Mineral Fibres and Asbestos Bodies in Human Lung Tissue: A Case Study
Abstract
1. Introduction
2. Materials and Methods
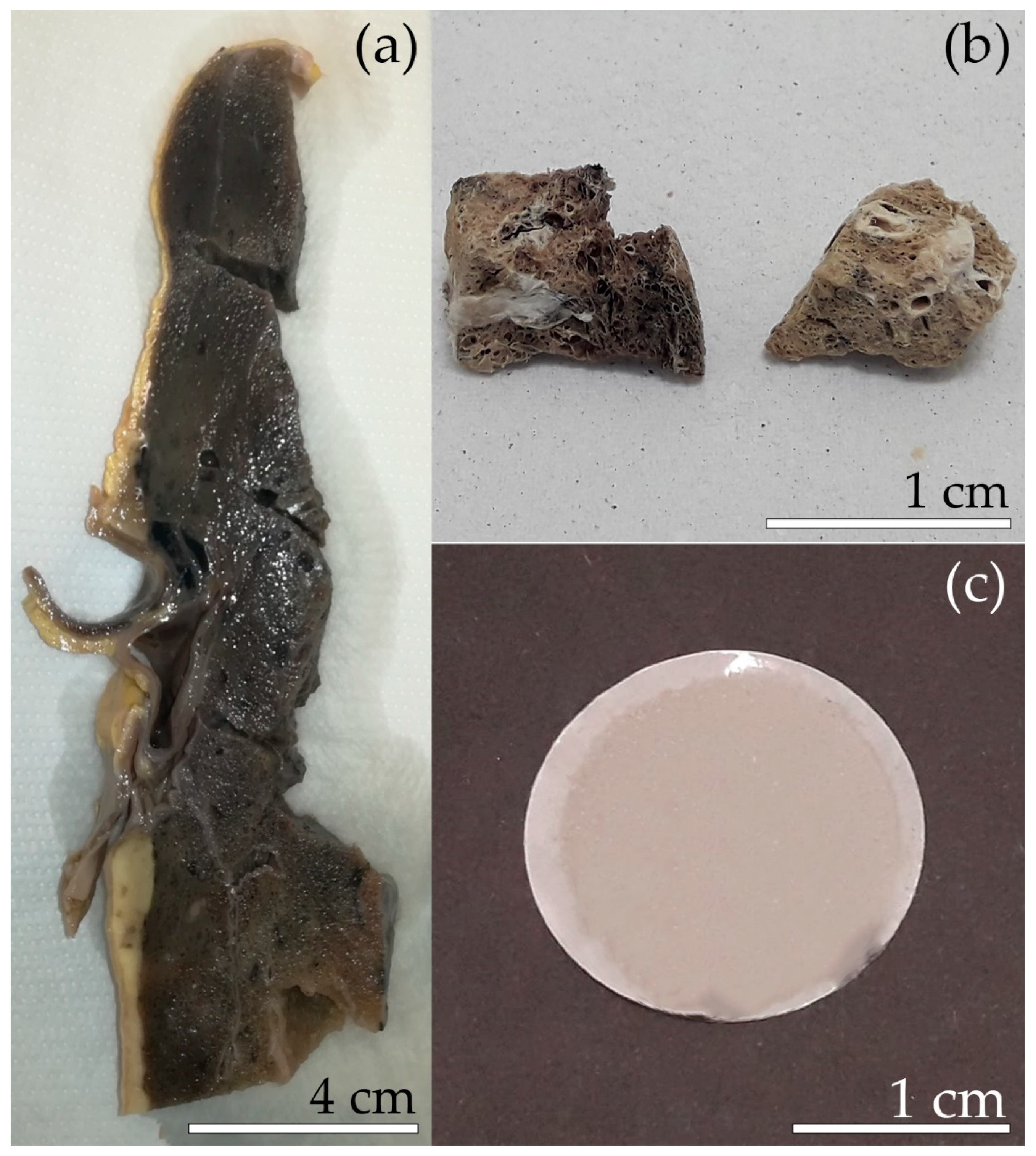
2.1. Description of the Male Subject
2.2. Separation of the Mineral Fibres from the Lung Tissue
2.3. SEM Investigation
2.4. TEM Investigation
3. Results and Discussion
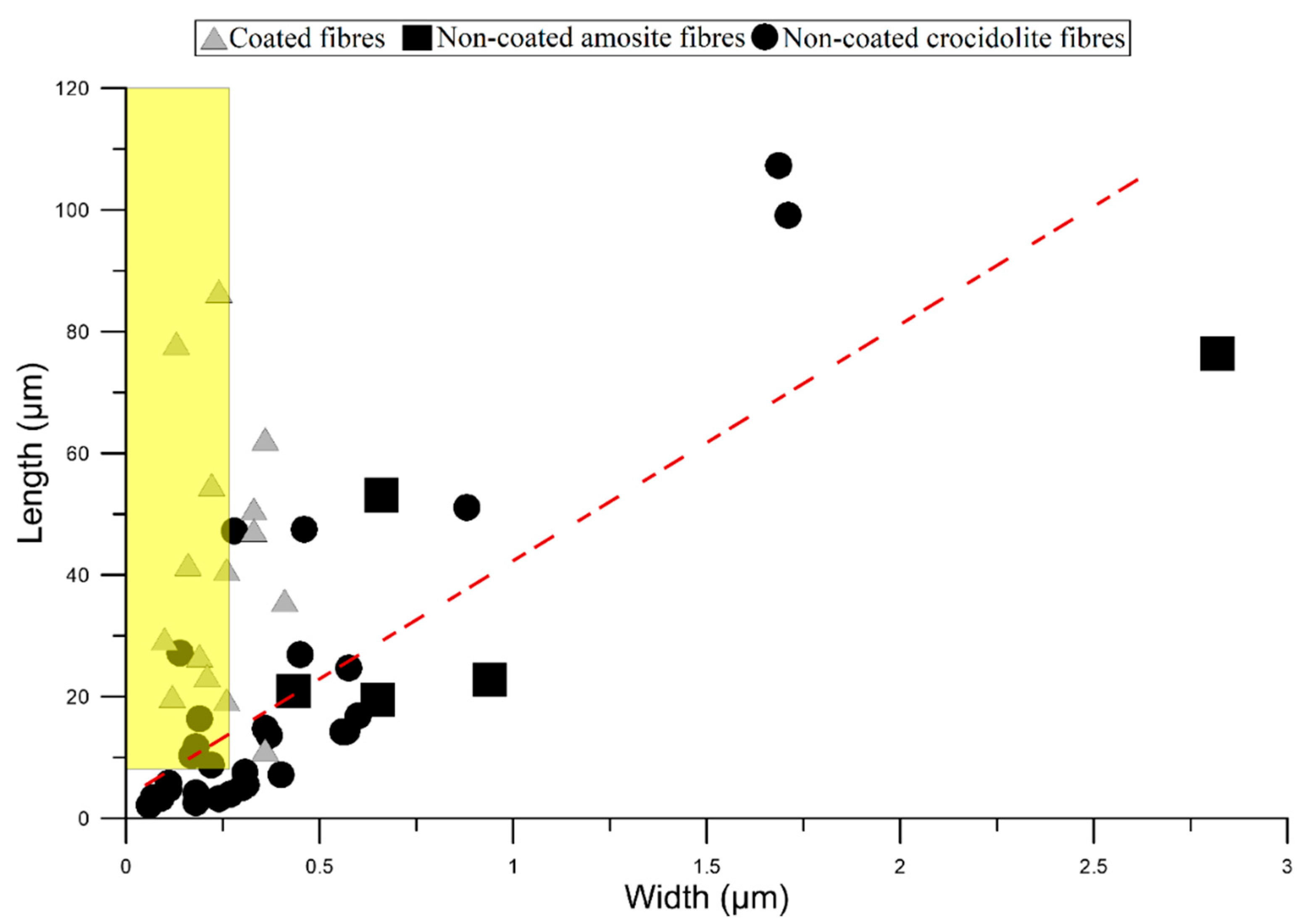
3.1. Mineral Fibres
3.2. Asbestos Bodies (ABs)
3.3. Comparison with Literature Data
3.4. New Insights in the Mechanism of Formation of ABs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alleman, J.E.; Mossman, B.T. Asbestos revisited. Sci. Am. 1997, 277, 54–57. [Google Scholar] [CrossRef]
- Gualtieri, A.F. Mineral fibre-based building materials and their health hazards. In Toxicity of Building Materials; Woodhead Publishing: Cambridge, UK, 2012; pp. 166–195. [Google Scholar]
- Dichicco, M.C.; Paternoster, M.; Rizzo, G.; Sinisi, R. Mineralogical asbestos assessment in the southern Apennines (Italy): A review. Fibers 2019, 7, 24. [Google Scholar] [CrossRef]
- Schumacher, J.C.; Welch, M.D.; Hawthorne, F.C.; Oberti, R.; Harlow, G.E.; Maresch, W.V.; Martin, R.F. Nomenclature of the amphibole supergroup. Am. Miner. 2012, 97, 2031–2048. [Google Scholar]
- Ballirano, P.; Bloise, A.; Lezzerini, M.; Pacella, A.; Perchiazzi, N.; Dogan, M.; Dogan, A.; Gualtieri, A. The crystal structure of mineral fibres. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Mineralogical Society of Great Britain and Ireland: London, UK, 2017; pp. 17–64. [Google Scholar]
- Gualtieri, A.F. Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Mineralogical Society of Great Britain and Ireland: London, UK, 2017; pp. 1–533. [Google Scholar]
- Niklinski, J.; Niklinska, W.; Chyczewska, E.; Laudanski, J.; Naumnik, W.; Chyczewski, L.; Pluygers, E. The epidemiology of asbestos-related diseases. Lung Cancer 2004, 45, S7–S15. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Asbestos (chrysotile, amosite, crocidolite, tremolite, actinolite, and anthophyllite). IARC Monogr Eval Carcinog Risks Hum. C 2012, 100, 219–309. [Google Scholar]
- Capella, S.; Belluso, E.; Bursi, N.; Tibaldi, E.; Mandrioli, D.; Belpoggi, F.; Gualtieri, A. In vivo biological activity of mineral fibres. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Mineralogical Society of Great Britain and Ireland: London, UK, 2017; pp. 307–345. [Google Scholar]
- Asgharian, B.; Hofmann, W.; Miller, F. Mucociliary clearance of insoluble particles from the tracheobronchial airways of the human lung. J. Aerosol Sci. 2001, 32, 817–832. [Google Scholar] [CrossRef]
- Lehnert, B.E. Pulmonary and thoracic macrophage subpopulations and clearance of particles from the lung. Environ. Heal. Perspect. 1992, 97, 17–46. [Google Scholar] [CrossRef]
- Schlosser, P.; Asgharian, B.; Medinsky, M. Inhalation Exposure and Absorption of Toxicants. In Comprehensive Toxicology; Elsevier BV: Amsterdam, The Netherlands, 2010; pp. 75–109. [Google Scholar]
- Donaldson, K.; Murphy, F.A.; Duffin, R.; Poland, C.A. Asbestos, carbon nanotubes and the pleural mesothelium: A review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part. Fibre Toxicol. 2010, 7, 5. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Determination of Airborne Fibre Number Concentrations: A Recommended Method, by Phase Contrast Optical Microscopy (Membrane Filter Method); World Health Organization: Geneva, Switzerland, 1997; pp. 1–53. [Google Scholar]
- Gualtieri, A.F. Towards a quantitative model to predict the toxicity/pathogenicity potential of mineral fibers. Toxicol. Appl. Pharmacol. 2018, 361, 89–98. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Pollastri, S.; Gandolfi, N.B.; Gualtieri, M.L. In vitro acellular dissolution of mineral fibres: A comparative study. Sci. Rep. 2018, 8, 7071. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Gandolfi, N.B.; Pollastri, S.; Burghammer, M.; Tibaldi, E.; Belpoggi, F.; Pollok, K.; Langenhorst, F.; Vigliaturo, R.; Dražić, G. New insights into the toxicity of mineral fibres: A combined in situ synchrotron μ-XRD and HR-TEM study of chrysotile, crocidolite, and erionite fibres found in the tissues of Sprague-Dawley rats. Toxicol. Lett. 2017, 274, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Bursi Gandolfi, N.; Gualtieri, A.F.; Pollastri, S.; Tibaldi, E.; Belpoggi, F. Assessment of asbestos body formation by high resolution FEG–SEMafter exposure of Sprague–Dawley rats to chrysotile, crocidolite, or erionite. J. Hazard. Mater. 2016, 306, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.K.; Kanz, M.F. Asbestos bodies in children’s lungs: An association with sudden infant death syndrome and bronchopulmonary dysplasia. Arch. Pathol. Lab. Med. 1988, 112, 514–518. [Google Scholar] [PubMed]
- Pooley, F. Asbestos Bodies, Their formation, Composition and Character. Environ. Res. 1972, 5, 363–379. [Google Scholar] [CrossRef]
- Davis, J.M.G. Electron-Microscope Studies of Asbestosis in Man and Animals. Ann. N. Y. Acad. Sci. 1965, 132, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Churg, J. Structure and development of the asbestos body. Am. J. Pathol. 1969, 55, 79–107. [Google Scholar] [PubMed]
- Suzuki, Y.; Churg, J. Formation of the asbestos body a comparative study with three types of asbestos. Environ. Res. 1970, 3, 107–118. [Google Scholar] [CrossRef]
- Koerten, H.K.; Hazekamp, J.; Kroon, M.; Daems, W.T. Asbestos body formation and iron accumulation in mouse peritoneal granulomas after the introductionof crocidolite asbestos fibres. Am. J. Pathol. 1990, 136, 141–157. [Google Scholar] [PubMed]
- Morgan, A.; Holmes, A. Concentrations and dimensions of coated and uncoated asbestos fibres in the human lung. Occup. Environ. Med. 1980, 37, 25–32. [Google Scholar] [CrossRef]
- Mossman, B.; Pugnaloni, A.; Gualtieri, A. In vitro biological activity and mechanisms of lung and pleural cancers induced by mineral fibres. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Mineralogical Society of Great Britain and Ireland: London, UK, 2017; pp. 261–306. [Google Scholar]
- Pollastri, S.; Gualtieri, A.F.; Vigliaturo, R.; Ignatyev, K.; Strafella, E.; Pugnaloni, A.; Croce, A. Stability of mineral fibres in contact with human cell cultures. An in situ μXANES, μXRD and XRF iron mapping study. Chemosphere 2016, 164, 547–557. [Google Scholar] [CrossRef]
- Bernstein, D.M.; Rogers, R.; Smith, P.; Chevalier, J. The Toxicological Response of Brazilian Chrysotile Asbestos: A Multidose Subchronic 90-Day Inhalation Toxicology Study with 92-Day Recovery to Assess Cellular and Pathological Response. Inhal. Toxicol. 2006, 18, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.; Dunnigan, J.; Hesterberg, T.; Brown, R.; Velasco, J.A.L.; Barrera, R.; Hoskins, J.; Gibbs, A. Health risk of chrysotile revisited. Crit. Rev. Toxicol. 2013, 43, 154–183. [Google Scholar] [CrossRef] [PubMed]
- Langer, A.M.; Nolan, R.P. Chrysotile Biopersistence in the Lungs of Persons in the General Population and Exposed Workers. Environ. Heal. Perspect. 1994, 102, 235. [Google Scholar]
- Pooley, F. An examination of the fibrous mineral content of asbestos lung tissue from the Canadian chrysotile mining industry. Environ. Res. 1976, 12, 281–298. [Google Scholar] [CrossRef]
- Kolanjiyil, A.V.; Kleinstreuer, C.; Kleinstreuer, N.C.; Pham, W.; Sadikot, R.T. Mice-to-men comparison of inhaled drug-aerosol deposition and clearance. Respir. Physiol. Neurobiol. 2019, 260, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Ménache, M.G.; Miller, F.J.; Raabe, O.G. Particle Inhalability Curves for Humans and Small Laboratory Animals*. Ann. Occup. Hyg. 1995, 39, 317–328. [Google Scholar] [CrossRef]
- Barbieri, P.G.; Mirabelli, D.; Somigliana, A.; Cavone, D.; Merler, E. Asbestos Fibre Burden in the Lungs of Patients with Mesothelioma Who Lived Near Asbestos-Cement Factories. Ann. Occup. Hyg. 2012, 56, 660–670. [Google Scholar]
- National Institute of Mental Health (NIMH). ImageJ. 2018. Available online: https://imagej.nih.gov/ij/ (accessed on 19 September 2018).
- Gatan. Gatan Microscopy Suite Software. 2015. Available online: http://www.gatan.com/products/temanalysis/gatan-microscopy-suite-software (accessed on 2 August 2019).
- Koerten, H.K.; de Bruijn, J.D.; Daems, W.T.H. The Formation of Asbestos Bodies by Mouse Peritoneal Macropages. An In Vitro Study. Am. J. Pathol. 1990, 137, 121–133. [Google Scholar]
- Della Ventura, G.; Vigliaturo, R.; Gieré, R.; Pollastri, S.; Gualtieri, A.F.; Iezzi, G. Infra Red Spectroscopy of the Regulated Asbestos Amphiboles. Minerals 2018, 8, 413. [Google Scholar] [CrossRef]
- Hawthorne, F.C. The crystal chemistry of the amphiboles. VIII. The crystal structure and site chemistry of fluor-riebeckite. Can. Miner. 1978, 16, 187–194. [Google Scholar]
- Rinaudo, C.; Croce, A.; Musa, M.; Fornero, E.; Allegrina, M.; Trivero, P.; Bellis, D.; Sferch, D.; Toffalorio, F.; Veronesi, G.; et al. Study of Inorganic Particles, Fibers, and Asbestos Bodies by Variable Pressure Scanning Electron Microscopy with Annexed Energy Dispersive Spectroscopy and Micro-Raman Spectroscopy in Thin Sections of Lung and Pleural Plaque. Appl. Spectrosc. 2010, 64, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.G. The ultra-structure of asbestos bodies from human lung. Brit. J. Exp. Pathol. 1964, 45, 642–646. [Google Scholar]
- Richter, G.W. Electron Microscopy of Hemosiderin: Presence of Ferritin and Occurrence of Crystalline Lattices in Hemosiderin Deposits. J. Cell Boil. 1958, 4, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Pascolo, L.; Gianoncelli, A.; Schneider, G.; Salomé, M.; Schneider, M.; Calligaro, C.; Kiskinova, M.; Melato, M.; Rizzardi, C. The interaction of asbestos and iron in lung tissue revealed by synchrotron-based scanning X-ray microscopy. Sci. Rep. 2013, 3, 1123. [Google Scholar] [CrossRef] [PubMed]
- Jian, N.; Dowle, M.; Horniblow, R.D.; Tselepis, C.; Palmer, R.E. Morphology of the ferritin iron core by aberration corrected scanning transmission electron microscopy. Nanotechnology 2016, 27, 46. [Google Scholar] [CrossRef]
- Bardelli, F.; Veronesi, G.; Capella, S.; Bellis, D.; Charlet, L.; Cedola, A.; Belluso, E. New insights on the biomineralisation process developing in human lungs around inhaled asbestos fibres. Sci. Rep. 2013, 7, 44862. [Google Scholar] [CrossRef] [PubMed]
- Oury, T.D.; Sporn, T.A.; Roggli, V.L. Pathology of Asbestos-Associated Diseases; Springer: Berlin, Germany, 2014; p. 357. [Google Scholar]
- Drits, V.A.; Sakharov, A.; Salyn, L.; Manceau, A. Structural model of ferrihydrite. Clay. Miner. 1993, 28, 185–207. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Venturelli, P. In situ study of the goethite-hematite phase transformation by real time synchroton powder diffraction. Am. Mineral. 1990, 84, 895–904. [Google Scholar] [CrossRef]
- Carlson, L.; Schwertmann, U. Natural ferrihydrites in surface deposits from Finland and their association with silica. Geochim. Cosmochim. Acta 1980, 45, 421–429. [Google Scholar] [CrossRef]
- Cudennec, Y.; Lecerf, A. The transformation of ferrihydrite into goethite or hematite, revisited. J. Solid State Chem. 2006, 179, 716–722. [Google Scholar] [CrossRef]
- Davis, J. Asbestos dust as a nucleation center in the calcification of old fibrous tissue lesions, and the possible association of this process to the formation of asbestos bodies. Exp. Mol. Pathol. 1970, 12, 133–147. [Google Scholar] [CrossRef]
- Roggli, V.L. The So-called Short-Fiber Controversy: Literature Review and Critical Analysis. Arch. Pathol. Lab. Med. 2015, 139, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.F.; Layard, M.; Tegeris, A.; Miller, E.; May, M.; Morgan, E.; Smith, A. Relation of Particle Dimension to Carcinogenicity in Amphibole Asbestoses and Other Fibrous Minerals. J. Natl. Cancer Inst. 1981, 67, 965–975. [Google Scholar]
- Churg, A. Asbestos lung burden and disease pattern in man. Rev. Mineral. Geochem. 1993, 28, 409–426. [Google Scholar]
- Roggli, V.L.; Green, C.L. Dimensions of elongated mineral particles: A study of more than 570 fibers from more than 90 cases with implications for pathogenicity and classification as asbestiform vs. cleavage fragments. Ultrastruct. Pathol. 2019, 43, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.W.; Crump, K.S. A Meta-Analysis of Asbestos-Related Cancer Risk That Addresses Fiber Size and Mineral Type. Crit. Rev. Toxicol. 2008, 38, 49–73. [Google Scholar] [CrossRef] [PubMed]
- Gunter, M.E.; Belluso, E.; Mottana, A. Amphiboles: Environmental and Health Concerns. Rev. Miner. Geochem. 2007, 67, 453–516. [Google Scholar] [CrossRef]
- Mace, M.L.; McLemore, T.L.; Roggli, V.; Brinkley, B.; Greenberg, S.D. Scanning electron microscopic examination of human asbestos bodies. Cancer Lett. 1980, 9, 95–104. [Google Scholar] [CrossRef]
- Krombach, F.; Münzing, S.; Allmeling, A.-M.; Gerlach, J.T.; Behr, J.; Dörger, M. Cell Size of Alveolar Macrophages: An Interspecies Comparison. Environ. Heal. Perspect. 1997, 105, 1261. [Google Scholar]
- Suzuki, Y. Interaction of asbestos with alveolar cells. Environ. Heal. Perspect. 1974, 9, 241–252. [Google Scholar] [CrossRef]
- Botham, S.K.; Holt, P.F. The mechanism of formation of asbestos bodies. J. Pathol. Bacteriol. 1968, 96, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Padmore, T.; Stark, C.; Turkevich, L.A.; Champion, J.A. Quantitative analysis of the role of fiber length on phagocytosis and inflammatory response by alveolar macrophages. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Fujihara, N.; Nishimura, T.; Funabashi, H.; Hirota, R.; Ikeda, T.; Kuroda, A. Live-cell imaging of macrophage phagocytosis of asbestos fibers under fluorescence microscopy. Genes Environ. 2019, 41, 14. [Google Scholar] [CrossRef] [PubMed]
- Fubini, B.; Barceló, F.; Areán, C.O. Ferritin Adsorption on Amosite Fibers: Possible Implications in the Formation and Toxicity of Asbestos Bodies. J. Toxicol. Environ. Heal. Part A 1997, 52, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Munro, H.N.; Linder, M.C. Ferritin: Structure, biosynthesis, and role in iron metabolism. Physiol. Rev. 1978, 58, 317–396. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Levi, S. Ferritin, Iron Homeostasis, and Oxidative Damage. Free Radic. Biol. Med. 2002, 4, 457–463. [Google Scholar] [CrossRef]
- Cozzi, A.; Santambrogio, P.; Levi, S.; Arosio, P. Iron detoxifying activity of ferritin. Effects of H and L human apoferritins on lipid peroxidation in vitro. FEBS Lett. 1990, 277, 119–122. [Google Scholar] [CrossRef]
- Lee, M.; Arosio, P.; Cozzi, A.; Chasteen, N.D. Identification of the EPR-Active Iron-Nitrosyl Complexes in Mammalian Ferritins. Biochemistry 1994, 33, 3679–3687. [Google Scholar] [CrossRef]
- Pérez-Arellano, J.L.; García, J.E.L.; Macías, M.C.G.; Gómez, F.G.; López, A.J.; De Castro, S. Hemosiderin-laden macrophages in bronchoalveolar lavage fluid. Acta Cytol. 1992, 36, 26–30. [Google Scholar]
- Roy, S.; Ray, M.R.; Basu, C.; Lahiri, P.; Lahiri, T. Abundance of siderophages in sputum: Indicator of an adverse lung reaction to air pollution. Acta Cytol. 2001, 45, 958–964. [Google Scholar] [CrossRef]
- Becroft, D.M.; Lockett, B.K. Intra-alveolar pulmonary siderophages in sudden infant death: A marker for previous imposed suffocation. Pathology 1997, 29, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Churg, A.; Roggli, V.L. Review: Ferruginous Bodies: Implications in the Mechanism of Fiber and Particle Toxicity. Toxicol. Pathol. 2004, 32, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Pascolo, L.; Borelli, V.; Canzonieri, V.; Gianoncelli, A.; Birarda, G.; Bedolla, D.E.; Salomé, M.; Vaccari, L.; Calligaro, C.; Cotte, M.; et al. Differential protein folding and chemical changes in lung tissues exposed to asbestos or particulates. Sci. Rep. 2015, 5, 12129. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, A.F.; Di Giuseppe, D.; Zoboli, A.; Lusvardi, G.; Lassinantti Gualtieri, M.; Vigliaturo, R.; Gieré, R.; Bonasoni, P.; Moliterni, A.; Altomare, A.; et al. The lifelong biopersistence of asbestos in the human lungs at the atomic scale. In preparation for IUCrJ.










| Percentiles * | ||||||||
|---|---|---|---|---|---|---|---|---|
| Morphometry | Min | 15th | 25th | 50th | 75th | 90th | Max | σn−1 |
| L (µm) | 2.17 | 4.96 | 7.45 | 19.8 | 47.3 | 61.6 | 107 | 26.2 |
| W (µm) | 0.06 | 0.13 | 0.18 | 0.28 | 0.44 | 0.66 | 2.82 | 0.47 |
| L/W | 13.5 | 24.5 | 29.5 | 55.2 | 141 | 243 | 601 | 107 |
| Element Weight Percent | ||||||
|---|---|---|---|---|---|---|
| Crocidolite | Fe/Si | Fe | Si | Al | Mg | Na |
| Mean (n = 6) | 1.53 | 49.1 | 33.1 | 3.12 | 4.03 | 10.6 |
| σn−1 | 0.45 | 8.45 | 3.82 | 1.54 | 0.91 | 5.48 |
| Amosite | Fe/Si | Fe | Si | Al | Mg | |
| Mean (n = 4) | 2.18 | 60.6 | 30.7 | 1.06 | 6.63 | |
| σn−1 | 0.78 | 9.12 | 8.26 | 0.78 | 1.56 | |
| Element Weight Percent | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Asbestos bodies | Subject | Descriptive Statistics | Fe | Si | Mg | Ca | P | Na | K | Al |
| Present Study | Human | Mean (n = 6) | 55.9 | 19.9 | 2.58 | 2.87 | 11.3 | 3.20 | 1.58 | 3.97 |
| Max | 68.2 | 41.2 | 7.85 | 4.69 | 24.6 | 6.16 | 2.00 | 7.75 | ||
| Min | 24.7 | 6.19 | 0.64 | 1.18 | 1.56 | 0.30 | 0.94 | 0.55 | ||
| σn−1 | 15.6 | 13.6 | 2.71 | 1.48 | 8.73 | 1.96 | 0.46 | 2.55 | ||
| Reference [40] | Human | Mean (n = 3) | 41.0 | 33.7 | 2.89 | 5.99 | 3.94 | 11.4 | 1.12 | 0.82 |
| Max | 53.5 | 43.9 | 2.90 | 6.98 | 5.70 | 13.7 | 1.12 | 1.31 | ||
| Min | 28.6 | 22.8 | 2.87 | 4.87 | 2.52 | 8.94 | 1.12 | 1.16 | ||
| σn−1 | 10.2 | 8.6 | 0.02 | 0.87 | 1.32 | 1.96 | - | 0.59 | ||
| Reference [18] | Rat | Mean (n = 11) | 4.57 | 1.82 | 0.35 | 16.4 | 6.99 | - | - | - |
| Max | 10.6 | 3.86 | 0.80 | 21.0 | 10.3 | - | - | - | ||
| Min | 1.99 | 0.99 | 0.07 | 8.83 | 3.46 | - | - | - | ||
| σn−1 | 2.63 | 0.98 | 0.18 | 4.34 | 1.99 | - | - | - | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Giuseppe, D.; Zoboli, A.; Vigliaturo, R.; Gieré, R.; Bonasoni, M.P.; Sala, O.; Gualtieri, A.F. Mineral Fibres and Asbestos Bodies in Human Lung Tissue: A Case Study. Minerals 2019, 9, 618. https://doi.org/10.3390/min9100618
Di Giuseppe D, Zoboli A, Vigliaturo R, Gieré R, Bonasoni MP, Sala O, Gualtieri AF. Mineral Fibres and Asbestos Bodies in Human Lung Tissue: A Case Study. Minerals. 2019; 9(10):618. https://doi.org/10.3390/min9100618
Chicago/Turabian StyleDi Giuseppe, Dario, Alessandro Zoboli, Ruggero Vigliaturo, Reto Gieré, Maria Paola Bonasoni, Orietta Sala, and Alessandro Francesco Gualtieri. 2019. "Mineral Fibres and Asbestos Bodies in Human Lung Tissue: A Case Study" Minerals 9, no. 10: 618. https://doi.org/10.3390/min9100618
APA StyleDi Giuseppe, D., Zoboli, A., Vigliaturo, R., Gieré, R., Bonasoni, M. P., Sala, O., & Gualtieri, A. F. (2019). Mineral Fibres and Asbestos Bodies in Human Lung Tissue: A Case Study. Minerals, 9(10), 618. https://doi.org/10.3390/min9100618






