Key Factors Affecting Strength Development of Steel Slag-Dredged Soil Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Steel Slags and Dredged Soils
2.1.2. Preparation of Steel Slag-Dredged Soil Mixtures
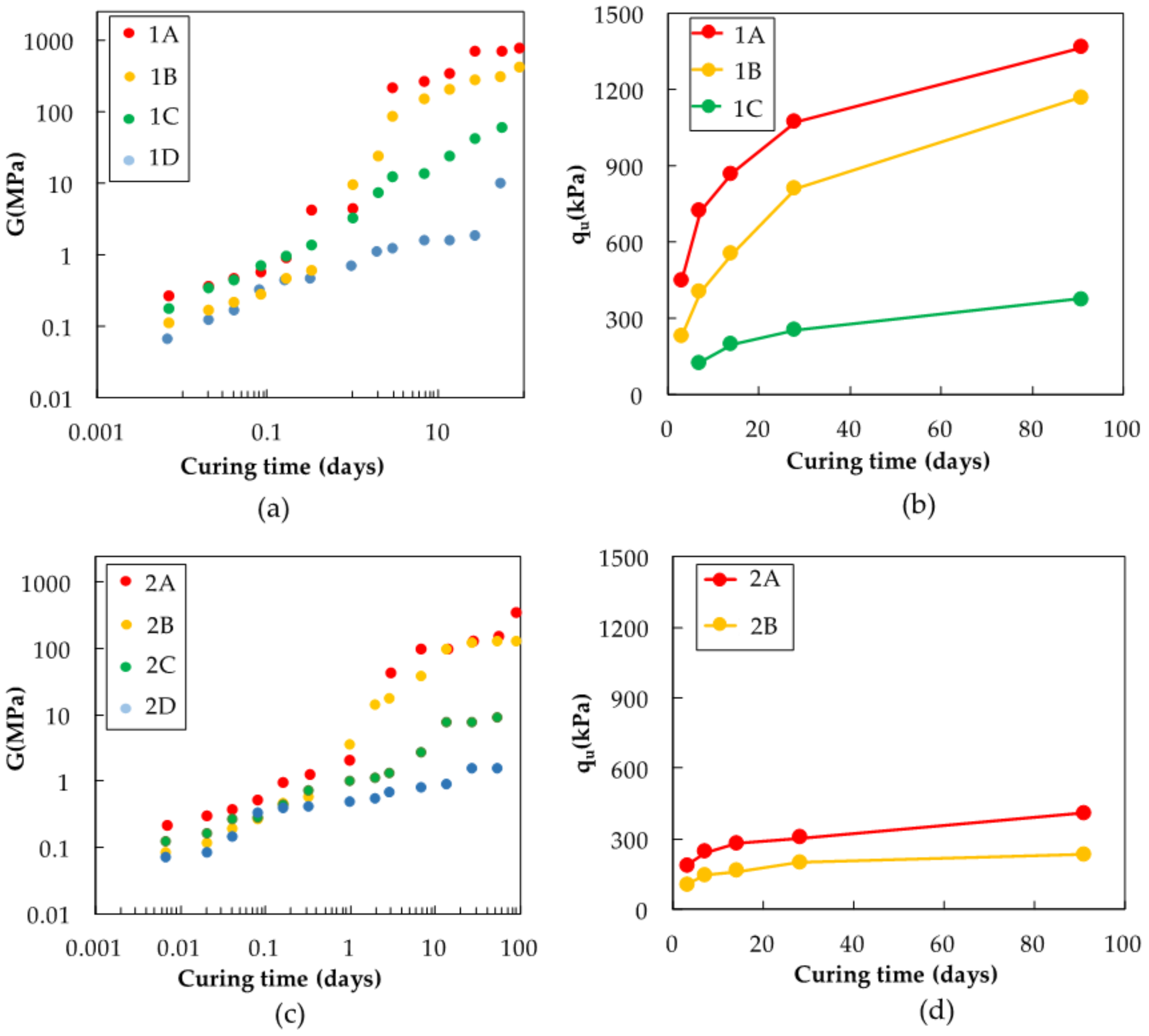
2.1.3. Physical Descriptions of Steel Slag-Dredged Soil Mixtures
2.2. Methods
2.2.1. Characterization of Starting Materials
2.2.2. Analyses of Steel Slag-Dredged Soil Mixtures
2.2.3. Analyses of the Solution Chemistry of Pore Water
3. Results
3.1. Characterization of the Starting Materials
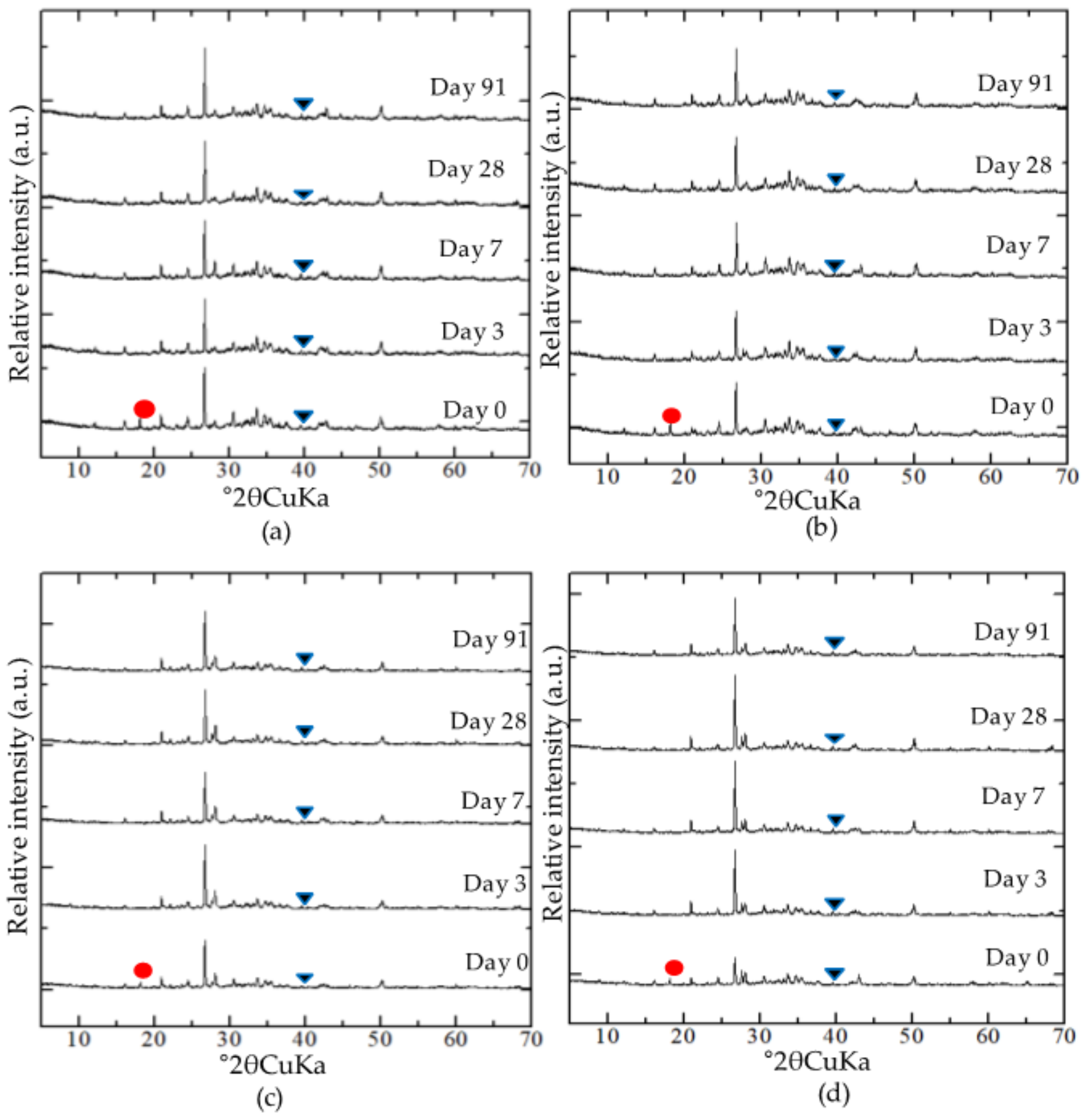
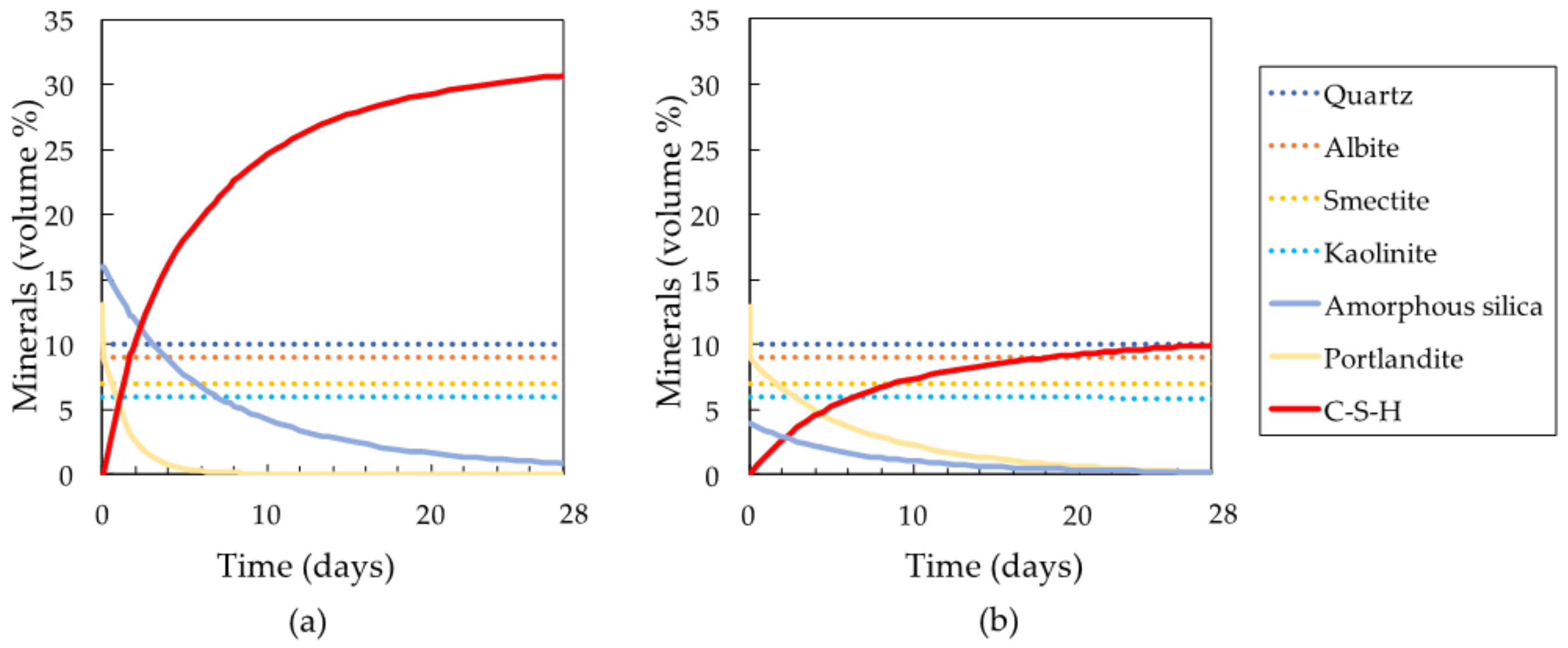
3.2. Secondary Phase Formation in the Mixtures
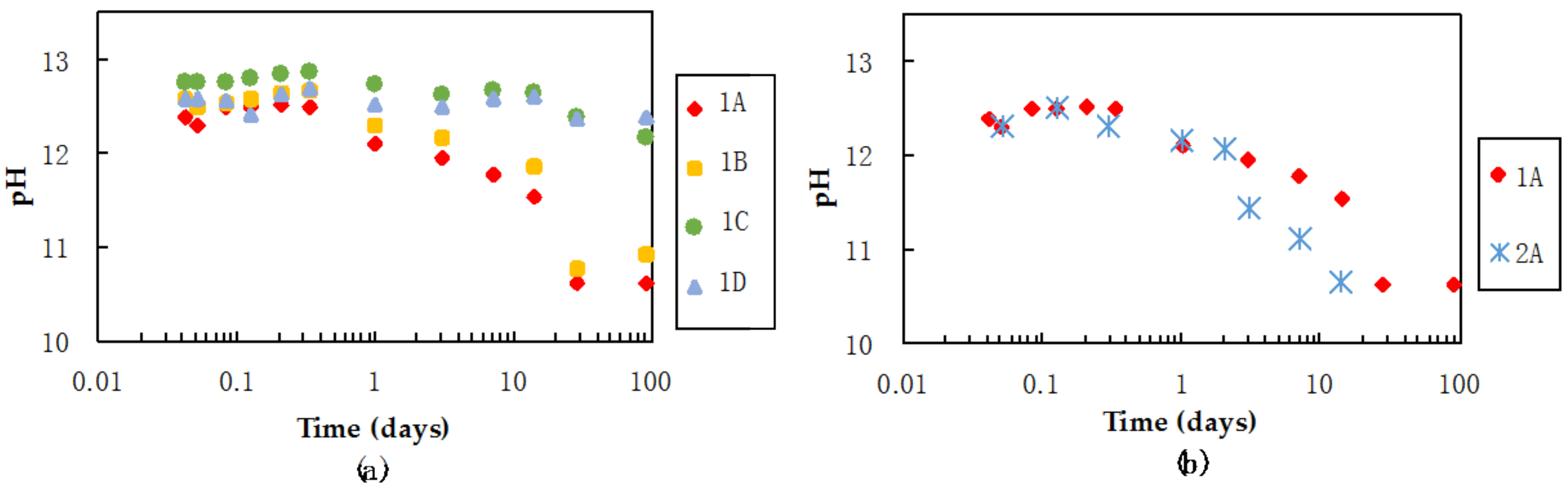
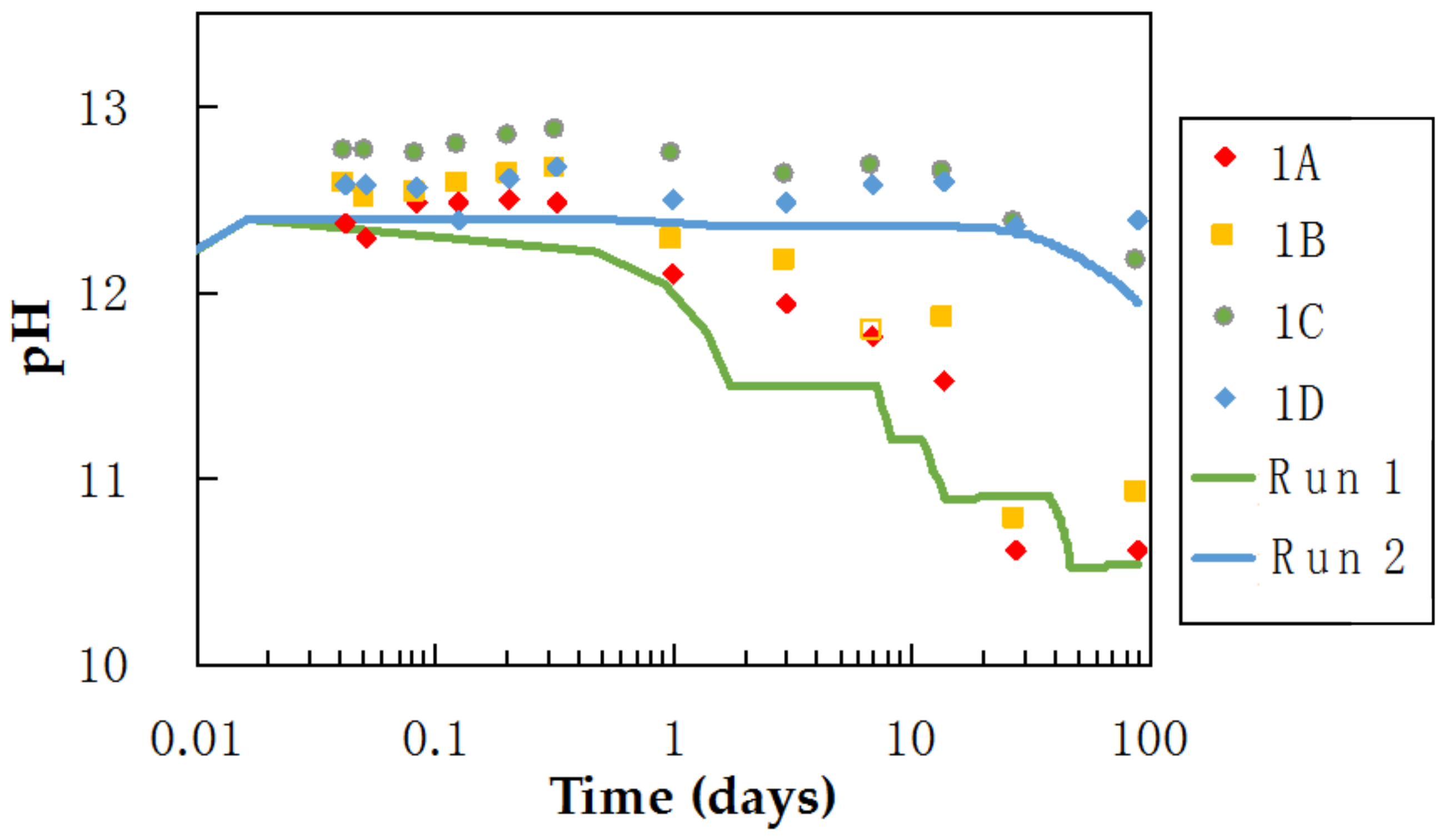
3.3. pH and Ca Concentrations of Pore Water in Mixtures during Curing
4. Discussion
4.1. Candidates for Factors Affecting the Formation of C-S-H
4.1.1. Factor (a): Calcium Ion Supply as a Factor in C-S-H Formation
4.1.2. Factor (b): pH of Pore Water in the Mixtures as a Factor in C-S-H Formation
4.1.3. Factor (c): Silica Supply as a Factor in C-S-H Formation
4.2. Key Factors That Are Important in the Formation of C-S-H
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Standard specification for coal fly ash and raw or calcined natural pozzolan for use as a mineral admixture in concrete. In American Society for Testing and Materials; ASTM International: West Conshohocken, PA, USA, 2008.
- Davraz, M.; Gunduz, L. Engineering properties of amorphous silica as a new natural pozzolan for use in concrete. Cem. Concr. Res. 2005, 35, 1251–1261. [Google Scholar] [CrossRef]
- Phoo-Ngernkham, T.; Chindaprasirt, P.; Sata, V.; Sinsiri, T. High calcium fly ash geopolymer containing diatomite as additive. Indian J. Eng. Mater. Sci. 2013, 20, 310–318. [Google Scholar]
- Horii, K.; Kato, T.; Sugahara, K.; Tsutsumi, N.; Kitano, Y. Overview of iron/steel slag application and development of new utilization technologies. Nippon Steel Sumitomo Technol. Rep. 2015, 109, 5–11. [Google Scholar]
- Lirer, S.; Liguori, B.; Capasso, I.; Flora, A.; Caputo, D. Mechanical and chemical properties of composite materials made of dredged sediments in a fly-ash based geopolymer. J. Environ. Manag. 2017, 191, 1–7. [Google Scholar] [CrossRef] [PubMed]
- UNESCO International Sediment Initiative. In Sediment Issues & Sediment Management in Large River Basins Interim Case Study Synthesis Report; UNESCO: Paris, France, 2011.
- Amundson, R.; Berhe, A.A.; Hopmans, J.W.; Olson, C.; Sztein, A.E.; Sparks, D.L. Soil and human security in the 21st century. Science 2015, 348, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Xu, G.; Cheng, H.; Wang, J.; Wan, Y.; Chen, H. An overview of utilization of steel slag. Procedia Environ. Sci. 2012, 16, 791–801. [Google Scholar] [CrossRef]
- Miraoui, M.; Zentar, R.; Abriak, N.E. Road material basis in dredged sediment and basic oxygen furnace steel slag. Constr. Build. Mater. 2012, 30, 309–319. [Google Scholar] [CrossRef]
- Kiso, E.; Tsujii, M.; Ito, K.; Nakagawa, M.; Gomyo, M.; Nagatome, T. Method of dredged soil improvement by mixing with converter steel-making slag. Kaiyo Kaihatsu Ronbunshu 2008, 24, 327–332. [Google Scholar]
- Kamon, M.; Tomohisa, S.; Sawa, K. On stabilization of hedoro by using cement group hardening materials. Zairyo 1989, 432, 1092–1097. [Google Scholar] [CrossRef]
- Kang, G.; Tsuchida, T.; Kim, Y.; Baek, W. Influence of humic acid on the strength behavior of cement-treated clay during various curing stages. J. Mater. Civ. Eng. 2017, 29, 1–18. [Google Scholar] [CrossRef]
- Fukushima, M.; Yamamoto, M.; Komai, T.; Yamamoto, K. Studies of structural alterations of humic acids from conifer bark residue during composting by pyrolysis-gas chromatography/mass spectrometry using tetramethylammonium hydroxide (TMAH-py-GC/MS). J. Anal. Appl. Pyrolysis 2009, 86, 200–206. [Google Scholar] [CrossRef]
- Weerakoon, N.; Nishimura, S.; Sato, H.; Toda, K.; Sato, T.; Arai, Y. Laboratory estimation of stiffness and strength mobilization in steel slag-mixed dredged clays with curing time. In Proceedings of the 3rd International Conference on Ground Improvement and Ground Control, Hangzhou, China, 27–29 October 2017. [Google Scholar]
- Sato, H.; Nishimura, S.; Sato, T.; Toda, K.; Arai, Y. Characteristics and interpretation of development of strength and stiffness for early-age Calcia-stabilized dredged soils. In Proceedings of the Japanese Geotechnical Society, Hokkaido Branch, Japan, 28–29 January 2016; pp. 4–5. [Google Scholar]
- Mortlock, R.A.; Froelich, P.N. A simple method for the rapid determination of biogenic opal in pelagic marine sediments. Deep Sea Res. Part A Oceanogr. Res. Pap. 1989, 36, 1415–1426. [Google Scholar] [CrossRef]
- Kodama, H. Identification and quantification of non-crystalline inorganic minerals in soils by selective chemical dissolution method. Chisitsu News 1995, 496, 26–35. [Google Scholar]
- Vlcek, J.; Tomkova, V.; Ovcacikova, H.; Ovcacik, F.; Topinkova, M.; Matejka, V. Slags from steel production: Properties and their utilization. Metallurgy 2013, 52, 329–333. [Google Scholar]
- Richardson, I.G. Nature of C-S-H in hardened cements. Cem. Concr. Res. 1999, 29, 1131–1147. [Google Scholar] [CrossRef]
- Blanc, P.; Bourbon, X.; Lassin, A.; Gaucher, E.C. Chemical model for cement-based materials: Temperature dependence of thermodynamic functions for nanocrystalline and crystalline C-S-H phases. Cem. Concr. Res. 2010, 40, 851–866. [Google Scholar] [CrossRef]
- Bates, R.G.; Bower, V.E.; Smith, E.R. Calcium hydroxide as a highly alkaline pH standard. J. Res. Natl. Bur. Stand. 1956, 56, 305–312. [Google Scholar] [CrossRef]
- Tipping, E.; Hurley, M.A. A unifying model of cation binding by humic substances. Geochim. Cosmochim. Acta 1992, 56, 3627–3641. [Google Scholar] [CrossRef]
- Zhu, W.; Chiu, C.F.; Zhang, C.L.; Zeng, K.L. Effect of humic acid on the behaviour of solidified dredged material. Can. Geotech. J. 2009, 46, 1093–1099. [Google Scholar] [CrossRef]
- Hoch, A.R.; Reddy, M.M.; Aiken, G.R. Calcite crystal growth inhibition by humic substances with emphasis on hydrophobic acids from the Florida Everglades. Geochim. Cosmochim. Acta 2000, 64, 61–72. [Google Scholar] [CrossRef]
- Tremblay, H.; Duchesne, J.; Locat, J.; Leroueil, S. Influence of the nature of organic compounds on fine soil stabilization with cement. Can. Geotech. J. 2002, 39, 535–546. [Google Scholar] [CrossRef]
- Atkinson, A.; Everitt, N.; Guppy, R. Evolution of pH in a Radwaste Repository: Internal Reactions between Concrete Constituents; AERE-R12939; UKAEA Harwell Lab.: Harwell, UK, 1988. [Google Scholar]
- Brady, V.P.; Walther, V.J. Kinetics of quartz dissolution at low temperatures. Chem. Geol. 1990, 82, 253–264. [Google Scholar] [CrossRef]
- Chou, L.; Wollast, R. Steady-state kinetics and dissolution mechanisms of albite. Am. J. Sci. 1985, 285, 963–993. [Google Scholar] [CrossRef]
- Sato, T.; Kuroda, M.; Yokoyama, S.; Tsutsui, M.; Fukushi, K.; Tanaka, T.; Nakayama, S. Dissolution mechanism and kinetics of smectite under alkaline condition. In Proceedings of the International Workshop on Bentonite-Cement Interaction in Repository Environments, Tokyo, Japan, 14–16 April 2004; pp. 38–41. [Google Scholar]
- Huertas, F.J.; Chou, L.; Wollast, R. Mechanism of kaolinite dissolution at room temperature and pressure Part II: Kinetic study. Geochim. Cosmochim. Acta 1999, 63, 3261–3275. [Google Scholar] [CrossRef]
- Niibori, Y.; Kunita, M.; Tochiyama, O.; Chida, T. Dissolution rates of amorphous silica in highly alkaline solution. J. Nucl. Sci. Technol. 2000, 37, 349–357. [Google Scholar] [CrossRef]










| Soil Particle Density (g/cm3) | Liquid Limit | Plastic Limit | Content of Fine Particles (<0.075 mm) (%) | |
|---|---|---|---|---|
| Soil A | 2.777 | 73.4 | 28.4 | 83.2 |
| Soil B | 2.737 | 89.8 | 37.1 | 99.3 |
| Soil C | 2.709 | 44.1 | 29.1 | 91.1 |
| Soil D | 2.707 | 66.2 | 30.7 | 58.6 |
| Mineral Dissolution Rate (mol/cm2·s) | Surface Area (cm2/g) | Reference | Vol % | ||
|---|---|---|---|---|---|
| Run 1 | Run 2 | ||||
| Quartz | 5.37 × 10−15 | 1110 | Brady and Walther (1990) [27] | 10 | 10 |
| Albite | 4.17 × 10−15 | 750 | Chou and Wollast (1985) [28] | 9 | 9 |
| Smectite | 1.97 × 10−16 | 53,000 | Sato et al. (2004) [29] | 7 | 7 |
| Kaolinite | 3.31 × 10−16 | 81,600 | Huertas (1999) [30] | 6 | 6 |
| Amorphous Silica | 9.40 × 10−12 | 5000 (Adjusted) | Niibori et al. (2000) [31] | 16 | 4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toda, K.; Sato, H.; Weerakoon, N.; Otake, T.; Nishimura, S.; Sato, T. Key Factors Affecting Strength Development of Steel Slag-Dredged Soil Mixtures. Minerals 2018, 8, 174. https://doi.org/10.3390/min8050174
Toda K, Sato H, Weerakoon N, Otake T, Nishimura S, Sato T. Key Factors Affecting Strength Development of Steel Slag-Dredged Soil Mixtures. Minerals. 2018; 8(5):174. https://doi.org/10.3390/min8050174
Chicago/Turabian StyleToda, Kanako, Haruna Sato, Nilan Weerakoon, Tsubasa Otake, Satoshi Nishimura, and Tsutomu Sato. 2018. "Key Factors Affecting Strength Development of Steel Slag-Dredged Soil Mixtures" Minerals 8, no. 5: 174. https://doi.org/10.3390/min8050174
APA StyleToda, K., Sato, H., Weerakoon, N., Otake, T., Nishimura, S., & Sato, T. (2018). Key Factors Affecting Strength Development of Steel Slag-Dredged Soil Mixtures. Minerals, 8(5), 174. https://doi.org/10.3390/min8050174






