A Comparison of Mineralogical and Thermal Storage Characteristics for Two Types of Stone Coal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the SA/SC Composites
2.3. Characterization
3. Results
3.1. Elemental Composition
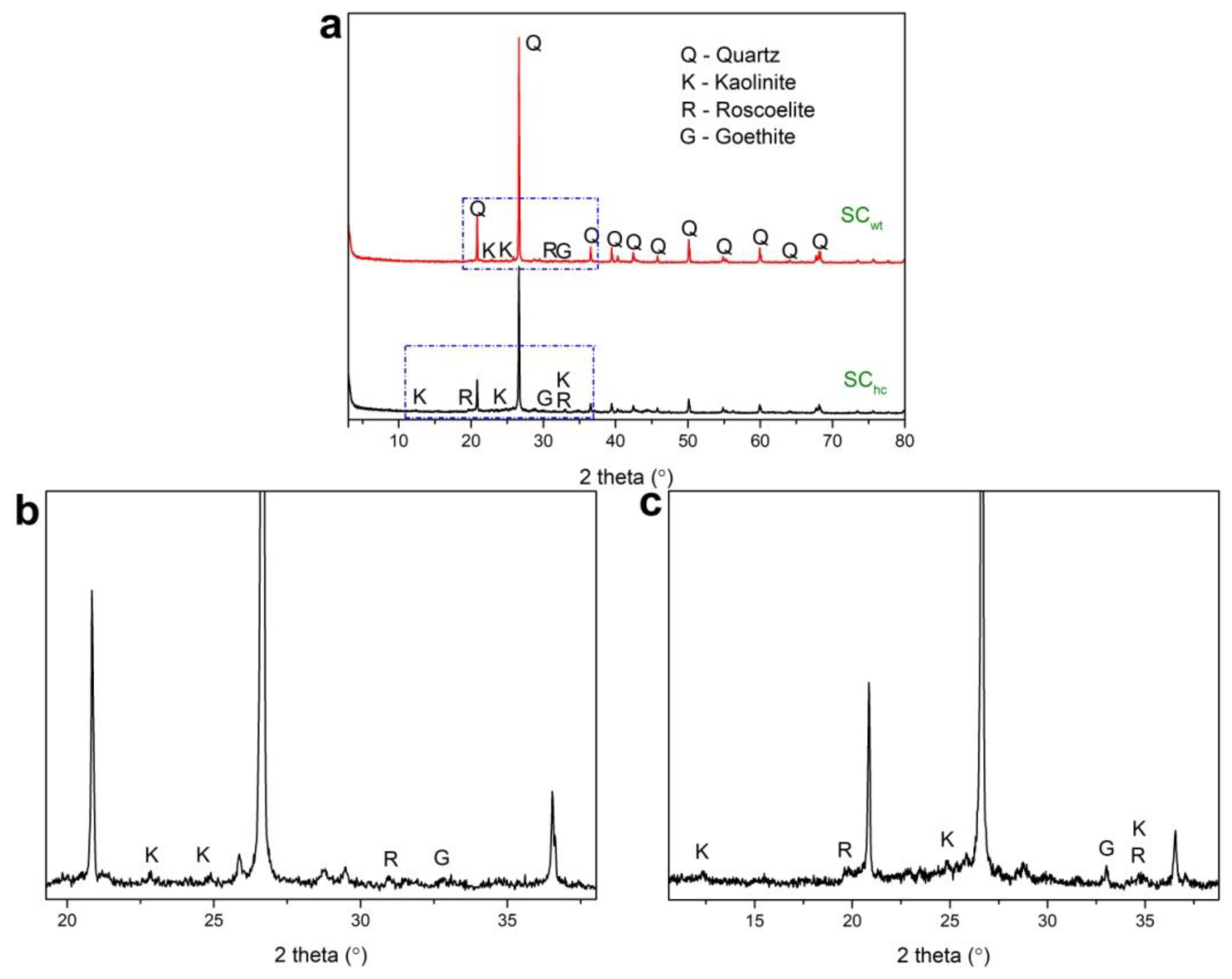
3.2. Mineralogical Composition
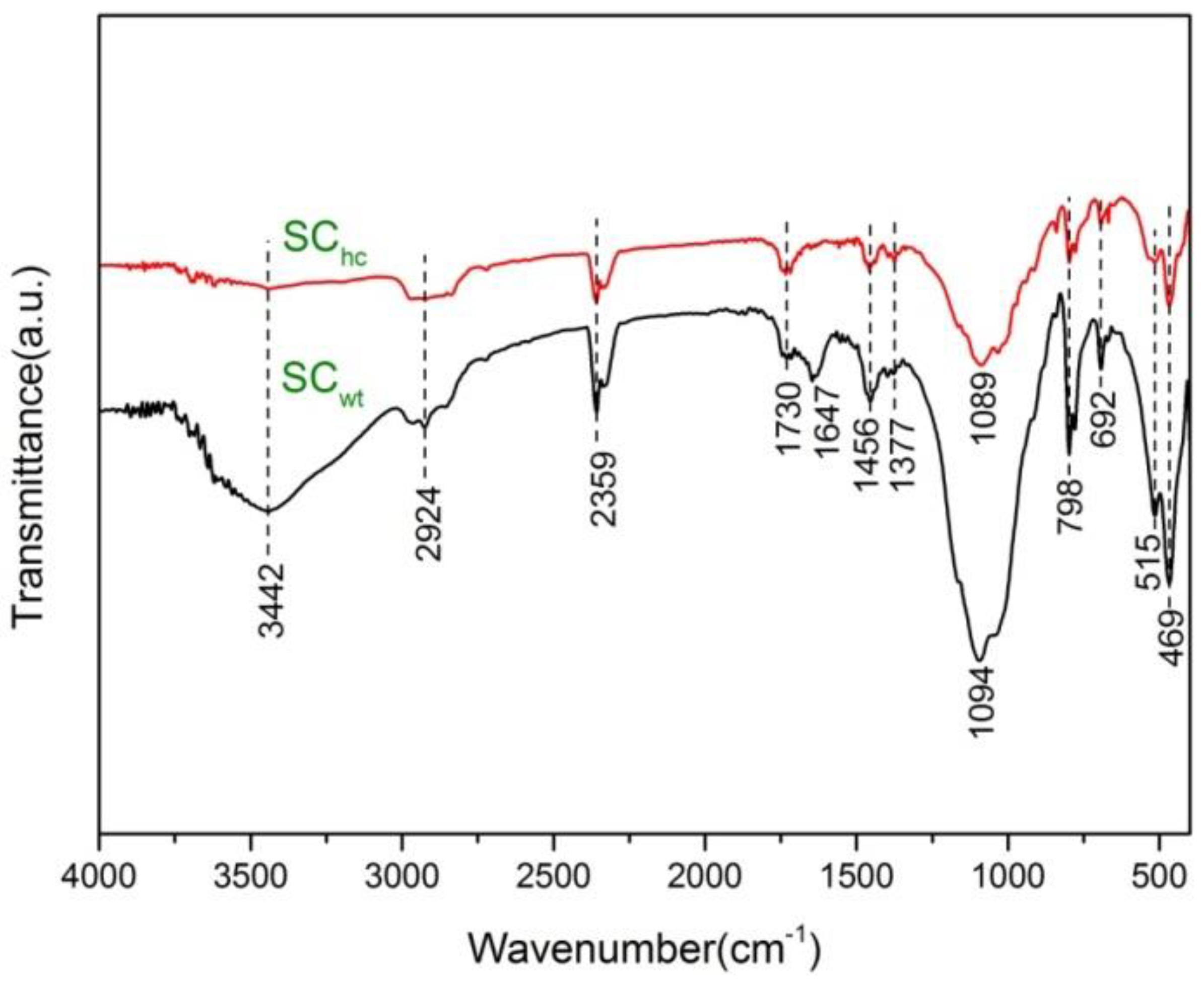
3.3. FTIR Analysis
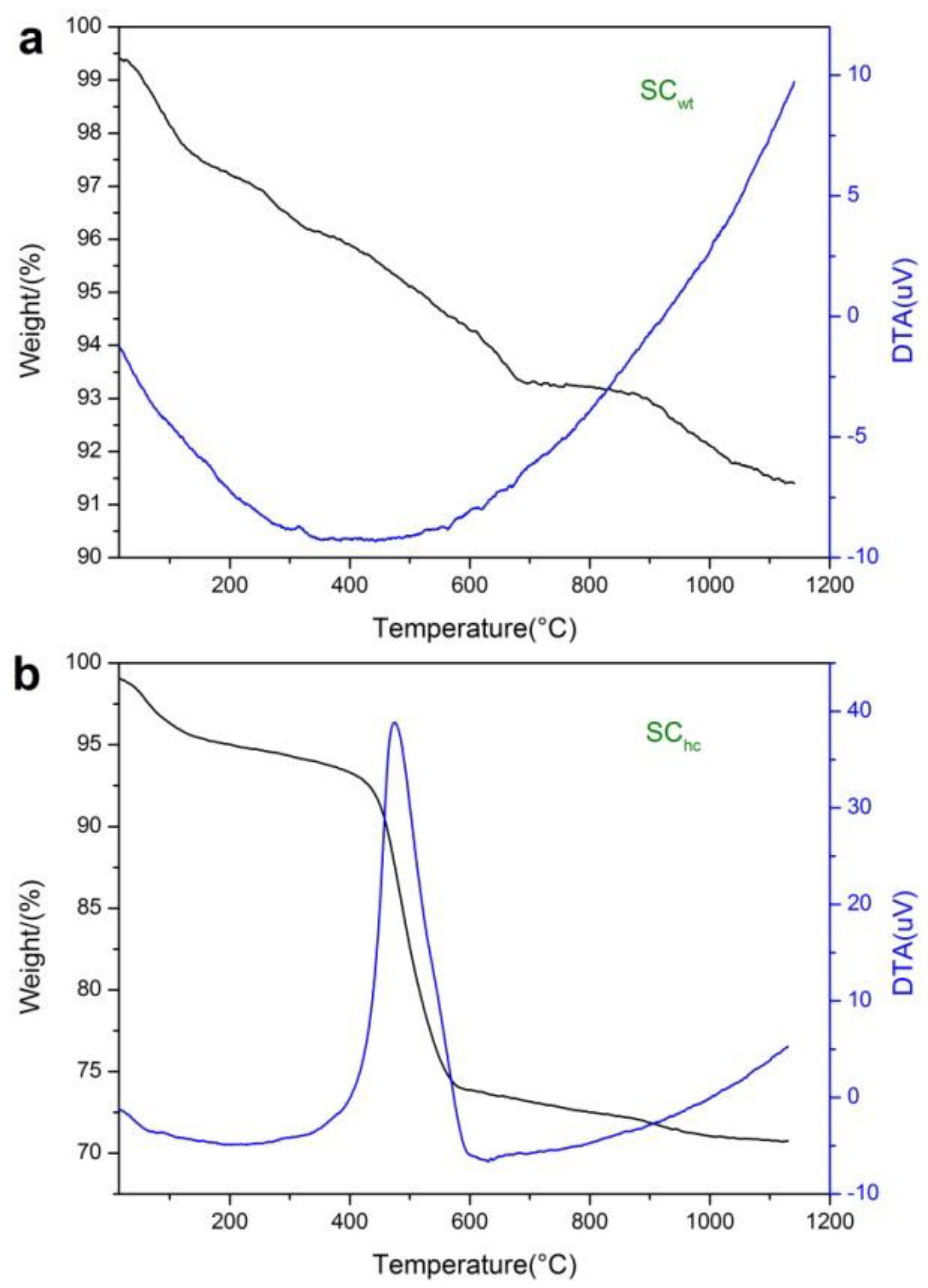
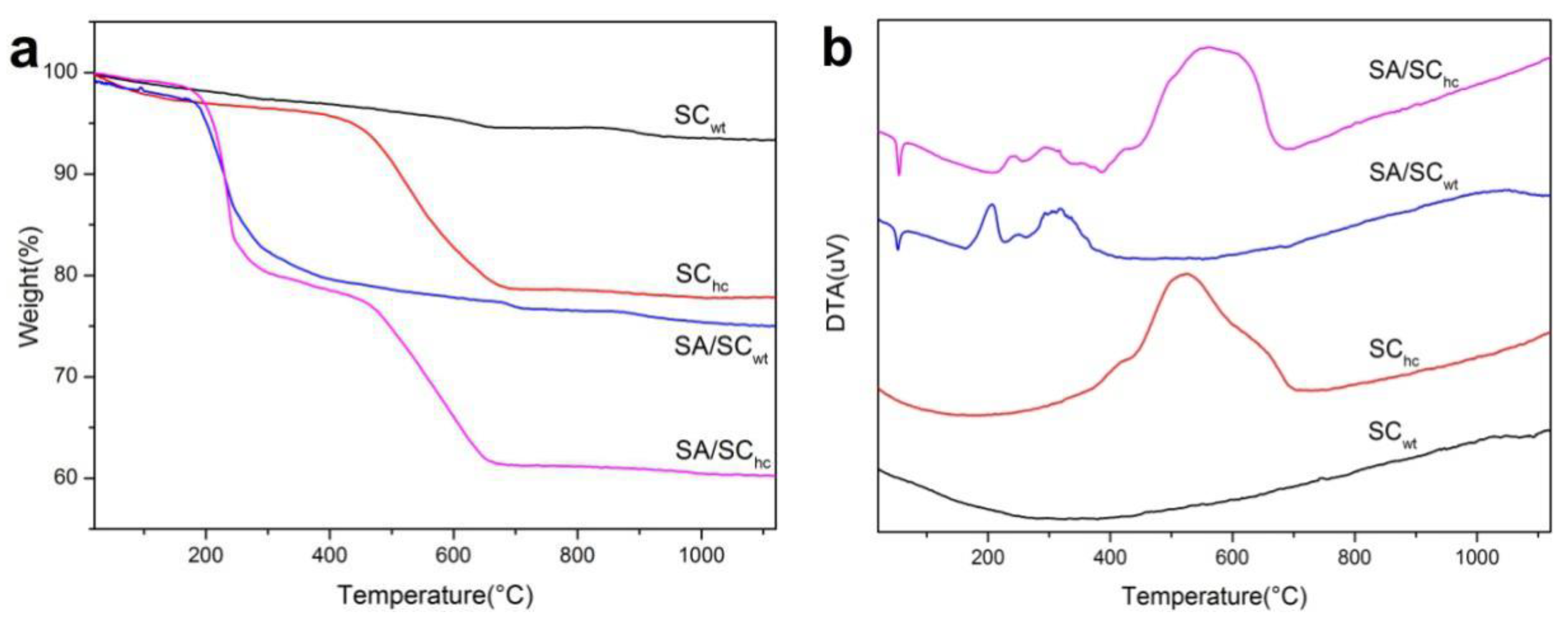
3.4. TG-DTA Analysis
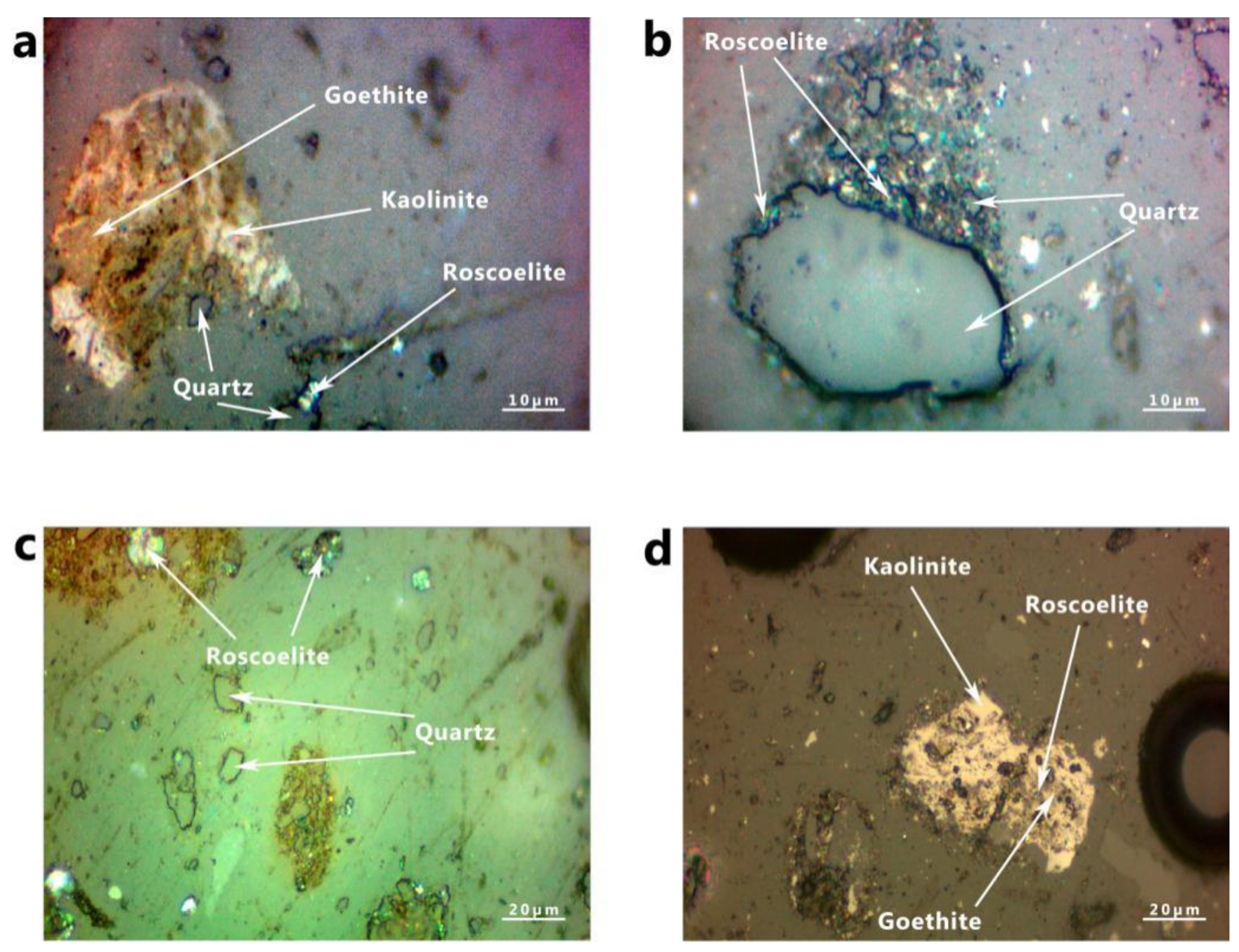
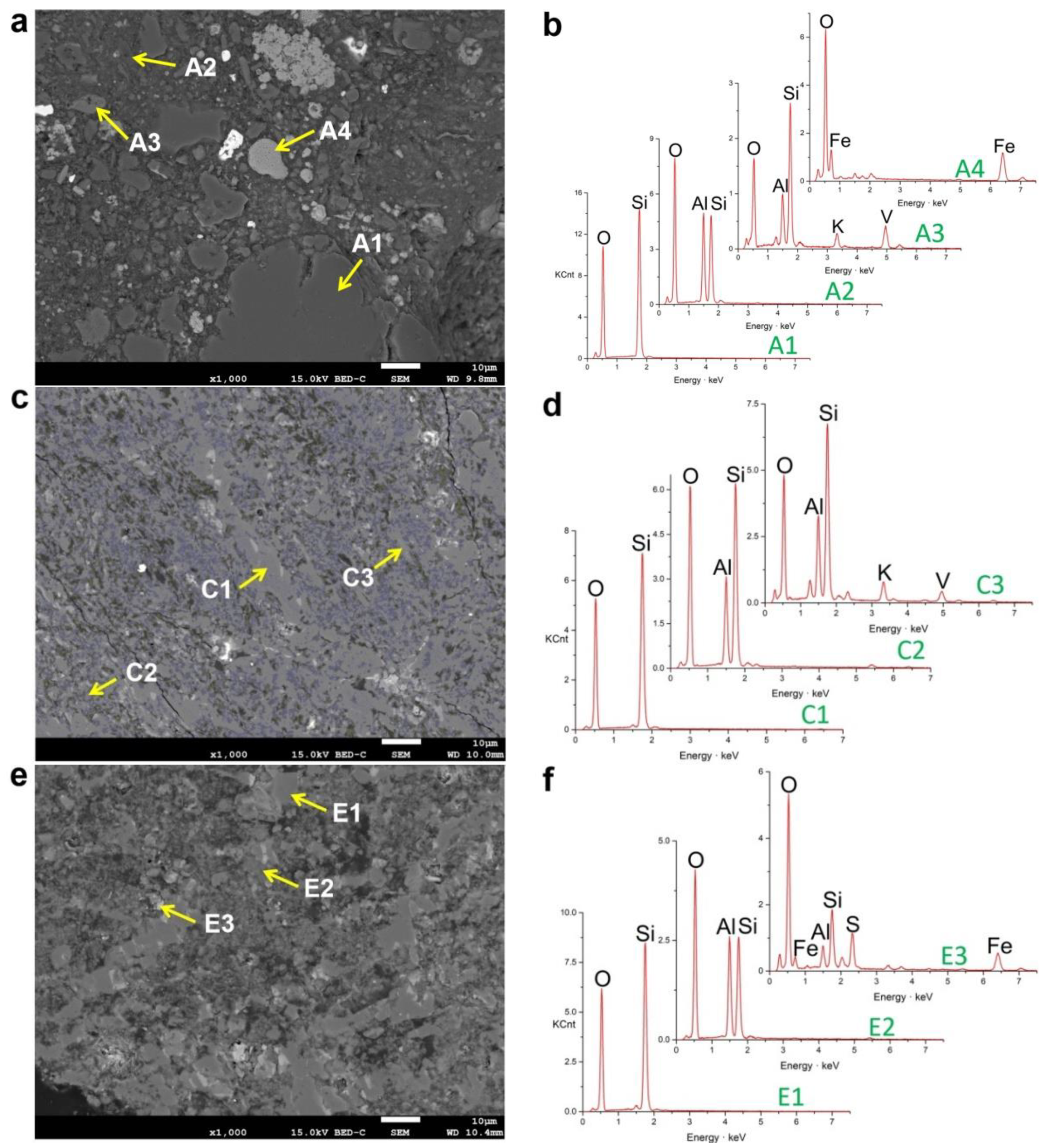
3.5. Reflected Light Microscopy Images Analysis
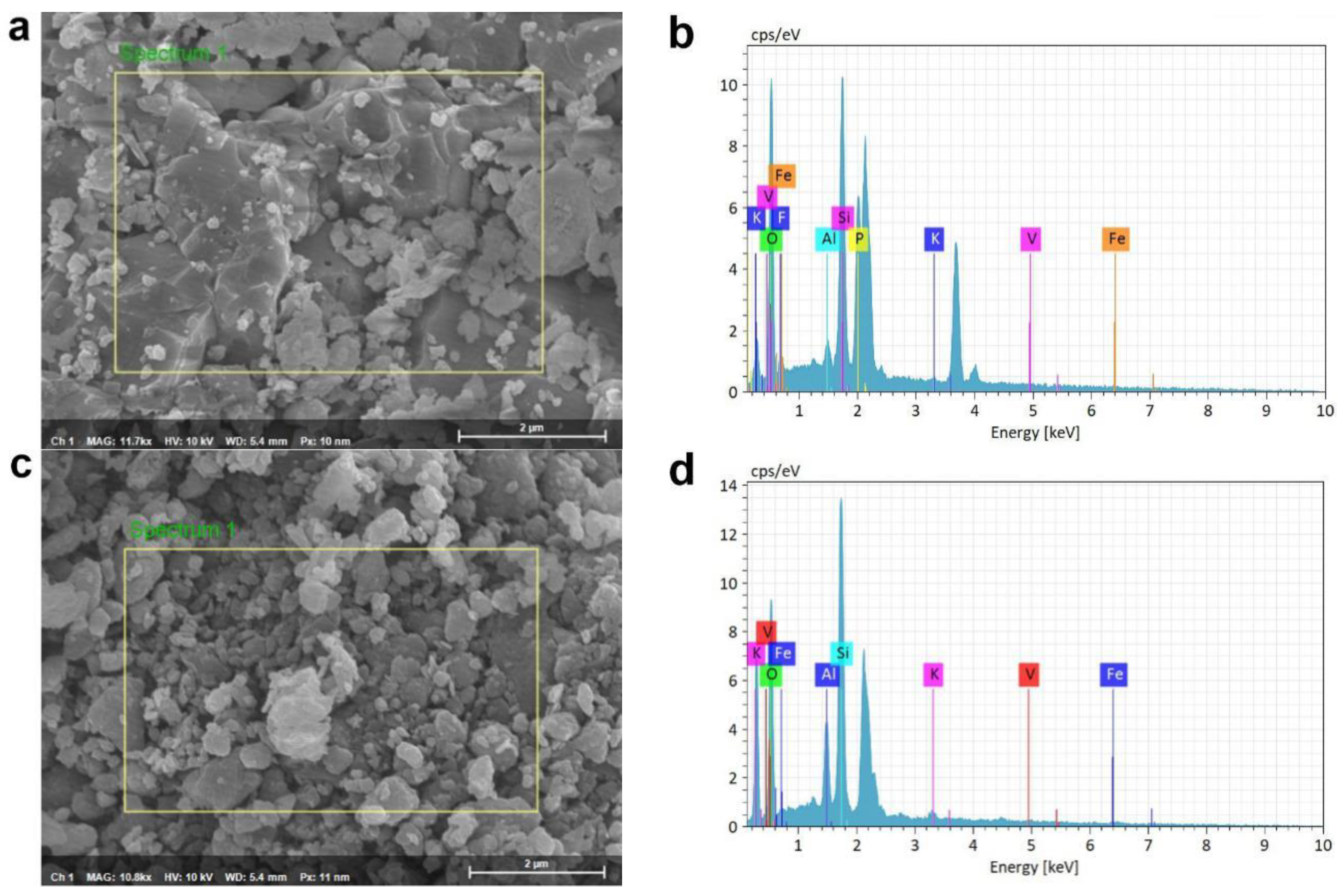
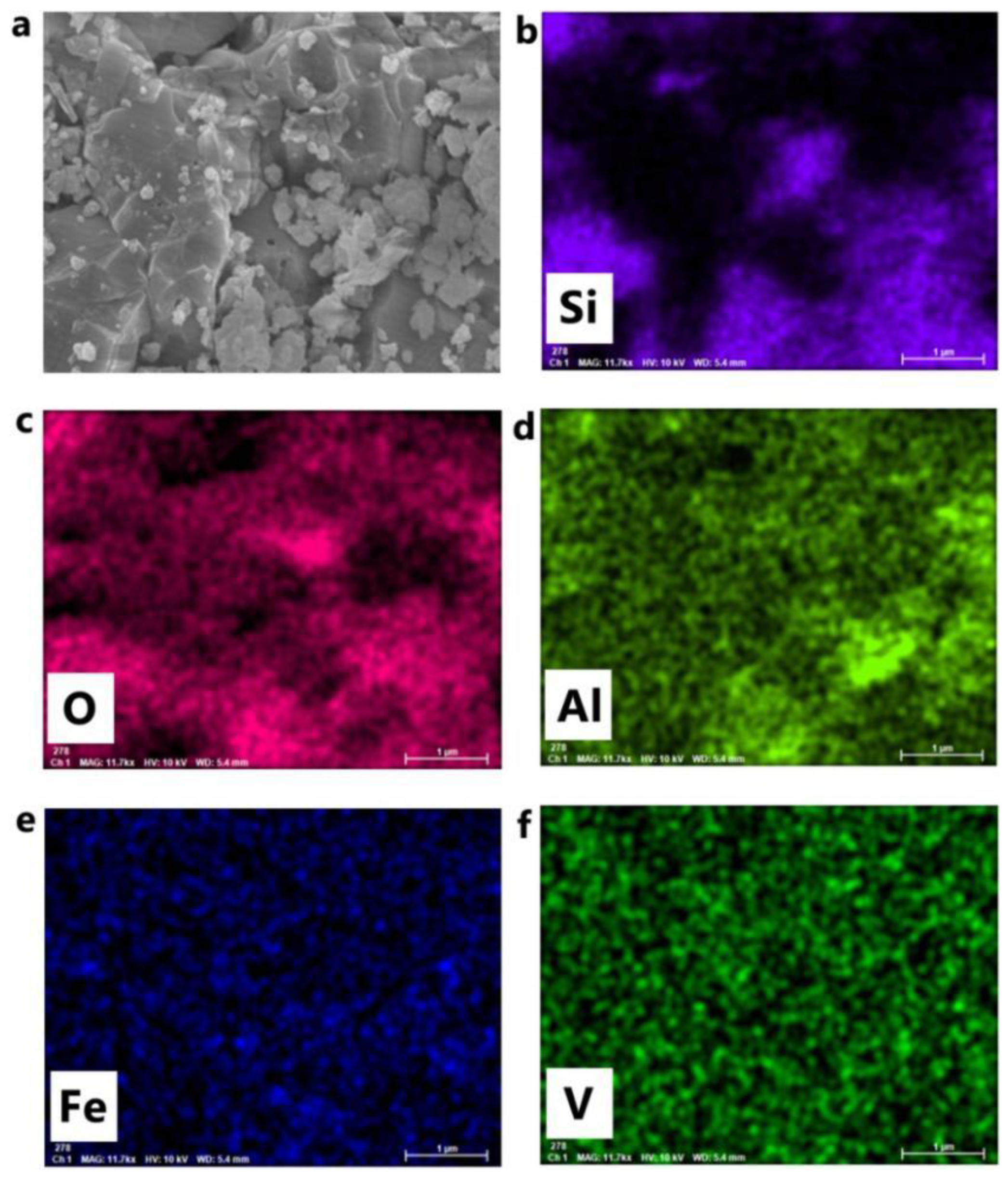
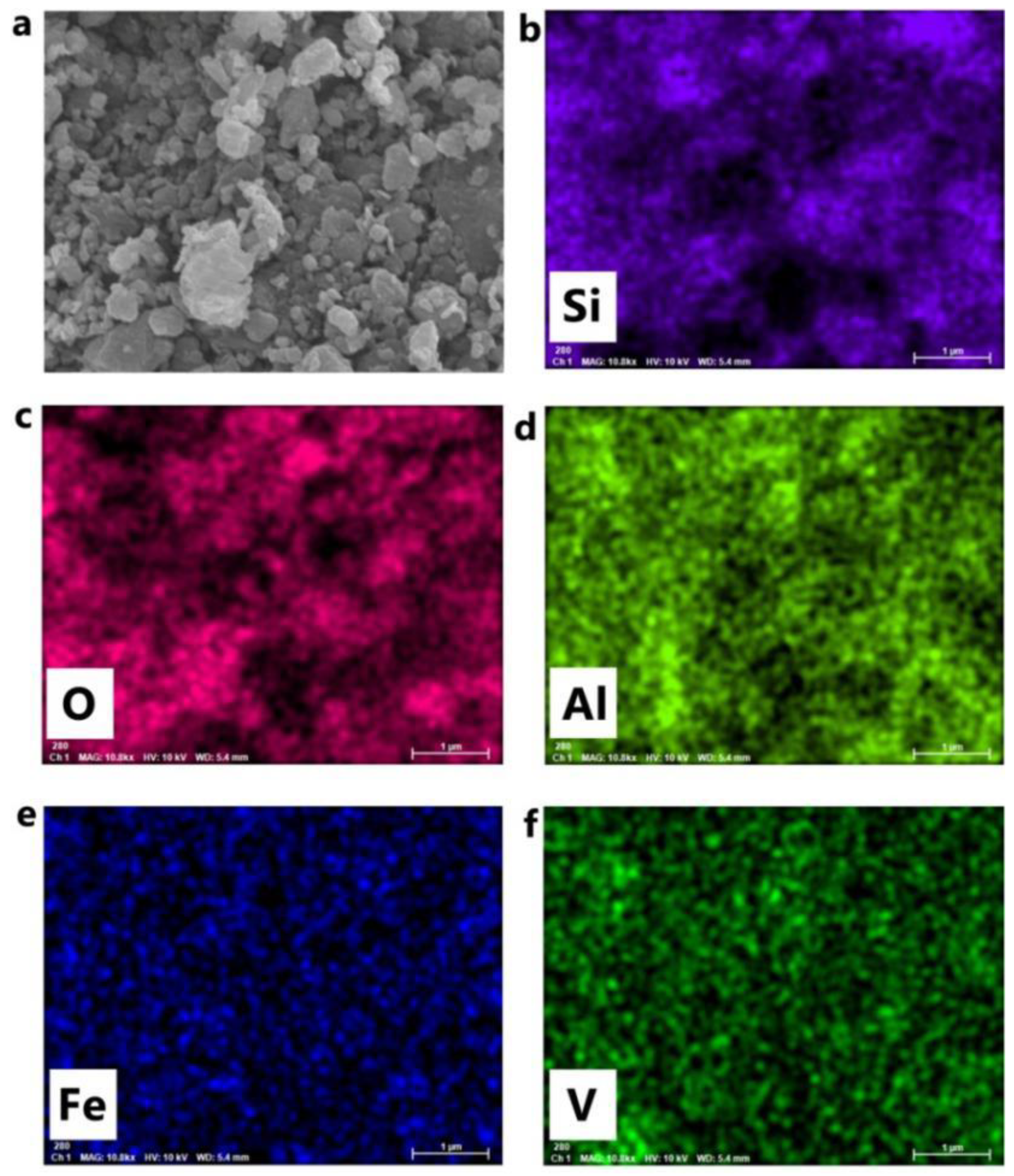
3.6. SEM-EDS Analysis
3.7. The Thermal Conductivity of Stone Coal
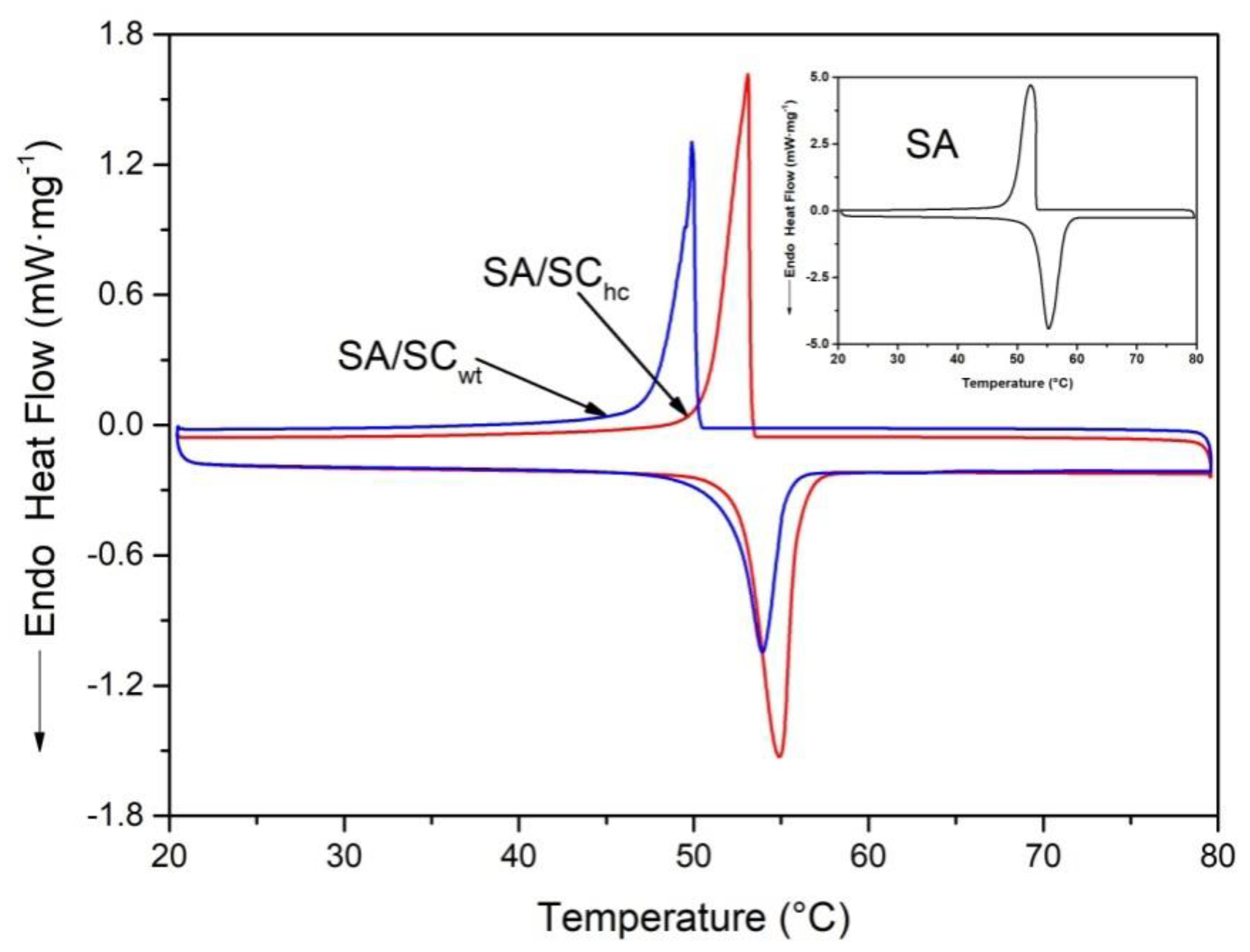
3.8. Thermal Stability and Loading Capacity
3.9. Thermal Storage Behavior of Composite Phase Change Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- He, Z.; Li, M.; Li, Y.; Wang, L.; Zhu, J.; Meng, W.; Li, C.; Zhou, H.; Dai, L. Electrospun nitrogen-doped carbon nanofiber as negative electrode for vanadium redox flow battery. Appl. Surf. Sci. 2019, 469, 423–430. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, M.; Wu, X.; Wu, X.; Zeng, F.; Li, Y.; Duan, S.; Fan, D.; Yang, Y.; Wu, X. The excellent electrochemical performances of ZnMn2O4/Mn2O3: The composite cathode material for potential aqueous zinc ion batteries. J. Electroanal. Chem. 2019, 832, 69–74. [Google Scholar] [CrossRef]
- Cui, J.; Wu, X.; Yang, S.; Li, C.; Tang, F.; Chen, J.; Chen, Y.; Xiang, Y.; Wu, X.; He, Z. Cryptomelane-Type KMn8O16 as Potential Cathode Material—for Aqueous Zinc Ion Battery. Front. Chem. 2018, 6, 352. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, Y.; Zhu, J.; Li, B.; Li, C.; Wang, L.; Meng, W.; He, Z.; Dai, L. Synthesis and performance of a graphene decorated NaTi2(PO4)3/C anode for aqueous lithium-ion batteries. J. Alloys Compd. 2019, 791, 176–183. [Google Scholar] [CrossRef]
- Yi, Z.; Huang, J.; Cen, C.; Chen, X.; Zhou, Z.; Tang, Y.; Wang, B.; Yi, Y.; Wang, J.; Wu, P. Nanoribbon-ring cross perfect metamaterial graphene multi-band absorber in THz range and the sensing application. Results Phys. 2019, 14, 102367. [Google Scholar] [CrossRef]
- He, X.; Sun, Z.; Zou, Q.; Wu, L.; Jiang, J. Electrochemical Behavior of Co(II) Reduction for Preparing Nanocrystalline Co Catalyst for Hydrogen Evolution Reaction from 1-ethyl-3-methylimidazolium Bisulfate and Ethylene Glycol System. J. Electrochem. Soc. 2019, 166, D57–D64. [Google Scholar] [CrossRef]
- Papanicolaou, C.; Kotis, T.; Foscolos, A.; Goodarzi, F. Coals of Greece: A review of properties, uses and future perspectives. Int. J. Coal Geol. 2004, 58, 147–169. [Google Scholar] [CrossRef]
- Dai, S.; Guo, W.; Nechaev, V.P.; French, D.; Ward, C.R.; Spiro, B.F.; Finkelman, R.B. Modes of occurrence and origin of mineral matter in the Palaeogene coal (No. 19-2) from the Hunchun Coalfield, Jilin Province, China. Int. J. Coal Geol. 2018, 189, 94–110. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Zhang, Q.-P. Recovery of vanadium and carbon from low-grade stone coal by flotation. Trans. Nonferrous Met. Soc. China 2015, 25, 3767–3773. [Google Scholar] [CrossRef]
- Fang, S.; Xu, L.; Wu, H.; Tian, J.; Lu, Z.; Sun, W.; Hu, Y. Adsorption of Pb(II)/benzohydroxamic acid collector complexes for ilmenite flotation. Miner. Eng. 2018, 126, 16–23. [Google Scholar] [CrossRef]
- Shu, K.; Xu, L.; Wu, H.; Fang, S.; Wang, Z.; Xu, Y.; Zhang, Z. Effects of ultrasonic pre-treatment on the flotation of ilmenite and collector adsorption. Miner. Eng. 2019, 137, 124–132. [Google Scholar] [CrossRef]
- Huang, Z.; Cheng, C.; Liu, Z.; Zeng, H.; Feng, B.; Zhong, H.; Luo, W.; Hu, Y.; Guo, Z.; He, G.; et al. Utilization of a new Gemini surfactant as the collector for the reverse froth flotation of phosphate ore in sustainable production of phosphate fertilizer. J. Clean. Prod. 2019, 221, 108–112. [Google Scholar] [CrossRef]
- Wu, H.; Tian, J.; Xu, L.; Fang, S.; Zhang, Z.; Chi, R. Flotation and adsorption of a new mixed anionic/cationic collector in the spodumene-feldspar system. Miner. Eng. 2018, 127, 42–47. [Google Scholar] [CrossRef]
- Fang, S.; Xu, L.; Wu, H.; Shu, K.; Xu, Y.; Zhang, Z.; Chi, R.; Sun, W. Comparative studies of flotation and adsorption of Pb(II)/benzohydroxamic acid collector complexes on ilmenite and titanaugite. Powder Technol. 2019, 345, 35–42. [Google Scholar] [CrossRef]
- Dai, S.; Zheng, X.; Wang, X.; Finkelman, R.B.; Jiang, Y.; Ren, D.; Yan, X.; Zhou, Y. Stone coal in China: A review. Int. Geol. Rev. 2018, 60, 736–753. [Google Scholar] [CrossRef]
- Xie, T.; Liao, T.; Liu, C.; Xu, L.; Yang, J.; Zhu, Q.; Wang, J.; Zhang, X. Synthesis of multifunctional photocatalyst vanadium oxide/activated carbon via in situ utilization of stone coal ore. Ceram. Int. 2019, 45, 4934–4944. [Google Scholar] [CrossRef]
- Wang, S.; Liu, G.; Hu, P.; Zhou, Y.; Ke, Y.; Li, C.; Chen, J.; Cao, T.; Long, Y. Largely lowered transition temperature of a VO2/carbon hybrid phase change material with high thermal emissivity switching ability and near infrared regulations. Adv. Mater. Interfaces 2018. [Google Scholar] [CrossRef]
- Zhang, Y.; Cen, C.; Liang, C.; Yi, Z.; Chen, X.; Li, M.; Zhou, Z.; Tang, Y.; Yi, Y.; Zhang, G. Dual-band switchable terahertz metamaterial absorber based on metal nanostructure. Results Phys. 2019, 14, 102422. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, L.; Cheng, G.; Wang, P.; Zhang, T.; Li, C.; Jiang, Y.; He, Z.; Dai, L.; Wang, L. Preparation of carbon nanosheet by molten salt route and it’s application in catalyzing VO2+/VO2+ redox reaction. J. Electrochem. Soc. 2019, 166, A953–A959. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, S.; Liu, T.; Chen, T.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Bao, S.; Chen, T.; Liu, X. Effect of Stone Coal Chemical Composition on Sintering Behavior during Roasting. Ind. Eng. Chem. Res. 2013, 53, 157–163. [Google Scholar] [CrossRef]
- He, D.; Feng, Q.; Zhang, G.; Ou, L.; Lu, Y. An environmentally-friendly technology of vanadium extraction from stone coal. Miner. Eng. 2007, 20, 1184–1186. [Google Scholar] [CrossRef]
- Wang, T.; Xu, L.; Liu, C.; Zhang, Z. Calcified roasting-acid leaching process of vanadium from low-grade vanadium-containing stone coal. Chin. J. Geochem. 2014, 33, 163–167. [Google Scholar] [CrossRef]
- Bian, Y.; Zhang, Y.M.; Bao, S.; Zhao, Y. Analytical Method for Vanadium Occurrence State in Stone Coal and Corresponding Chemical Explanation. Min. Metall. Eng. 2013, 33, 62–70. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, F.; Zhang, H.; Cui, L.; Yu, J.; Xu, G. Extraction of vanadium from stone coal by roasting in a fluidized bed reactor. Fuel 2015, 142, 180–188. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.; Huang, J.; Liu, T.; Yuan, Y.; Xue, N. Eco-Friendly Leaching and Separation of Vanadium over Iron Impurity from Vanadium-Bearing Shale Using Oxalic Acid as a Leachant. ACS Sustain. Chem. Eng. 2017, 6, 1900–1908. [Google Scholar] [CrossRef]
- Gao, Z.; Fan, R.; Ralston, J.; Sun, W.; Hu, Y. Surface broken bonds: An efficient way to assess the surface behaviour of fluorite. Miner. Eng. 2019, 130, 15–23. [Google Scholar] [CrossRef]
- Gao, Z.; Xie, L.; Cui, X.; Hu, Y.; Sun, W.; Zeng, H. Probing Anisotropic Surface Properties and Surface Forces of Fluorite Crystals. Langmuir 2018, 34, 2511–2521. [Google Scholar] [CrossRef]
- Li, C.; Gao, Z. Tune surface physicochemical property of fluorite particles by regulating the exposure degree of crystal surfaces. Min. Eng. 2018, 128, 123–132. [Google Scholar] [CrossRef]
- Tian, M.; Gao, Z.; Sun, W.; Han, H.; Sun, L.; Hu, Y. Activation role of lead ions in benzohydroxamic acid flotation of oxide minerals: New perspective and new practice. J. Colloid Interface Sci. 2018, 529, 150–160. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H. Composite of Coal-Series Kaolinite and Capric–Lauric Acid as Form-Stable Phase-Change Material. Energy Technol. 2015, 3, 77–83. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H. Stearic acid hybridizing coal–series kaolin composite phase change material for thermal energy storage. Appl. Clay Sci. 2014, 101, 277–281. [Google Scholar] [CrossRef]
- Lin, H.; Li, G.; Dong, Y.; Li, J. Effect of pH on the release of heavy metals from stone coal waste rocks. Int. J. Min. Process. 2017, 165, 1–7. [Google Scholar] [CrossRef]
- Li, C.; Xie, B.; Chen, J.; Chen, Z.; Sun, X.; Gibb, S.W. H2O2-microwave treated graphite stabilized stearic acid as a composite phase change material for thermal energy storage. RSC Adv. 2017, 7, 52486–52495. [Google Scholar] [CrossRef]
- Li, C.; Xie, B.; Chen, J. Graphene-decorated silica stabilized stearic acid as a thermal energy storage material. RSC Adv. 2017, 7, 30142–30151. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Wang, L.; Cao, X.; Liu, R.; Xu, L.; Han, H. Flotation technology and adsorption mechanism of vanadium extraction from stone coal. Chin. J. Nonferrous Metals 2012, 22, 2069–2074. [Google Scholar]
- Li, M.; Liu, P.; Wang, F.; Cui, R. Study on Process Mineralogy of a Stone Coal Vanadium Ore in Hubei. Conserv. Util. Miner. Resour. 2013, 3, 41–46. [Google Scholar] [CrossRef]
- Li, C.; Ouyang, J.; Yang, H. Novel sensible thermal storage material from natural minerals. Phys. Chem. Miner. 2013, 40, 681–689. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Huang, J.; Liu, T.; Yuan, Y.; Huang, X. Influence of Mechanical Activation on Mineral Properties and Process of Acid Leaching from Stone Coal. Chin. J. Rare Met. 2014, 38. [Google Scholar] [CrossRef]
- Fleet, M.E.; Liu, X. Carbonate apatite type A synthesized at high pressure: New space group (P3) and orientation of channel carbonate ion. J. Solid State Chem. 2003, 174, 412–417. [Google Scholar] [CrossRef]
- Li, C.; Zeng, L.; Fu, H.; Chen, J.; He, J.; He, Z. Mineralogical and chemical characteristics of the lead-zinc tailing and contaminated soil from the mine tailing pond in Hunan Province (China). Physicochem. Probl. Miner. Process. 2017, 53, 1133–1147. [Google Scholar] [CrossRef]
- Lin, H.; Wang, X.; Dong, Y.; Xu, X.; Zhang, Y. Bioleaching of Vanadium-Bearing Stone Coal by Heterotrophic Bacteria. Chin. J. Rare Met. 2017, 41. [Google Scholar] [CrossRef]
- Li, C.; Fu, L.; Ouyang, J.; Tang, A.; Yang, H. Kaolinite stabilized paraffin composite phase change materials for thermal energy storage. Appl. Clay Sci. 2015, 115, 212–220. [Google Scholar] [CrossRef]
- Wu, H.; Wei, C.; Fan, G.; Li, M.; Deng, Z.; Ge, H. Occurrence of Vanadium in High Carbon Stone Coal and Priority of Preparation Process. J. Kunming Univ. Sci. Technol. (Sci. Technol.) 2008, 33. [Google Scholar] [CrossRef]
- Shutong, X.; Wen, S.; Yican, L.; Laili, J.; Shouyuan, J.; Okay, A.I.; Sengör, A.M.C. Diamond from the Dabie Shan Metamorphic Rocks and Its Implication for Tectonic Setting. Science 1992, 256, 80–82. [Google Scholar] [CrossRef]
- van Panhuys-Sigler, M.; Trewin, N.H.; Still, J. Roscoelite associated with reduction spots in Devonian red beds, Gamrie Bay, Banffshire. Scott. J. Geol. 1996, 32, 127–132. [Google Scholar] [CrossRef]
- Dai, S.; Xie, P.; Jia, S.; Ward, C.R.; Hower, J.C.; Yan, X.; French, D. Enrichment of U-Re-V-Cr-Se and rare earth elements in the Late Permian coals of the Moxinpo Coalfield, Chongqing, China: Genetic implications from geochemical and mineralogical data. Ore Geol. Rev. 2017, 80, 1–17. [Google Scholar] [CrossRef]
- Yang, G.; Yuan, Y.; Shen, Y.; Qu, Y. Analysis on thermal conductivity of cretaceous sandstone associated with microstructure. Coal Eng. 2015, 47, 82–85. [Google Scholar] [CrossRef]
- Li, C.; Xie, B.; Chen, D.; Chen, J.; Li, W.; Chen, Z.; Gibb, S.W.; Long, Y. Ultrathin graphite sheets stabilized stearic acid as a composite phase change material for thermal energy storage. Energy 2019, 166, 246–255. [Google Scholar] [CrossRef]
- Li, C.; Xie, B.; Chen, J.; He, Z.; Chen, Z.; Long, Y. Emerging mineral-coupled composite phase change materials for thermal energy storage. Energy Convers. Manag. 2019, 183, 633–644. [Google Scholar] [CrossRef]
- Li, C.; Xie, B.; He, Z.; Chen, J.; Long, Y. 3D structure fungi-derived carbon stabilized stearic acid as a composite phase change material for thermal energy storage. Renew. Energy 2019, 140, 862–873. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Xie, B.; Zhao, X.; Chen, J.; Chen, Z.; Long, Y. Stearic acid/expanded graphite as a composite phase change thermal energy storage material for tankless solar water heater. Sustain. Cities Soc. 2019, 44, 458–464. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.; Xie, B.; Ma, H.; Chen, J. Enhanced properties of diatomite-based composite phase change materials for thermal energy storage. Renew. Energy 2020, 147, 265–274. [Google Scholar] [CrossRef]
| Na | Mg | Al | Si | P | SO3 | Cl | K | Ca | |
| SCwt | 764.19 | 5646 | 41,876.47 | 308,186.67 | 4366.20 | 18,700 | 18,000 | 8546.81 | 20,642.86 |
| SChc | 487.45 | 2588.4 | 45,837.53 | 243,231.8 | 2841.96 | 567 | 0 | 10,652.81 | 2068.57 |
| Ti | V | Cr | Cu | Zn | As | Se | Rb | Sr | |
| SCwt | 1362 | 8182.42 | 841.58 | 616 | 1348.15 | 143.94 | 35.59 | 27.43 | 725.15 |
| SChc | 1468.8 | 5479.98 | 9713.74 | 393.6 | 95.49 | 237.12 | 0 | 33.83 | 445.08 |
| Y | Zr | Mo | Ba | Pb | Co | Mn | Fe | Ni | |
| SCwt | 126.02 | 63.63 | 46.67 | 30,623.53 | 176.37 | 15.73 | 123.94 | 38,745 | 330.4 |
| SChc | 57.5 | 46.61 | 42.67 | 20,451.50 | 41.77 | 11.8 | 179.72 | 21,886.9 | 815.77 |
| Sample | Loadage (β, %) | Melting Temperature (Tm, °C) | Freezing Temperature (Tf, °C) | Latent Heat of Melting (ΔHm, J g−1) | Latent Heat of Freezing (ΔHf, J g−1) | Theoretic Values of ΔHm (ΔHth, J g−1) | Crystallinity of SA (Fc, %) | Efficient Energy per Unit Mass of SA (Eef, J g−1) |
|---|---|---|---|---|---|---|---|---|
| SA | 100 | 52.91 | 53.10 | 190.2 | 191.5 | - | 100 | - |
| SA/SCwt | 16.63 | 52.18 | 50.15 | 29.21 | 30.55 | 31.63 | 92.35 | 175.64 |
| SA/SChc | 17.40 | 52.81 | 53.24 | 33.02 | 33.17 | 33.09 | 99.78 | 189.78 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Ma, H.; Xie, B.; Zhang, B.; Zhao, X.; Wang, M.; He, Z.; Li, W.; Chen, J. A Comparison of Mineralogical and Thermal Storage Characteristics for Two Types of Stone Coal. Minerals 2019, 9, 594. https://doi.org/10.3390/min9100594
Li C, Ma H, Xie B, Zhang B, Zhao X, Wang M, He Z, Li W, Chen J. A Comparison of Mineralogical and Thermal Storage Characteristics for Two Types of Stone Coal. Minerals. 2019; 9(10):594. https://doi.org/10.3390/min9100594
Chicago/Turabian StyleLi, Chuanchang, Huan Ma, Baoshan Xie, Bo Zhang, Xinbo Zhao, Mengfan Wang, Zhangxing He, Wei Li, and Jian Chen. 2019. "A Comparison of Mineralogical and Thermal Storage Characteristics for Two Types of Stone Coal" Minerals 9, no. 10: 594. https://doi.org/10.3390/min9100594
APA StyleLi, C., Ma, H., Xie, B., Zhang, B., Zhao, X., Wang, M., He, Z., Li, W., & Chen, J. (2019). A Comparison of Mineralogical and Thermal Storage Characteristics for Two Types of Stone Coal. Minerals, 9(10), 594. https://doi.org/10.3390/min9100594





