Minerals and Enrichment of W, Rb, and Cs in Late Permian Coal from Meitian Mine, Meitian Coalfield, Southern China by Magmatic Hydrothermal Fluids
Abstract
1. Introduction
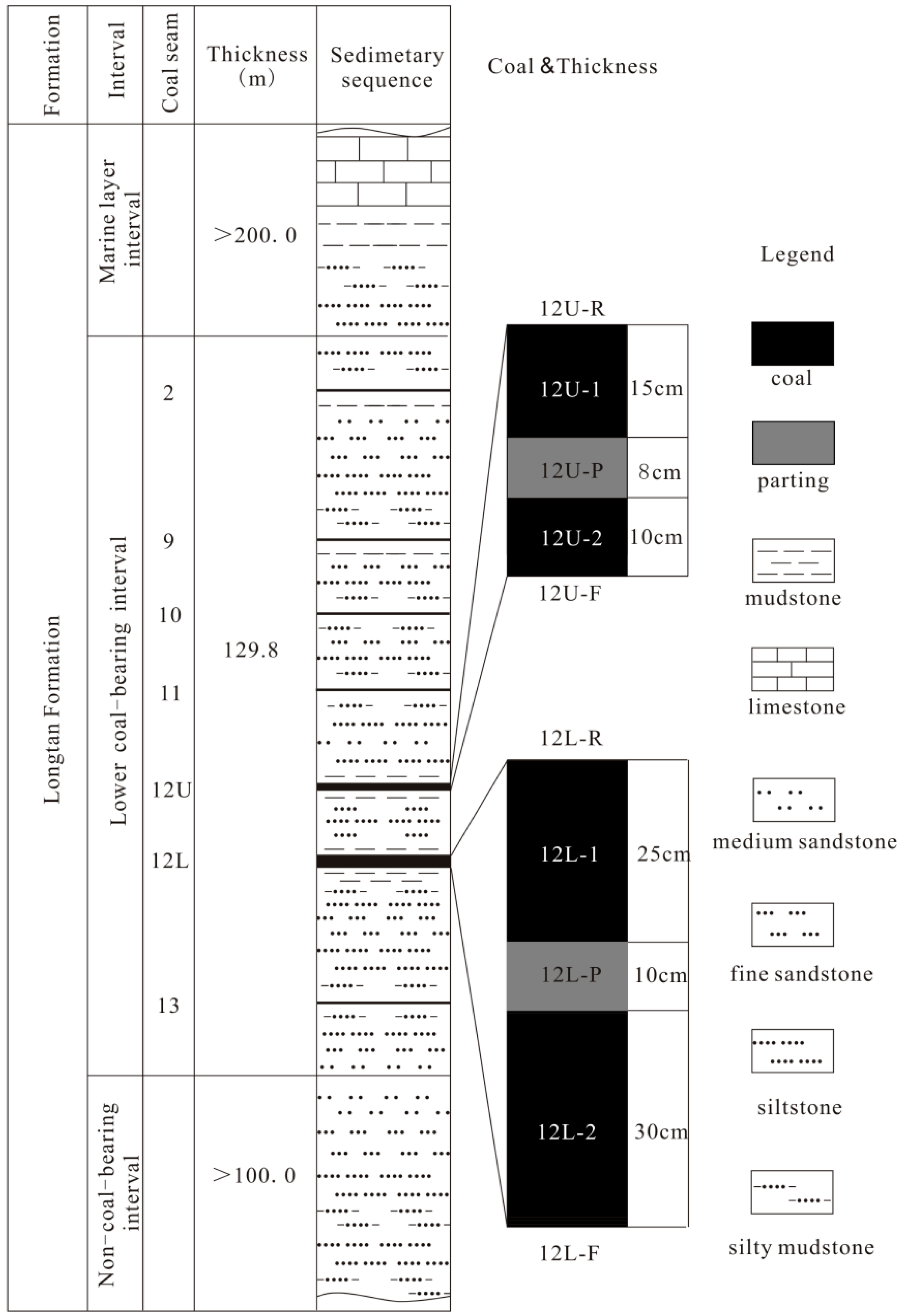
2. Geological Setting
3. Material and Analytical Methods
4. Results
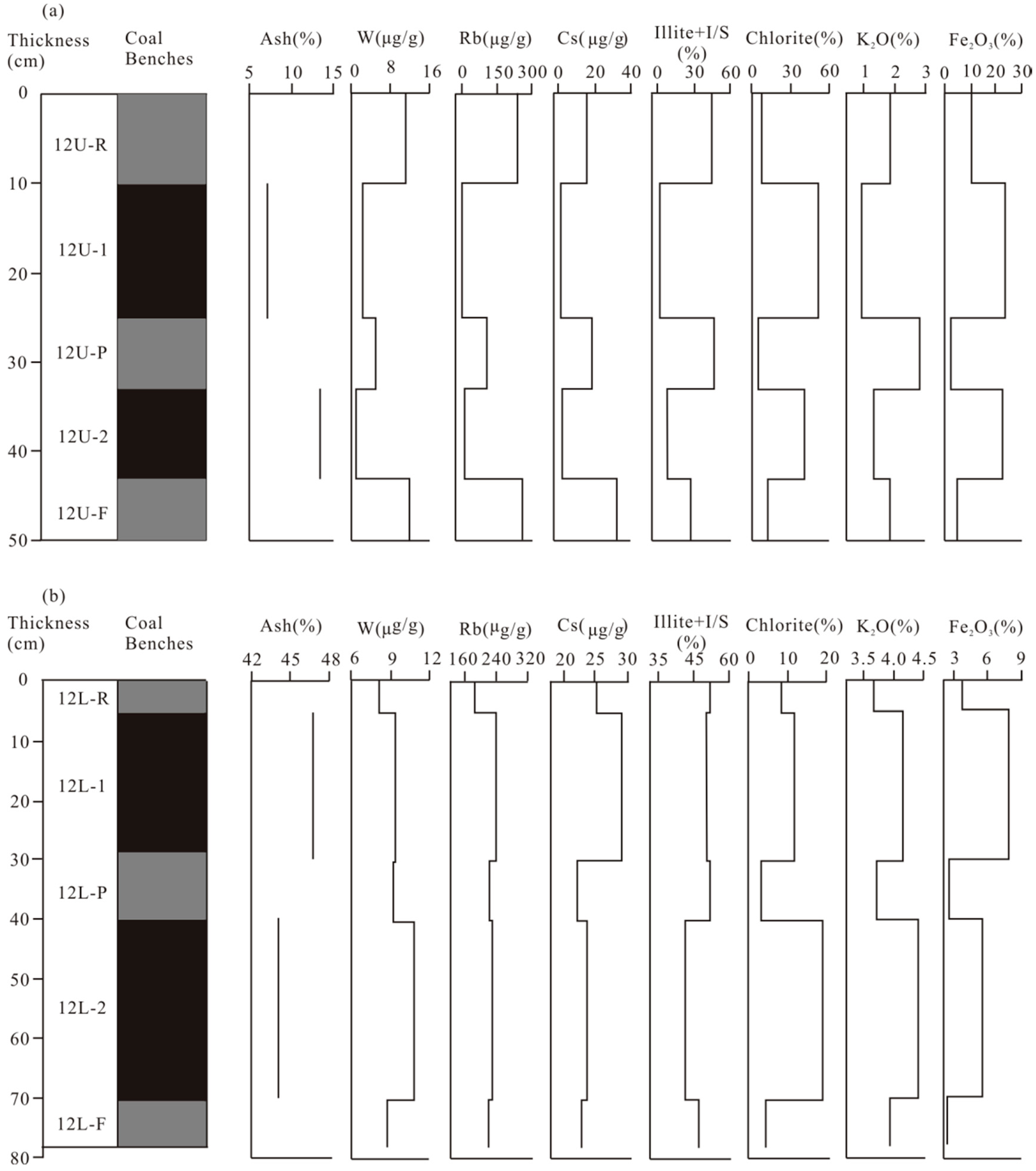
4.1. Coal Chemistry and Vitrinite Reflectance
4.2. Geochemistry
4.2.1. Major Element Oxides
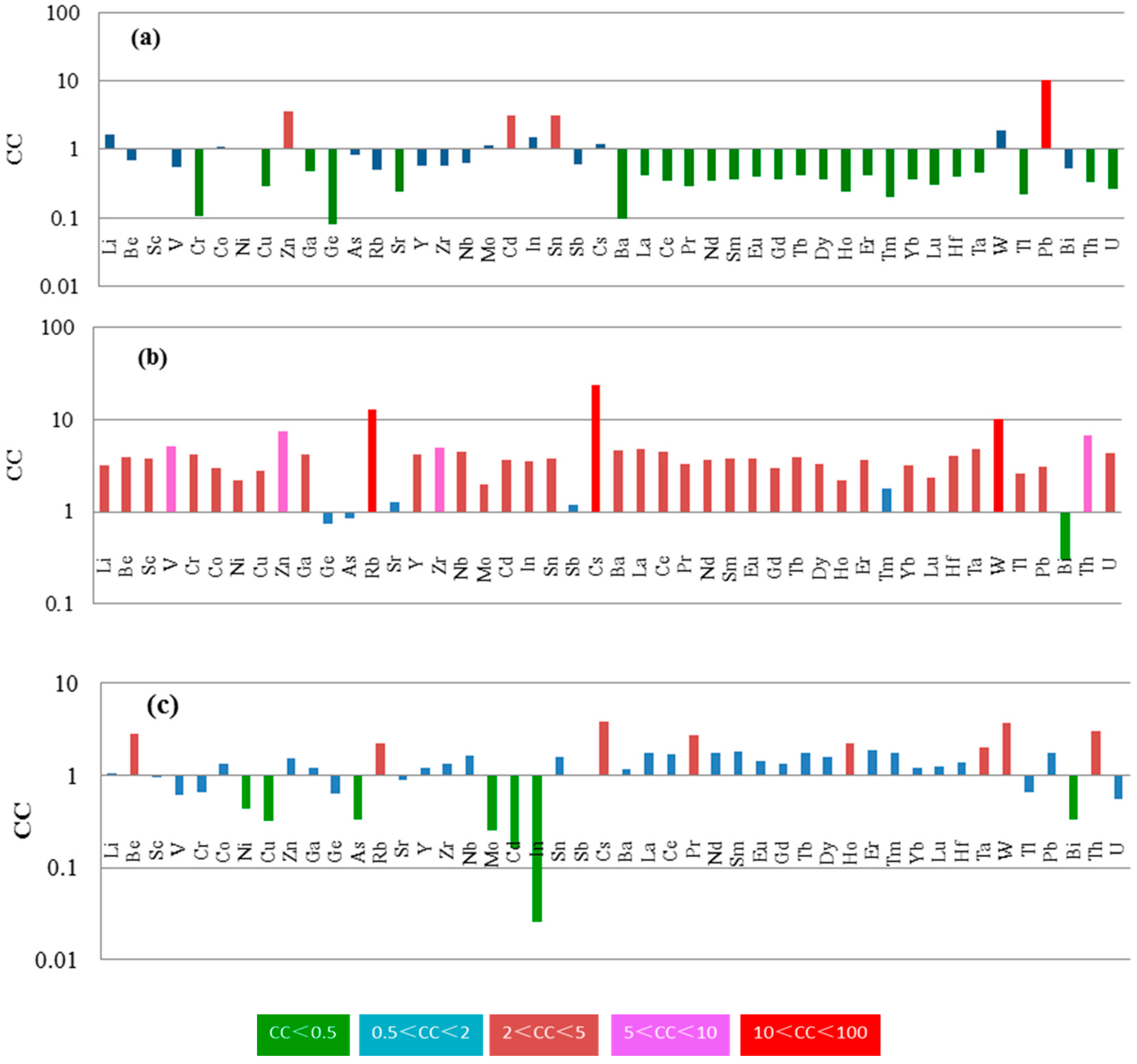
4.2.2. Trace Elements
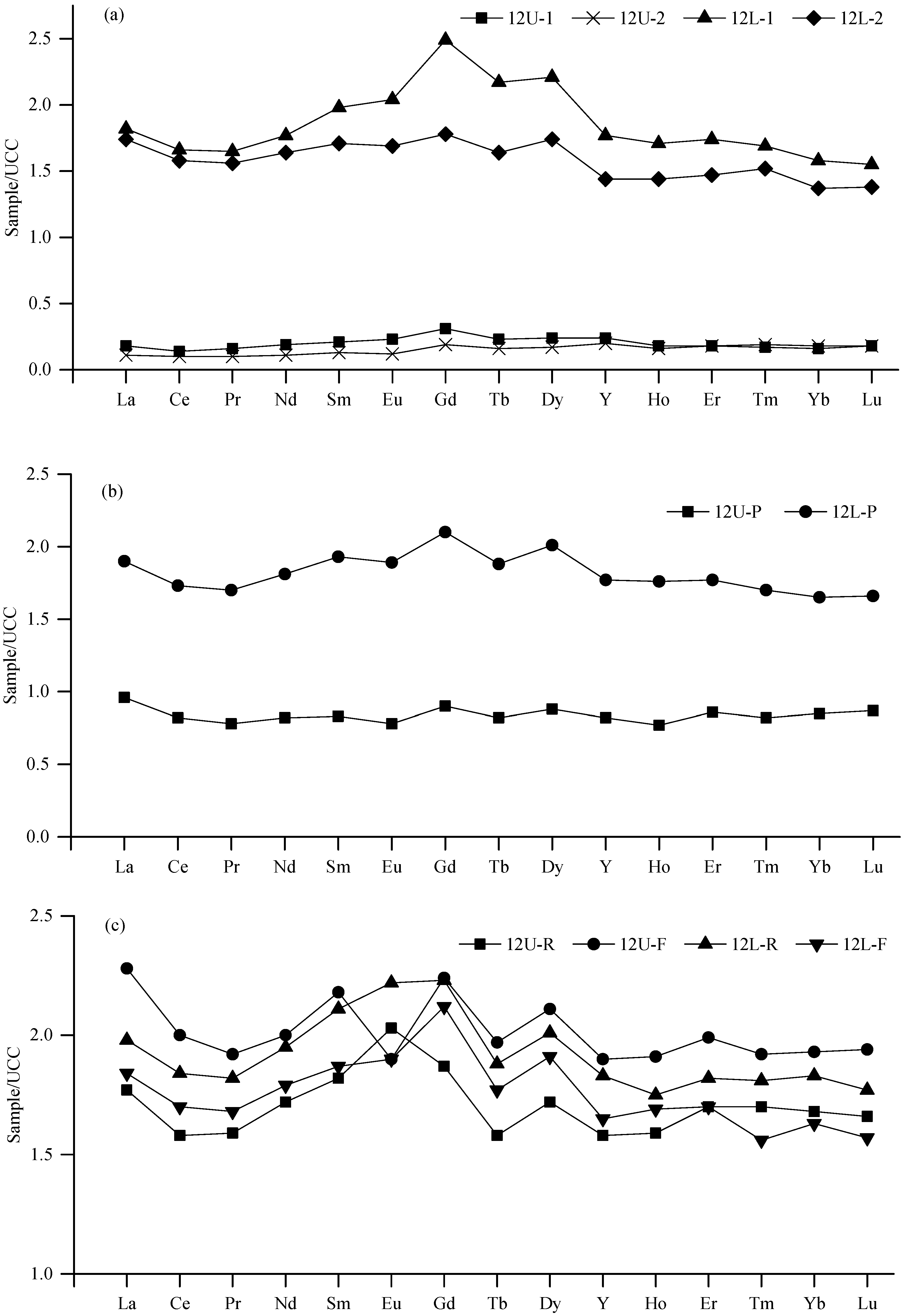
4.2.3. Rare Earth Elements and Yttrium (REY)
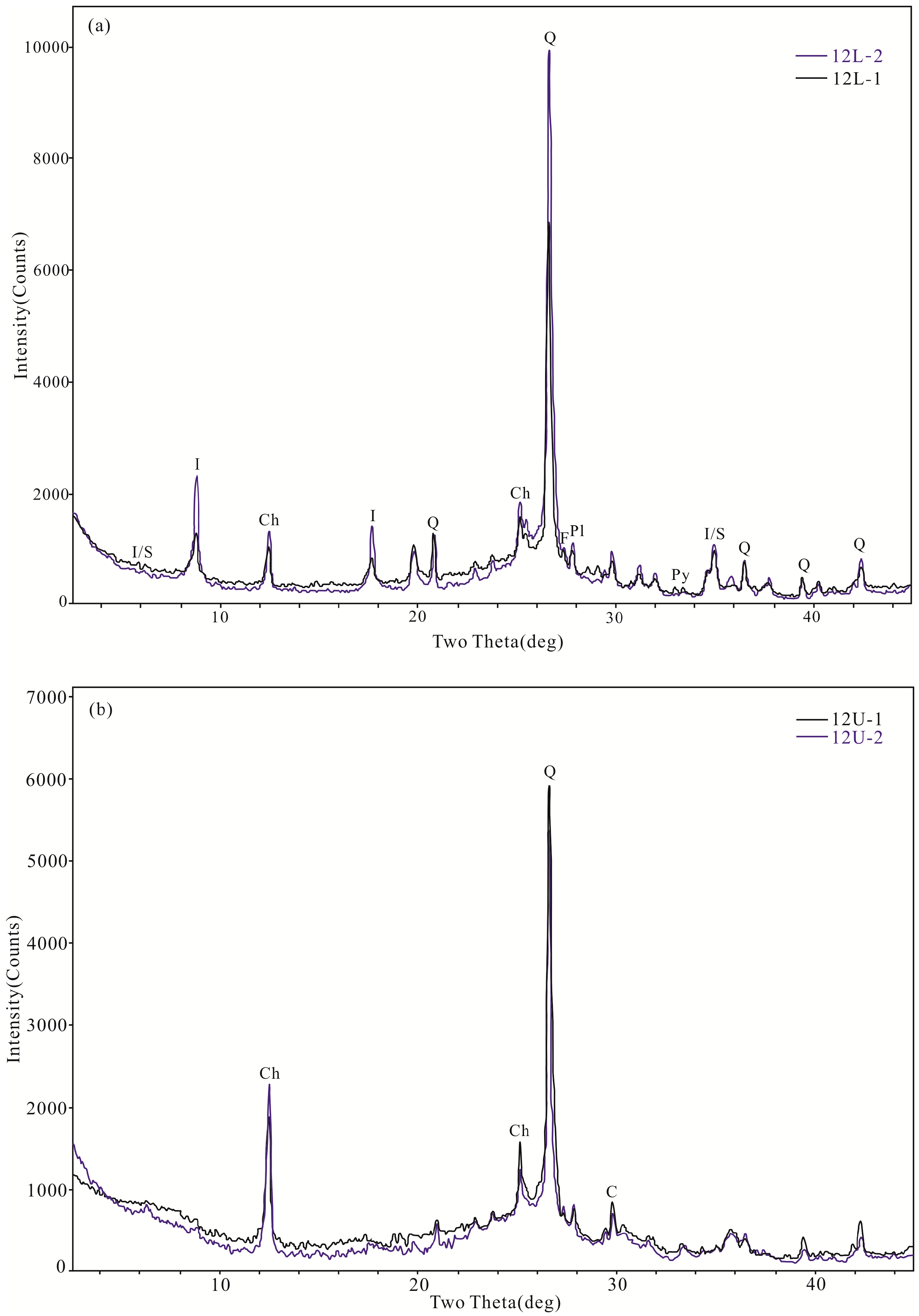
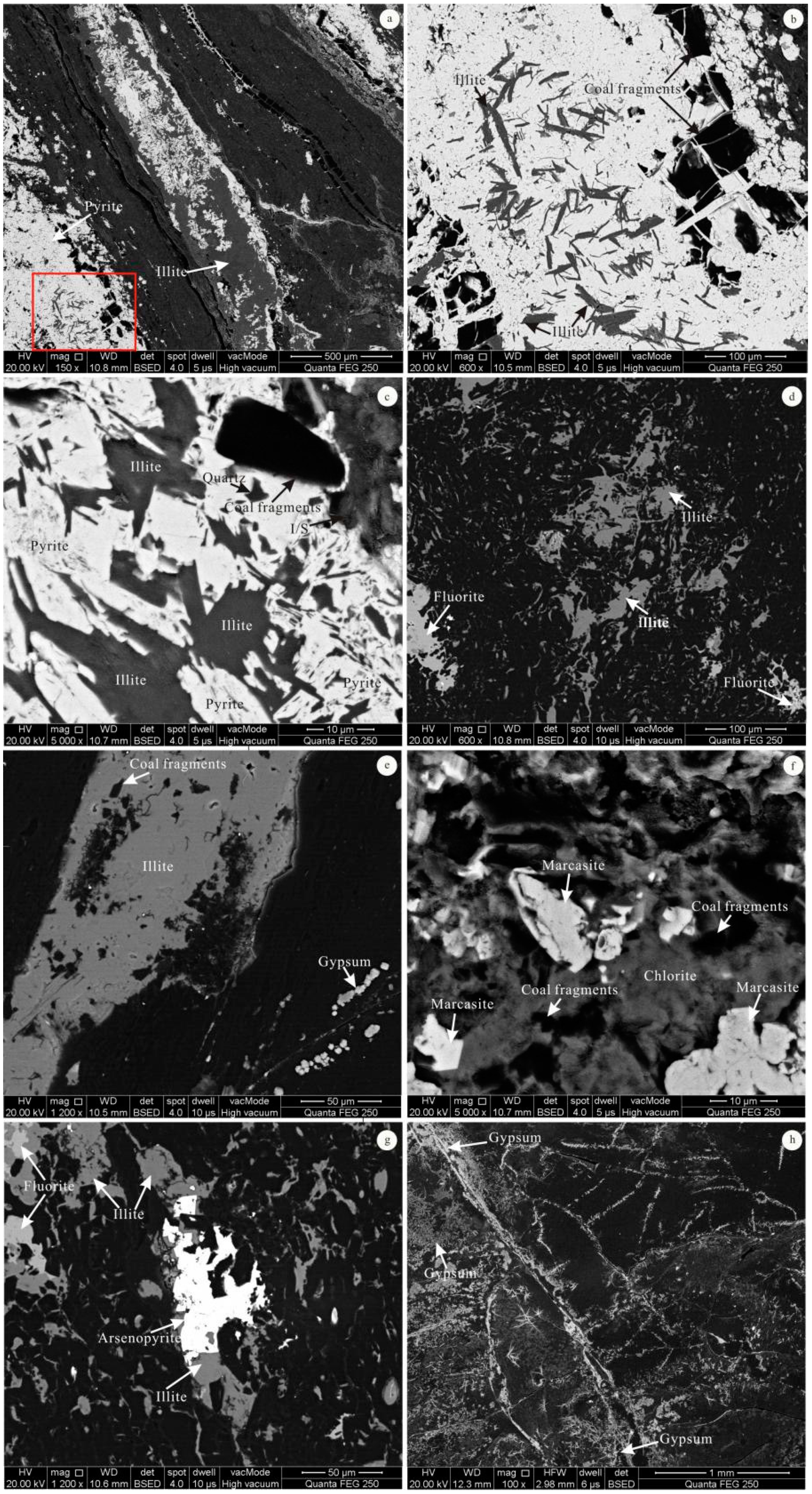
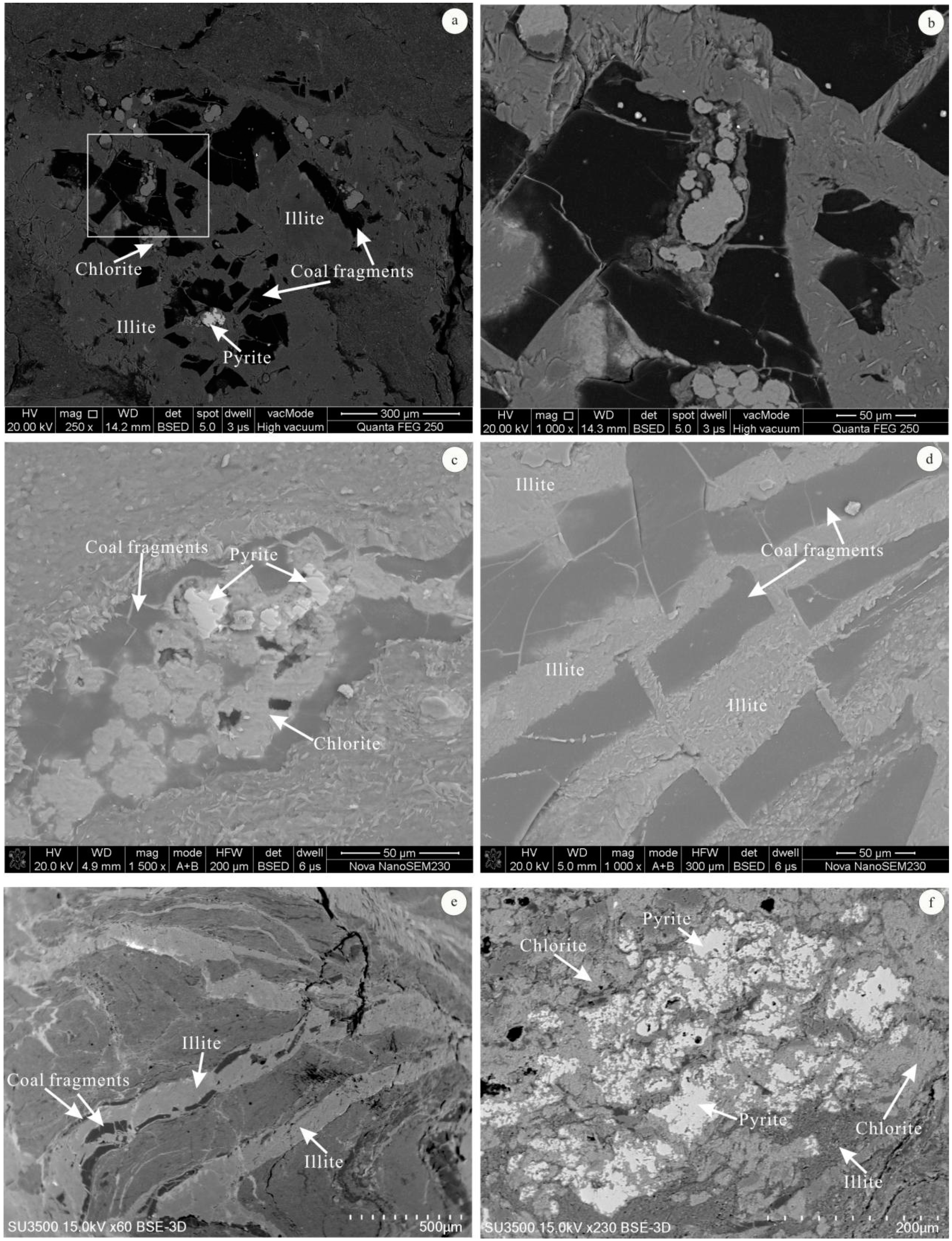
4.3. Minerals in the Coal
4.4. Modes of Occurrence of Minerals
5. Discussion
5.1. Enrichment Origin of Elements in the Coal: Magmatic Hydrothermal Fluids
5.2. Minerals Originating from Magmatic Hydrothermal Fluids
5.3. Mode of Occurrence of Rb, Cs, W, Pb, Zn and Cd
5.3.1. Rubidium
5.3.2. Cesium
5.3.3. Tungsten
5.3.4. Pb, Zn and Cd
5.4. Favorable Conditions for the Enrichment of W, Rb, and Cs in Coal
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dai, S.; Ren, D.; Chou, C.-L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Dai, S.; Wang, P.; Ward, C.R.; Tang, Y.; Song, X.; Jiang, J.; Hower, J.C.; Li, T.; Seredin, V.V.; Wagner, N.J.; et al. Elemental and mineralogical anomalies in the coal-hosted Ge ore deposit of Lincang, Yunnan, southwestern China: Key role of N2-CO2-mixed hydrothermal solutions. Int. J. Coal Geol. 2015, 152, 19–46. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Xiao, L.; Li, Y.; Liu, S.; Li, Z.; Zhao, B.; Ma, J.; Chu, G.; Gao, P.; et al. Significant enrichment of Ga, Rb, Cs, REEs and Y in the Jurassic No. 6 coal in the Iqe Coalfield, northern Qaidam Basin, China—A hidden gem. Ore Geol. Rev. 2017, 83, 1–13. [Google Scholar] [CrossRef]
- Seredin, V.V.; Finkelman, R.B. Metalliferous coals: A review of the main genetic and geochemical types. Int. J. Coal Geol. 2008, 76, 253–289. [Google Scholar] [CrossRef]
- Dai, S.; Xie, P.; Jia, S.; Ward, C.R.; Hower, J.C.; Yan, X.; French, D. Enrichment of U–Re–V–Cr–Se and rare earth elements in the Late Permian coals of the Moxinpo Coalfield, Chongqing; China: Genetic implications from geochemical and mineralogical data. Ore Geol. Rev. 2017, 80, 1–17. [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S. Coal deposits as potential alternative sources for lanthanides and yttrium. Int. J. Coal Geol. 2012, 94, 67–93. [Google Scholar] [CrossRef]
- Hower, J.C.; Granite, E.J.; Mayfield, D.B.; Lewis, A.S.; Finkelman, R.B. Notes on contributions to the science of rare earth element enrichment in coal and coal combustion byproducts. Minerals 2016, 6, 32. [Google Scholar] [CrossRef]
- Dai, S.; Finkelman, R.B. Coal as a promising source of critical elements: Progress and future prospects. Int. J. Coal Geol. 2018, 186, 155–164. [Google Scholar] [CrossRef]
- Querol, X.; Alastuey, A.; Lopez-Soler, A.; Plana, F.; Fernandez-Turiel, J.L.; Zeng, R.; Xu, W.; Zhuang, X.; Spiro, B. Geological controls on the mineral matter and trace elements of coals from the Fuxin Basin, Liaoning province, northeast China. Int. J. Coal Geol. 1997, 34, 89–109. [Google Scholar] [CrossRef]
- Finkelman, R.B.; Bostick, N.H.; Dulong, F.T.; Senftle, F.E.; Thorpe, A.N. Influence of an igneous intrusion on the inorganic geochemistry of a bituminous coal from Pitkin County, Colorado. Int. J. Coal Geol. 1998, 36, 223–241. [Google Scholar] [CrossRef]
- Gurba, L.W.; Weber, C.R. Effects of igneous intrusions on coalbed methane potential, Gunnedah Basin, Australia. Int. J. Coal Geol. 2001, 46, 113–131. [Google Scholar] [CrossRef]
- Jiang, J.; Cheng, Y.; Wang, L.; Li, W.; Wang, L. Petrographic and geochemical effects of sill intrusions on coal and their implications for gas outbursts in the Wolonghu Mine, Huaibei Coalfield, China. Int. J. Coal Geol. 2011, 88, 55–66. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D. Effects of igneous intrusions on coal petrology, pore-fracture and coalbed methane characteristics in Hongyang, Handan and Huaibei Coalfields, north China. Int. J. Coal Geol. 2012, 96–97, 72–81. [Google Scholar] [CrossRef]
- Salmachi, A.; Rajabi, M.; Reynolds, P.; Yarmohammadtooski, Z.; Wainman, C. The effect of magmatic intrusions on coalbed methane reservoir characteristics: A case study from the Hoskissons coalbed, Gunnedah Basin, Australia. Int. J. Coal Geol. 2016, 165, 278–289. [Google Scholar] [CrossRef]
- Walker, R.; Mastalerz, M.; Brassell, S.; Elswick, E.; Hower, J.C. Chemistry of thermally altered high volatile bituminous coals from southern Indiana. Int. J. Coal Geol. 2007, 71, 2–14. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Y.; Chen, S.; Wang, H. The effects of igneous intrusions on coal-bed macerals, maturity, and adsorption. Energy Sources Part A 2017, 39, 58–66. [Google Scholar] [CrossRef]
- Golab, A.N.; Carr, P.F. Changes in geochemistry and mineralogy of thermally altered coal, Upper Hunter Valley, Australia. Int. J. Coal Geol. 2004, 57, 197–210. [Google Scholar] [CrossRef]
- Suárez-Ruiz, I.; Flores, D.; Marques, M.M.; Martinez-Tarazona, M.R.; Pis, J.; Rubiera, F. Geochemistry, mineralogy and technological properties of coals from Rio Maior (Portugal) and Peñarroya (Spain) Basins. Int. J. Coal Geol. 2006, 67, 171–190. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D. Effects of magmatic intrusion on mineralogy and geochemistry of coals from the Fengfeng−Handan Coalfield, Hebei, China. Energy Fuels 2007, 21, 1663–1673. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, G.; Qi, C.; Zhang, Y.; Wong, M. The use of sequential extraction to determine the distribution and modes of occurrence of mercury in Permian Huaibei coal, Anhui Province, China. Int. J. Coal Geol. 2008, 73, 139–155. [Google Scholar] [CrossRef]
- Dai, S.; Zou, J.; Jiang, Y.; Ward, C.R.; Wang, X.; Li, T.; Xue, W.; Liu, S.; Tian, H.; Sun, X.; et al. Mineralogical and geochemical compositions of the Pennsylvanian coal in the Adaohai Mine, Daqingshan Coalfield, Inner Mongolia, China: Modes of occurrence and origin of diaspore, gorceixite, and ammonian illite. Int. J. Coal Geol. 2012, 94, 250–270. [Google Scholar] [CrossRef]
- Chen, J.; Liu, G.; Li, H.; Wu, B. Mineralogical and geochemical responses of coal to igneous intrusion in the Pansan Coal Mine of the Huainan Coalfield, Anhui, China. Int. J. Coal Geol. 2014, 124, 11–35. [Google Scholar] [CrossRef]
- Goodarzi, F.; Cameron, A.R. Organic petrology and elemental distribution in thermally altered coals from Telkwa; British Columbia. Energy Sources 1990, 12, 315–343. [Google Scholar] [CrossRef]
- Wang, Y.; Mo, J.; Ren, D. Distribution of minor and trace elements in magmatic hydrothermal metamorphic coal of Meitian Coal Mine, Hunan Province. Geochimica 1999, 28, 289–296, (In Chinese with English abstract). [Google Scholar]
- Ward, C.R. Analysis, origin and significance of mineral matter in coal: An updated review. Int. J. Coal Geol. 2016, 165, 1–27. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, M.; Li, Y.; Zhao, Z.; Gao, J. Genetic types, mineralization styles, and geodynamic settings of Mesozoic tungsten deposits in South China. J. Asian Earth Sci. 2017, 137, 109–140. [Google Scholar] [CrossRef]
- Li, Z.; Hu, R.; Yang, J.; Peng, J.; Li, X.; Bi, X. He, Pb and S isotopic constraints on the relationship between the A-type Qitianling granite and the Furong tin deposit, Hunan Province, China. Lithos 2007, 97, 161–173. [Google Scholar] [CrossRef]
- Zhao, K.; Jiang, S.; Yang, S.; Dai, B.; Lu, J. Mineral chemistry, trace elements and Sr–Nd–Hf isotope geochemistry and petrogenesis of Cailing and Furong granites and mafic enclaves from the Qitianling batholith in the Shi-Hang zone, South China. Gondwana Res. 2012, 22, 310–324. [Google Scholar] [CrossRef]
- Yang, Z. Correlation of the coal rock bed in the Meitian coalfield. Coal Geol. Explor. 1983, 2, 11–15, (In Chinese with English abstract). [Google Scholar]
- ASTM Standards D3173-11. Test Method for Moisture in the Analysis Sample of Coal and Coke; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- ASTM Standards D3174-11. Annual Book of ASTM Standards. Test Method for Ash in the Analysis Sample of Coal and Coke; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- ASTM Standards D3175-11. Test Method for Volatile Matter in the Analysis Sample of Coal and Coke; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- ASTM Standards D3177-02. Test Method for Total Sulur in the Analysis Sample of Coal and Coke (Reapproved 2007); ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- ASTM Standards D2798-05. Annual Book of ASTM Standards. Test Method for Microscopical Determination of the Vitrinite Reflectance of Coal, Gaseous Fuels, Coal and Coke; ASTM International: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Han, T.; Zhu, X.; Li, K.; Jiang, L.; Zhao, C.; Wang, Z. Metal sources for the polymetallic Ni–Mo–PGE mineralization in the black shales of the Lower Cambrian Niutitang Formation, South China. Ore Geol. Rev. 2015, 67, 158–169. [Google Scholar] [CrossRef]
- Chou, C.-L. Sulfur in coals: A review of geochemistry and origins. Int. J. Coal Geol. 2012, 100, 1–13. [Google Scholar] [CrossRef]
- ASTM Standards D388-12. Standards Classification of Coal by Rank; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Classification for Quality of Coal: Part 1. GB/T 15224.1-2018; Standards Press of China: Beijing, China, 2018. (In Chinese)
- Dai, S.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Xing, Y.; Zhang, W. Enrichment of U–Se–Mo–Re–V in coals preserved within marine carbonate successions: Geochemical and mineralogical data from the Late Permian Guiding Coalfield, Guizhou, China. Miner. Depos. 2015, 50, 159–186. [Google Scholar] [CrossRef]
- Ketris, M.P.; Yudovich, Y. Estimations of Clarkes for Carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-formations, Transvaal Supergroup, South Africa. Precambrian Res. 1996, 79, 37–55. [Google Scholar] [CrossRef]
- Dai, S.; Graham, I.T.; Ward, C.R. A review of anomalous rare earth elements and yttrium in coal. Int. J. Coal Geol. 2016, 159, 82–95. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell: Oxford, UK, 1985; p. 312. [Google Scholar]
- Cobb, J.; Steele, J.D.; Treworgy, C.G.; Ashby, J.F. The abundance of zinc and cadmium in sphalerite-bearing coals in Illinois. In Illinois Mineral Note; Illinois State Geological Survey: Champaign, IL, USA, 1980; Volume 74, p. 33. [Google Scholar]
- Dawson, G.K.W.; Golding, S.D.; Esterle, J.S.; Massarotto, P. Occurrence of minerals within fractures and matrix of selected Bowen and Ruhr Basin coals. Int. J. Coal Geol. 2012, 94, 150–166. [Google Scholar] [CrossRef]
- Dai, S.; Hower, J.C.; Ward, C.R.; Guo, W.; Song, H.; O’Keefe, J.M.K.; Xie, P.; Hood, M.M.; Yan, X. Elements and phosphorus minerals in the middle Jurassic inertinite-rich coals of the Muli Coalfield on the Tibetan Plateau. Int. J. Coal Geol. 2015, 144–145, 23–47. [Google Scholar] [CrossRef]
- Eskenazy, G.M. The geochemistry of tungsten in Bulgarian coals. Int. J. Coal Geol. 1982, 2, 99–111. [Google Scholar] [CrossRef]
- Dai, S.; Wang, X.; Seredin, V.V.; Hower, J.C.; Ward, C.R.; O’Keefe, J.M.K.; Huang, W.; Li, T.; Li, X.; Liu, H.; et al. Petrology, mineralogy, and geochemistry of the Ge-rich coal from the Wulantuga Ge ore deposit, Inner Mongolia, China: New data and genetic implications. Int. J. Coal Geol. 2012, 90–91, 72–99. [Google Scholar] [CrossRef]
- Li, X.; Hu, R.; Bi, X.; Peng, J. Geochemistry and tin metallogenic potential for Qitianling granite mass in Southern Hunan. J. Jinlin Univ. 2010, 40, 80–92, (In Chinese with English abstract). [Google Scholar]
- He, H.; Wang, D.; Su, X.; Zhang, Y.; Wang, G.; Li, J.; Zhao, B.; Li, J. Geochemical characteristics and ore potential of rare metal elements of the Qitianlin batholith in South Hunan, China. Geoteetonica Metallog. 2014, 38, 366–374, (In Chinese with English abstract). [Google Scholar]
- Kisch, H.J.; Taylor, G.H. Metamorphism and alteration near an intrusive coal contract. Econ. Geol. 1966, 61, 343–361. [Google Scholar] [CrossRef]
- Dai, S.; Chou, C.-L.; Yue, M.; Luo, K.; Ren, D. Mineralogy and geochemistry of a Late Permian coal in the Dafang Coalfield, Guizhou, China: Influence from siliceous and iron-rich calcic hydrothermal fluids. Int. J. Coal Geol. 2005, 61, 241–258. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, W.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Song, W.; Wang, X.; Li, X.; Zhao, L.; Kang, H.; et al. Factors controlling geochemical and mineralogical compositions of coals preserved within marine carbonate successions: A case study from the Heshan Coalfield, southern China. Int. J. Coal Geol. 2013, 109–110, 77–100. [Google Scholar] [CrossRef]
- Dai, S.; Xie, P.; French, D.; Ward, C.R.; Graham, I.T.; Yan, X.; Guo, W. The occurrence of buddingtonite in super-high-organic-sulphur coals from the Yishan Coalfield, Guangxi, southern China. Int. J. Coal Geol. 2018, 195, 347–361. [Google Scholar] [CrossRef]
- Xie, L.; Wang, R.; Chen, J.; Zhu, J. Mineralogical evidence for magmatic and hydrothermal processes in the Qitianling oxidized tin-bearing granite (Hunan, South China): EMP and (MC)-LA-ICPMS investigations of three types of titanite. Chem. Geol. 2010, 276, 53–68. [Google Scholar] [CrossRef]
- Pei, X.; Qian, Z.; Shi, G. A mineralogical study of the Chuncheon nephrite, South Korea. Acta Petrol. Mineral. 2011, 30, 89–94, (In Chinese with English abstract). [Google Scholar]
- Finkelman, R.B.; Palmer, C.A.; Wang, P. Quantification of the modes of occurrence of 42 elements in coal. Int. J. Coal Geol. 2018, 185, 138–160. [Google Scholar] [CrossRef]
- Yudovich, Y.; Ketris, M. Valuable Trace Elements in Coal; RAS: Ekaterinburg, Russia, 2006; p. 538.
- Palmer, C.A.; Filby, R.H. Distribution of trace elements in coal from the Powhatan No. 6 mine, Ohio. Fuel 1984, 63, 318–328. [Google Scholar] [CrossRef]
- Qin, S.; Lu, Q.; Li, Y.; Wang, J.; Zhao, Q.; Gao, K. Relationships between trace elements and organic matter in coals. J. Geochem. Explor. 2018, 188, 101–110. [Google Scholar] [CrossRef]
- Swaine, D.J. Trace Elements in Coal; Butterworths: London, UK, 1990. [Google Scholar]
- Seredin, V.V. Anomalous concentrations of trace elements in the Spetsugli Germanium Deposit (Pavlovka Brown Coal Deposit; southern Primorye): Communication 2. Rubidium and Cesium. Lithol. Miner. Resour. 2003, 38, 233–241. [Google Scholar] [CrossRef]
- Seredin, V.V.; Danilcheva, Y.A.; Magazina, L.O.; Sharova, I.G. Ge-bearing coals of the Luzanovka Graben, Pavlovka brown coal deposit, southern Primorye. Lithol. Miner. Resour. 2006, 41, 280–301. [Google Scholar] [CrossRef]
- Etschmann, B.; Liu, W.; Li, K.; Dai, S.; Reith, F.; Falconer, D.; Kerr, G.; Paterson, D.; Howard, D.; Kappen, P.; et al. Enrichment of germanium and associated arsenic and tungsten in coal and roll-front uranium deposits. Chem. Geol. 2017, 463, 29–49. [Google Scholar] [CrossRef]
- Kortenski, J.; Sotirov, A. Trace and major element content and distribution in Neogene lignite from the Sofia Basin; Bulgaria. Int. J. Coal Geol. 2002, 52, 63–82. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Chou, C.-L.; Li, S.; Jiang, Y. Mineralogy and geochemistry of the no. 6 coal (Pennsylvanian) in the Junger Coalfield, Ordos Basin, China. Int. J. Coal Geol. 2006, 66, 253–270. [Google Scholar] [CrossRef]
- Finkelman, R.B. Modes of occurrence of potentially hazardous elements in coal: Levels of confidence. Fuel Process. Technol. 1994, 39, 21–34. [Google Scholar] [CrossRef]
- Mastalerz, M.; Drobniak, A. Arsenic, cadmium, lead, and zinc in the Danville and Springfield coal members (Pennsylvanian) from Indiana. Int. J. Coal Geol. 2007, 71, 37–53. [Google Scholar] [CrossRef]
- Linnen, R.L. The solubility of Nb-Ta-Zr-Hf-W in granitic melts with Li and Li+F: Constraints for mineralization in rare metal granites and pegmatites. Econ. Geol. 1998, 93, 1013–1025. [Google Scholar] [CrossRef]
- O’Neill, H.S.C.; Berry, A.J.; Eggins, S.M. The solubility and oxidation state of tungsten in silicate melts: Implications for the comparative chemistry of W and Mo in planetary differentiation processes. Chem. Geol. 2008, 255, 346–359. [Google Scholar] [CrossRef]
- Che, X.; Linnen, R.L.; Wang, R.; Aseri, A.; Thibault, Y. Tungsten solubility in evolved granitic melts: An evaluation of magmatic wolframite. Geoteetoniea Metallog. 2013, 106, 84–98. [Google Scholar] [CrossRef]
- Lackschewitz, K.S.; Devey, C.W.; Stoffers, P.; Botz, R.; Eisenhauer, A.; Kummetz, M.; Schmidt, M.; Singer, A. Mineralogical, geochemical and isotopic characteristics of hydrothermal alteration processes in the active; submarine, felsic-hosted PACMANUS field, Manus basin, Papua New Guinea. Geoteetoniea Metallog. 2004, 68, 4405–4427. [Google Scholar] [CrossRef]
- Alekandre, P.; Kyser, K.; Polito, P.; Thomas, D. Alteration mineralogy and stable isotope geochemistry of paleoproterozoic basement hosted unconformity-type uranium deposits in the Athabasca Basin, Canada. Econ. Geol. 2005, 100, 1547–1563. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Wang, S.; Liu, Y.; Qin, W. Metallognic mechanism of Dayingezhuang gold deposit, northwestern Jiaodong Peninsula: Geochemistry constrains from the gold bearing pyrite typomorph and sulfur isotope. Acta Petrol. Sin. 2016, 32, 2451–2464, (In Chinese with English abstract). [Google Scholar]
- Inoue, A.; Kitagawa, R. Morphological characteristics of illitic clay minerals from a hydrothermal system. Am. Mineral. 1994, 79, 700–711. [Google Scholar]
- Jin, Z.; Zhu, J.; Ji, J.; Li, F.; Lu, X. Two origins of illite at the dexing porphyry Cu deposit; east China: Implications for ore-forming fluid constraint on illite crystallinity. Clays Clay Miner. 2002, 50, 381–387. [Google Scholar]
- Brockamp, O.; Schlegel, A.; Wemmer, K. Complex hydrothermal alteration and illite K-Ar ages in Upper Visean molasse sediments and magmatic rocks of the Variscan Badenweiler-Lenzkirch suture zone, Black Forest, Germany. Int. J. Earth Sci. 2015, 104, 683–702. [Google Scholar] [CrossRef]





| Coal Seam | Sample No. | Thickness (cm) | Mad | Ad | Vdaf | St,d | Ro, ran ± Stdev |
|---|---|---|---|---|---|---|---|
| 12U | 12U-1 | 15 | 2.58 | 7.07 | 11.18 | 0.38 | 2.64 ± 0.35 |
| 12U-2 | 10 | 4.82 | 13.44 | 8.15 | 0.43 | 2.96 ± 0.24 | |
| 12L | 12L-1 | 25 | 2.39 | 46.80 | 8.19 | 0.64 | 2.89 ± 0.41 |
| 12L-2 | 30 | 6.13 | 44.14 | 6.62 | 0.52 | 3.45 ± 0.16 | |
| 12U | Av | 12.5 | 3.70 | 10.26 | 9.67 | 0.41 | 2.80 ± 0.23 |
| 12L | Av | 27.5 | 4.26 | 45.47 | 7.41 | 0.58 | 3.17 ± 0.46 |
| Sample No. | LOI | SiO2 | TiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | P2O5 | SiO2/Al2O3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 12U-R | 28.71 | 59.75 | 0.94 | 15.02 | 10.17 | 0.69 | 2.35 | 0.50 | 1.86 | 0.28 | 3.98 |
| 12U-1 | 92.96 | 36.82 | 1.06 | 11.89 | 23.84 | 0.76 | 3.89 | 0.51 | 0.98 | 0.36 | 3.10 |
| 12U-P | 30.52 | 68.28 | 1.08 | 21.41 | 2.60 | 0.62 | 2.40 | 0.50 | 2.81 | 0.35 | 3.19 |
| 12U-2 | 86.60 | 36.76 | 0.80 | 13.26 | 21.97 | 0.57 | 1.45 | 0.50 | 1.31 | 0.44 | 2.77 |
| 12U-F | 19.89 | 67.96 | 0.80 | 12.00 | 4.89 | 0.74 | 4.24 | 0.49 | 1.86 | 0.31 | 5.66 |
| 12U-WA | 90.42 | 36.80 | 0.96 | 12.44 | 23.09 | 0.68 | 2.91 | 0.51 | 1.11 | 0.39 | 2.96 |
| 12L-R | 24.12 | 66.43 | 1.05 | 25.68 | 3.72 | 1.04 | 0.70 | 0.50 | 3.67 | 0.35 | 2.59 |
| 12L-1 | 53.27 | 54.94 | 1.17 | 30.58 | 7.83 | 0.95 | 1.01 | 0.52 | 4.17 | 0.40 | 1.80 |
| 12L-P | 23.85 | 66.29 | 1.15 | 24.54 | 2.56 | 0.72 | 0.85 | 0.51 | 3.72 | 0.34 | 2.70 |
| 12L-2 | 55.90 | 57.28 | 1.27 | 31.86 | 5.54 | 1.16 | 1.29 | 0.53 | 4.43 | 0.37 | 1.80 |
| 12L-F | 26.12 | 66.13 | 1.14 | 26.69 | 2.35 | 1.08 | 1.00 | 0.51 | 3.94 | 0.36 | 2.48 |
| 12L-WA | 54.70 | 56.22 | 1.22 | 31.28 | 6.58 | 1.06 | 1.16 | 0.53 | 4.31 | 0.38 | 1.80 |
| China a | n.d. | 8.47 | 0.33 | 5.98 | 4.85 | 0.22 | 1.23 | 0.16 | 0.19 | 0.09 | 1.42 |
| Element | 12U Beaches | 12L Beaches | World | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12U-R | 12U-1 | 12U-P | 12U-2 | 12U-F | 12U-WA | 12L-R | 12L-1 | 12L-P | 12L-2 | 12L-F | 12L-WA | ||
| Li | 33 | 17 | 31 | 33 | 51 | 23 | 103 | 59 | 29 | 33 | 34 | 45 | 14 |
| Be | 4.9 | 0.81 | 5.2 | 2.2 | 9.1 | 1.4 | 7.4 | 8.9 | 7.2 | 6.8 | 6.7 | 7.8 | 2.0 |
| Sc | 14 | bdl | 4.3 | bdl | 15 | bdl | 16 | 14 | 14 | 14 | 14 | 14 | 3.7 |
| V | 144 | 16 | 57 | 16 | 134 | 16 | 123 | 131 | 141 | 153 | 126 | 143 | 28 |
| Cr | 69 | 1.0 | 29 | 2.9 | 78 | 1.8 | 75 | 72 | 70 | 70 | 66 | 71 | 17 |
| Co | 17 | 1.2 | 15 | 14 | 29 | 6.5 | 21 | 16 | 26 | 20 | 28 | 18 | 6.0 |
| Ni | 37 | 15 | 22 | 20 | 37 | 17 | 26 | 37 | 50 | 37 | 43 | 37 | 17 |
| Cu | 40 | 3.8 | 5.8 | 5.9 | 9.8 | 4.7 | 55 | 47 | 40 | 41 | 44 | 44 | 16 |
| Zn | 122 | 100 | 59 | 105 | 84 | 102 | 379 | 196 | 247 | 218 | 412 | 208 | 28 |
| Ga | 25 | 2.1 | 12 | 4.1 | 26 | 2.9 | 27 | 25 | 28 | 26 | 25 | 25 | 6.0 |
| Ge | 1.7 | 0.12 | 0.87 | 0.30 | 2.0 | 0.19 | 2.4 | 1.7 | 1.7 | 1.9 | 2.0 | 1.8 | 2.4 |
| As | 7.4 | 1.5 | 14 | 15 | 20 | 6.9 | 2.8 | 5.6 | 3.5 | 8.0 | 4.3 | 6.9 | 8.3 |
| Rb | 235 | 4.8 | 111 | 16 | 256 | 9.1 | 184 | 242 | 221 | 228 | 225 | 234 | 18 |
| Sr | 141 | 20 | 124 | 29 | 254 | 24 | 230 | 145 | 125 | 116 | 208 | 129 | 100 |
| Y | 35 | 5.2 | 18 | 4.4 | 42 | 4.9 | 40 | 39 | 39 | 32 | 37 | 35 | 8.4 |
| Zr | 169 | 22 | 102 | 20 | 274 | 21 | 243 | 174 | 212 | 186 | 187 | 181 | 36 |
| Nb | 17 | 2.9 | 11 | 2.0 | 27 | 2.5 | 24 | 18 | 21 | 18 | 20 | 18 | 4.0 |
| Mo | 5.6 | 2.8 | 2.8 | 1.8 | 4.9 | 2.4 | 4.0 | 6.7 | 3.8 | 2.2 | 5.9 | 4.2 | 2.1 |
| Cd | 0.40 | 0.42 | 0.55 | 0.91 | 0.72 | 0.62 | 1.1 | 0.59 | 0.98 | 0.84 | 1.4 | 0.73 | 0.20 |
| In | 0.11 | 0.03 | 0.08 | 0.10 | 0.16 | 0.06 | 0.11 | 0.15 | 0.13 | 0.13 | 0.11 | 0.14 | 0.04 |
| Sn | 5.1 | 0.20 | 11 | 10 | 23 | 4.3 | 11 | 5.0 | 6.2 | 5.4 | 5.4 | 5.2 | 1.4 |
| Sb | 7.4 | 0.53 | 1.6 | 0.73 | 6.0 | 0.61 | 3.7 | 1.9 | 0.82 | 0.58 | 1.7 | 1.2 | 1.0 |
| Cs | 15 | 0.67 | 18 | 2.3 | 32 | 1.3 | 25 | 29 | 22 | 24 | 23 | 26 | 1.1 |
| Ba | 770 | 10 | 304 | 21 | 722 | 14 | 543 | 682 | 766 | 696 | 788 | 690 | 150 |
| La | 53 | 5.3 | 29 | 3.2 | 68 | 4.5 | 60 | 55 | 57 | 52 | 55 | 53 | 11 |
| Ce | 101 | 9.2 | 53 | 6.2 | 128 | 8.0 | 118 | 106 | 111 | 101 | 109 | 103 | 23 |
| Pr | 11 | 1.1 | 5.6 | 0.73 | 14 | 0.97 | 13 | 12 | 12 | 11 | 12 | 11 | 3.4 |
| Nd | 45 | 4.9 | 21 | 2.9 | 52 | 4.1 | 51 | 46 | 47 | 43 | 47 | 44 | 12 |
| Sm | 8.2 | 0.94 | 3.7 | 0.61 | 9.8 | 0.81 | 9.5 | 8.9 | 8.7 | 7.9 | 8.4 | 8.3 | 2.2 |
| Eu | 1.8 | 0.21 | 0.68 | 0.11 | 1.7 | 0.17 | 2.0 | 1.8 | 1.7 | 1.5 | 1.7 | 1.6 | 0.43 |
| Gd | 7.1 | 1.2 | 3.4 | 0.74 | 8.5 | 1.0 | 8.5 | 9.5 | 8.0 | 6.8 | 8.1 | 8.0 | 2.7 |
| Tb | 1.0 | 0.15 | 0.52 | 0.10 | 1.3 | 0.13 | 1.2 | 1.4 | 1.2 | 1.1 | 1.1 | 1.2 | 0.31 |
| Dy | 6.0 | 0.85 | 3.1 | 0.61 | 7.4 | 0.75 | 7.0 | 7.7 | 7.0 | 6.1 | 6.7 | 6.8 | 2.1 |
| Ho | 1.3 | 0.15 | 0.62 | 0.13 | 1.5 | 0.14 | 1.4 | 1.4 | 1.4 | 1.2 | 1.4 | 1.3 | 0.57 |
| Er | 3.9 | 0.40 | 2.0 | 0.42 | 4.6 | 0.41 | 4.2 | 4.0 | 4.1 | 3.4 | 3.9 | 3.7 | 1.0 |
| Tm | 0.56 | 0.06 | 0.27 | 0.06 | 0.63 | 0.06 | 0.60 | 0.56 | 0.56 | 0.50 | 0.52 | 0.53 | 0.30 |
| Yb | 3.7 | 0.34 | 1.9 | 0.39 | 4.3 | 0.36 | 4.0 | 3.5 | 3.6 | 3.0 | 3.6 | 3.2 | 1.0 |
| Lu | 0.53 | 0.06 | 0.28 | 0.06 | 0.62 | 0.06 | 0.57 | 0.50 | 0.53 | 0.44 | 0.50 | 0.47 | 0.20 |
| Hf | 4.7 | 0.46 | 2.7 | 0.50 | 7.5 | 0.48 | 6.8 | 4.9 | 5.3 | 4.8 | 5.0 | 4.9 | 1.2 |
| Ta | 1.4 | 0.17 | 0.87 | 0.10 | 2.2 | 0.14 | 2.1 | 1.5 | 1.7 | 1.4 | 1.7 | 1.5 | 0.30 |
| W | 11 | 2.3 | 5.1 | 1.1 | 12 | 1.8 | 8.0 | 9.4 | 9.2 | 11 | 8.6 | 10 | 0.99 |
| Tl | 3.7 | 0.12 | 0.64 | 0.15 | 1.5 | 0.13 | 1.1 | 1.6 | 1.6 | 1.4 | 1.6 | 1.5 | 0.58 |
| Pb | 58 | 128 | 61 | 45 | 75 | 95 | 63 | 45 | 20 | 13 | 26 | 28 | 9.0 |
| Bi | 0.35 | 0.81 | 0.30 | 0.20 | 0.50 | 0.57 | 0.45 | 0.54 | 0.25 | 0.17 | 0.32 | 0.34 | 1.1 |
| Th | 22 | 1.1 | 10 | 0.96 | 29 | 1.1 | 27 | 22 | 24 | 22 | 23 | 22 | 3.2 |
| U | 13 | 0.51 | 3.4 | 0.50 | 7.9 | 0.51 | 7.4 | 11 | 6.7 | 6.0 | 7.3 | 8.1 | 1.9 |
| Sample No. | REY | LREY | MREY | HREY | LaN/LuN | LaN/SmN | GdN/LuN | Eu/Eu* | Ce/Ce* | Gd/Gd* | YN/HoN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 12U-R | 279.48 | 218.39 | 51.12 | 9.97 | 1.07 | 0.97 | 1.13 | 1.16 | 0.94 | 1.13 | 1.01 |
| 12U-1 | 30.07 | 21.45 | 7.61 | 1.01 | 0.95 | 0.85 | 1.64 | 1.10 | 0.85 | 1.36 | 1.27 |
| 12U-P | 142.67 | 111.88 | 25.79 | 5 | 1.10 | 1.16 | 1.03 | 0.94 | 0.94 | 1.10 | 1.06 |
| 12U-2 | 20.72 | 13.68 | 5.98 | 1.06 | 0.58 | 0.80 | 1.04 | 0.88 | 0.91 | 1.30 | 1.24 |
| 12U-F | 344.03 | 271.69 | 60.73 | 11.61 | 1.18 | 1.05 | 1.16 | 0.91 | 0.95 | 1.10 | 1.00 |
| 12U-WA | 26.33 | 18.34 | 6.96 | 1.03 | 0.80 | 0.83 | 1.40 | 1.03 | 0.87 | 1.35 | 1.28 |
| 12L-R | 320.35 | 250.59 | 58.97 | 10.79 | 1.11 | 0.94 | 1.25 | 1.09 | 0.97 | 1.14 | 1.05 |
| 12L-1 | 296.59 | 227.4 | 59.28 | 9.91 | 1.17 | 0.92 | 1.59 | 1.00 | 0.95 | 1.18 | 1.03 |
| 12L-P | 302.76 | 235.8 | 56.77 | 10.19 | 1.15 | 0.98 | 1.27 | 0.99 | 0.96 | 1.11 | 1.00 |
| 12L-2 | 270.37 | 214.82 | 47.07 | 8.48 | 1.27 | 0.99 | 1.29 | 1.00 | 0.96 | 1.07 | 1.00 |
| 12L-F | 295.45 | 231.1 | 54.49 | 9.86 | 1.18 | 0.99 | 1.36 | 1.04 | 0.97 | 1.18 | 1.00 |
| 12L-WA | 282.17 | 220.42 | 52.62 | 9.13 | 1.21 | 0.97 | 1.43 | 1.00 | 0.95 | 1.13 | 1.02 |
| World | 68.61 | 51.6 | 13.94 | 3.07 | 0.015 | 0.22 | 1.23 | 0.16 | 0.19 | 0.092 | 1.42 |
| Sample No. | Quartz | K-Feldspar | Plagioclase | Calcite | Pyrite | Kaolinite | Illite | I/S | Chlorite |
|---|---|---|---|---|---|---|---|---|---|
| 12U-R | 39.0 | 0.5 | 4.1 | 3.2 | 45.8 | 7.4 | |||
| 12U-1 | 35.6 | 0.8 | 3.6 | 10.9 | 0.5 | 50.4 | |||
| 12U-P | 39.7 | 0.9 | 4.9 | 2.5 | 46.8 | 5.2 | |||
| 12U-2 | 34.9 | 3.6 | 4.8 | 5.2 | 2.4 | 4.9 | 41.2 | ||
| 12U-F | 48.6 | 0.2 | 4.1 | 7.3 | 26.7 | 13.1 | |||
| 12L-R | 34.7 | 1.4 | 3.3 | 52.1 | 8.5 | ||||
| 12L-1 | 26.8 | 2.0 | 2.0 | 0.6 | 8.9 | 31.6 | 16.5 | 11.7 | |
| 12L-P | 39.0 | 1.2 | 2.8 | 1.0 | 52.6 | 3.4 | |||
| 12L-2 | 28.5 | 1.9 | 4.2 | 4.9 | 41.7 | 18.8 | |||
| 12L-F | 39.2 | 1.4 | 3.1 | 4.1 | 47.5 | 4.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Z.; Cao, Y.; Jiang, W.; Zhou, P.; Huang, Y.; Liu, J. Minerals and Enrichment of W, Rb, and Cs in Late Permian Coal from Meitian Mine, Meitian Coalfield, Southern China by Magmatic Hydrothermal Fluids. Minerals 2018, 8, 504. https://doi.org/10.3390/min8110504
Xiao Z, Cao Y, Jiang W, Zhou P, Huang Y, Liu J. Minerals and Enrichment of W, Rb, and Cs in Late Permian Coal from Meitian Mine, Meitian Coalfield, Southern China by Magmatic Hydrothermal Fluids. Minerals. 2018; 8(11):504. https://doi.org/10.3390/min8110504
Chicago/Turabian StyleXiao, Zhenghui, Yunjiang Cao, Wei Jiang, Ping Zhou, Yanran Huang, and Jisong Liu. 2018. "Minerals and Enrichment of W, Rb, and Cs in Late Permian Coal from Meitian Mine, Meitian Coalfield, Southern China by Magmatic Hydrothermal Fluids" Minerals 8, no. 11: 504. https://doi.org/10.3390/min8110504
APA StyleXiao, Z., Cao, Y., Jiang, W., Zhou, P., Huang, Y., & Liu, J. (2018). Minerals and Enrichment of W, Rb, and Cs in Late Permian Coal from Meitian Mine, Meitian Coalfield, Southern China by Magmatic Hydrothermal Fluids. Minerals, 8(11), 504. https://doi.org/10.3390/min8110504





