vermiculite and chlorite. Figure 1 displays these different groups and their subgroups.
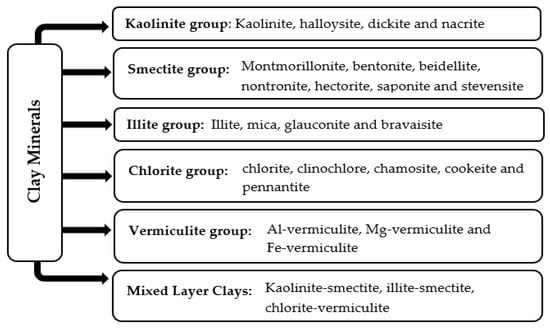
Figure 1.
Classification of clay minerals.
As presented in Figure 1, the first clay mineral group consists of kaolinite and its polymorphs, i.e., halloysite, dickite, and nacrite. These clay minerals are formed from the acid hydrothermal alteration of aluminosilicates, chiefly feldspars. While they share the same molecular formula, Al2Si2O5(OH)4, each clay has its own color, structure, and applications. Kaolinite, commonly known as kaolin, is the main compound in this group.
The smectites issued from volcanic ashes form the second group of clay minerals. Montmorillonite, with the chemical composition (Na, Ca)0.3 (Al, Mg)2 Si4 O10 (OH)2. n (H2O), is the most recognized smectite clay. Additionally, other related minerals with almost the same structure of montmorillonite were explored. Examples include bentonite, beidellite, nontronite, hectorite, saponite, and stevensite, having varying chemical formulae and belonging to the smectite group.
On the other hand, the third group is the illites, including illite, mica, glauconite, and bravaisite. The illite clay, with the chemical formula K0.65Al2 (Al0.65Si3.35O10) (OH)2, is derived from slight metamorphic alterations of muscovite and feldspars in sedimentary rocks. Further, the chlorites, including clinochlore, chamosite, cookeite, and pennantite, form the fourth class of clay minerals. The chlorites, with the general molecular formula (Mg, Fe)5Al (Si3Al) O10(OH)8, are produced by mafic minerals such as pyroxene, amphibole, and biotite. Lastly, the fifth group is the vermiculites, with the chemical formula (Mg,Fe,Al)3(Al,Si)4O10(OH)2 4H2O. They are typically formed from weathering and hydrothermal changes in micas, chiefly biotite, under acidic conditions. As per the dominant metal ions, the vermiculites are classified into Al-vermiculite, Mg-vermiculite, and Fe-vermiculite.
In addition to these acknowledged clay mineral groups, another class of so-called mixed-layer clays has emerged as an output for specific environmental and geological conditions (diagenesis and critical thermal or pressure alterations of shales). The mixed-layer clays consist of two or more types of clay minerals regularly or randomly ordered within the same crystal structure. The most popular ones are Kaolinite–smectite, illite–smectite and chlorite–vermiculite.
Taking into consideration all these clay mineral groups (Figure 1), it is worth investigating their crystalline structures and inherent physicochemical properties to provide a deep insight into their possible applications.
2.2. Structure of Clay Minerals
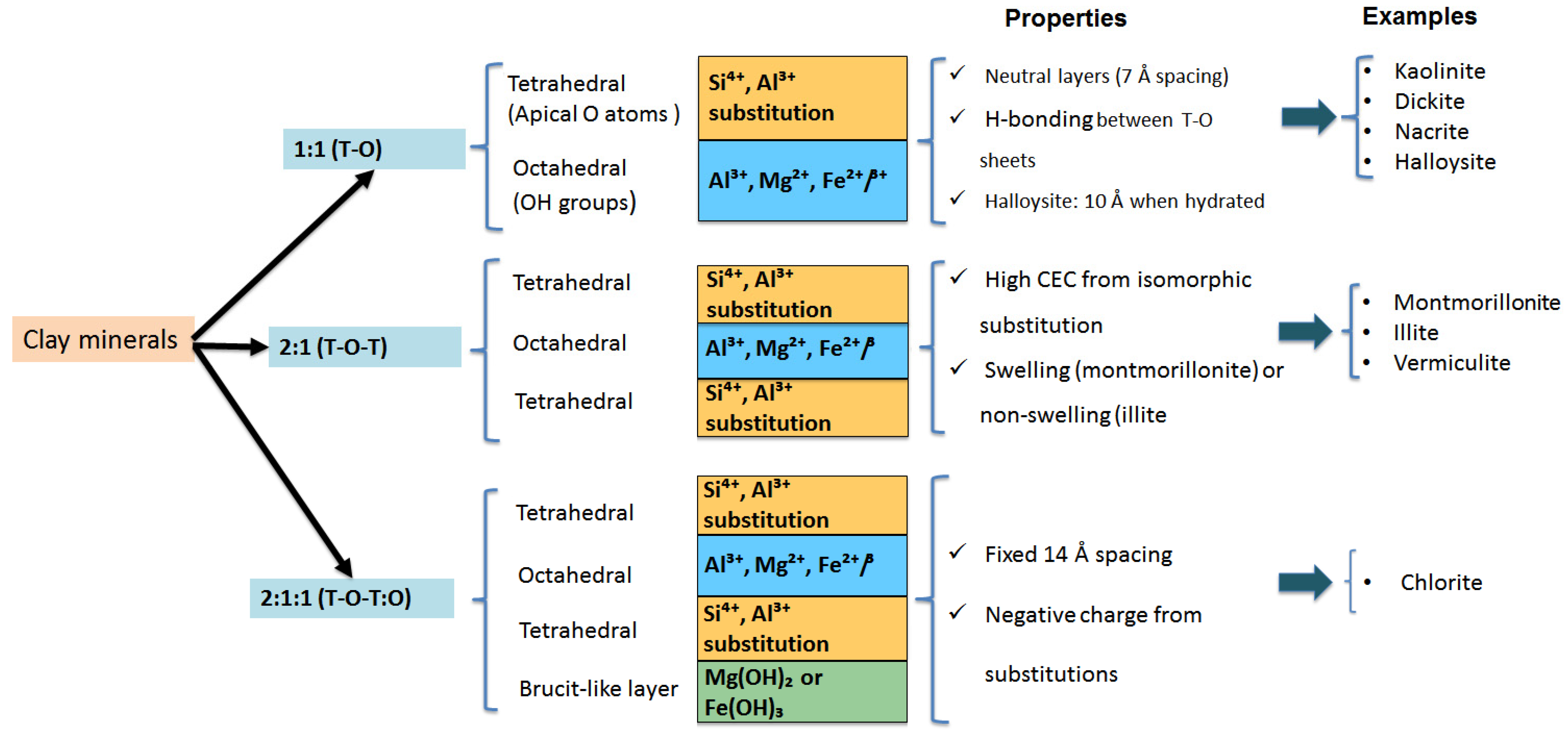
Clay minerals typically show a layered structure composed of a determinant arrangement of tetrahedral and octahedral sheets. As their names suggest, the tetrahedral sheet is constituted by silicate tetrahedra, while the octahedral layer is formed of octahedra enclosing metal cations like Al3+ and Fe3+ at their centers bonded to oxygen or hydroxyl ions set on their six vertices. Under environmental and geological conditions, the different interactions occurring between the tetrahedral and octahedral layers as well as with other molecules in the medium (water, organic molecules, metal ions, etc.) confer the clay minerals with well-defined crystalline structures. Therefore, they are classified according to the number and arrangement of their tetrahedral and octahedral sheets. The phyllosilicates are present under different common structures, namely, 1:1 (T-O), 2:1 (T-O-T), and 2:1:1 (T-O-T:O) (Figure 2).
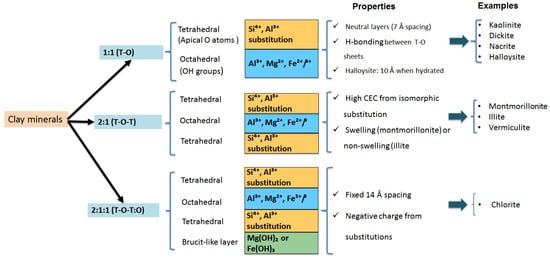
Figure 2.
Structural classification of clay minerals.
The 1:1 (T-O) clay mineral type refers to the arrangement of one tetrahedral and one octahedral layer in the clay structure. The kaolinite group members (kaolinite, dickite, nacrite, and halloysite) are 1:1 aluminosilicate clay. For example, kaolinite is composed of a structure in which one tetrahedral sheet of one layer faces one octahedral sheet of the next. Despite their disordered stacking, the layers are electrostatically neutral and linked via hydrogen bonds between an apical oxygen atom in the tetrahedral sheet and a hydroxyl (OH) in the octahedral sheet. Their thickness and interlayer spacing are about 7 Ǻ. Similarly, dickite and nacrite exhibit similar structures to kaolinite with slight differences (more ordered and compacted layers). However, in contrast to the platy form of kaolinite clay, the halloysite clay shows a tubular structure with a higher layer thickness of 10 Ǻ due to the presence of water in its interlayer, thus allowing its swelling.
On the other hand, the second common clay structure, so-called 2:1 (T-O-T), is composed of one octahedral layer sandwiched between two tetrahedral layers. The main well-known phyllosilicates having such structure are the smectite (montmorillonite, bentonite, etc.), illite, and vermiculite groups. For instance, each montmorillonite layer is formed of one octahedral sheet (composed of Al3+ or Mg2+) sequestered between two tetrahedral ones (Si4+). These latter interconnect with the octahedral sheet via apical oxygens. The two octahedral and tetrahedral layers are separated by an interlayer of 14 Ǻ. According to the valence of their metal cations, the octahedral sheets can be either dioctahedral sheets involving trivalent cations (Al3+, Fe3+, Cr3+) or trioctahedral sheets holding divalent cations (Mg2+, Fe2+, Ni2+). Thus, montmorillonite and illite serve as dioctahedral clays, while vermiculite is a trioctahedral clay. Interestingly, some replacement in the central metal cations may occur in both tetrahedral and octahedral sheets through isomorphic substitution. Thus, the Si4+ of the tetrahedral sheet may be replaced by Al3+ or Fe3+. Likewise, the Al3+ in the octahedral sheet may be replaced by Mg2+, hence leading to a negative charge on the layer surface. This negative charge can be balanced by exchangeable cations (Na+, K+) present in the interlayer.
Otherwise, the 2:1:1 (T-O-T:O) clay structure type consists of two tetrahedral layers embedded between two octahedral layers. As an example, chlorite clays are arranged in a 2:1:1 (T-O-T:O) structure. They exhibit a 2:1 (T-O-T) structure interspersed with a supplementary brucite-like hydroxide sheet. Additionally, similarly to the smectite structure, chlorite minerals possess an interlayer thickness of 14 Ǻ and have negative charge, which is compensated for by the substitution of metal ions (Al, Mg, Fe, and Si) in each structure of chlorite minerals.
To sum up, each clay mineral exhibits a precise structure that would certainly confer upon it distinctive physicochemical features. Building on the understanding of clay mineral properties and functionalization methods, the following section delves into the different strategies used for immobilizing enzymes onto these versatile supports, highlighting how these methods influence enzymatic activity, reusability, and performance in wastewater treatment applications.
2.3. Properties of Clay Minerals
Clay minerals have been extensively used in many industrial applications thanks to their unique physicochemical, mechanical, and thermal properties [31]. They have a small particle size less than 2 μm and a large specific surface area, increasing their adsorption ability. Based on their layered structure, clay minerals show a cationic exchange capacity [33], which refers to the number of exchangeable cations required to balance the negative charge induced by the isomorphic substitution. Notably, clays of the 2:1 type, namely, smectite and vermiculite, have received keen interest among others thanks to their high CEC (more than 100 meq/100 g, at pH 7, for both smectite and vermiculite), hence underscoring their high reactivity and flexibility. Due to the weak electrostatic attraction between the negatively charged layers and the cations in the interlayer space, water can easily enter in the 2:1 clay mineral structure, consequently leading to an excellent swelling capacity. In fact, the more the interlayer hydration increases, the more significantly the layers expand and are easily exfoliated. As a result, clays of the 2:1 type, mainly smectite clays, exhibit high plasticity, boosting their smooth deformation for ceramic applications [34].
However, in contrast to smectite and vermiculite clays, illite clays belonging to the 2:1 clay class possess totally different properties. Although they have the same structure, an illite differs from the others in its interlayer cations, which are mainly K+ ions. These latter are responsible for the distinct illite clay features. Unlike the exchangeable Ca2+ and Mg2+ cations present in smectite and vermiculite interlayers, the K+ ions are non-exchangeable because of their ionic radius (1.38 Ǻ), relatively close to that of oxygen (1.40 Ǻ), resulting in strong electrostatic attraction between layers. Therefore, illite layers become firmly held together, impeding water interference in the structure. Consequently, these clays have little swelling capacity. In addition, they are non-expandable and less plastic.
Otherwise, compared to 2:1 clays, the 1:1 clays, namely, the kaolinite group, have a lower surface area and a limited CEC (3–15 meq/100 g, at pH 7) due to the lack of or little isomorphic substitution that may occur in its structure, in which tetrahedral and octahedral layers are stacked and tightly interconnected via strong hydrogen bonds. As a result, the kaolinite layers are non-expandable, hindering water absorption. Accordingly, the 1:1 clay does not swell in water. Hence, improving the structure flexibility and adsorption capability is challenging for such clay minerals. Moreover, owing to their rigid structure, kaolinite clays are characterized by their mechanical and thermal stability. Interestingly, once subjected to high temperature, they do not shrink, contrarily to the other clay types that shrink without decomposing or combusting. Thus, it is worth noting that all clay classes show an exceptional heat tolerance, guaranteeing their structural alteration and maintaining mineral composition.
In summary, all these outstanding properties clearly indicate that clay minerals could be prime materials for versatile applications (drug delivery, ceramics, soil conditioning, and wastewater treatment) owing to their high thermal and mechanical stability, arranged structural framework, and surface functionalities, permitting their modification for specific applications [35,36,37].
2.4. Functionalization of Clay Minerals
The functionalization of clay minerals consists of chemical or physical modifications of their surface to enhance their inherent properties or introduce new functionalities for specific applications [38]. Indeed, their distinctive characteristics, including their layered structure, high surface area, cation exchange capacity, and swelling ability, contribute to the reactivity of clay minerals and make them amenable for any modifications [35]. As summarized in Table 1, the functionalization of clay minerals can be conducted by different methods including chemical and thermal activation [39], intercalation [40], pillaring [41], metal oxide deposition [42], and grafting [43].
According to the target functionality, chemical activation consists of the treatment of clay minerals using acids, bases, or surfactants. Acid activation takes place using acid solutions such as hydrochloric acid (HCl), nitric acid (HNO3), and sulfuric acid (H2SO4) [44]. Due to their high cation exchange capacity, chiefly in montmorillonite, cations of the octahedral sheet (Al3+, Mg2+) are exchanged with the proton (H+) of the acid activating agent, leading to the dissolution of clay layers and creation of acid sites [45]. Consequently, the modified clays’ surface area is increased, thus improving their adsorption capacity. The efficacy of such treatment depends on the acidity of the used activating agent [46]. Thus, sulfuric acid has been widely used owing to its high acidity and neutralization affinity after the activation process [37]. For instance, Kangmennaa et al. [47] found that acid activation improved the clay’s adsorption capacity, which enabled significant methylene blue removal (80%) from wastewater. Likewise, in dealing with an Algerian bentonite, the addition of a sulfuric acid modifier enhanced its adsorption capacity for phenolic pollutants (phenol and 4-chlorophenol) and led to their uptake from wastewater (the removal efficiency was more than 95% for both contaminants) [47]. Furthermore, the alkaline activation of clay minerals is conducted using bases like sodium hydroxide (NaOH), potassium hydroxide (KOH), and ammonium hydroxide (NH4OH). These base modifiers carrying hydroxyl (OH) groups allow a high cation exchange with the interlayer cations, mainly Na+, hence resulting in the expansion of the interlayer space and an increase in charge density [48].
Otherwise, clay hydrophobicity could be adjusted using surfactants, mainly quaternary ammonium salts [49]. The surfactant’s cations offer positive charge, counteracting that of clay layers. Consequently, the isomorphic substitution increases and results in the interlayer expanding [49]. Hence, surfactants alter the physicochemical properties of clay minerals, making them hydrophobic and chemically compatible [50]. On the other hand, the thermal activation of clay through heating causes some alterations in its structure through increasing its surface area and pore volume. New active binding sites appear and foster their adsorption capability [39].
Another approach for clay mineral functionalization is the intercalation method. It is based on the insertion of molecules or polymers into the interlayer space of clay minerals [35]. The intercalation process serves as a physical adsorption modifying the physicochemical properties of the clay surface without altering its main structure due to the weak electrostatic van der Waals bonding [51]. For example, despite its rigid and tightly bound structure, the intercalation of polar solvents like hydrazine, urea, and dimethyl sulfoxide (DMSO) between the kaolinite layers reduces their interaction and leads to their expansion, which improves the surface area and promotes an increase in active sites, thus improving the kaolinite’s adsorption capacity [52]. Almost the same features were found for organosilane-modified montmorillonite clay [53]. The interaction of organosilanes in the interlamellar space of montmorillonite clay gives rise to a cube-like structure having an increased interlayer space and exhibiting a high metal-binding efficiency [53].
In addition, along with the intercalation of organic/inorganic polymers between their layers, clay minerals can be functionalized through a pillaring process which aims to improve the thermal and mechanical properties of a clay mineral [41]. The intercalation of a pillaring agent such as poly(hydroxo) metal cations into the interlayer space of clay minerals results in a thermally stable, bidimensional, and mesoporous clay material having an interlayer region separating its parent layers [54]. The efficiency of pillaring mainly depends on the characteristics of the host clay, the pillaring agent, and the experimental conditions [41]. In fact, if the host clay possesses a large surface area, and a high surface charge and CEC, the intercalation of the pillaring agent occurs easily by cation exchange, leading to the formation of chemically and thermally stable pillared clay [55]. Thus, it is worth noting that most of the host clays investigated were smectite clays. In this respect, Liu et al. [56] had successfully prepared an iron-pillared montmorillonite using the iron oxides as a pillaring agent obtained after an appropriate treatment of ferric nitrate [52]. Based on their novel physicochemical properties, pillared clays could act as adsorbents, thermal insulators, and membranes [41].
Additionally, besides the use of polyoxycations as pillaring agents, another method for clays’ modification consists of coating with metal oxides (TiO2, ZnO, Fe3O4) on their surfaces [42]. This process improves the properties and photocatalytic activity of the metal oxide-based photocatalysts and opens the way for clays’ functionalization. As demonstrated in the study of Rapsomanikis et al. [57], halloysite-supported TiO2 nanoparticles form a mesoporous TiO2-halloysite film having an enhanced photocatalytic activity for azo dye removal.
Furthermore, grafting is the most widely used approach to modify the inherent characteristics of clay minerals. It refers to the attachment of organic, inorganic, or biological compounds onto the surface of clay minerals [58]. Due to their functional groups (hydroxyl (OH), amine (NH2), and carboxylic (COOH)), the polymers interact with the clay surface molecules via covalent bonding. For example, the grafting of silylating agents onto the montmorillonite clay surface has been widely studied [59]. Once intercalated into the surface, the silane molecules react at the broken edges and at the interlayer clay surfaces. As a result, they exhibited a parallel bilayer arrangement in montmorillonite clay [59]. Hence, the grafting technique modified the clay’s structural properties via broadening its interlamellar space to enhance its compatibility with other polymers and increase its adsorption capacity [60]. In addition, grafting kaolinite with a methoxy group modified its mechanical properties [61]. It became softer and more easily delaminated than the native kaolinite clay [61].
Interestingly, out of all the clay functionalization methods, the grafting process has received great attention among researchers owing to its versatile applications in adsorption, catalysis, and drug delivery [62]. Moreso, unlike the other techniques of clay modification, the grafting process can be adapted for all clay types and shows significant biocompatibility for all biopolymers such as drugs, proteins, and enzymes.
In fact, clay minerals have emerged as exceptional support for enzyme immobilization in wastewater treatment applications. Their unique layered aluminosilicate structure, exemplified by montmorillonite (specific surface area: 50–800 m2/g) and kaolinite (10–30 m2/g), provides abundant binding sites through cation exchange capacities ranging from 10 to 150 meq/100g [63,64]. These natural materials demonstrate remarkable thermal stability (maintaining their structure up to 300 °C) and pH tolerance (functional from pH 2 to 12), making them robust platforms for enzymatic processes in variable wastewater conditions [64].

Table 1.
Functionalization methods for clay minerals.
Table 1.
Functionalization methods for clay minerals.
| Method | Principle | Aims | Applications | References |
|---|---|---|---|---|
| Chemical Activation | Treatment with acids (HCl, H2SO4), bases (NaOH, NH4OH), or surfactants (quaternary ammonium salts) |
|
| [37,44,45,46,47,48,49,50] |
| Thermal Activation | Heating clays (generally at 300–800 °C) |
| Improved adsorption of organic pollutants | [39] |
| Intercalation | Insertion of polar solvents (DMSO, urea) or polymers into interlayers |
| Enhanced dye and heavy metals adsorption | [40,51,52,53] |
| Pillaring | Insertion of poly(hydroxo) metal cations (Fe3+, Al3+) to create stable pillars |
| Acts as adsorbents, thermal insulators and membranes | [41,54,55,56,57,58] |
| Metal Oxide Coating | Deposition of TiO2, ZnO, and Fe2O3, on clay surfaces |
| Significant azo dyes degradation | [42,57] |
| Grafting | Covalent bonding of silanes or polymers (-OH, NH2 groups) to clay surfaces |
| Versatile applications in adsorption, catalysis and drug delivery | [43,58,62] |
| Enzyme Immobilization | Clay as a support for enzymes (laccase, peroxidase) |
| Removal of diverse wastewater pollutants (dyes, heavy metals and pharmaceuticals) | [63,64] |
3. Enzyme Immobilization on Clay Minerals
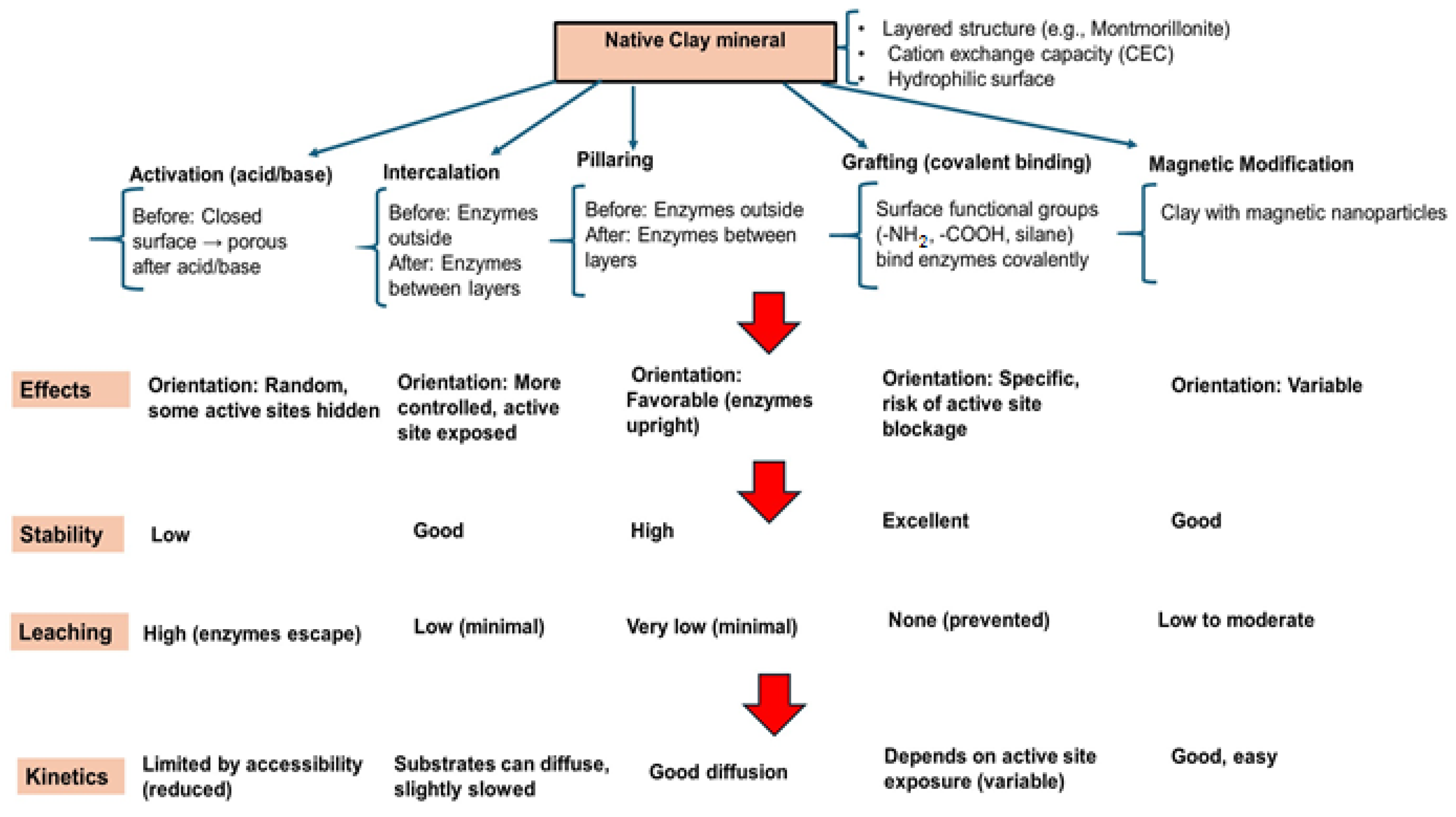
Enzyme immobilization on clay minerals is not only a method for physically anchoring enzymes but also a strategic approach for enhancing catalytic performance, prolonging operational life, and enabling recovery and reuse in wastewater treatment. Compared to free enzymes, immobilized forms can exhibit up to five-fold-higher stability and retain more than 70% of their activity after 10 operational cycles [65]. The high surface area, layered structure, and ion-exchange capacity of clays create abundant binding sites and protective microenvironments that improve the enzyme’s resistance to denaturation, enabling 2–3 times greater stability in organic solvents and under extreme pH or temperature conditions [66]. Immobilized enzyme–clay systems operate via a dual-action mechanism: (i) the enzymatic transformation of organic pollutants through degradation, oxidation, or hydrolysis into less toxic or more biodegradable forms, and (ii) the clay-mediated removal of pollutants and reaction intermediates, including heavy metals, via adsorption, ion exchange, or complexation. The choice of immobilization strategy must balance stability, catalytic activity, mass transfer efficiency, and reusability depending on the application. A schematic overview of the principal immobilization routes and their characteristics is provided in Figure 3.
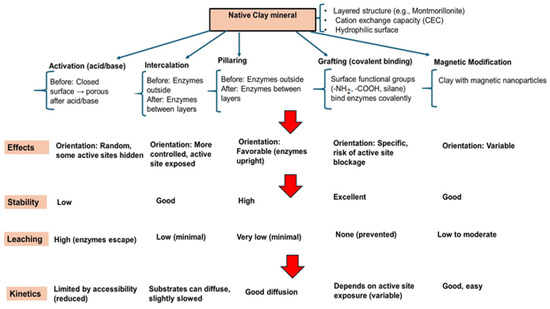
Figure 3.
Comparative schematic of common methods for enzyme immobilization on clay minerals and their functional outcomes.
3.1. Methods of Enzyme Immobilization on Clay Minerals
The immobilization of enzymes on clay minerals can be achieved through several strategies, each influencing the catalytic activity, stability, and reusability of the enzyme–clay complexes. The choice of method depends on the nature of the enzyme, the type of clay mineral, and the intended application in wastewater treatment or biocatalysis. The most common methods involve physical adsorption, covalent bonding immobilization, entrapment in clay gel matrices, crosslinking with a clay support, and intercalation in layered clays.
3.1.1. Physical Adsorption (Non-Covalent Immobilization)
Physical adsorption is the simplest and most widely used technique for enzyme immobilization on clay minerals. It relies on weak, non-covalent interactions such as van der Waals forces, hydrogen bonding, and electrostatic attractions between the enzyme and the surface of the clay. Montmorillonite, due to its high specific surface area and negatively charged layers, is especially effective in adsorbing positively charged enzyme molecules under appropriate pH conditions. Enzyme immobilization is optimized at pH 4–5 for laccase (pI~4.2) to align with montmorillonite’s negative layers [23]. This method offers several advantages: it is simple to implement, is cost-effective, and does not require chemical modification of either the enzyme or the support material. However, the primary disadvantage lies in the weak binding forces involved, which can result in enzyme leaching during repeated use, thereby reducing long-term operational stability. A practical example is the immobilization of laccase on bentonite, which demonstrated retained catalytic activity over five cycles [67] and showed good efficiency in the removal of dyes from wastewater.
3.1.2. Covalent Bonding Immobilization
In this method, enzymes are chemically bonded to functionalized clay surfaces through covalent interactions, offering a more stable and durable immobilization compared to physical adsorption. This approach typically involves the surface modification of the clay using coupling agents such as glutaraldehyde, silanes, or carbodiimide reagents, which facilitate the formation of covalent bonds between functional groups on the clay and reactive sites on the enzyme. Interestingly, glutaraldehyde crosslinks lysine residues (–NH2) to clay hydroxyl groups (–OH), while silane grafting forms covalent Si–O–Si bonds [68]. Covalent attachment greatly enhances enzyme retention and operational stability, particularly under harsh environmental conditions such as extreme pH or temperature. The main advantage of this method is the strong binding that effectively prevents enzyme leaching, thereby ensuring consistent catalytic performance over multiple uses. However, a potential drawback is that the chemical bonding process may alter the enzyme’s conformation, potentially leading to partial loss of its catalytic activity. According to Sheldon and van Pelt [68], covalent immobilization is widely used for its robustness and is especially advantageous when long-term operational stability is required in industrial or environmental applications. Among these techniques, covalent bonding stands out when enzyme retention is critical under industrial conditions. However, softer approaches like entrapment may be preferable when preserving enzyme conformation is essential.
3.1.3. Entrapment in Clay Gel Matrices
Entrapment involves encapsulating the enzyme within a clay–polymer matrix, typically using materials such as alginate or polyacrylamide blended with clay minerals. In this technique, the enzyme is physically confined within the matrix without forming direct chemical bonds with the clay, allowing for the diffusion of substrates and products while maintaining a stable and protective environment for the enzyme. One of the main advantages of entrapment is its ability to minimize enzyme leaching, which contributes to improved reusability and prolonged activity. Additionally, the encapsulating matrix offers protection against environmental stressors such as pH fluctuations and extreme temperatures. However, this method may also present certain limitations, particularly related to mass transfer, as the matrix can restrict substrate access to the active sites of the enzyme. Therefore, optimizing the porosity and composition of the matrix is often necessary to enhance performance. A representative example is the entrapment of crosslinked cellulose colloids in alginate beads, which improved cellulose hydrolysis and maintained high activity over repeated use [69].
3.1.4. Crosslinking with Clay Supports
Crosslinking involves the use of bifunctional reagents, such as glutaraldehyde, to form intermolecular bridges between enzyme molecules and the clay support, resulting in a more rigid and stable immobilized system. This method is frequently employed in combination with other techniques like physical adsorption or covalent bonding to enhance the overall mechanical strength and operational stability of the enzyme–clay complex. One of the key advantages of crosslinking is its ability to improve the durability of the immobilized enzymes, making them more resistant to denaturation and degradation under harsh environmental conditions, such as high temperatures or extreme pH. However, a potential drawback of this method is the risk of enzyme inactivation, as the crosslinking reagents may alter the enzyme’s active conformation or obstruct access to its catalytic site. A notable example is the immobilization of laccase on bentonite via glutaraldehyde-mediated crosslinking, which retained approximately 75% of its initial activity even after 40 consecutive uses and demonstrated strong storage stability over 30 days at 4 °C [70].
3.1.5. Intercalation in Layered Clays
In this technique, enzymes are inserted into the interlayer spaces of expandable clay minerals such as montmorillonite, a process known as intercalation. By confining the enzyme molecules within the layered structure of the clay, this method offers enhanced protection against denaturing agents, including organic solvents, high temperatures, and extreme pH levels. The spatial confinement helps maintain the enzyme’s native conformation, thereby increasing its stability and longevity during repeated use. One of the primary advantages of intercalation is the high degree of stability it imparts to the immobilized enzyme, along with effective shielding from harsh environmental factors. However, the technique is inherently limited to relatively small enzymes that can fit within the narrow interlayer spacing of the clay, which may restrict its broader applicability. Generally, enzymes with a molecular weight lower than 50 kDa require clay interlayer expansion via pillaring for intercalation [71]. A well-documented example involves the intercalation of α-amylase, glucoamylase, and invertase into acid-activated montmorillonite K10. The successful intercalation of all three enzymes was confirmed by X-ray diffraction (XRD), with the immobilized forms exhibiting significantly higher activity and storage stability compared to their free counterparts, even after prolonged storage or continuous reactor operation. For instance, XRD analysis of α-amylase-intercalated montmorillonite revealed an expansion in the d-spacing (from 12 Å to 18 Å), while FTIR spectroscopy demonstrated preserved amide I and II bands (1650 cm−1 and 1540 cm−1, respectively), indicating minimal conformational changes in the enzyme structure [71].
3.2. Comparative Analysis of Immobilization Techniques
Five principal immobilization techniques have been employed for clay–enzyme composites, each with distinct advantages and limitations (Table 2). Physical adsorption, while operationally simple, typically shows moderate enzyme retention (50%–70% over five cycles) due to leaching, making it cost-effective (5–10 USD/g) for batch dye treatment but unsuitable for continuous systems [67,72]. Covalent approaches, particularly glutaraldehyde crosslinking, achieve superior retention (>80% after 15 cycles) through stable amine bonding, though the higher costs (20–50 USD/g) and occasional activity reduction (15%–40%) may limit scalability [33,71]. Notably, intercalation demonstrates exceptional stability (>90% retention) by shielding enzymes within clay layers, yet its very high cost (>80 USD/g) and size restrictions (<50 kDa enzymes) constrain widespread adoption [23,71]. For most industrial applications, hybrid adsorption–crosslinking systems offer the optimal balance, maintaining 70%–90% activity over at least 10 cycles at moderate cost (15–30 USD/g), particularly for complex wastewater streams containing multiple pollutant classes [70,73].

Table 2.
Performance metrics and cost analysis of clay-based enzyme immobilization methods under operational conditions.
4. Applications of Enzymes Immobilized on Clay Minerals for Pollutant Removal from Wastewater
Enzymes immobilized on clay minerals have been widely studied for their potential to degrade and remove a broad range of wastewater pollutants. Their enhanced stability, reusability, and tolerance to harsh environmental conditions make them promising tools for sustainable treatment. In the present section, applications are organized by pollutant category (dyes, pharmaceuticals and personal care products, heavy metals), highlighting the most effective enzyme–clay combinations and their relevant immobilization approaches.
4.1. Removal of Dyes
Enzyme–clay composites have been extensively investigated for the removal of synthetic dyes from wastewater (Table 3). For example, laccase immobilized on bentonite by adsorption removed 86% of azo dyes [74], while adsorption on montmorillonite achieved 90% degradation of anthraquinone dyes [72]. Intercalated laccase in modified clays removed 88% of methylene blue [67], and peroxidase covalently bound to kaolin showed 81% and 91% degradation of Congo red and reactive black dyes, respectively [33]. Laccase immobilized on activated clay demonstrated 85% removal efficiency for brilliant blue dye [65], while crosslinking on montmorillonite enabled 87% removal of RB5 dye with enhanced stability [73]. Other studies reported 84% removal of acid yellow dye by laccase covalently attached to kaolinite [75] and 82% removal of navy-blue dye using peroxidase entrapped in nanoclay [33].
These findings highlight that adsorption and covalent immobilization are among the most efficient strategies for dye degradation. Adsorption methods are operationally simple and cost-effective but often suffer from enzyme leaching during repeated use, whereas covalent bonding ensures stronger stability under harsh conditions but may cause partial enzyme inactivation [63]. Smectite clays such as montmorillonite and bentonite generally outperform kaolinite due to their higher cation exchange capacity and expandable interlayer spacing, which enhance enzyme stabilization [23,64]. Crosslinking and intercalation approaches provide improved reusability and long-term activity, suggesting that hybrid systems combining adsorption with crosslinking could achieve a balance between efficiency and cost [70,74]. Importantly, clays can adsorb toxic aromatic amines generated during azo dye degradation, reducing bioavailability and environmental risks [72,76]. However, further studies on byproduct fate are required for large-scale applications.
4.1.1. Degradation of Pharmaceuticals and Personal Care Products (PPCPs)
Immobilized enzymes on clay supports have also been applied to the degradation of pharmaceuticals and personal care products (PPCPs) (Table 4). Laccase immobilized on hydrothermally treated stevensite removed 95% of tetracycline within 8 h, retaining 80% activity after 10 cycles [73]. Laccase entrapped in alginate–montmorillonite beads removed 78% of ibuprofen, showing stability across pH 2–12 without enzyme leaching [77]. Peroxidase covalently bound to organobentonite degraded 88% of 17β-estradiol in a continuous flow system [78], while laccase immobilized on Fe3O4@clay nanoparticles achieved 85% removal of diclofenac, with 75% activity retained after 15 cycles [74]. Other reported systems include kaolin–chitosan–peroxidase hybrids degrading propranolol (82%) [79], laccase in layered double hydroxides degrading carbamazepine (70%) [23], and peroxidase immobilized on graphene–clay composites achieving 91% triclosan removal [79].
The performance of these systems indicates that clay functionalization plays a crucial role in enhancing the enzymatic degradation of pharmaceuticals. Hydrothermally treated clays (stevensite) provide larger surface areas and improved enzyme–pollutant interactions, while hybrid and magnetic composites improve recyclability and operational stability [73,74]. Differences in degradation efficiency across compounds reflect enzyme substrate specificity and pollutant recalcitrance (e.g., carbamazepine vs. tetracycline) [23,78]. Continuous-flow bioreactor studies with immobilized enzymes demonstrate the feasibility of scaling up [78], though most systems remain at laboratory scale. Future research should focus on testing in real wastewater matrices to account for complex pollutant interactions and operational challenges.
4.1.2. Heavy Metal Detoxification
Although enzymes do not directly degrade metals, enzyme–clay composites exhibit high efficiency in heavy metal removal primarily due to the sorption capacity of clays (Table 5). The enzyme provides synergistic value by degrading organic co-pollutants that compete for sorption sites and, for specific metals, facilitating detoxification via redox transformations. Fe3O4@bentonite–laccase composites achieved 95% removal of Pb2+ in 2 h [80]. Thiol-modified clay–peroxidase systems reached a 98% removal efficiency for Hg2+ through strong chelation [81]. Kaolinite–cellulase complexes eliminated 80% of Cd2+ [14], while clay–chitosan–laccase systems reduced Cr6+ to Cr3+ with 88% efficiency [82]. Iron oxide–clay–peroxidase composites oxidized As3+ to As5+ with 85% removal efficiency [61], and organoclay–lipase removed 75% of Cu2+ [73].
These results illustrate the synergistic role of clay matrices and immobilized enzymes. While clay minerals adsorb and sequester metal ions, enzymes can facilitate redox transformations for certain metals, enhancing detoxification [61,75]. Functionalized clays (e.g., thiol, chitosan, Fe3O4) demonstrate superior affinity for toxic metals, reinforcing the importance of surface modification [37,82]. Moreover, the dual functionality of these composites, removing organic pollutants enzymatically and inorganic metals via adsorption, makes them particularly effective for complex industrial effluents [74]. However, concerns remain about the possible leaching of structural cations (Fe3+, Al3+) from clays under acidic conditions, which requires careful monitoring during practical applications [80].

Table 3.
Dye removal by enzymes immobilized on clay mineral.
Table 3.
Dye removal by enzymes immobilized on clay mineral.
| Dye | Enzyme/Support | Immobilization Method | Removal Effciency (%) | Initial Concentration (mg/L) | Enzyme Units/Loading | pH | Temperature (°C) | Mediator | Reuse Cycles/Leaching | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Azo | Laccase/Bentonite | Adsorption | 86 | 50 | NR | 5 | 25 | ABTS | 5 cycles (70% retained) | [74] |
| Anthraquinone | Laccase/Montmorillonite | Adsorption | 90 | 100 | 0.8 mg/g | 6 | 30 | NR | 7 cycles (80% retained) | [72] |
| Methylene Blue | Laccase/Modified Clay | Intercalation | 88 | 25 | 1.2 mg/g | 4.5 | 25 | HOBT | NR | [67] |
| Congo Red | Peroxidase/Kaolin | Covalent | 89 | 100 | NR | 7 | 30 | H2O2 | 10 cycles (85% retained) | [33] |
| Reactive Black | Peroxidase/Kaolin | Adsorption | 91 | 200 | NR | 7 | 25 | H2O2 | NR | [33] |
| Brilliant Blue | Laccase/Activated Clay | Adsorption | 85 | NR | NR | 5 | 25 | NR | 6 cycles (75% retained) | [70] |
| Blue RB5 | Laccase/Montmorillonite | Crosslinking | 87 | 100 | NR | 6 | 25 | NR | 8 cycles (82% retained) | [74] |
| Acid Yellow | Laccase/Kaolinite | Covalent | 84 | 50 | NR | 5 | 30 | ABTS | NR | [76] |
| Navy Blue | Peroxidase/Nanoclay | Entrapment | 82 | NR | NR | 6 | 25 | H2O2 | 4 cycles (65% retained) | [33] |
| Fuchsine Pink | Laccase/Bentonite | Adsorption | 89 | 80 | 0.6 mg/g | 6 | 25 | NR | 5 cycles (70% retained) | [70] |
NR: Not Reported.

Table 4.
PPCP removal by enzymes immobilized on clay mineral.
Table 4.
PPCP removal by enzymes immobilized on clay mineral.
| PPCP Category | Enzyme/Clay System | Immobilization Method | Removal Efficiency (%) | Time (h) | Initial Conc. (mg/L) | Enzyme Units/Loading | pH | Temperature (°C) | Mediator | Reuse Cycles/Leaching | Key Findings | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotics (Tetracycline) | Laccase/Stevensite-Clay | Hydrothermal | 95 | 8 | NR | NR | 3–9 | 25 | NR | 10 cycles (80% retained) | Stable over wide pH range; high reusability | [73] |
| NSAIDs (Ibuprofen) | Laccase/Alginate–Montmorillonite | Entrapment | 78 | 12 | NR | NR | 2–12 | 25–30 | NR | No leaching observed | Broad pH tolerance; good stability | [77] |
| Hormones (17β-Estradiol) | Peroxidase/Organobentonite | Covalent (Glutaraldehyde) | 88 | 6 | NR | NR | NR | 30 | NR | NR | 90% degradation in continuous flow reactor | [78] |
| Analgesics (Diclofenac) | Laccase/Fe3O4@Clay | Magnetic | 85 | 8 | NR | NR | NR | 25 | NR | 15 cycles (75% retained) | Easy recovery due to magnetism | [74] |
| β-Blockers (Propranolol) | Peroxidase/Kaolin–Chitosan | Hybrid | 82 | 10 | NR | NR | NR | 25 | NR | NR | Chitosan enhances adsorption | [79] |
| Antidepressants (Carbamazepine) | Laccase/Layered Double Hydroxide | Intercalation | 70 | 24 | NR | NR | NR | 25 | NR | Stable across matrices | Slow degradation, good matrix tolerance | [23] |
| Antimicrobials (Triclosan) | Peroxidase/Clay–Graphene | Composite | 91 | 5 | NR | NR | NR | 25–30 | NR | NR | Strong synergistic oxidation–adsorption effect | [82] |
| X-ray Contrast Media | Laccase/Porous Si–Al Clay | Entrapment | 65 | 48 | NR | NR | NR | 25 | NR | NR | Effective for persistent compounds | [78] |
| Stimulants (Caffeine) | Laccase/Bentonite | Adsorption | 75 | 6 | NR | NR | NR | 25 | NR | 30% enzyme leaching | Cost-effective but leaching issue | [73] |
| Antiepileptics (Gabapentin) | Cellulase/Kaolinite | Physical | 60 | 18 | NR | NR | NR | 25 | NR | NR | Low efficiency but scalable | [14] |
NR: Not Reported.

Table 5.
Heavy metal detoxification process using enzymes immobilized on clay mineral.
Table 5.
Heavy metal detoxification process using enzymes immobilized on clay mineral.
| Heavy Metal | Clay–Enzyme System | Mechanism/Immobilization Method | Removal Efficiency (%) | Time (h) | Capacity (mg/g) | Initial Conc. (mg/L) | Enzyme Units/Loading | Immobilization Yield (%) | pH | Temperature (°C) | Mediator | Reuse Cycles/Leaching | Key Advantages | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pb2+ | Fe3O4@Bentonite–Laccase | Adsorption + Redox (Magnetic) | 95 | 2 | 180 | NR | NR | NR | 6–7 | 25 | NR | 6 cycles (80% retained) | Easy magnetic recovery | [83] |
| Hg2+ | Thiol–Modified Clay–Peroxidase | Chelation | 98 | 3 | 210 | NR | NR | NR | 6–8 | 25–30 | NR | NR | Ultra-high affinity via thiol groups | [81] |
| Cd2+ | Kaolinite–Cellulase | Adsorption | 80 | 5 | 95 | NR | NR | NR | 7 | 25 | NR | NR | Removes both organics and metals | [14] |
| Cr6+ | Clay–Chitosan–Laccase | Reduction to Cr3+ (Covalent/Hybrid) | 88 | 4 | 150 | NR | NR | NR | 3–5 | 30 | H2O2 | 8 cycles (75% retained) | Chitosan enhances binding + redox | [78] |
| As3+ | Iron Oxide–Clay–Peroxidase | Oxidation to As5+ (Redox) | 85 | 6 | 120 | NR | NR | NR | 7–8 | 25–30 | NR | 5 cycles (70% retained) | Detoxification via oxidation | [66] |
| Cu2+ | Organoclay–Lipase | Adsorption | 75 | 8 | 80 | NR | NR | NR | NR | NR | NR | NR | Low-cost sorbent | [80] |
| Zn2+ | Montmorillonite–Amylase | Ion Exchange | 70 | 10 | 65 | NR | NR | NR | 6–7 | 25 | NR | NR | Works under mild conditions | [71] |
| Ni2+ | Porous Si–Al Clay–Laccase | Adsorption | 82 | 7 | 110 | NR | NR | NR | 6 | 25 | NR | NR | High surface area | [78] |
| Co2+ | Graphene–Clay–Peroxidase | Adsorption + Redox | 90 | 5 | 170 | NR | NR | NR | 6–8 | 25–30 | NR | NR | Enhanced conductivity | [82] |
| Mn2+ | Biochar–Clay–Cellulase | Adsorption | 78 | 9 | 85 | NR | NR | NR | 6–7 | 25 | NR | NR | Eco-friendly composite | [79] |
NR: Not Reported.
4.2. Advantages of Clay Mineral Supports in Enzyme Applications
Clay minerals offer several unique advantages as support for enzyme immobilization, particularly in wastewater treatment applications. Their high enzyme-loading capacity and stable immobilization are attributed to their large specific surface area, layered structure, and strong cation exchange capacity (CEC). For instance, smectites (CEC: 80–150 meq/100 g) demonstrate superior laccase loading (0.8 mg/g) compared to kaolinite (CEC: 3–15 meq/100 g; 0.2 mg/g) due to enhanced electrostatic interactions. Additionally, montmorillonite’s expandable interlayer spacing (~14 Å) accommodates and stabilizes enzyme conformation, whereas the rigid structure of kaolinite (~7 Å) may impose spatial constraints, particularly for larger enzymes (>50 kDa) [21]. Interestingly, clay minerals are naturally abundant, low-cost, and chemically stable under a wide range of environmental conditions. These properties make them ideal for industrial-scale use, especially in low-resource or decentralized wastewater treatment facilities.
One of the main benefits of enzyme immobilization on clays is the enhanced operational stability of the biocatalyst. Immobilized enzymes are more resistant to thermal and chemical denaturation, allowing them to maintain activity across a broad pH range and under exposure to inhibitors typically present in complex wastewater matrices. Furthermore, clay-based systems facilitate the easier separation and recovery of enzymes from reaction mixtures, reducing downstream processing costs and supporting multiple reuse cycles. The incorporation of magnetic components, such as Fe3O4 nanoparticles, into clay structures improves the recyclability of enzyme supports, enabling magnetic recovery with minimal activity loss [75,79]. Hybrid clay composites that combine clay with graphene, chitosan, or carbon nanotubes further enhance mechanical strength, increase enzyme–substrate interactions, and improve pollutant removal efficiency.
Importantly, clay minerals also possess intrinsic adsorption capabilities for various organic and inorganic contaminants. This dual functionality, combining enzymatic degradation and physical adsorption, makes them particularly effective for treating complex effluents containing both persistent organic pollutants and heavy metals.
4.3. Fate and Toxicity of Enzymatic Degradation Byproducts
Enzyme–clay composites achieve high pollutant degradation efficiencies, but the formation and fate of intermediate byproducts remain a critical concern. The laccase-catalyzed breakdown of azo dyes such as Congo Red generates aromatic amines, some of which exhibit carcinogenic potential. Thanks to their adsorptive capacity, clay minerals—particularly montmorillonite, with a high cation exchange capacity—can effectively sequester these intermediates, reducing their bioavailability and toxicity [67,84]. In pharmaceutical transformation, the peroxidase-mediated oxidation of tetracycline produces low-toxicity oligomers through radical coupling. However, the incomplete degradation of endocrine disruptors (e.g., bisphenol A) may lead to persistent quinoid structures that require further treatment [69,83]. Recent studies indicate that these quinones can also be adsorbed by clay matrices, although their long-term stability is still under investigation. In the case of heavy metals, immobilized laccase supports chelation processes, while acidic conditions (pH < 4) can promote the leaching of structural ions such as Fe3+ and Al3+ from clay lattices [80]. These findings highlight both the promise and the challenges of enzymatic wastewater treatment. While clay minerals mitigate toxicity by adsorbing harmful intermediates, the risk of persistent or mobile byproducts underscores the need for further optimization. The dual functionality of clay–enzyme systems involving the simultaneous immobilization of metals and potential release of structural ions demands the careful control of operational conditions, especially pH. To ensure environmental safety, several research gaps must be addressed: (i) the comprehensive identification of transformation products using advanced analytical techniques (e.g., LC-QTOF-MS), (ii) long-term ecotoxicity assessments of residual byproducts, and (iii) the evaluation of byproduct fate in continuous-flow systems. Hybrid approaches that integrate enzyme–clay composites with advanced oxidation processes (e.g., enzyme–clay–ozonation) appear particularly promising for achieving the complete mineralization of recalcitrant intermediates [14,23]
5. Challenges and Future Perspectives in Enzyme Immobilization on Clay Minerals for Wastewater Treatment
Despite the numerous advantages of clay-based enzyme systems, several challenges must be overcome to fully realize their potential in practical wastewater treatment applications. One major limitation is the partial loss of enzyme activity during or after immobilization. Techniques involving covalent bonding or chemical modification of the support surface can lead to conformational changes in the enzyme, thereby reducing its catalytic performance [72]. Conversely, physically adsorbed enzymes often suffer from desorption or leaching during repeated operation, especially under fluctuating pH or ionic strength conditions typical of real wastewater environments [85]. Mass transfer resistance is another critical issue, particularly in densely loaded or poorly porous systems. The diffusion of substrates into the clay matrix and access to active sites may be hindered, which limits reaction efficiency. Additionally, natural clays can show batch-to-batch variability in mineral composition, porosity, and surface chemistry, leading to inconsistencies in immobilization efficiency and catalytic behavior. The regeneration and reusability of enzyme–clay composites remain challenging due to inevitable activity losses during recovery processes. While mild buffer washing preserves over 80% of the enzymatic activity, it significantly extends the processing time by requiring twice as many cycles compared to harsher methods. In contrast, more aggressive approaches like 0.1 M HCl washing leach 40%–50% of adsorbed enzymes, and thermal regeneration above 60 °C denatures nearly 70% of the enzymatic activity [65]. Although clay supports themselves are inherently reusable, repeated exposure to chemical or thermal regeneration gradually degrades both the structural integrity of the clay matrix and the stability of the immobilized enzymes. This cumulative damage ultimately reduces the composite’s operational lifespan, highlighting the need to balance regeneration efficiency with long-term stability for sustainable applications. To address these challenges, future research should focus on developing advanced functionalized clays and hybrid materials that improve enzyme binding strength while preserving activity. Surface grafting with specific linkers, co-immobilization strategies, or the inclusion of biocompatible polymers can help optimize enzyme orientation and reduce activity loss. Magnetic nanoclay systems, graphene–clay composites, and chitosan-modified supports are promising next-generation materials with enhanced mechanical and catalytic performance. Furthermore, testing with real wastewater is essential to investigate pollutant interactions and properly assess the performance of clay mineral-immobilized enzymes under practical conditions. To enhance depollution performance, the integration of clay–enzyme bioreactors with complementary treatment technologies such as membrane filtration, electrochemical oxidation, or photocatalysis could improve overall removal efficiency for a wider range of pollutants. Critical to these hybrid systems will be maintaining clay support integrity, as smectite-based matrices typically withstand 15–20 cycles before showing structural degradation, while kaolinite maintains stability for more than 50 cycles [44,56,64]. The standardized monitoring of clay crystallinity (by XRD) and cation exchange capacity retention should accompany performance evaluations. Finally, for successful real-world implementation, large-scale field trials using actual municipal and industrial wastewater are essential to rigorously evaluate long-term stability, operational performance, and techno-economic viability before full-scale deployment. For example, Rodríguez-Couto [80] demonstrated that immobilized laccase in a packed-bed reactor removed 60%–67% of Bisphenol A (200 mg/L) from industrial wastewater in both batch and continuous modes. Similarly, another study tested an immobilized laccase-mediator system in a packed-bed reactor for treating real textile effluent in continuous operation [78]. However, the reactor volume was limited, highlighting the need for larger-scale studies to assess the practical feasibility of this approach. Moreover, comprehensive lifecycle assessments and cost–benefit analyses are needed to validate their economic viability against conventional methods. Achieving cost parity remains critical, as hybrid clays must become more competitive with widely used alternatives like activated carbon (1–5 USD/g) [10]. Policy interventions, such as tax motivations for bio-based water treatments, could help bridge this gap while promoting sustainable solutions. Pilot projects in industrial zones can prove the technology’s effectiveness and speed up adoption. Collaborative frameworks involving governments, industries, and researchers are essential to accelerate justifiable deployment, ensuring alignment with sustainable development goals for water management and public health protection.
Beyond laboratory-scale demonstrations, a major challenge lies in translating these systems into practical wastewater treatment technologies, which requires the careful consideration of reactor design and operational parameters.
Design Considerations for Bioreactor Scale-Up
Transitioning enzyme–clay composites from batch experiments to industrial wastewater treatment requires careful bioreactor design, addressing inherent challenges in mass transfer, fouling, and long-term stability. Reactor Configuration: While batch reactors are ideal for initial proof-of-concept studies, continuous-flow systems are essential for scale-up. Packed-bed reactors (PBRs), where the clay composite is fixed in a column, offer high catalyst loading and efficient contact time, making them suitable for low-turbidity effluents [86]. However, they are highly susceptible to clogging from suspended solids. In contrast, fluidized-bed reactors (FBRs), where the composite is kept suspended by the upward flow of wastewater, minimize fouling and are ideal for treating complex waste streams or for use with magnetic composites (e.g., Fe3O4@clay-enzyme) that can be easily recovered and fluidized [87]. Addressing Mass Transfer and Fouling: The layered structure of clays, while beneficial for adsorption, can impose diffusional limitations on substrate access to the immobilized enzyme [23]. Optimizing the clay particle size (e.g., using larger granules to reduce the pressure drop or finer particles to increase the surface area) is a critical design trade-off. Furthermore, fouling leading to the blocking of active sites and pores by organic/inorganic matter is a primary concern for long-term operation [88]. This can be mitigated by employing robust pre-treatment steps (e.g., screening, coagulation) and by designing composites with anti-fouling surface coatings, such as grafting hydrophilic polymers like polyethylene glycol (PEG) to create a hydration barrier [89]. Operational Stability and Regeneration: The economic viability of the process hinges on the composite’s operational lifespan. As demonstrated in Table 2, the immobilization method directly dictates stability; covalent bonding is often preferred for continuous systems due to its superior resistance to leaching [71]. Implementing pH and temperature control units before the reactor inlet is crucial to maintain enzymatic activity within the optimal range for the specific enzyme–clay system [90]. Furthermore, protocols for in situ regeneration must be developed. This could involve periodic back-flushing with buffer or mild solvents to desorb recalcitrant pollutants and foulants from the clay surface without denaturing the enzyme, thereby restoring catalytic activity [91]. Hybrid Systems for Enhanced Performance: For treating real wastewater containing diverse pollutant classes, standalone enzymatic treatment may be insufficient. Integrating enzyme–clay bioreactors with complementary technologies presents a robust solution. A pre-treatment membrane filtration step can remove solids to prevent fouling of the subsequent bioreactor [92]. Post-treatment with advanced oxidation processes (AOPs) can mineralize recalcitrant intermediates generated by enzymatic action [15]. Furthermore, as explored in this review, the inherent properties of magnetic composites facilitate easy catalyst recovery and reuse, significantly improving process economics [73]. In conclusion, successful scale-up will not rely on a single breakthrough but on a systems engineering approach that optimizes reactor choice, mitigates mass transfer issues, implements clever regeneration strategies, and embraces hybrid technology integration. Pilot-scale studies using real industrial effluent are the next essential step to validate these design considerations and conduct rigorous techno-economic analyses [85].
6. Conclusions
The immobilization of enzymes onto clay minerals offers a sustainable and versatile strategy for wastewater treatment, effectively overcoming the inherent limitations of free enzymes. Utilizing various techniques such as adsorption, covalent bonding, and intercalation, clay minerals significantly enhance enzyme stability, catalytic activity, and reusability across diverse environmental conditions. Their intrinsic characteristics, high surface area, ion exchange capacity, and thermal stability make them ideal supports for biocatalytic applications.
As evidenced by studies on a wide range of pollutants including dyes, pharmaceuticals, and heavy metals, enzyme–clay composites consistently outperform free enzymes by combining catalytic degradation with adsorption capabilities. Progress in functionalization methods and the development of hybrid materials (such as magnetic, polymeric, and carbon-based composites) further broaden their applicability and improve operational durability.
However, challenges remain, notably enzyme leaching, variability in clay structure, and mass transfer limitations. Future efforts should focus on designing advanced, multifunctional clay supports with tailored surface chemistries, improved enzyme-loading efficiencies, and seamless integration into scalable bioreactor systems. Additionally, real-world validation under continuous flow conditions and comprehensive techno-economic analyses are essential to facilitate industrial adoption.
In summary, enzyme immobilization on clay minerals represents a highly promising, eco-friendly, and cost-effective platform for advancing next-generation wastewater treatment technologies.
Author Contributions
Conceptualization, F.B.R.; investigation, N.S., W.M., B.O., M.K., and F.B.R.; data curation, N.S., W.M., B.O., M.K., and F.B.R.; writing—original draft preparation, N.S., W.M., B.O., Z.A., and F.B.R.; writing—review and editing, W.M. and M.K.; supervision, W.M., M.K., Z.A., and F.B.R.; project administration, F.B.R.; funding acquisition, Z.A. and W.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Deanship of Scientific Research at the University of Bisha-Saudi Arabia, through the Fast-Track Research Support Program.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are thankful to the Deanship of Graduate Studies and Scientific Research at University of Bisha for supporting this work through the Fast-Track Research Support Program.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yazdan, M.M.S.; Ahad, M.T.; Jahan, I.; Mazumder, M. Review on the Evaluation of the Impacts of Wastewater Disposal in Hydraulic Fracturing Industry in the United States. Technologies 2020, 8, 67. [Google Scholar] [CrossRef]
- United Nations. The What, Why and How of the World Water Crisis. 2023. Available online: https://sdgs.un.org/sites/default/files/2023-03/Why-What-How-of-Water-Crisis-Web.pdf (accessed on 14 July 2025).
- Richardson, S.D.; Manasfi, T. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2024, 96, 8184–8219. [Google Scholar] [CrossRef]
- Ansari, Z.H.; Bista, U. Hazards Associated with Industrial Effluents and Its Mitigation Strategies. In Anthropogenic Environmental Hazards: Compensation and Mitigation; Pathak, P., Srivastava, R.R., Ilyas, S., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 89–117. ISBN 978-3-031-41013-0. [Google Scholar]
- Iyiola, A.O.; Ipinmoroti, M.O.; Akingba, O.O.; Ewutanure, J.S.; Setufe, S.B.; Bilikoni, J.; Ofori-Boateng, E.; Wangboje, O.M. Organic Chemical Pollutants Within Water Systems and Sustainable Management Strategies. In Water Crises and Sustainable Management in the Global South; Izah, S.C., Ogwu, M.C., Loukas, A., Hamidifar, H., Eds.; Springer Nature: Singapore, 2024; pp. 211–251. ISBN 978-981-97-4966-9. [Google Scholar]
- Nwankwo, C.E.I.; Okeke, E.S.; Umeoguaju, F.U.; Ejeromedoghene, O.; Adedipe, D.T.; Ezeorba, T.P.C. Addressing Emerging Contaminants in Agriculture Affecting Plant–Soil Interaction: A Review on Bio-Based and Nano-Enhanced Strategies for Soil Health and Global Food Security (GFS). Discov. Toxicol. 2025, 2, 4. [Google Scholar] [CrossRef]
- Lin, L.; Yang, H.; Xu, X. Effects of Water Pollution on Human Health and Disease Heterogeneity: A Review. Front. Environ. Sci. 2022, 10, 880246. [Google Scholar] [CrossRef]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. Chem. Eng. 2022, 6, 8. [Google Scholar] [CrossRef]
- Yuan, C.; Croft, K.; de Nicola, S.; Davis, A.P.; Kjellerup, B.V. Treatment of Polycyclic Aromatic Hydrocarbons (PAHs) and Polychlorinated Biphenyls (PCBs) in Stormwater Using Polishing Columns with Biochar and Granular Activated Carbon. Chemosphere 2025, 372, 144107. [Google Scholar] [CrossRef]
- Othmani, B.; Gamelas, J.A.F.; Mendes, C.V.T.; Rasteiro, M.G.; Khadhraoui, M. Green Flocculants from Cactus Cladodes: Physicochemical Characterization and Assessment of Their Flocculating Activity for Crystal Violet Dye Removal. Water Air Soil Pollut. 2024, 235, 458. [Google Scholar] [CrossRef]
- Chai, J.; Zheng, J.; Yu, H.; Chai, F.; Tian, M. Recyclable and Selective PVDF-Based Multifunctional Molecular Imprinted Membranes for the Removal of Bisphenol A. Sep. Purif. Technol. 2024, 342, 127002. [Google Scholar] [CrossRef]
- Corbalán, M.; Da Silva, C.; Barahona, A.; Huiliñir, C.; Guerrero, L. Nitrification–Autotrophic Denitrification Using Elemental Sulfur as an Electron Donor in a Sequencing Batch Reactor (SBR): Performance and Kinetic Analysis. Sustainability 2024, 16, 4269. [Google Scholar] [CrossRef]
- Feng, S.; Guo, W.; Ding, A.; Parsa, S.M.; Pan, J.; Cheng, D.; Van Tung, T.; Ngo, H.H. Enzyme Sources in Wastewater Treatment: Their Influence on Enzymatic Bioremediation and Large-Scale Applications. Chem. Eng. J. 2025, 510, 161891. [Google Scholar] [CrossRef]
- Zhang, J.; White, J.C.; Lowry, G.V.; He, J.; Yu, X.; Yan, C.; Dong, L.; Tao, S.; Wang, X. Advanced Enzyme-Assembled Hydrogels for the Remediation of Contaminated Water. Nat. Commun. 2025, 16, 3050. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; El Gaayda, J.; Ait Akbour, R.; Nidheesh, P.V.; Hamdani, M. Removal of Organic Pollutants from Wastewater by Advanced Oxidation Processes and Its Combination with Membrane Processes. Chem. Eng. Process. 2021, 169, 108631. [Google Scholar] [CrossRef]
- Guzmán-Rasillo, J.; Ochoa-Terán, A.; López-Maldonado, E.A.; Pérez-Sicairos, S.; Trujillo-Navarrete, B.; López-Martínez, L.M.; García-Elías, J.; Sandoval-Hernandez, P.A.; Quiroz, M.M. Carboxyl-Functionalized Bis (Carbamoylcarboxylic) Acid Ligands as a Novel Alternative for Hazardous Metal Ions Removal by Coagulation-Flocculation. J. Mol. Struct. 2025, 1336, 142107. [Google Scholar] [CrossRef]
- Rahman, N.H.A.; Murugesu, K.; Rahman, R.A.; Mohamad, Z.; Jaafar, J.; Illias, R.M.; Sukmawati, D.; Syukri, M.S.M. A Brief Review of Immobilized Oxidoreductase Enzymes for the Removal of Endocrine-Disrupting Chemicals from Wastewater. J. Bioprocess. Biomass Technol. 2023, 2, 1–11. [Google Scholar] [CrossRef]
- Pandey, K.; Singh, B.; Pandey, A.K.; Badruddin, I.J.; Pandey, S.; Mishra, V.K.; Jain, P.A. Application of Microbial Enzymes in Industrial Waste Water Treatment. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1243–1254. [Google Scholar] [CrossRef]
- Feng, S.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Cheng, D.; Varjani, S.; Lei, Z.; Liu, Y. Roles and Applications of Enzymes for Resistant Pollutants Removal in Wastewater Treatment. Bioresour. Technol. 2021, 335, 125278. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi Nejad, Z.; Borghei, S.M.; Yaghmaei, S. Kinetic Studies of Bisphenol A in Aqueous Solutions by Enzymatic Treatment. Int. J. Environ. Sci. Technol. 2019, 16, 821–832. [Google Scholar] [CrossRef]
- Daniel, R.M.; Dines, M.; Petach, H.H. The Denaturation and Degradation of Stable Enzymes at High Temperatures. Biochem. J. 1996, 317, 1–11. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Coupling Chemical Modification and Immobilization to Improve the Catalytic Performance of Enzymes. Adv. Synth. Catal. 2011, 353, 2216–2238. [Google Scholar] [CrossRef]
- An, N.; Zhou, C.H.; Zhuang, X.Y.; Tong, D.S.; Yu, W.H. Immobilization of Enzymes on Clay Minerals for Biocatalysts and Biosensors. Appl. Clay Sci. 2015, 114, 283–296. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Sun, J.; Yendluri, R.; Liu, K.; Guo, Y.; Lvov, Y.; Yan, X. Enzyme-Immobilized Clay Nanotube–Chitosan Membranes with Sustainable Biocatalytic Activities. Phys. Chem. Chem. Phys. 2017, 19, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Gonzalez, R.; Horton, A.; Li, T. Immobilization of Enzymes by Polymeric Materials. Catalysts 2021, 11, 1211. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Wong, J.R.; Tan, K.W.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S.; Ganesan, P. Immobilization of Cellulase Enzyme on Functionalized Multiwall Carbon Nanotubes. J. Mol. Catal. B Enzym. 2014, 107, 124–131. [Google Scholar] [CrossRef]
- Hung, B.-Y.; Kuthati, Y.; Kankala, R.K.; Kankala, S.; Deng, J.-P.; Liu, C.-L.; Lee, C.-H. Utilization of Enzyme-Immobilized Mesoporous Silica Nanocontainers (IBN-4) in Prodrug-Activated Cancer Theranostics. Nanomaterials 2015, 5, 2169–2191. [Google Scholar] [CrossRef]
- Azhagapillai, P.; Gopalsamy, K.; Othman, I.; Alhatti, N.I.; Abu Haija, M.; Ashraf, S.S. Immobilization of Soybean Peroxidase Enzyme on Hierarchical Zeolite-Ordered Mesoporous Carbon Nanocomposite and Its Activity. RSC Adv. 2025, 15, 5781–5794. [Google Scholar] [CrossRef]
- Guisan, J.M.; Fernandez-Lorente, G.; Rocha-Martin, J.; Moreno-Gamero, D. Enzyme Immobilization Strategies for the Design of Robust and Efficient Biocatalysts. Curr. Opin. Green Sustain. Chem. 2022, 35, 100593. [Google Scholar] [CrossRef]
- Belghazdis, M.; Hachem, E.-K. Clay and Clay Minerals: A Detailed Review. Int. J. Recent Technol. Appl. Sci. (IJORTAS) 2022, 4, 54–75. [Google Scholar] [CrossRef]
- Özdemir, F.; Yalçinkaya, Z. Examination of The Immobilization and Kinetics of The Laccase Enzyme on Various Clay Minerals. MAS J. Appl. Sci. 2023, 8, 286–306. [Google Scholar] [CrossRef]
- Saphy, A.; Tijero, M.; García-Delgado, C.; Ortega, A.; Zamora, S.; Ruiz, A.I.; Eymar, E.; Cuevas, J.; Fernández, R. Biogeofilter with Hydrothermal Treated Stevensite Clay and Laccase Enzymes for Retention and Degradation of Tetracycline. Minerals 2022, 12, 1631. [Google Scholar] [CrossRef]
- Singh, N.B. Clays and Clay Minerals in the Construction Industry. Minerals 2022, 12, 301. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Riela, S. The Use of Some Clay Minerals as Natural Resources for Drug Carrier Applications. J. Funct. Biomater. 2018, 9, 58. [Google Scholar] [CrossRef]
- Huang, A.; He, J.; Feng, J.; Huang, C.; Yang, J.; Mo, W.; Su, X.; Zou, B.; Ma, S.; Lin, H. A High-Efficiency Clay Mineral Based Organic Photocatalysttowards Photodegradation of Butyl Xanthate in Mineral Processing Wastewater. Sep. Purif. Technol. 2024, 349, 127880. [Google Scholar] [CrossRef]
- Ndé, H.S.; Tamfuh, P.A.; Clet, G.; Vieillard, J.; Mbognou, M.T.; Woumfo, E.D. Comparison of HCl and H2SO4 for the Acid Activation of a Cameroonian Smectite Soil Clay: Palm Oil Discolouration and Landfill Leachate Treatment. Heliyon 2019, 5, e02926. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.M.; Abdella, E.M.; Hamed, R.R.; Salah, S.M.; Fahmy, H.M. Synthetic Nanoclays: Synthesis, Modifications, Polymer Integration, and Physicochemical Characterization. In Functionalized Nanoclays; Micro and Nano Technologies; Mallakpour, S., Hussain, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 25–44. ISBN 978-0-443-15894-0. [Google Scholar]
- Heller-Kallai, L. Thermally Modified Clay Minerals. In Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 289–308. [Google Scholar]
- Deng, Y.; Dixon, J.B.; White, G.N. Intercalation and Surface Modification of Smectite by Two Non-Ionic Surfactants. Clays Clay Miner. 2003, 51, 150–161. [Google Scholar] [CrossRef]
- Ishii, R.; Nakatsuji, M.; Ooi, K. Preparation of Highly Porous Silica Nanocomposites from Clay Mineral: A New Approach Using Pillaring Method Combined with Selective Leaching. Microporous Mesoporous Mater. 2005, 79, 111–119. [Google Scholar] [CrossRef]
- Fatimah, I.; Fadillah, G.; Yanti, I.; Doong, R. Clay-Supported Metal Oxide Nanoparticles in Catalytic Advanced OxidationProcesses: A Review. Nanomaterials 2022, 12, 825. [Google Scholar] [CrossRef]
- Funes, I.G.A.; Peralta, M.E.; Pettinari, G.R.; Carlos, L.; Parolo, M.E. Facile Modification of Montmorillonite by Intercalation and Grafting: The Study of the Binding Mechanisms of a Quaternary Alkylammonium Surfactant. Appl. Clay Sci. 2020, 195, 105738. [Google Scholar] [CrossRef]
- Komadel, P. Acid ActivatedClays: Materials in Continuous Demand. Appl. Clay Sci. 2016, 131, 84–99. [Google Scholar] [CrossRef]
- Ismadji, S.; Soetaredjo, F.E.; Ayucitra, A. Modification of Clay Minerals for Adsorption Purpose. In Clay Materials for Environmental Remediation; Ismadji, S., Soetaredjo, F.E., Ayucitra, A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 39–56. ISBN 978-3-319-16712-1. [Google Scholar]
- Sarkar, B.; Rusmin, R.; Ugochukwu, U.C.; Mukhopadhyay, R.; Manjaiah, K.M. Modified Clay Minerals for Environmental Applications. In Modified Clay and Zeolite Nanocomposite Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–127. [Google Scholar]
- Kangmennaa, A.; Acquah, S.; Forkuo, R.B.; Adusei, J.K.; Atongo, G.A.; Amarh, F.A.; Opoku, F.; Agorku, E.S. Methylene Blue Dye Adsorption on Ghana’s Activated Clay from Teleku Bukazo. J. Dispers. Sci. Technol. 2025, 46, 807–820. [Google Scholar] [CrossRef]
- Messaid, B.; Djemai, I.; Boudouh, I.; Robustillo, M.D. Assessment of the Performance of an Acid-Activated Composite Made of Na-Montmorillonite Algerian Clay to Remove Phenol and 4-Chlorophenol from Water. Desalin. Water Treat. 2025, 322, 101088. [Google Scholar] [CrossRef]
- Xi, Y.; Mallavarapu, M.; Naidu, R. Preparation, Characterization of Surfactants Modified Clay Minerals and Nitrate Adsorption. Appl. Clay Sci. 2010, 48, 92–96. [Google Scholar] [CrossRef]
- Johnston, C.T.; Santagata, M.; Sasar, M. Physical and Chemical Properties of Layered Clay Mineral Particle Surfaces. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2022; Volume 10, pp. 125–167. [Google Scholar]
- Wu, N.; Wu, L.; Liao, L.; Lv, G. Organic Intercalation of Structure Modified Vermiculite. J. Colloid Interface Sci. 2015, 457, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Detellier, C. Functional Kaolinite. Chem. Rec. 2018, 18, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Balomenou, G.; Stathi, P.; Enotiadis, A.; Gournis, D.; Deligiannakis, Y. Physicochemical Study of Amino-Functionalized Organosilicon Cubes Intercalated in Montmorillonite Clay: H-Binding and Metal Uptake. J. Colloid Interface Sci. 2008, 325, 74–83. [Google Scholar] [CrossRef]
- Cheng, J.; Yu, S.M.; Zuo, P. Horseradish Peroxidase Immobilized on Aluminum-Pillared Interlayered Clay for the Catalytic Oxidation of Phenolic Wastewater. Water Res. 2006, 40, 283–290. [Google Scholar] [CrossRef]
- Vallejo, C.A.; Galeano, L.A.; Trujillano, R.; Vicente, M.Á.; Gil, A. Preparation of Al/Fe-PILC Clay Catalysts from Concentrated Precursors: Enhanced Hydrolysis of Pillaring Metals and Intercalation. RSC Adv. 2020, 10, 40450–40460. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, C.; Wei, H.; Yuan, W.; Li, K. Adsorption of Levofloxacin onto an Iron-Pillared Montmorillonite (Clay Mineral): Kinetics, Equilibrium and Mechanism. Appl. Clay Sci. 2015, 118, 301–307. [Google Scholar] [CrossRef]
- Rapsomanikis, A.; Papoulis, D.; Panagiotaras, D.; Kaplani, E.; Stathatos, E. NanocrystallineTiO2 and Halloysite Clay Mineral Composite Films Prepared by Sol–Gel Method: Synergistic Effect and the Case of Silver Modification to the Photocatalytic Degradation of Basic Blue-41 AzoDye in Water. Glob. NEST J. 2014, 16, 485–498. [Google Scholar]
- Dedzo, G.K.; Detellier, C. Clay Minerals—Ionic Liquids, Nanoarchitectures, and Applications. Adv. Funct. Mater. 2018, 28, 1703845. [Google Scholar] [CrossRef]
- He, H.; Duchet, J.; Galy, J.; Gérard, J.-F. Grafting of Swelling Clay Materials with 3-Aminopropyltriethoxysilane. J. Colloid Interface Sci. 2005, 288, 171–176. [Google Scholar] [CrossRef]
- Letaief, S.; Detellier, C. Application of Thermal Analysis for the Characterisation of Intercalated and Grafted Organo-Kaolinite Nanohybrid Materials. J. Therm. Anal. Calorim. 2011, 104, 831–839. [Google Scholar] [CrossRef]
- Machida, S.; Idota, N.; Sugahara, Y. Interlayer Grafting of Kaolinite Using Trimethylphosphate. Dalton Trans. 2019, 48, 11663–11673. [Google Scholar] [CrossRef]
- Scholtzová, E.; Tunega, D. Prediction of Mechanical Properties of Grafted Kaolinite—A DFT Study. Appl. Clay Sci. 2020, 193, 105692. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. General Introduction: Clays, Clay Minerals, and Clay Science. In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 1–19. [Google Scholar]
- Murray, H.H. Applied Clay Mineralogy: Occurrences, Processing and Applications of Kaolins, Bentonites, Palygorskite-Sepiolite, and Common Clays; Elsevier: Amsterdam, The Netherlands, 2006; Volume 2. [Google Scholar]
- Bilal, M.; Asgher, M.; Parra-Saldivar, R.; Hu, H.; Wang, W.; Zhang, X.; Iqbal, H.M.N. Immobilized Ligninolytic Enzymes: An Innovative and Environmental Responsive Technology to Tackle Dye-Based Industrial Pollutants—A Review. Sci. Total Environ. 2017, 576, 646–659. [Google Scholar] [CrossRef]
- Zhou, C.H.; Keeling, J. Fundamental and Applied Research on Clay Minerals: From Climate and Environment to Nanotechnology. Appl. Clay Sci. 2013, 74, 3–9. [Google Scholar] [CrossRef]
- Arıca, M.Y.; Altıntas, B.; Bayramoğlu, G. Immobilization of Laccase onto Spacer-Arm Attached Non-Porous Poly (GMA/EGDMA) Beads: Application for Textile Dye Degradation. Bioresour. Technol. 2009, 100, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme Immobilisation in Biocatalysis: Why, What and How. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.S.; Yang, K.-L. Entrapment of Cross-Linked Cellulase Colloids in Alginate Beads for Hydrolysis of Cellulose. Colloids Surf. B Biointerfaces 2016, 145, 862–869. [Google Scholar] [CrossRef]
- Alsoufi, M.A. Use of Immobilized Laccase in Bioremediation of Phenolic Compounds Which Causes Environmental Pollution. J. Biodivers. Environ. Sci. 2018, 12, 370–377. [Google Scholar]
- Sanjay, G.; Sugunan, S. Acid Activated Montmorillonite: An Efficient Immobilization Support for Improving Reusability, Storage Stability and Operational Stability of Enzymes. J. Porous Mater. 2008, 15, 359–367. [Google Scholar] [CrossRef]
- Iqhrammullah, M.; Fahrina, A.; Chiari, W.; Ahmad, K.; Fitriani, F.; Suriaini, N.; Safitri, E.; Puspita, K. Laccase Immobilization Using Polymeric Supports for Wastewater Treatment: A Critical Review. Macromol. Chem. Phys. 2023, 224, 2200461. [Google Scholar] [CrossRef]
- Morsy, S.A.G.Z.; Ahmad Tajudin, A.; Ali, M.S.M.; Shariff, F.M. Current Development in Decolorization of SyntheticDyes by Immobilized Laccases. Front. Microbiol. 2020, 11, 572309. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wang, J.; Li, Y.; Chen, X.; Liu, Y. Enzymes Immobilized on SuperparamagneticFe3O4@ClaysNanocomposites: Preparation, Characterization, and a New Strategy for the Regeneration of Supports. J. Phys. Chem. C 2011, 115, 6350–6359. [Google Scholar] [CrossRef]
- Cameselle, C.; Pazos, M.; Lorenzo, M.; Sanromán, M. Enhanced Decolourisation Ability of Laccase towards Various Synthetic Dyes by an Electrocatalysis Technology. Biotechnol. Lett. 2003, 25, 603–606. [Google Scholar] [CrossRef]
- Rodríguez-Couto, S. Immobilized-Laccase Bioreactors for Wastewater Treatment. Biotechnol. J. 2024, 19, 2300354. [Google Scholar] [CrossRef]
- Mehandia, S.; Ahmad, S.; Sharma, S.C.; Arya, S.K. Decolorization and detoxification of textile effluent by immobilized laccase-ACS into chitosan-clay composite beads using a packed bed reactor system: An ecofriendly approach. J. Water Process Eng. 2022, 47, 102662. [Google Scholar] [CrossRef]
- Jiang, B.; Li, Y.; Wang, H.; Jia, L.; Huang, F.; Hu, X. Application of a New Type of Si–Al Porous Clay Material as a Solid Phase Support for Immobilizing Acidovorax Sp. PM3 to Treat Domestic Sewage. Adsorpt. Sci. Technol. 2019, 37, 729–744. [Google Scholar] [CrossRef]
- Al-Maqdi, K.A.; Elmerhi, N.; Athamneh, K.; Bilal, M.; Alzamly, A.; Ashraf, S.S.; Shah, I. Challenges and Recent Advances in Enzyme-Mediated Wastewater Remediation—A Review. Nanomaterials 2021, 11, 3124. [Google Scholar] [CrossRef]
- Dong, H.; Li, J.; Li, Y.; Hu, L.; Luo, D. Improvement of Catalytic Activity and Stability of Lipase by Immobilization on Organobentonite. Chem. Eng. J. 2012, 181, 590–596. [Google Scholar] [CrossRef]
- Wang, P.; Shen, X.; Qiu, S.; Zhang, L.; Ma, Y.; Liang, J. Clay-Based Materials for Heavy Metals Adsorption: Mechanisms, Advancements, and Future Prospects in Environmental Remediation. Crystals 2024, 14, 1046. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, S.; Shen, Y.; Jiang, X.; Lv, H.; Han, C.; Liu, W.; Zhao, Q. A Critical Review of Clay Mineral-Based Photocatalysts for Wastewater Treatment. Catalysts 2024, 14, 575. [Google Scholar] [CrossRef]
- Alali, A.F.; Almojil, S.F.; Almohana, A.I.; Almoalimi, K.T. Highly Reusable Bentonite Clay@Biochar@Fe3O4 Nanocomposite for Hg(II) Removal from Synthetic and Real Wastewater. Environ. Sci. Pollut. Res. 2023, 30, 72484–72502. [Google Scholar] [CrossRef] [PubMed]
- Mohidem, N.A.; Mohamad, M.; Rashid, M.U.; Norizan, M.N.; Hamzah, F.; Mat, H. Recent Advances in Enzyme Immobilisation Strategies: An Overview of Techniques and Composite Carriers. J. Compos. Sci. 2023, 7, 488. [Google Scholar] [CrossRef]
- Tzialla, A.A.; Pavlidis, I.V.; Felicissimo, M.P.; Rudolf, P.; Gournis, D.; Stamatis, H. Lipase Immobilization on Smectite Nanoclays: Characterization and Application to the Epoxidation of α-Pinene. Bioresour. Technol. 2010, 101, 1587–1594. [Google Scholar] [CrossRef]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient Immobilisation of Industrial Biocatalysts: Criteria and Constraints for the Selection of Organic Polymeric Carriers and Immobilisation Methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for EnzymeImmobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N.; Hu, H.; Wang, W.; Zhang, X. Development of Horseradish Peroxidase-Based Cross-Linked Enzyme Aggregates and Their Environmental Exploitation for Bioremediation Purposes. J. Environ. Manage. 2017, 188, 137–143. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme Immobilization by Adsorption: A Review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying Enzyme Activity and Selectivity by Immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Industrial Applications of Immobilized Enzymes—A Review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Judd, S. The Status of Membrane Bioreactor Technology. Trends Biotechnol. 2008, 26, 109–116. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).