Microscopic Blue Sapphire in Nelsonite from the Western Adirondack Mountains of New York State, USA
Abstract
:1. Introduction
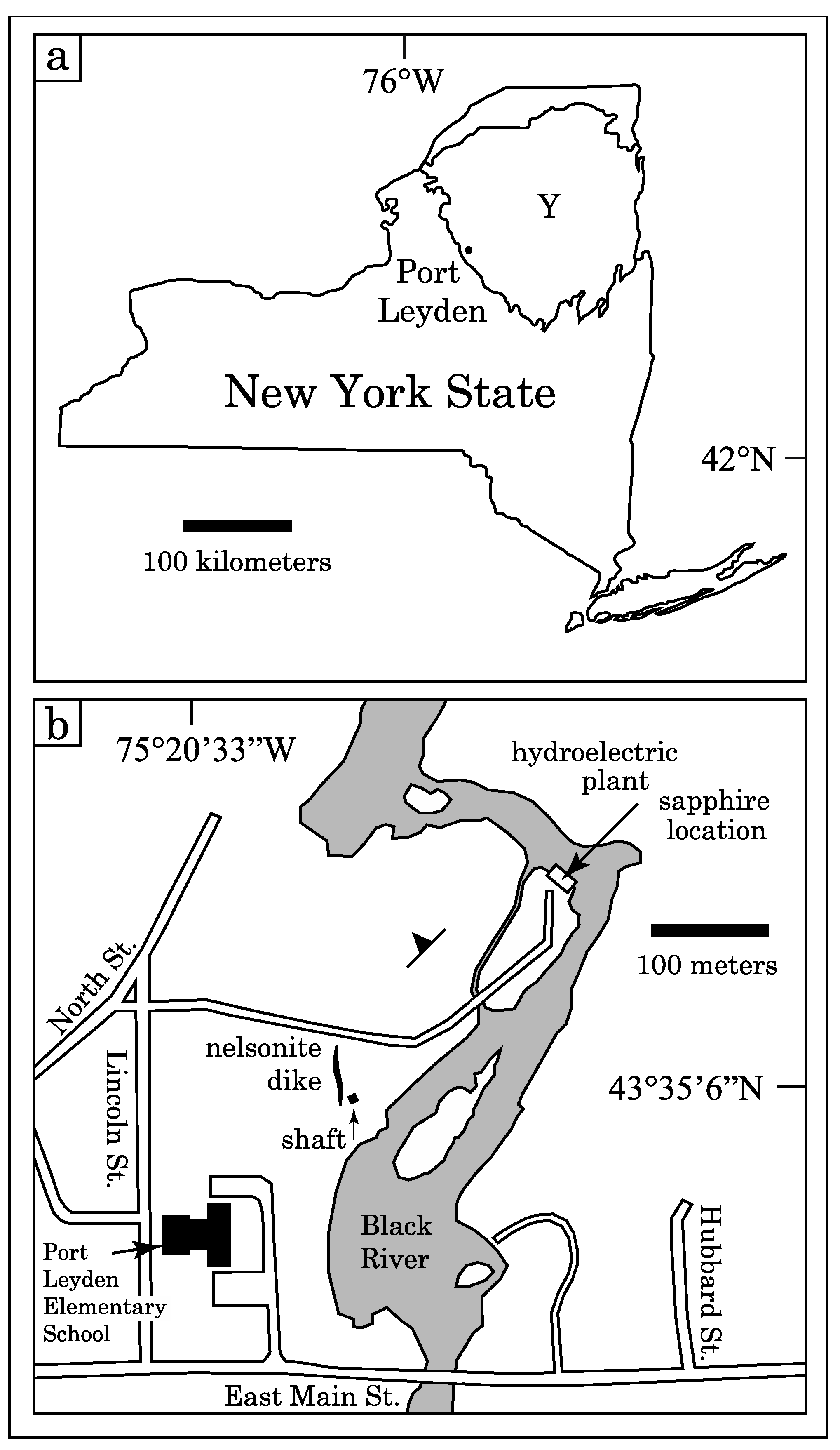
2. Study Area
3. Geologic Setting
4. Petrography




5. Sapphire Composition
| Color | Clear | Clear | Blue | Blue | Blue | Blue | Blue | Blue |
|---|---|---|---|---|---|---|---|---|
| Analysis | 1-007 | 1-016 | 1-001 | 1-009 | 1-013 | 1-020 b | 2-031 a | 2-05 |
| SiO2 | 0.024 | --- | 0.002 | 0.014 | --- | --- | 0.017 | --- |
| TiO2 | 0.002 | 0.011 | 0.470 | 0.334 | 0.359 | 0.251 | 0.226 | 0.219 |
| Al2O3 | 99.507 | 99.556 | 97.729 | 98.200 | 99.454 | 99.993 | 99.90 | 100.281 |
| FeO | 0.706 | 0.750 | 0.770 | 0.589 | 0.685 | 0.591 | 0.762 | 0.383 |
| Cr2O3 | 0.020 | --- | 0.079 | 0.072 | 0.038 | 0.006 | 0.018 | 0.051 |
| V2O3 | 0.020 | 0.042 | 0.075 | 0.010 | 0.077 | 0.024 | 0.013 | 0.022 |
| MgO | 0.013 | --- | --- | 0.001 | 0.015 | --- | --- | --- |
| Ga2O3 | --- | --- | 0.056 | 0.097 | 0.026 | --- | 0.052 | 0.041 |
| Total | 100.292 | 100.359 | 99.181 | 99.317 | 100.654 | 100.865 | 100.990 | 100.997 |
| Atoms per formula unit b | ||||||||
| Si | 0.00041 | 0.00000 | 0.00003 | 0.00024 | 0.00000 | 0.00000 | 0.00029 | 0.00000 |
| Ti | 0.00003 | 0.00014 | 0.00608 | 0.00431 | 0.00457 | 0.00319 | 0.00287 | 0.00278 |
| Al | 1.99154 | 1.99214 | 1.98172 | 1.98610 | 1.98534 | 1.98978 | 1.98764 | 1.99128 |
| Fe | 0.01003 | 0.01065 | 0.01108 | 0.00845 | 0.00970 | 0.00835 | 0.01076 | 0.00540 |
| Cr | 0.00027 | 0.00000 | 0.00107 | 0.00098 | 0.00051 | 0.00008 | 0.00024 | 0.00068 |
| V | 0.00027 | 0.00057 | 0.00103 | 0.00014 | 0.00105 | 0.00032 | 0.00018 | 0.00030 |
| Mg | 0.00066 | 0.00000 | 0.00000 | 0.00003 | 0.00038 | 0.00000 | 0.00000 | 0.00000 |
| Ga | 0.00000 | 0.00000 | 0.00062 | 0.00107 | 0.00028 | 0.00000 | 0.00056 | 0.00044 |
| Cations | 2.00320 | 2.00350 | 2.00165 | 2.00131 | 2.00184 | 2.00172 | 2.00253 | 2.00087 |
| Ti c | 0.30 | 1.30 | 35.43 | 33.78 | 32.03 | 27.64 | 21.06 | 33.99 |

6. Sapphire Optical Spectroscopy

7. Origin of the Sapphires
7.1. Petrologic Considerations
7.2. Trace Element Considerations:

8. Significance and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simonet, C.; Fritsch, E.; Lasnier, B. A classification of gem corundum deposits aimed towards gem exploration. Ore Geol. Rev. 2008, 34, 127–133. [Google Scholar] [CrossRef]
- Giuliani, G.; Ohnenstetter, D.; Fallick, A.; Groat, L.; Fagan, A. The geology and genesis of gem corundum deposits. In Geology of Gem Deposits, 2nd ed.; Groat, L., Ed.; Mineralogical Association of Canada Short Course Series 44; Mineralogical Association of Canada: Quebec City, QC, Canada, 2014; pp. 29–112. [Google Scholar]
- Ortega-Gutierrez, F.; Martiny, B.M.; Morán-Zenteno, D.J.; Reyes-Salas, A.M.; Solé-Viñas, J. Petrology of very high temperature crustal xenoliths in the Puente Negro intrusion: A sapphire-spinel-bearing Oligocene andesite, Mixteco terrane, southern Mexico. Rev. Mex. Cienc. Geol. 2011, 28, 593–629. [Google Scholar]
- Philpotts, A.R. Origin of certain iron-titanium oxide and apatite rocks. Econ. Geol. 1967, 62, 303–315. [Google Scholar] [CrossRef]
- Dymek, R.F.; Owens, B.E. Petrogenesis of apatite-rich rocks (nelsonites and oxide-apatite gabbronorites) associated with massif anorthosites. Econ. Geol. 2001, 96, 797–815. [Google Scholar] [CrossRef]
- Charlier, B.; Namur, O.; Bolle, O.; Latypov, R.; Duchesne, J.C. Fe–Ti–V–P ore deposits associated with Proterozoic massif-type anorthosites and related rocks. Earth Sci. Rev. 2015, 141, 56–81. [Google Scholar] [CrossRef]
- Duchesne, J.C.; Liégeois, J.P. The origin of nelsonite and high-Zr ferrodiorite associated with Proterozoic anorthosite. Ore Geol. Rev. 2015, 71, 40–56. [Google Scholar] [CrossRef]
- Darling, R.S.; Florence, F.P. Apatite light rare earth chemistry of the Port Leyden nelsonite, Adirondack Highlands, NY: Implications for the origin of nelsonite in anorthosite suite rocks. Econ. Geol. 1995, 90, 964–968. [Google Scholar] [CrossRef]
- Rivers, T. Assembly and preservation of lower, mid, and upper orogenic crust in the Grenville Province—Implications for the evolution of large hot long-duration orogens. Precambrian Res. 2008, 167, 237–259. [Google Scholar] [CrossRef]
- McLelland, J.M.; Selleck, B.W.; Bickford, M.E. Review of the Proterozoic evolution of the Grenville Province, its Adirondack outlier, and the Mesoproterozoic inliers of the Appalachians. In From Rodinia to Pangea: Lithotectonic Record of the Appalachian Region. Geological Society of America Memoir 206; Tollo, R.P., Bartholomew, M.J., Hibbard, J.P., Karabinos, P.M., Eds.; Geological Society of America: Boulder, CO, USA, 2010; pp. 21–49. [Google Scholar]
- Florence, F.P.; Darling, R.S.; Orrell, S.E. Moderate pressure metamorphism and anatexis due to anorthosite intrusion, western Adirondack Highlands, New York. Contrib. Miner. Petrol. 1995, 121, 424–436. [Google Scholar] [CrossRef]
- Darling, R.S. Zircon-bearing, crystallized melt inclusions in peritectic garnet from the western Adirondack Mountains, New York State, USA. Geofluids 2013, 13, 453–459. [Google Scholar] [CrossRef]
- Darling, R.S.; Florence, F.P.; Lester, G.W.; Whitney, P.R. Petrogenesis of prismatine-bearing metapelitic gneiss along the Moose River, west-central Adirondacks, New York. In Proterozoic Tectonic Evolution of the Grenville Orogen in North America. Geological Society of America Memoir 197; Tollo, R.P., Corriveau, L., McLelland, J., Bartholomew, M.J., Eds.; Geological Society of America: Boulder, CO, USA, 2004; pp. 325–336. [Google Scholar]
- Storm, L.C.; Spear, F.S. Application of the titanium-in-quartz thermometer in pelitic migmatites from the Adirondack Highlands, New York. J. Metamorph. Geol. 2009, 27, 479–494. [Google Scholar] [CrossRef]
- Darling, R.S. Geology of the Black River Valley and the Western Adirondacks. In Field Trip Guidebook for the 84th Annual Meeting of the New York State Geological Association, 28–30, September 2012, Clinton, NY, USA; Rayne, T., Ed.; New York State Geological Association, 2012; pp. A1–A22. [Google Scholar]
- White, J.S. Boehmite exsolution in corundum. Am. Mineral. 1979, 64, 1300–1302. [Google Scholar]
- Klapper, H. Generation and propagation of defects during crystal growth. In Springer Handbook of Crystal Growth; Dhanaraj, G., Byrappa, K., Prasad, V., Dudley, M., Eds.; Springer: Berlin, Germany, 2010; pp. 93–132. [Google Scholar]
- Voudouris, P.; Mavrogonatos, C.; Graham, I.; Giuliani, G.; Melfos, V.; Karampelas, S.; Karantoni, V.; Wang, K.; Tarantola, A.; Zaw, K.; et al. Gem Corundum Deposits of Greece: Geology, Mineralogy and Genesis. Minerals 2019, 9, 49. [Google Scholar] [CrossRef]
- Grapes, R.; Palmer, K. (Ruby—Sapphire)—Chromian Mica—Tourmaline Rocks from Westland, New Zealand. J. Petrol. 1996, 37, 293–315. [Google Scholar] [CrossRef]
- Izokh, A.E.; Smirnov, S.Z.; Egorova, V.V.; Anh, T.T.; Kovyazin, S.V.; Phuong, N.T.; Kalinina, V.V. The conditions of formation of sapphire and zircon in the areas of alkali-basaltoid volcanism in Central Vietnam. Rus. Geol. Geophys. 2010, 51, 719–733. [Google Scholar] [CrossRef]
- Meyer, H.; Mitchell, R. Sapphire-bearing ultramafic lamprophyre from Yogo, Montana: A ouachitite. Can. Miner. 1988, 26, 81–88. [Google Scholar]
- Simonet, C.; Paquette, J.L.; Pin, C.; Lasnier, B.; Fritsch, E. The Dusi (Garba Tula) sapphire deposit, Central Kenya—A unique Pan-African corundum-bearing monzonite. J. Afr. Earth Sci. 2004, 38, 401–410. [Google Scholar] [CrossRef]
- Sorokina, E.S.; Karampelas, S.; Nishanbaev, T.P.; Nikandrov, S.N.; Semiannikov, B.S. Sapphire megacrysts in syenite pegmatites from the Ilmen Mountains, South Urals, Russia: New mineralogical data. Can. Miner. 2017, 55, 823–843. [Google Scholar] [CrossRef]
- Spencer, K.J.; Lindsley, D.H. A solution model for coexisting iron–titanium oxides. Am. Miner. 1981, 66, 1189–1201. [Google Scholar]
- Emmett, J.L.; Scarratt, K.; McClure, S.F.; Moses, T.; Douthit, T.R.; Hughes, R.; Novak, S.; Shigley, J.E.; Wang, W.; Bordelon, O.; et al. Beryllium diffusion of ruby and sapphire. Gems Gemol. 2003, 39, 84–135. [Google Scholar] [CrossRef]
- Nassau, K. The origins of color in minerals. Am. Miner. 1978, 63, 219–229. [Google Scholar]
- Burns, R.G. Intervalence transitions in mixed-valence minerals of iron and titanium. Annu. Rev. Earth Planet. Sci. 1981, 9, 345–383. [Google Scholar] [CrossRef]
- Fritsch, E.; Rossman, G.R. An update on color in gems. Part II. Colors caused by charge transfers and color centers. Gems Gemol. 1988, 24, 3–15. [Google Scholar] [CrossRef]
- Rossman, G.R. Optical spectroscopy. Rev. Miner. Geochem. 2014, 78, 371–398. [Google Scholar] [CrossRef]
- Sutherland, F.L.; Duroc-Danner, J.M.; Meffre, S. Age and origin of gem corundum and zircon megacrysts from the Mercaderes–Rio Mayo area, South-west Colombia, South America. Ore Geol. Rev. 2008, 34, 155–168. [Google Scholar] [CrossRef]
- Shore, M.; Fowler, A.D. Oscillatory zoning in minerals; a common phenomenon. Can. Miner. 1996, 34, 1111–1126. [Google Scholar]
- Upton, B.G.J.; Hinton, R.W.; Aspen, P.; Finch, A.; Valley, J.W. Megacrysts and associated xenoliths: Evidence for migration of geochemically enriched melts in the upper mantle beneath Scotland. J. Petrol. 1999, 40, 935–956. [Google Scholar] [CrossRef]
- Keller, D.S.; Ague, J.J. High-pressure granulite facies metamorphism (~1.8 GPa) revealed in silica-undersaturated garnet-spinel-corundum gneiss, Central Maine Terrane, Connecticut, USA. Am. Miner. 2018, 103, 1851–1868. [Google Scholar]
- Bohlen, S.R.; Essene, E.J. Igneous pyroxenes from metamorphosed anorthosite massifs. Contrib. Miner. Petrol. 1978, 65, 433–442. [Google Scholar] [CrossRef]
- Morse, S.A. A partisan review of Proterozoic anorthosites. Am. Miner. 1982, 67, 1087–1100. [Google Scholar]
- Ranson, W.A. Complex exsolution in inverted pigeonite; exsolution mechanisms and temperatures of crystallization and exsolution. Am. Miner. 1986, 71, 1322–1336. [Google Scholar]
- Powers, R.E.; Bohlen, S.R. The role of synmetamorphic igneous rocks in the metamorphism and partial melting of metasediments, Northwest Adirondacks. Contrib. Miner. Petrol. 1985, 90, 401–409. [Google Scholar] [CrossRef]
- Chiarenzelli, J.; Lupulescu, M.; Robinson, G.; Bailey, D.; Singer, J. Age and origin of silicocarbonate pegmatites of the Adirondack Region. Minerals 2019, 9, 508. [Google Scholar] [CrossRef]
- Florence, F.P. Melt forming reactions at the Port Leyden nelsonite-pelitic gneiss contact: A disequilibrium record of tectonic history. Geol. Soc. Am. Abstr. Programs 1997, 29, 45. [Google Scholar]
- Turnock, A.C.; Eugster, H.P. Fe-Al Oxides: Phase Relationships below 1000 °C. J. Petrol. 1962, 3, 533–565. [Google Scholar] [CrossRef]
- Peucat, J.J.; Ruffault, P.; Fritsch, E.; Bouhnik-Le Coz, M.; Simonet, C.; Lasnier, B. Ga/Mg ratio as a new geochemical tool to differentiate magmatic from metamorphic blue sapphires. Lithos 2007, 98, 261–274. [Google Scholar] [CrossRef]
- Sutherland, F.L.; Zaw, K.; Meffre, S.; Giuliani, G.; Fallick, A.E.; Graham, I.T.; Webb, G.B. Gem-corundum megacrysts from east Australian basalt fields: Trace elements, oxygen isotopes and origins. Aust. J. Earth Sci. 2009, 56, 1003–1022. [Google Scholar] [CrossRef]
- Sutherland, F.; Zaw, K.; Meffre, S.; Yui, T.F.; Thu, K. Advances in trace element “fingerprinting” of gem corundum, ruby and sapphire, Mogok area, Myanmar. Minerals 2015, 5, 61–79. [Google Scholar] [CrossRef]
- Uher, P.; Giuliani, G.; Szakall, S.; Fallick, A.; Strunga, V.; Vaculovic, T.; Ozdin, D.; Greganova, M. Sapphires related to alkali basalts from the Cerová Highlands, Western Carpathians (southern Slovakia): Composition and origin. Geol. Carpath. 2012, 63, 71–82. [Google Scholar] [CrossRef]
- Chiarenzelli, J.; Lupulescu, M.; Thern, E.; Cousens, B. Tectonic implications of the discovery of a Shawinigan ophiolite (Pyrites Complex) in the Adirondack Lowlands. Geosphere 2011, 7, 333–356. [Google Scholar] [CrossRef]
- Wang, K.K.; Graham, I.T.; Lay, A.; Harris, S.J.; Cohen, D.R.; Voudouris, P.; Belousova, E.; Giuliani, G.; Fallick, A.E.; Greig, A. The origin of a new pargasite-schist hosted ruby deposit from Paranesti, Northern Greece. Can. Miner. 2017, 55, 535–560. [Google Scholar] [CrossRef]
- Charlier, B.; Namur, O.; Malpas, S.; De Marneffe, C.; Duchesne, J.-C.; Vander Auwera, J.; Bolle, O. Origin of the giant Allard Lake ilmenite ore deposit (Canada) by fractional crystallization, multiple magma pulses and mixing. Lithos 2010, 117, 119–134. [Google Scholar] [CrossRef]
- Morisset, C.E.; Scoates, J.S.; Weis, D.; Sauvé, M.; Stanaway, K.J. Rutile-bearing ilmenite deposits associated with the Proterozoic Saint-Urbain and Lac Allard anorthosite massifs, Grenville Province, Quebec. Can. Miner. 2010, 48, 821–849. [Google Scholar] [CrossRef]
- Liu, T.C.; Presnall, D.C. Liquidus phase relationships on the join anorthite–forsterite–quartz at 20 kbar with applications to basalt petrogenesis and igneous sapphirine. Contrib. Miner. Petrol. 1990, 104, 735–742. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darling, R.S.; Gordon, J.L.; Loew, E.R. Microscopic Blue Sapphire in Nelsonite from the Western Adirondack Mountains of New York State, USA. Minerals 2019, 9, 633. https://doi.org/10.3390/min9100633
Darling RS, Gordon JL, Loew ER. Microscopic Blue Sapphire in Nelsonite from the Western Adirondack Mountains of New York State, USA. Minerals. 2019; 9(10):633. https://doi.org/10.3390/min9100633
Chicago/Turabian StyleDarling, Robert S., Jessica L. Gordon, and Ellis R. Loew. 2019. "Microscopic Blue Sapphire in Nelsonite from the Western Adirondack Mountains of New York State, USA" Minerals 9, no. 10: 633. https://doi.org/10.3390/min9100633
APA StyleDarling, R. S., Gordon, J. L., & Loew, E. R. (2019). Microscopic Blue Sapphire in Nelsonite from the Western Adirondack Mountains of New York State, USA. Minerals, 9(10), 633. https://doi.org/10.3390/min9100633




