Pre-Concentration of Vanadium from Stone Coal by Gravity Using Fine Mineral Spiral
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Procedure
2.3. Test Methods
- The chemical composition of the stone coal was detected by the Axios advanced X-ray fluorescence (XRF) analyzer (PANalytical B.V., Almelo, The Netherlands).
- Optical microscopy study was performed using Leica DMLP polarization microscope (Leica, Wetzlar, Germany).
- The X-ray diffraction (XRD) analysis was conducted by using D/Max-RB X-ray diffraction meter (Rigaku, Akishima, Japan) with Cu Kα radiation.
- The vanadium valences of the raw ore were measured on ZDJ-4A automatic potentiometric titrimeter (Shanghai INESA Scientific Instrument Ltd., Shanghai, China) by ammonium ferrous sulfate method [25].
- Sizing analysis was conducted on sub-samples of feed and test products using Mastersizer 2000 laser particle characterization system (Malvern Instruments Ltd., Malvern, UK) and laboratory wet screening method.
- The determination of vanadium grade was measured in accordance with Test Methods of Vanadium in Coal Standard (GB/T 19226-2003) [26].
3. Results and Discussion
3.1. Mineralogical Study of the Raw Ore
3.1.1. Chemical Composition Analysis
3.1.2. Mineral Composition Analysis
3.1.3. Occurrence of Vanadium
3.2. Selective Grinding
3.3. Gravity Separation
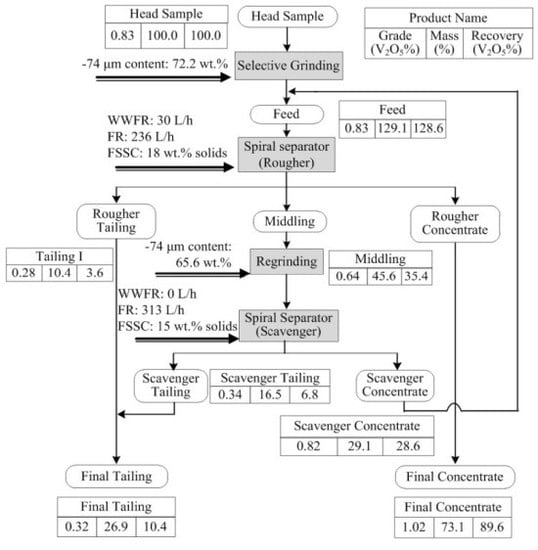
3.4. Flowsheet Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bin, Z.Y. Progress of the research on extraction of vanadium pentoxide from stone coal and market of the V2O5. Hunan Nonferrous Metals 2006, 22, 16–20. (In Chinese) [Google Scholar]
- Cai, Z.L.; Zhang, Y.M.; Liu, T.; Huang, J. Mechanisms of vanadium recovery from stone coal by novel BaCO3/CaO composite additive roasting and acid leaching technology. Minerals 2016, 6, 26. [Google Scholar]
- Wang, M.Y.; Wang, X.W. Research status and prospect of vanadium leaching processes from stone coal. Chin. J. Rare Metals 2010, 1, 90–97. [Google Scholar]
- Xue, N.N.; Zhang, Y.M.; Liu, T.; Huang, J. Study of the dissolution behavior of muscovite in stone coal by oxygen pressure acid leaching. Metall. Mater. Trans. B 2015, 47, 1–8. [Google Scholar]
- Zhao, Y.L.; Zhang, Y.M.; Liu, T.; Chen, T.J.; Bian, Y.; Bao, S.X. Pre-concentration of vanadium from stone coal by gravity separation. Int. J. Miner. Process. 2013, 121, 1–5. [Google Scholar]
- Zhang, Y.M.; Bao, S.X.; Liu, T.; Huang, J.; Chen, T.J. Research status and prospect of vanadium extraction from stone coal in China. Nonferrous Metals Extr. Metall. Sect. 2015, 2, 24–30. (In Chinese) [Google Scholar]
- Zhu, X.B.; Zhang, Y.M.; Huang, J.; Liu, T.; Wang, Y. A kinetics study of multi-stage counter-current circulation acid leaching of vanadium from stone coal. Int. J. Miner. Process. 2012, 114–117, 1–6. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Bao, S.X.; Liu, T.; Chen, T.J.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Hu, Y.J.; Bao, S.X. Vanadium emission during roasting of vanadium-bearing stone coal in chlorine. Miner. Eng. 2012, 30, 95–98. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.M.; Liu, T.; Huang, J.; Wang, Y. Effect of impurities on vanadium purification from acid leaching solution of stone coal with solvent extraction process. Nonferrous Metals Extr. Metall. 2013, 5, 27–30. (In Chinese) [Google Scholar]
- Li, J.; Ma, J. Technical study of mechanical separation to discard tailings of vanadium ore contained in black rock series. Nonferrous Metals Process. Sect. 2010, 4, 25–28. [Google Scholar]
- Sun, W.; Wang, L.; Cao, X.F.; Liu, R.Q.; Xu, L.H.; Han, H.S. Flotation technology and adsorption mechanism of vanadium extraction from stone coal. Chin. J. Nonferrous Metals 2012, 22, 2069–2074. [Google Scholar]
- Wang, L.; Sun, W.; Liu, R.Q.; Gu, X.C. Flotation recovery of vanadium from low-grade stone coal. Trans. Nonferrous Met. Soc. China 2014, 24, 1145–1151. [Google Scholar]
- Xiang, P.; Feng, Q.M.; Niu, Y.J.; Pan, A.X. Enrichment of vanadium from stone coal in Aksu vanadium mine by ore dressing method. Mater. Res. Appl. 2010, 4, 65–70. (In Chinese) [Google Scholar]
- Gu, X.C.; Sun, W.; Liu, R.Q.; Song, S.B.; Chen, X.Z. Study on mineral processing of a decarburized stone coal in Hunan. Nonferrous Metals Process. Sect. 2014, 5, 67–71. (In Chinese) [Google Scholar]
- Wu, H.L.; Zhao, W.; Li, M.T.; Deng, Z.G.; Ge, H.W.; Wei, C. New craft study on enriching vanadium by means of priority coal flotation from high carbon stone-coal. J. Chin. Rare Earth Soc. 2008, 26, 530–533. [Google Scholar]
- Bian, Y.; Zhang, Y.M.; Ren, L.Y.; Bao, S.X.; Zhao, Y.L.; Liu, X. New process for calcite flotation from high calcium and carbon-type vanadium-bearing stone coal. Chin. J. Rare Met. 2014, 4, 693–702. [Google Scholar]
- Mishara, B.K.; Tripathy, A. A preliminary study of particle separation in spiral concentrators using DEM. Int. J. Miner. Process. 2010, 94, 192–195. [Google Scholar]
- Das, S.K.; Godivalla, K.M.; Panda, L.; Bhattacharya, K.K.; Singh, R.; Mehrotra, S.P. Mathematical modeling of separation characteristics of coal-washing spiral. Int. J. Miner. Process. 2007, 84, 118–132. [Google Scholar] [CrossRef]
- Richards, R.G.; MacHunter, D.M.; Gates, P.J.; Palmer, M.K. Gravity separation of ultra-fine (−0.1 mm) minerals using spiral separators. Miner. Eng. 2000, 13, 65–77. [Google Scholar] [CrossRef]
- Liu, H.Z. Development of BL1500—A spiral chute and its application in tailings retreatment. Mining Metall. 2001, 10, 24–28. (In Chinese) [Google Scholar]
- Liu, X.; Zhang, Y.M.; Liu, T.; Cai, Z.; Chen, T.J.; Sun, K. Beneficiation of a sedimentary phosphate ore by a combination of spiral gravity and direct-reverse flotation. Minerals 2016, 6, 38. [Google Scholar]
- Kapur, P.C.; Meloy, T.P. Spirals observed. Int. J. Miner. Process. 1998, 53, 15–28. [Google Scholar]
- Zhang, Y.M.; Liu, H.Z. Development and application of out-limited H/D helical chute. Multipurp. Util. Miner. Resour. 2000, 5, 43–46. (In Chinese) [Google Scholar]
- Bao, S.X.; Zhang, Y.M.; Huang, J.; Yang, X.; Hu, Y.J. Determination of vanadium valency in roasted stone coal by separate dissolve-potentiometric titration method. MRS Proc. 2012, 1380. [Google Scholar] [CrossRef]
- General Administration of Quality Supervision, Inspection and Quarantine of China. Determination of Vanadium in Coal; GB/T 19226-2003; Standard Press of China: Beijing, China, 2003. (In Chinese)
- Wu, L.; Bai, G.; Yuan, Z. Minerals and Rocks; Chemical Industry Press of China: Beijing, China, 2010; pp. 170–210. (In Chinese) [Google Scholar]
- Zhu, Y.M.; Han, Y.X.; Wang, Z.H.; Tian, W.L. Selective grinding of low grade bauxite in ball mill grinding. Metal Mine 2009, 6, 60–63. [Google Scholar]
- Zhang, G.F.; Feng, Q.M.; Chen, Q.Y.; Zhang, P.M. Study on grinding media of selective grinding of bauxite. J. Cent. South Univ. Sci. Technol. 2004, 4, 552–556. [Google Scholar]
- Liu, J.; Zhang, Y.M.; Huang, J.; Liu, T.; Yuan, Y.Z.; Huang, X.B. Influence of mechanical activation on mineral properties and process of acid leaching from stone coal. Chin. J. Rare Metals 2014, 38, 115–122. [Google Scholar]
- Ou, L.M.; Feng, Q.M.; Lu, Y.P.; Zhang, G.F. Bauxite cracking mode and selective separation of aluminum and silicon minerals. Metal Mine 2005, 2, 28–32. (In Chinese) [Google Scholar]
- Li, Y.C. Test on recleaning of tailing from air-separated fragmental mica. Non Metallic Mines 2002, 25, 39–40. (In Chinese) [Google Scholar]







| Height | Pitch (P) | Outer Diameter (D) | Inner Radius | Radial Width | Trough Slope Angle (θ) |
|---|---|---|---|---|---|
| 850 mm | 144 mm | 400 mm | 16 mm | 184 mm | 8° |
| Component | V2O5 | Al2O3 | SiO2 | Fe2O3 | P2O5 | CaO | K2O | Na2O | C | S |
|---|---|---|---|---|---|---|---|---|---|---|
| % | 0.81 | 9.12 | 57.99 | 5.83 | 1.18 | 6.05 | 4.82 | 0.34 | 4.96 | 2.17 |
| Mineral | Muscovite and Illite | Quartz | Pyrite | Calcite | Coal |
| Content (%) | 20.5 | 40.5 | 6.6 | 7.1 | 4.3 |
| Density (g/cm3) | 2.76–3.10 and 2.6–2.9 | 2.55–2.65 | 4.9–5.2 | 2.6–2.8 | - |
| Mohs Scale | 2–3(001) and 1–2 | 7 | 6.0–6.5 | 3 | - |
| Mineral | Kaolinite | Apatite | Hematite | Other | |
| Content (%) | 4.7 | 2.9 | 2.0 | 11.4 | |
| Density (g/cm3) | 2.6–2.7 | 3.18–3.21 | 5.0–5.3 | - | |
| Mohs Scale | 1–3 | 5 | 5–6 | - |
| V2O5 | SiO2 | K2O | Al2O3 | MgO | CaO | FeO | Mineral |
|---|---|---|---|---|---|---|---|
| 0.00 | 98.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | Quartz |
| 4.19 | 49.80 | 9.46 | 28.31 | 2.74 | 0.03 | 0.16 | Muscovite |
| 2.81 | 30.72 | 9.72 | 28.33 | 0.49 | 0.10 | 0.05 | Illite |
| 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 59.27 | Pyrite |
| 0.00 | 0.06 | 0.00 | 0.00 | 2.05 | 54.88 | 0.27 | Calcite |
| Products | Stream No. | Yield (%) | Assay Value (wt %) | ||||
|---|---|---|---|---|---|---|---|
| V2O5 | CaO | Fe2O3 | P2O5 | SiO2 | |||
| Tailing | 1 | 2.8 | 0.21 | 6.87 | 13.26 | 2.57 | 35.50 |
| 2 | 3.7 | 0.26 | 6.86 | 13.64 | 2.47 | 40.30 | |
| Total | 6.5 | 0.24 | 6.86 | 13.48 | 2.51 | 38.22 | |
| Middling | 3 | 6.7 | 0.46 | 6.41 | 9.09 | 2.08 | 53.62 |
| 4 | 12.4 | 0.59 | 6.20 | 7.19 | 1.44 | 67.20 | |
| 5 | 16.7 | 0.71 | 6.05 | 4.17 | 1.00 | 65.49 | |
| Total | 35.9 | 0.62 | 6.17 | 6.14 | 1.35 | 63.85 | |
| Concentrate | 6 | 12.0 | 0.98 | 4.60 | 2.35 | 0.44 | 62.17 |
| 7 | 7.6 | 1.05 | 3.17 | 3.76 | 0.75 | 59.18 | |
| 8 | 4.8 | 1.19 | 3.70 | 3.5 | 0.82 | 53.9 | |
| 9 | 2.6 | 1.18 | 4.93 | 4.15 | 0.74 | 56.64 | |
| 10 | 2.9 | 1.03 | 6.24 | 4.03 | 0.79 | 60.11 | |
| 11 | 2.1 | 0.99 | 7.32 | 4.9 | 0.89 | 58.14 | |
| 12 | 5.6 | 0.98 | 7.32 | 5.43 | 1.05 | 53.10 | |
| 13 | 8.7 | 0.98 | 7.42 | 5.69 | 1.01 | 51.65 | |
| 14 | 11.4 | 0.98 | 8.96 | 5.53 | 0.97 | 49.78 | |
| Total | 57.6 | 1.02 | 6.08 | 4.32 | 0.81 | 55.69 | |
| Feed | - | 100.0 | 0.83 | 6.16 | 5.56 | 1.12 | 57.49 |
| Products | Size Fraction (μm) | Yield (wt %) | V2O5 % | ||
|---|---|---|---|---|---|
| Grade | Recovery | Total Recovery | |||
| Tailing | >74 | 41.8 | 0.27 | 43.5 | 0.9 |
| 56~74 | 27.7 | 0.25 | 26.8 | 0.6 | |
| 45~56 | 13.4 | 0.22 | 11.4 | 0.2 | |
| 38~45 | 9.9 | 0.24 | 9.2 | 0.2 | |
| 30~38 | 3.3 | 0.27 | 3.4 | 0.1 | |
| <30 | 3.9 | 0.38 | 5.8 | 0.1 | |
| Total | 100.0 | 0.26 | 100.0 | 2.1 | |
| Middling | >74 | 47.1 | 0.71 | 53.6 | 14.5 |
| 56~74 | 23.0 | 0.61 | 22.5 | 6.1 | |
| 45~56 | 10.8 | 0.47 | 8.1 | 2.2 | |
| 38~45 | 8.4 | 0.46 | 6.2 | 1.7 | |
| 30~38 | 3.7 | 0.44 | 2.6 | 0.7 | |
| <30 | 7.0 | 0.62 | 7.0 | 1.9 | |
| Total | 100.0 | 0.62 | 100.0 | 27.1 | |
| Concentrate | >74 | 14.5 | 1.17 | 16.7 | 11.8 |
| 56~74 | 8.0 | 1.07 | 8.5 | 6.0 | |
| 45~56 | 8.3 | 1.01 | 8.2 | 5.8 | |
| 38~45 | 9.7 | 1.11 | 10.6 | 7.5 | |
| 30~38 | 8.2 | 0.99 | 8.0 | 5.7 | |
| <30 | 51.3 | 0.95 | 48.0 | 34.0 | |
| Total | 100.0 | 1.02 | 100.0 | 70.8 | |
| Products | Yield (%) | Grade of V2O5 (%) | Recovery of V2O5 (%) |
|---|---|---|---|
| Concentrate | 57.8 | 1.01 | 71.4 |
| Rougher Tailing | 6.4 | 0.25 | 2.0 |
| Rougher Middling | 35.8 | 0.63 | 26.6 |
| Scavenger Concentrate | 19.6 | 0.82 | 19.7 |
| Scavenger Tailing | 16.2 | 0.35 | 6.9 |
| Feed | 100.0 | 0.82 | 100.0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhang, Y.; Liu, T.; Cai, Z.; Sun, K. Pre-Concentration of Vanadium from Stone Coal by Gravity Using Fine Mineral Spiral. Minerals 2016, 6, 82. https://doi.org/10.3390/min6030082
Liu X, Zhang Y, Liu T, Cai Z, Sun K. Pre-Concentration of Vanadium from Stone Coal by Gravity Using Fine Mineral Spiral. Minerals. 2016; 6(3):82. https://doi.org/10.3390/min6030082
Chicago/Turabian StyleLiu, Xin, Yimin Zhang, Tao Liu, Zhenlei Cai, and Kun Sun. 2016. "Pre-Concentration of Vanadium from Stone Coal by Gravity Using Fine Mineral Spiral" Minerals 6, no. 3: 82. https://doi.org/10.3390/min6030082
APA StyleLiu, X., Zhang, Y., Liu, T., Cai, Z., & Sun, K. (2016). Pre-Concentration of Vanadium from Stone Coal by Gravity Using Fine Mineral Spiral. Minerals, 6(3), 82. https://doi.org/10.3390/min6030082