A Review of Boron-Bearing Minerals (Excluding Tourmaline) in the Adirondack Region of New York State
Abstract
1. Introduction
2. Materials and Methods
3. Boron Minerals in the Adirondack Mountains
3.1. Danburite CaB2Si2O8
3.1.1. Danburite History and Geologic Setting
3.1.2. Danburite Properties
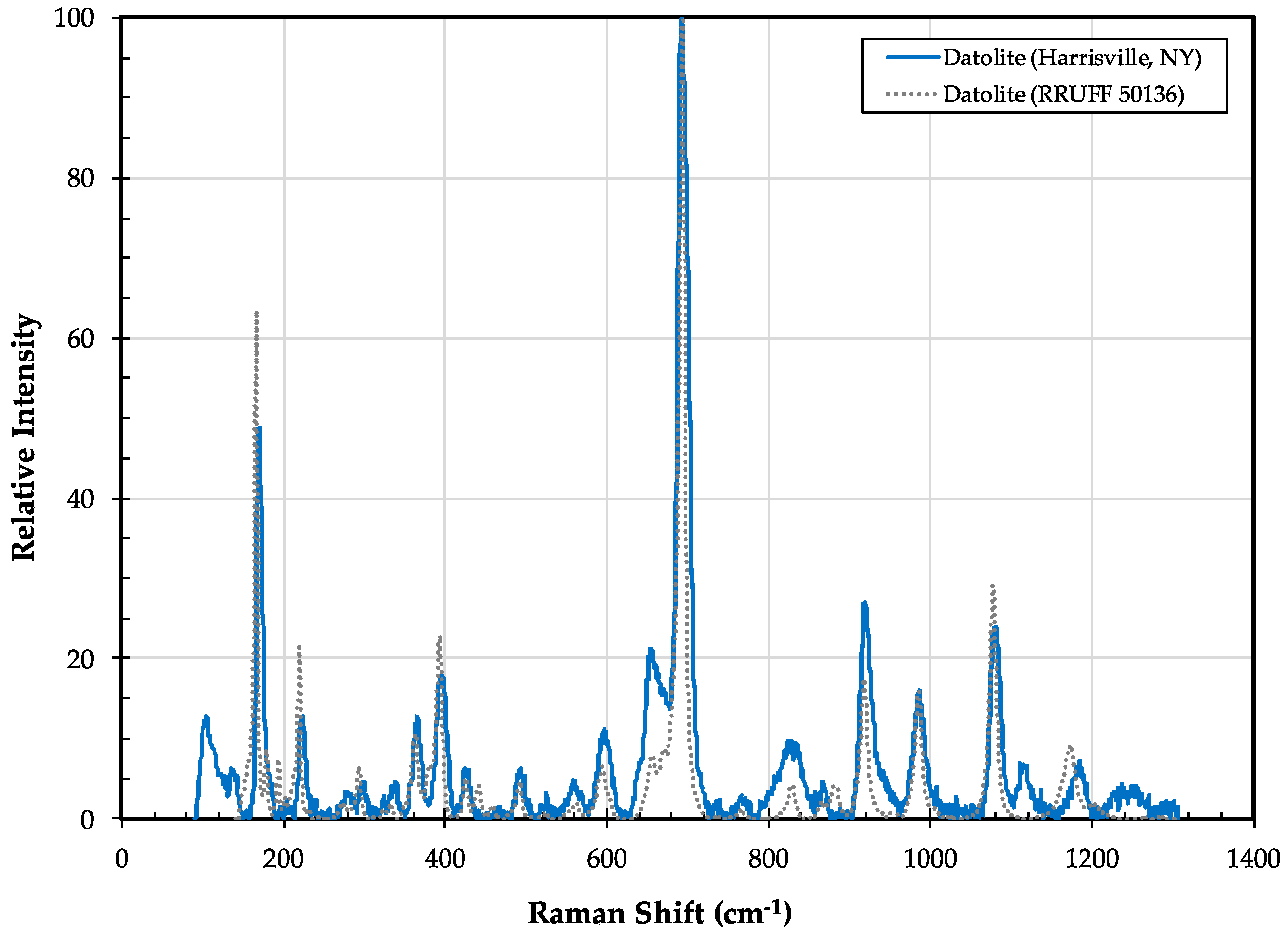
3.2. Datolite CaB(SiO4)(OH)
3.2.1. Datolite History and Geologic Setting
3.2.2. Datolite Properties
3.3. Dumortierite AlAl6BSi3O18
3.3.1. Dumortierite History and Geologic Setting
3.3.2. Dumortierite Properties
3.4. Grandidierite MgAl3O2(BO3)(SiO4)
3.4.1. Grandidierite History and Geologic Setting
3.4.2. Grandidierite Properties
3.5. Harkerite Ca12Mg4Al(BO3)3(SiO4)4(CO3)5·H2O
3.5.1. Harkerite History and Geologic Setting
3.5.2. Harkerite Properties
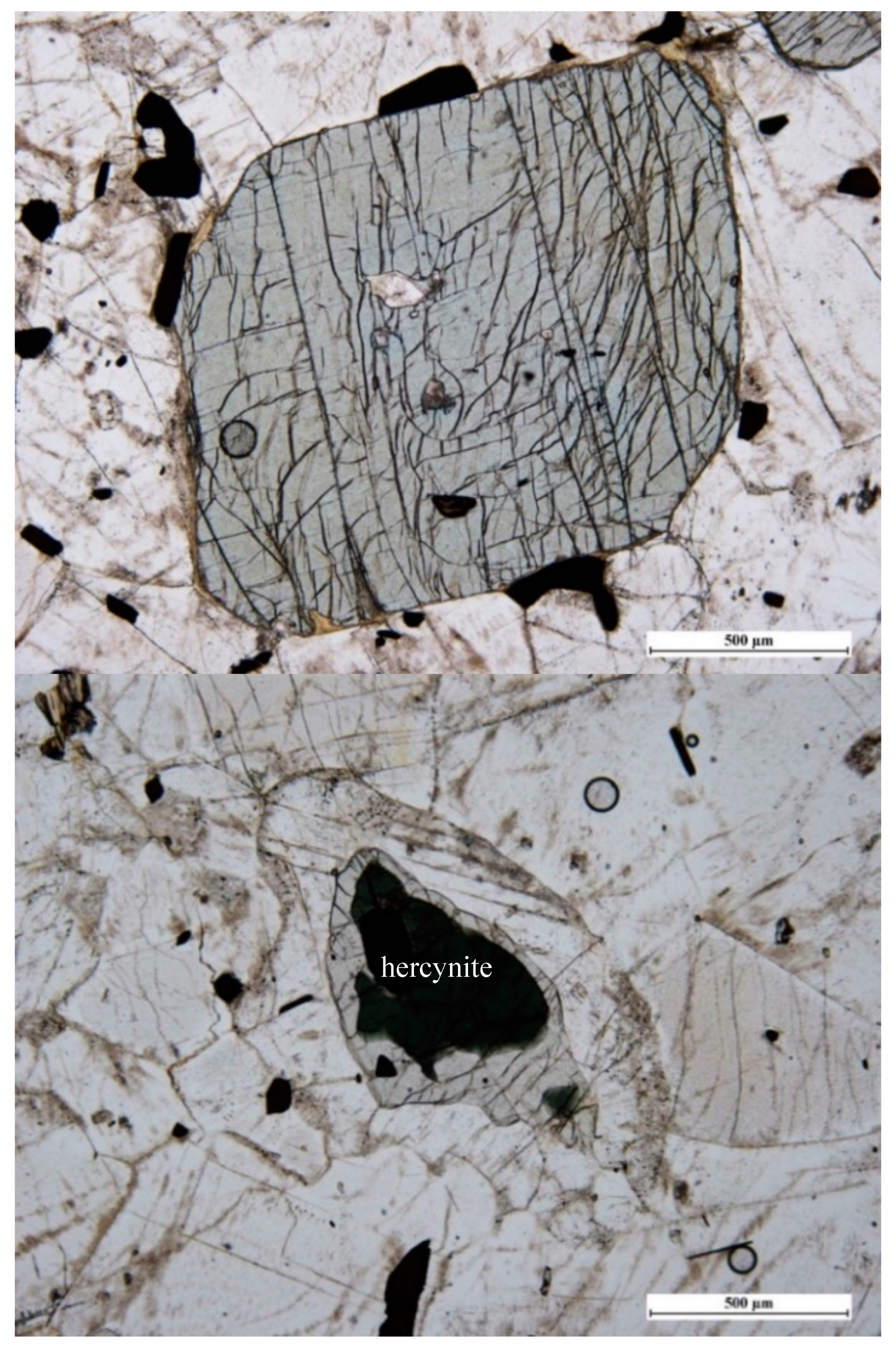
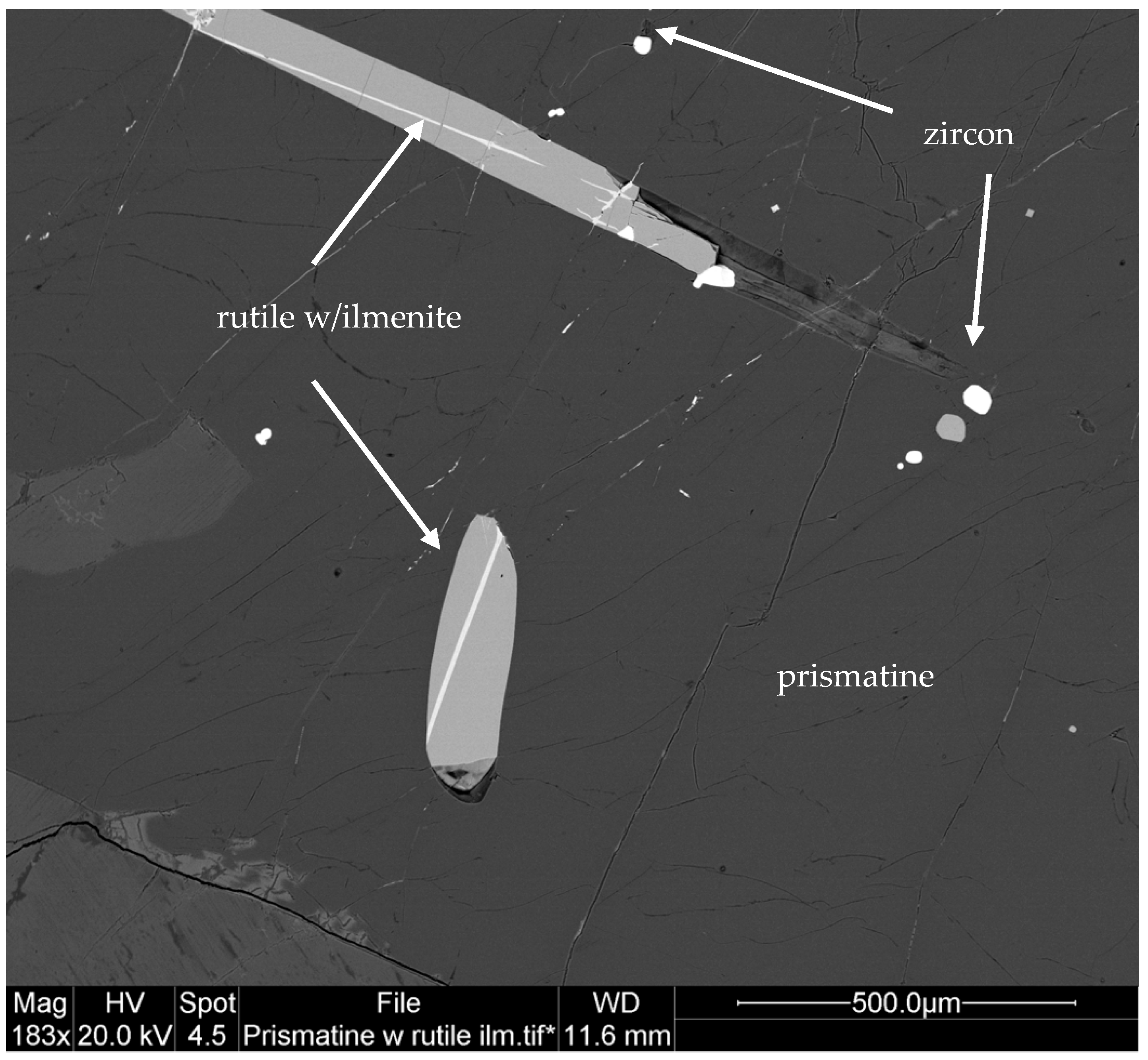
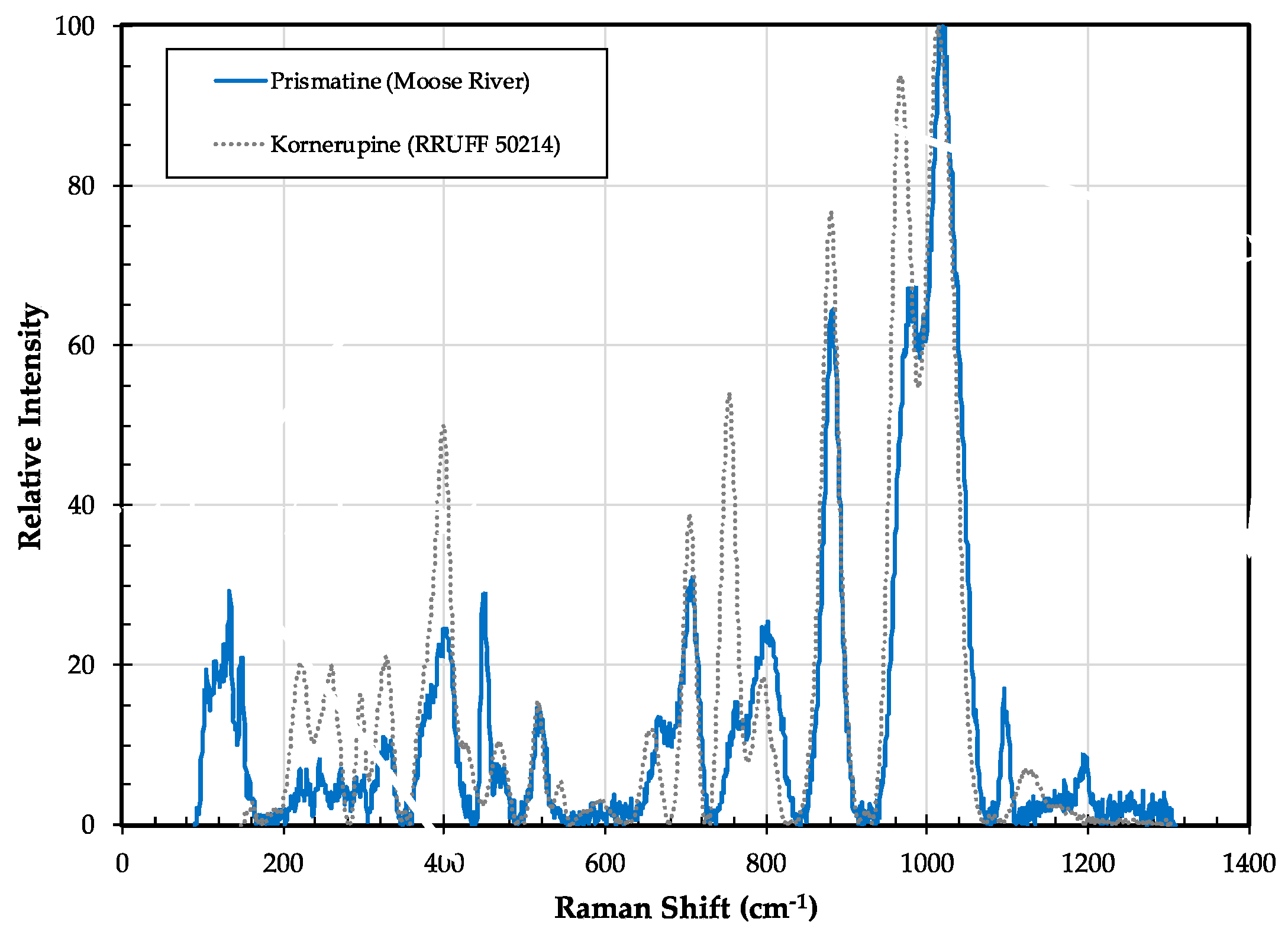
3.6. Kornerupine/Prismatine (□,Mg,Fe2+)(Mg,Fe2+)2(Al,Mg,Fe2+,Fe3+)7(Si,Al)4(Si,Al,B)O21(OH,F)/(□,Mg,Fe2+)(Mg,Fe2+)2(Al,Mg,Fe2+,Fe3+)7(Si,Al)4(B,Si,Al)O21(OH,F)
3.6.1. Kornerupine/Prismatine History and Geologic Setting
3.6.2. Kornerupine/Prismatine Properties
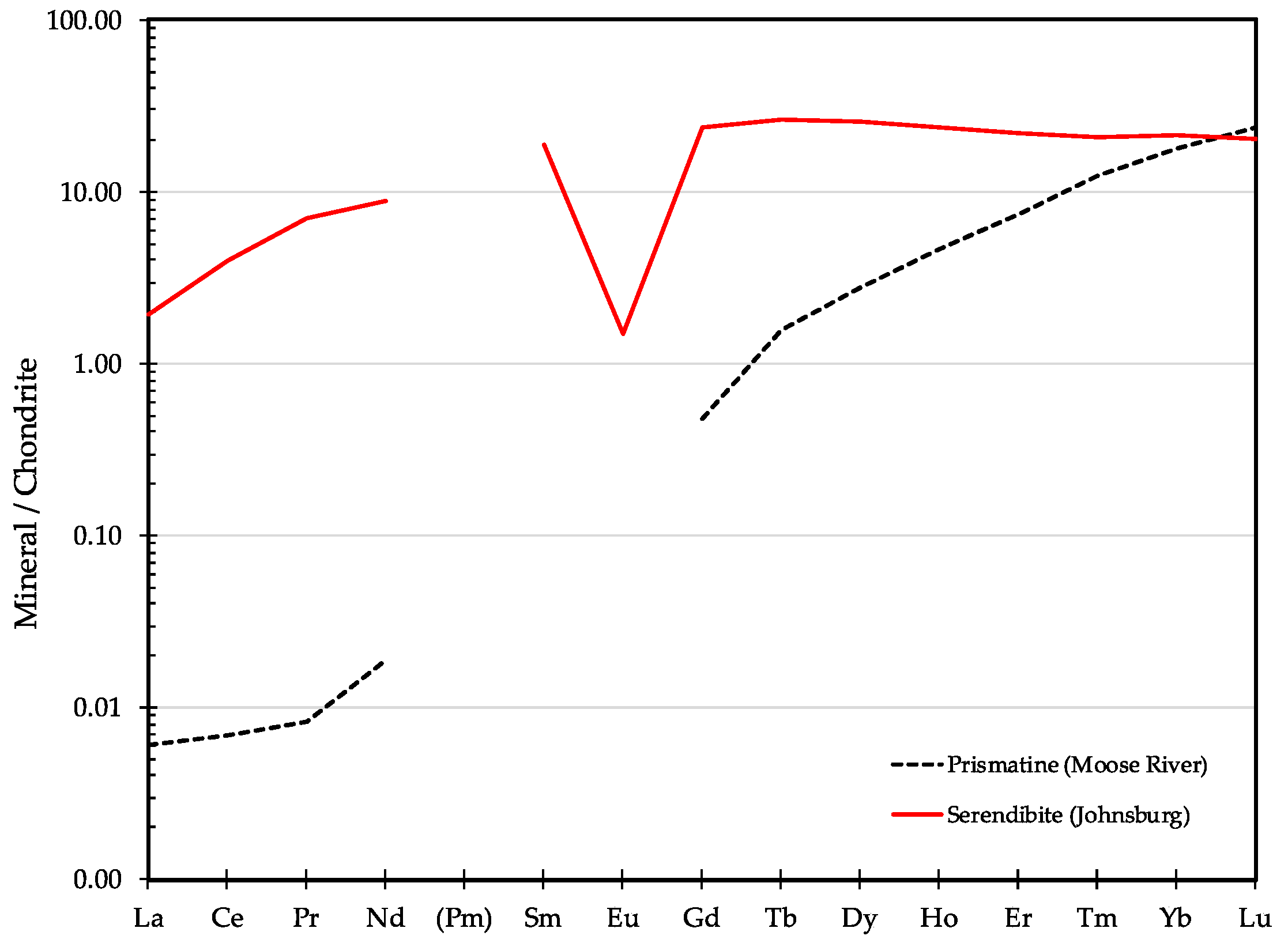
3.7. Serendibite Ca4[Mg6Al6]O4[Si6B3Al3O36]
3.7.1. Serendibite History and Geologic Setting
3.7.2. Serendibite Properties
3.8. Sinhalite MgAl(BO4)
3.8.1. Sinhalite History and Geologic Setting
3.8.2. Sinhalite Properties
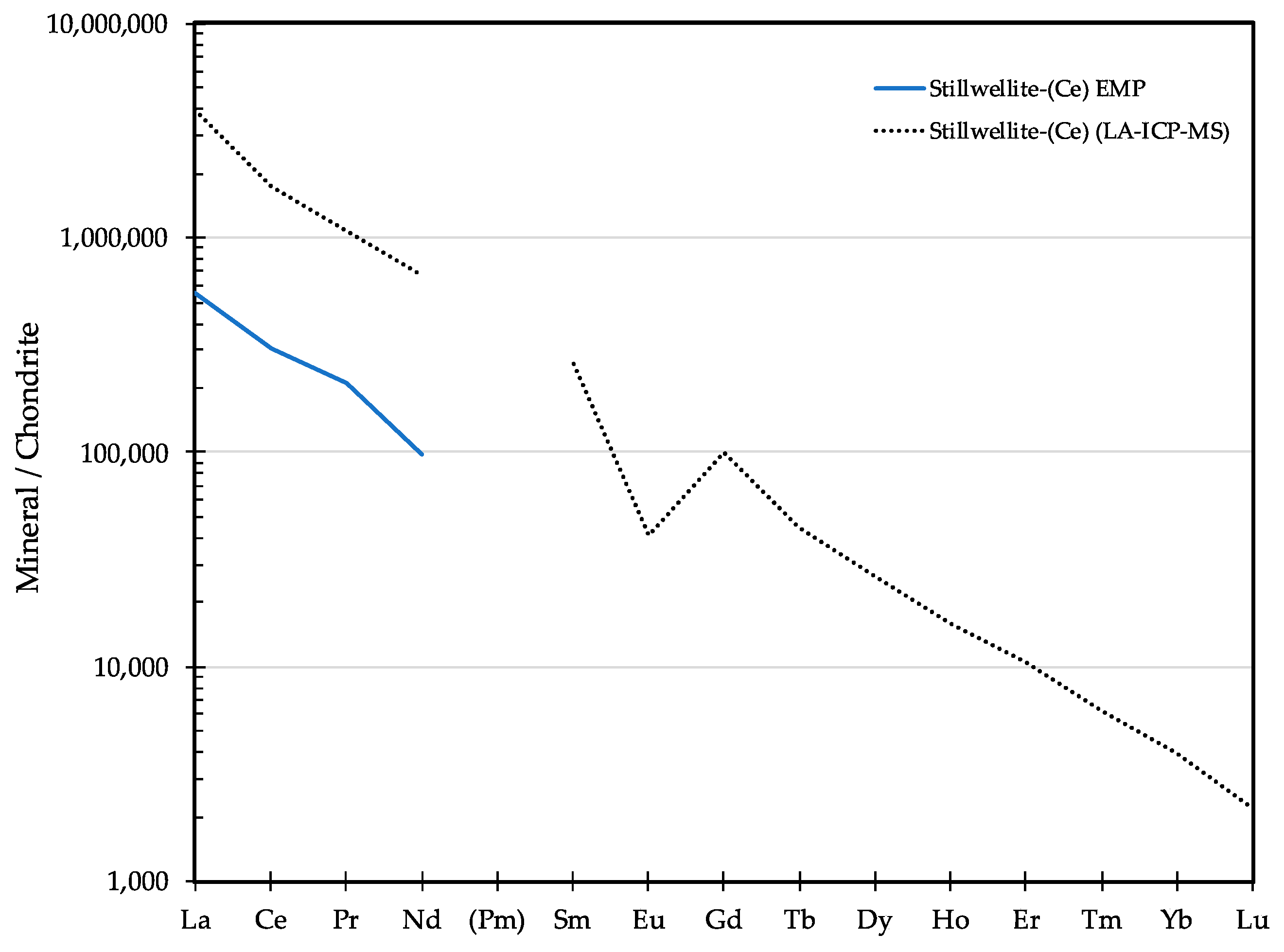
3.9. Stillwellite-(Ce) CeBSiO5
3.9.1. Stillwellite-(Ce) History and Geologic Setting
3.9.2. Stillwellite-(Ce) Properties
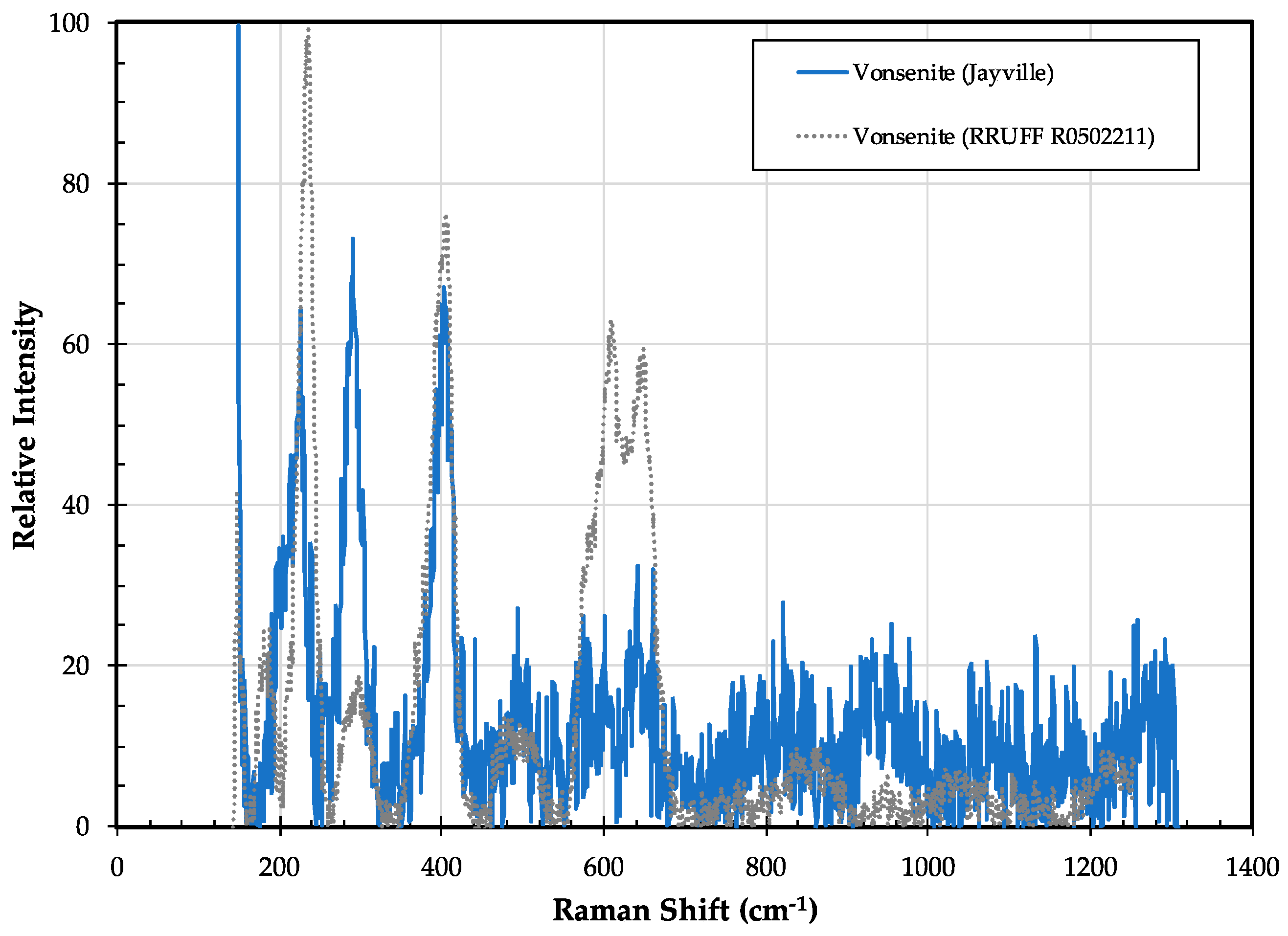
3.10. Vonsenite Fe2+2Fe3+(BO3)O2
3.10.1. Vonsenite History and Geologic Setting
3.10.2. Vonsenite Properties
3.11. Warwickite (Mg,Ti,Fe,Cr,Al)2O(BO3)
Warwickite History and Geologic Setting
4. Summary and Discussion
- Primary igneous origin
- (a)
- Granitic pegmatites
- Dumortierite (Ledge Mountain, Benson Mines, Batchellerville)
- (b)
- Anatectic melts
- Prismatine (Moose River)
- 2. Regional metamorphic origin
- (a)
- Al and Mg-rich ultramafic, metaigneous rocks
- Kornerupine (Warrensburg)
- (b)
- Al-poor, Mg-rich metasedimentary rocks
- Warwickite (Edwards, Balmat)
- (c)
- Ca and B-rich metasedimentary rocks
- Danburite (Russell)
- Serendibite (Russell)
- Skarn/pyrometasomatic origin
- Danburite (Macomb)
- Datolite (Valentine Mine)
- Harkerite (Cascade Slide)
- Serendibite (Johnsburg)
- Sinhalite (Johnsburg)
- (?) Vonsenite (Jayville)
- Retrograde/metasomatic origin
- Datolite (Russell, Macomb)
- Grandidierite (Johnsburg, Russell)
- Stillwellite-(Ce) (Mineville)
- (?) Vonsenite (Jayville)
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uluisik, I.; Karakaya, H.C.; Koc, A. The importance of boron in biological systems. J. Trace Elem. Med. Biol. 2018, 45, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Leeman, W.P.; Sisson, V.B. Geochemistry of boron and its implications for crustal and mantle processes. Rev. Mineral. 1996, 33, 645–707. [Google Scholar]
- Grew, E.S. Borosilicates (exclusive of tourmaline) and boron in rock-forming minerals in metamorphic environments. Rev. Mineral. 1996, 33, 387–502. [Google Scholar]
- Grew, E.S. Boron; from cosmic scarcity to 300 minerals. Elements 2017, 13, 225–229. [Google Scholar] [CrossRef]
- Grew, E.S.; Hystad, G.; Hazen, R.M.; Golden, J.; Krivovichev, S.V.; Gorelova, L.A. How many boron minerals occur in Earth’s upper crust? Am. Mineral. 2017, 102, 1573–1587. [Google Scholar] [CrossRef]
- Grew, E.S.; Krivovichev, S.V.; Hazen, R.M.; Hystad, G. Evolution of structural complexity in boron minerals. Can. Mineral. 2016, 54, 125–143. [Google Scholar] [CrossRef]
- Chamberlain, S.C.; Lupulescu, M.; Bailey, D.G.; Carlin, D.M., Jr. Classic danburite locality near Russell, St. Lawrence County, New York; new collecting and new research. Rocks Miner. 2011, 86, 175–176. [Google Scholar]
- Chamberlain, S.C.; Robinson, G.W.; Walter, M.R.; Chiarenzelli, J.R.; Lupulescu, M.V.; Bailey, D.G. Collector’s Guide to the Black Tourmaline of Pierrepont, New York; Schiffer Publishing, Ltd.: Atglen, PA, USA, 2016; p. 128. [Google Scholar]
- Dana, J.D. A System of Mineralogy; Including an Extended Treatise on Crystallography; with an Appendix Containing the Application of Mathematics to Crystallographic Investigation, and a Mineralogical Bibliography; Durrie, Peck, Herrick, and Noyes: New Haven, CT, USA, 1837; p. 571. [Google Scholar]
- Bosi, F. Tourmaline crystal chemistry. Am. Mineral. 2018, 103, 298–306. [Google Scholar] [CrossRef]
- Streepey, M.M.; Johnson, E.L.; Mezger, K.; van der Pluijm, B.A. Early history of the Carthage-Colton shear zone, Grenville Province, Northwest Adirondacks, New York (U.S.A.). J. Geol. 2001, 109, 479–492. [Google Scholar] [CrossRef]
- Isachsen, Y.W.; Wright, S.F.; Geraghty, E.P. Extent and Character of the Carthage-Colton Mylonite Zone, Northwest Adirondacks, New York; 02781670; U. S. Nuclear Regulatory Commission, Office of Nuclear Regulatory Research: Washington, DC, USA, 1981; p. 105.
- Chiarenzelli, J.; Selleck, B. Bedrock geology of the Adirondack Region. Adirond. J. Environ. Stud. 2016, 21, 19–42. [Google Scholar]
- Chiarenzelli, J.; Kratzmann, D.; Selleck, B.; deLorraine, W. Age and provenance of Grenville Supergroup rocks, Trans-Adirondack Baisn, constrained by detrital zircons. Geology 2015, 43, 183–186. [Google Scholar] [CrossRef]
- Rivers, T. Upper-crustal orogenic lid and mid-crustal core complexes; signature of a collapsed orogenic plateau in the hinterland of the Grenville Province. Can. J. Earth Sci. 2012, 49, 1–42. [Google Scholar] [CrossRef]
- Brush, G.J.; Dana, E.S. On crystallized danburite from Russell, Saint Lawrence County, New York. Am. J. Sci. 1880, 20, 111–118. [Google Scholar] [CrossRef]
- Clark, W. Danburite locality near Russell, New York. Rocks Miner. 1949, 24, 36–37. [Google Scholar]
- Jensen, D.E. Minerals of New York State; Ward Press: Rochester, NY, USA, 1978; p. 220. [Google Scholar]
- Butts, B.D.; Kelson, C.R. A mineralogical and geochemical investigation of a granitic pegmatite near Dekalb Junction, St. Lawrence County, New York. Abstr. Programs Geol. Soc. Am. 2012, 44, 113. [Google Scholar]
- Munschauer, R.W., II; Bailey, D.G. A mineralogical and historical study of the McLear Pegmatite, Dekalb Junction, New York. Rocks Miner. 2010, 85, 464. [Google Scholar]
- Sutherland, A.; Sutherland, S.; Robinson, G.W.; Lupulescu, M.; Bailey, D.G.; Chamberlain, S.C. New danburite locality discovered in the town of Macomb, St. Lawrence County, New York. Rocks Miner. 2017, 92, 180–184. [Google Scholar] [CrossRef]
- Edwards, R.L.; Essene, E.J. Pressure, temperature and C-O-H fluid fugacities across the amphibolite-granulite transition, NW Adirondack Mountains, NY. J. Petrol. 1988, 29, 39–72. [Google Scholar] [CrossRef]
- Chiarenzelli, J.; Lupulescu, M.; Robinson, G.; Bailey, D.; Singer, J. Age and origin of silicocarbonate pegmatites of the Adirondack region. Minerals 2019, 9, 508. [Google Scholar] [CrossRef]
- Beran, A. OH groups in nominally anhydrous framework structures: An infrared spectroscopic investigation of danburite and labradorite. Phys. Chem. Miner. 1987, 14, 441–445. [Google Scholar] [CrossRef]
- Grew, E.S.; Yates, M.G.; Delorraine, W.F. Serendibite from the Northwest Adirondack Lowlands, in Russell, New York, USA. Mineral. Mag. 1990, 54, 133–136. [Google Scholar] [CrossRef]
- Grew, E.S.; Yates, M.G.; Adams, P.M.; Kirkby, R.; Wiedenbeck, M. Harkerite and associated minerals in marble and skarn from Crestmore Quarry, Riverside County, California and Cascade Slide, Adirondack Mountains, New York. Can. Mineral. 1999, 37, 277–296. [Google Scholar]
- Darling, R.S.; Florence, F.P.; Lester, G.W.; Whitney, P.R. Petrogenesis of prismatine-bearing metapelitic gneisses along the Moose River, west-central Adirondacks, New York. Mem. Geol. Soc. Am. 2004, 197, 325–336. [Google Scholar]
- Larsen, E.S.; Schaller, W.T. Serendibite from Warren County, New York, and its paragenesis. Am. Mineral. 1932, 17, 457–465. [Google Scholar]
- Grew, E.S.; Yates, M.G.; Swihart, G.H.; Moore, P.B.; Marquez, N. The paragenesis of serendibite at Johnsburg, New York, USA; an example of boron enrichment in the granulite facies. In Progress in Metamorphic and Magmatic Petrology; Perchuk, L.L., Ed.; Cambridge University Press: Cambridge, UK, 1991; pp. 247–285. [Google Scholar]
- Leonard, B.F., III; Vlisidis, A.C. Vonsenite at the Jayville magnetite deposit, Saint Lawrence County, New York. Am. Mineral. 1961, 46, 786–811. [Google Scholar]
- Lupulescu, M.V.; Rowe, R.; Bailey, D.; Hawkins, M. Chernikovite, Fe-free warwickite, and dissakisite-(Ce) from New York State. Rocks Miner. 2014, 89, 542. [Google Scholar]
- Huong, L.T.-T.; Otter, L.M.; Forster, M.W.; Hauzenberger, C.A.; Krenn, K.; Alard, O.; Macholdt, D.S.; Weis, U.; Stoll, B.; Jochum, K.P. Femtosecond laser ablation ICP mass spectrometry and CNHS elemental analyzer reveal trace element characteristics of Danburite from Mexico, Tanzania, and Vietnam. Minerals 2018, 8, 234. [Google Scholar] [CrossRef]
- Best, S.P.; Clark, R.J.H.; Hayward, C.L.; Withnall, R. Polarized single-crystal Raman spectroscopy of danburite, CaB2Si2O8. J. Raman Spectrosc. 1994, 25, 557–563. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; W. De Gruyter: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Torrey, J. Analysis of datolite from Patterson, New Jersey. Am. J. Sci. Arts 1820, 2, 369. [Google Scholar]
- Rowley, E.B. Rare-earth pegmatite discovered in Adirondack Mountain area, Essex County, New York; Pt. 1. Rocks Miner. 1962, 37, 341–347. [Google Scholar] [CrossRef]
- Newland, D.H.; Cushing, H.P. Geology of the Gouverneur Quadrangle. Bull. N. Y. State Mus. Sci. Serv. 1925, 259, 122. [Google Scholar]
- Robinson, G.W. Famous mineral localities; De Kalb, New York. Mineral. Rec. 1990, 21, 535–541. [Google Scholar]
- Engel, A.E.J. The Precambrian Geology and Talc Deposits of the Balmat-Edwards District, Northwest Adirondack Mountains, New York; USGS Open-File Report #62–43; US Geological Survey: Reston, VA, USA, 1962.
- Chamberlain, S.C.; King, V.T.; Cooke, D.; Robinson, G.W.; Holt, W. Minerals of the Gouverneur Talc Company No. 4 quarry (Valentine Deposit); Town of Diana, Lewis County, New York. Rocks Miner. 1999, 74, 236–249. [Google Scholar] [CrossRef]
- Gerdes, M.L.; Valley, J.W. Fluid flow and mass transport at the Valentine wollastonite deposit, Adirondack Mountains, New York State. J. Metamorph. Geol. 1994, 12, 589–608. [Google Scholar] [CrossRef]
- Frost, R.L.; Xi, Y.; Scholz, R.; Lima, R.M.F.; Horta, L.F.C.; Lopez, A. Thermal analysis and vibrational spectroscopic characterization of the borosilicate mineral datolite—CaBSiO4(OH). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Zaccarini, F.; Morales-Ruano, S.; Scacchetti, M.; Garuti, G.; Heide, K. Investigation of datolite (CaB[SiO4/(OH)]) from basalts in the Northern Apennines ophiolites (Italy); genetic implications. Chem. Erde Geochem. 2008, 68, 265–277. [Google Scholar] [CrossRef]
- Pan, Y.; Fleet, M.E. Intrinsic and external controls on the incorporation of rare-earth elements in calc-silicate minerals. Can. Mineral. 1996, 34, 147–159. [Google Scholar]
- Schaller, W.T. Dumortierite. Am. J. Sci. 1905, 19, 211–224. [Google Scholar] [CrossRef]
- Seadler, A.R.; Lupulescu, M.V.; Bailey, D.G. Pegmatites of New York State: The Batchellerville Pegmatite. In New York State Geological Association, 84th Annual Meeting, Field Trip Guidebook; New York State Geological Association: Clinton, NY, USA, 2012; Volume 84. [Google Scholar]
- Lupulescu, M.V.; Bailey, D.G.; Hawkins, M.; Carl, J.D.; Chiarenzelli, J.R. The Benson Mines, St. Lawrence County, New York. Rocks Miner. 2014, 89, 118–131. [Google Scholar] [CrossRef]
- Gervais, S.M.; Metzger, E.P. Preliminary analysis of borosilicate minerals in pegmatitic leucosomes within aluminous granulites at Ledge Mountain, Central Adirondack Highlands, New York. In American Geophysical Union, Fall Meeting; American Geophysical Union: San Francisco, CA, USA, 2011; Volume V31F-2594. [Google Scholar]
- Pieczka, A.; Evans, R.J.; Grew, E.S.; Groat, L.A.; Ma, C.; Rossman, G.R. The dumortierite supergroup; I, A new nomenclature for the dumortierite and holtite groups. Mineral. Mag. 2013, 77, 2825–2839. [Google Scholar] [CrossRef][Green Version]
- Pieczka, A.; Evans, R.J.; Grew, E.S.; Groat, L.A.; Ma, C.; Rossman, G.R. The dumortierite supergroup; II, Three new minerals from the Szklary Pegmatite, SW Poland; Nioboholtite, (Nb0.6 0.4)Al6BSi3O18, titanoholtite, (Ti0.75 0.25)Al6BSi3O18, and szklaryite, Al6BAs33+O15. Mineral. Mag. 2013, 77, 2841–2856. [Google Scholar] [CrossRef]
- Grew, E.S. Boron and beryllium minerals in granulite-facies pegmatites and implications of beryllium pegmatites for the origin and evolution of the Archean Napier Complex of East Antarctica. Mem. Natl. Inst. Polar Res. Spec. Issue 1998, 53, 74–92. [Google Scholar]
- Huijsmans, J.P.P.; Barton, M.; van Bergen, M.J. A pegmatite containing Fe-rich grandidierite, Ti-rich dumortierite and tourmaline from the Precambrian, high-grade metamorphic complex of Rogaland, S.W. Norway. Neues Jahrb. Fuer Mineral. Abh. 1982, 143, 249–261. [Google Scholar]
- Carmichael, D.M. Genesis of grandidierite and kornerupine in metapelites by anatectic dehydration of tourmaline. Abstr. Programs Geol. Soc. Am. 1994, 26, 448–449. [Google Scholar]
- Nicollet, C. Occurrences of grandidierite, serendibite and tourmaline near Ihosy, Southern Madagascar. Mineral. Mag. 1990, 54, 131–133. [Google Scholar] [CrossRef]
- Carson, C.J.; Hand, M.; Dirks, P.H.G.M. Stable coexistence of grandidierite and kornerupine during medium pressure granulite facies metamorphism. Mineral. Mag. 1995, 59, 327–339. [Google Scholar] [CrossRef]
- MacGregor, J.R. Boron Isotopic Study of the Borosilicates Tourmaline, Prismatine and Grandidierite in Granulite Facies Paragneisses, from the Larsemann Hills, Prydz Bay, East Antarctica. Master’s Thesis, University of Maine, Orono, ME, USA, 2012. [Google Scholar]
- Herd, R.K.; Windley, B.F.; Ackermand, D. Grandidierite from a pelitic xenolith in the Haddo House Complex, NE Scotland. Mineral. Mag. 1984, 48, 401–406. [Google Scholar]
- van Bergen, M.J. Grandidierite from aluminous metasedimentary xenoliths within acid volcanics, a first record in Italy. Mineral. Mag. 1980, 43, 651–658. [Google Scholar] [CrossRef]
- Barbieri, M.; Cozzupoli, D.; Federico, M.; Fornaseri, M.; Merlino, S.; Orlandi, P.; Tolomeo, L. Harkerite from the Alban Hills, Italy. Lithos 1977, 10, 133–141. [Google Scholar] [CrossRef]
- Baillieul, T.A. The Cascade slide; a mineralogical investigation of a calc-silicate body on Cascade Mountain, Town of Keene, Essex County, New York. Master’s Thesis, University of Massachusetts at Amherst, Amherst, MA, USA, 1976. [Google Scholar]
- Valley, J.W.; Essene, E.J. Akermanite in the Cascade Slide xenolith and its significance for regional metamorphism in the Adirondacks. Contrib. Mineral. Petrol. 1980, 74, 143–152. [Google Scholar] [CrossRef][Green Version]
- Peck, W.H.; Selleck, B.W.; Valley, J.W.; Spicuzza, M.J.; Taylor, A.T. Emplacement and metamorphism of the Marcy Anorthosite; new constraints from geochronology and oxygen isotopes. Abstr. Programs Geol. Soc. Am. 2017, 49, #106-2. [Google Scholar]
- Valley, J.W. The conundrum at Cascade Slide; a petrologic/stable isotopic answer. Pap. Proc. Gen. Meet. Int. Mineral. Assoc. 1986, 253. [Google Scholar]
- Grew, E.S.; Cooper, M.A.; Hawthorne, F.C. Prismatine; revalidation for boron-rich compositions in the kornerupine group. Mineral. Mag. 1996, 60, 483–491. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Cooper, M.A.; Grew, E.S. The crystal chemistry of the kornerupine-prismatine series; III, Chemical relations. Can. Mineral. 2009, 47, 275–296. [Google Scholar] [CrossRef]
- Farrar, S.S. Mg-Al-B-rich facies associated with the Moon Mountain metanorthosite sill, southeastern Adirondacks, NY. Abstr. Programs Geol. Soc. Am. 1995, 27, 42–43. [Google Scholar]
- Farrar, S.S. Mg-Al-rich Adirondack Grenville ultramafics; anything similar in Southern Appalachians? Abstr. Programs Geol. Soc. Am. 2007, 39, 27. [Google Scholar]
- Farrar, S.S.; Babcock, L.G. A sapphirine+ kornerupine-bearing hornblende spinel peridotite associated with Adirondack anorthositic sill. Abstr. Programs Geol. Soc. Am. 1993, 25, 265. [Google Scholar]
- Farrar, S.S.; Miller, J.M. Southeastern Adirondack anorthositic sills and dikes with included ultramafic lenses. Abstr. Programs Geol. Soc. Am. 2005, 37, 22. [Google Scholar]
- Storm, L.C.; Spear, F.S. Application of the titanium-in-quartz thermometer to pelitic migmatites from the Adirondack Highlands, New York. J. Metamorph. Geol. 2009, 27, 479–494. [Google Scholar] [CrossRef]
- Cooper, M.A.; Hawthorne, F.C.; Grew, E.S. The crystal chemistry of the kornerupine-prismatine series; I, Crystal structure and site populations. Can. Mineral. 2009, 47, 233–262. [Google Scholar] [CrossRef]
- Carlson, W.D.; Gale, J.D.; Wright, K. Incorporation of Y and REEs in aluminosilicate garnet: Energetics from atomic simulation. Am. Mineral. 2014, 99, 1022–1034. [Google Scholar] [CrossRef]
- Wopenka, B.; Freeman, J.J.; Grew, E.S. Raman spectroscopic identification of B-free and B-rich kornerupine (prismatine). Am. Mineral. 1999, 84, 550–554. [Google Scholar] [CrossRef]
- Frost, R.L.; Lopes, A.; Xi, Y.; Scholz, R. A vibrational spectroscopic study of the silicate mineral kornerupine. Spectrosc. Lett. 2015, 48, 487–491. [Google Scholar] [CrossRef]
- Bohlen, S.R.; Valley, J.W.; Essene, E.J. Metamorphism in the Adirondacks; I, Petrology, pressure and temperature. J. Petrol. 1985, 26, 971–992. [Google Scholar] [CrossRef]
- Grice, J.D.; Belley, P.M.; Fayek, M. Serendibite, a complex borosilicate mineral from Pontiac, Quebec; description, chemical composition, and crystallographic data. Can. Mineral. 2014, 52, 1–14. [Google Scholar] [CrossRef]
- Claringbull, G.F.; Hey, M.H. Sinhalite (MgAlBO4), a new mineral. Mineral. Mag. 1952, 29, 841–849. [Google Scholar]
- Schaller, W.T.; Hildebrand, F.A. A second occurrence of the mineral sinhalite (2MgO·Al2O3·B2O3). Am. Mineral. 1955, 40, 1022–1031. [Google Scholar]
- Hayward, C.L.; Angel, R.J.; Ross, N.L. The structural redetermination and crystal chemistry of sinhalite, MgAlBO4. Eur. J. Mineral. 1994, 6, 313–321. [Google Scholar] [CrossRef]
- Edwards, A.B.; McAndrew, J. Stillwellite, a New Rare Earth Mineral from the Mary Kathleen Lease, Mount Isa District, Queensland; Commonwealth Scientific and Industrial Research Organization: Melbourne East, Australia, 1955; p. 3. [Google Scholar]
- Grice, J.D.; Gault, R.A. Johnsenite-(Cd), a new member of the eudialyte group from Mont Saint-Hilaire, Quebec, Canada. Can. Mineral. 2006, 44, 105–116. [Google Scholar] [CrossRef]
- Hirtopanu, P.; Andersen, C.J.; Fairhurst, J.R.; Jakab, G. Allanite-(Ce) and its associations, from the Ditrau alkaline intrusive massif, East Carpathians, Romania. Proc. Rom. Acad. Ser. B Chem. Life Sci. Geosci. 2013, 15, 59–74. [Google Scholar]
- Mei, L.; Larson, R.R.; Loferski, P.J.; Klemic, H. Analyses and Description of a Concentrate of Stillwellite from Mineville, Essex County, New York; OF 79-847; U. S. Geological Survey: Reston, VA, USA, 1979; p. 9.
- Lupulescu, M.V.; Pyle, J.M. The Fe-P-REE deposit at Mineville, Essex Co., NY; manifestations of Precambrian and Mesozoic fluid infiltration events. Abstr. Programs Geol. Soc. Am. 2005, 37, 4. [Google Scholar]
- Leonard, B.F., III; Vlisidis, A.C. Vonsenite from Saint Lawrence County, northwest Adirondacks, New York. Am. Mineral. 1960, 45, 439–442. [Google Scholar]
- Buddington, A.F.; Leonard, B.F. Ore Deposits of the Saint Lawrence County Magnetite District, Northwest Adirondacks, New York; P 0377; U. S. Geological Survey: Reston, VA, USA, 1964; p. 259. [Google Scholar]
- Hall, T.; Johnson, E.L.; Rosner, M. Analysis of the role and timing of fluid flow during ore synthesis and vonsenite crystallization in the Jayville iron deposit, NW Adirondack Mountains, NYS. Abstr. Programs Geol. Soc. Am. 2013, 45, 726. [Google Scholar]
- Johnson, E.L.; Chapman, D.; Valder, J. Fluid driven metamorphism along the Carthage-Colton mylonite zone; the role of late granite intrusion, and regional fluid flow during the waning stages of the Ottawan Orogeny; Adirondack Mountians, NYS. Abstr. Programs Geol. Soc. Am. 2005, 37, 387. [Google Scholar]
- Lupulescu, M.V.; Chiarenzelli, J.R.; Selleck, B.; McLelland, J.M.; Regan, S.P. LA-MC-ICP-MS U/Pb zircon geochronology of the Grenville-age iron deposits of New York. Abstr. Programs Geol. Soc. Am. 2016, 48, 31. [Google Scholar]
- Bonner, E.P.T.; Hughes, J.; Lupulescu, M.V.; Chiarenzelli, J. Vonsenite, 2FeO.FeBO3, a high-temperature mineral occurring in contact metamorphic deposits; crystal structure at room and liquid nitrogen temperatures. Green Mt. Geol. 2016, 43, 19. [Google Scholar]
- Lopez Ruiz, J.; Salvador Salvador, P. Chemical and crystallographic data for vonsenite from Burguillos del Cerro, Badajoz, Spain. Am. Mineral. 1971, 56, 2149–2151. [Google Scholar]
- Frost, R.L.; Scholz, R.; Lopez, A.; Xi, Y.; Belotti, F.M. Infrared and Raman Spectroscopic Characterization of the Borate Mineral Vonsenite. Spectrosc. Lett. 2014, 47, 512–517. [Google Scholar] [CrossRef][Green Version]
- Shepard, C.U. Notice of warwickite, a new mineral species. Am. J. Sci. 1838, 34, 313–315. [Google Scholar]
- Aleksandrov, S.M.; Troneva, M.A. Geochemistry of titanium and its modes of occurrence in metasomatically altered rocks at skarn deposits. Geochem. Int. 2003, 41, 21–37. [Google Scholar]
- Cotkin, S.J. Manganiferous dravite-uvite tourmaline, fluorine distribution, and talc formation, Northwest Adirondacks. Neues Jahrb. Fuer Mineral. Mon. 1989, 1989, 201–211. [Google Scholar]
- Dalton, C.T. Geochemical Characterization and Petrologic Implications of Grenville-Aged Tourmalines from the Adirondack Lowlands, St. Lawrence County, New York. Master’s Thesis, University of Akron, Akron, OH, USA, 2003. [Google Scholar]
- Lupulescu, M.; Rakovan, J. A new occurrence of tourmaline with tetrahedrally coordinated boron; rossmanite and associated minerals from Newcomb, Essex County, New York. Rocks Miner. 2009, 84, 366. [Google Scholar]
- Lupulescu, M.V. Tourmaline-group minerals in Grenville rocks from New York; relationship between composition and the geological setting. Abstr. Programs Geol. Soc. Am. 2007, 39, 41. [Google Scholar]
- Lupulescu, M.V.; Chiarenzelli, J.R. Geochemistry of tourmaline from some Adirondacks locations; indicator of the host environment. Guideb. N. Y. State Geol. Assoc. Meet. 2014, 86, 56–70. [Google Scholar]
- Swihart, G.H.; Moore, P.B. A reconnaissance of the boron isotopic composition of tourmaline. Geochim. Cosmochim. Acta 1989, 53, 911–916. [Google Scholar] [CrossRef]













| Sample ID | Map # | Locality Name | Town | County |
|---|---|---|---|---|
| Danburite CaB2Si2O8 | ||||
| DA-1 | 1 | Old Van Buskirk Farm | Russell | St. Lawrence |
| DA-2 | 2 | Downing Farm/Hickory Lake | Macomb | St. Lawrence |
| DA-3 | 3 | McLear Pegmatite | Dekalb | St. Lawrence |
| Datolite CaB(SiO4)(OH) | ||||
| DT-1 | 1 | Valentine Mine | Diana | Lewis |
| DT-2 | 2 | Old Van Buskirk Farm | Russell | St. Lawrence |
| DT-3 | 4 | Downing Farm/Hickory Lake | Macomb | St. Lawrence |
| DT-4 | 5 | Woodcock Mine | Fowler | St. Lawrence |
| DT-5 | 6 | McLear Pegmatite | Dekalb | St. Lawrence |
| DT-6 | 7 | Schroon Lake | Schroon Lake | Warren |
| Dumortierite AlAl6BSi3O18 | ||||
| DU-1 | 8 | Batchellerville Pegmatite | Edinburg | Saratoga |
| DU-2 | 9 | Benson Mines | Star Lake | St. Lawrence |
| DU-3 | 10 | Ledge Mountain | Indian Lake | Hamilton |
| Grandidierite MgAl3O2(BO3)(SiO4) | ||||
| G-1 | 11 | Armstrong Farm | Johnsburg | Warren |
| G-2 | 12 | St. Joe Drill Core | Russell | St. Lawrence |
| Harkerite Ca12Mg4Al(BO3)3(SiO4)4(CO3)5.H2O | ||||
| H-1 | 13 | Cascade Slide | Keene | Essex |
| Kornerupine (□,Mg,Fe2+)(Mg,Fe2+)2(Al,Mg,Fe2+,Fe3+)7(Si,Al)4(Si,Al,B)O21(OH,F) | ||||
| K-1 | 14 | Warrensburg | Warrensburg | Warren |
| Prismatine (□,Mg,Fe2+)(Mg,Fe2+)2(Al,Mg,Fe2+,Fe3+)7(Si,Al)4(B,Si,Al)O21(OH,F) | ||||
| P-1 | 15 | Moose River 1 | Lyonsdale | Lewis |
| P-2 | 16 | Moose River 2 | Lyonsdale | Lewis |
| Serendibite Ca4(Mg6Al6)O4(Si6B3Al3O36) | ||||
| SB-1 | 11 | Armstrong Farm | Johnsburg | Warren |
| SB-2 | 12 | St. Joe Drill Core | Russell | St. Lawrence |
| Sinhalite MgAl(BO4) | ||||
| SH-1 | 11 | Armstrong Farm | Johnsburg | Warren |
| SH-2 | 12 | St. Joe Drill Core | Russell | St. Lawrence |
| Stillwellite-(Ce) CeBSiO5 | ||||
| ST-1 | 17 | Mineville Iron Mine | Mineville | Essex |
| Vonsenite Fe2+2Fe3+(BO3)O2 | ||||
| V-1 | 18 | Jayville Mine | Jayville | St. Lawrence |
| V-2 | 19 | Clifton Mine | Clifton | St. Lawrence |
| Warwickite (Mg,Ti,Fe,Cr,Al)2O(BO3) | ||||
| W-1 | 20 | Balmat | Fowler | St. Lawrence |
| W-2 | 21 | Edwards Mine | Edwards | St. Lawrence |
| Mineral | Danburite | Danburite | Danburite | Datolite | Dumortierite | Grandidierite | Harkerite | Prismatine | Prismatine |
|---|---|---|---|---|---|---|---|---|---|
| Source | (a) | (b) | (c) | (d) | |||||
| Map ID | 1 | 1 | 2 | 4 | 8 | 12 | 13 | 16 | 16 |
| Locality | Russell | Russell | Macomb | Harrisville | Batcheller. | Russell | Cascade Sl. | Moose R. | Moose R. |
| Method | EMP | Wet | EMP | EMP | EMP | EMP | EMP/SIMS | EMP/SIMS | EMP |
| SiO2 | 49.19 | 48.23 | 49.15 | 37.15 | 31.43 | 20.81 | 15.90 | 30.60 | 29.74 |
| TiO2 | nd | nd | nd | 0.08 | 0.03 | 0.24 | 0.24 | ||
| Al2O3 | 0.02 | 0.47 | 0.03 | nd | 63.90 | 52.03 | 2.92 | 40.93 | 43.48 |
| Fe2O3T | tr | 0.97 | |||||||
| FeO * | 0.01 | 0.01 | 0.02 | 1.14 | 0.76 | 9.65 | 10.02 | ||
| MnO | nd | nd | nd | nd | 0.06 | 0.05 | 0.05 | ||
| MgO | 0.01 | nd | nd | 0.75 | 13.08 | 10.83 | 14.49 | 14.28 | |
| CaO | 24.18 | 23.24 | 24.27 | 36.43 | nd | 0.07 | 44.00 | 0.03 | 0.04 |
| Na2O | nd | nd | nd | nd | 0.01 | 0.04 | 0.03 | ||
| K2O | 0.01 | 0.01 | 0.01 | 0.01 | 0.05 | 0.02 | |||
| La2O3 | |||||||||
| Ce2O3 | |||||||||
| Pr2O3 | |||||||||
| Nd2O3 | |||||||||
| Sm2O3 | |||||||||
| Cr2O3 | |||||||||
| V2O3 | |||||||||
| Y2O3 | |||||||||
| ZrO2 | |||||||||
| ThO2 | |||||||||
| B2O3 | 26.58 | 26.93 | 26.53 | 20.84 | 2.86 | 7.43 | 5.87 | 3.54 | 2.10 |
| Li2O | 0.21 | ||||||||
| CO2 | 14.95 | ||||||||
| H2O | 5.55 | 0.90 | |||||||
| Cl | 1.24 | ||||||||
| TOTAL | 100.00 | 98.87 | 100.00 | 100.00 | 100.00 | 94.71 | 97.37 | 99.78 | 100.00 |
| Mineral | Serendibite | Serendibite | Serendibite | Sinhalite | Stillwell. | Vonsenite | Vonsenite | Warwickite | |
| Source | (e) | (f) | (g) | (h) | (i) | ||||
| Map ID | 11 | 11 | 12 | 11 | 17 | 18 | 18 | 21 | |
| Locality | Johnsburg | Johnsburg | Russell | Johnsburg | Mineville | Jayville | Jayville | Edwards | |
| Method | EMP | Wet | EMP | EMP | EMP | EMP | Wet | EMP | |
| SiO2 | 25.49 | 26.30 | 24.70 | 0.01 | 22.67 | 0.02 | |||
| TiO2 | 0.01 | 0.03 | nd | 0.06 | 0.13 | 21.89 | |||
| Al2O3 | 38.94 | 34.05 | 34.90 | 41.59 | 0.38 | 1.48 | 6.96 | ||
| Fe2O3T | 30.14 | ||||||||
| FeO * | 1.00 | 2.76 | 2.02 | 1.05 | nd | 86.36 | 54.04 | 0.01 | |
| MnO | 0.06 | 0.07 | 0.02 | 0.46 | 0.28 | 0.14 | |||
| MgO | 15.54 | 15.44 | 15.41 | 30.64 | 1.42 | 0.56 | 43.63 | ||
| CaO | 13.68 | 13.30 | 14.31 | nd | 0.03 | nd | |||
| Na2O | 1.31 | 0.57 | nd | ||||||
| K2O | 0.01 | nd | 0.01 | ||||||
| La2O3 | 21.55 | ||||||||
| Ce2O3 | 31.13 | ||||||||
| Pr2O3 | 2.99 | ||||||||
| Nd2O3 | 7.19 | ||||||||
| Sm2O3 | 0.38 | ||||||||
| Cr2O3 | 0.91 | ||||||||
| V2O3 | |||||||||
| Y2O3 | 0.01 | ||||||||
| ZrO2 | 0.76 | ||||||||
| ThO2 | 0.09 | ||||||||
| B2O3 | 3.96 | 8.37 | 7.44 | 27.68 | 13.92 | 11.29 | 13.37 | 25.70 | |
| Li2O | |||||||||
| CO2 | |||||||||
| H2O | |||||||||
| Cl | 0.04 | ||||||||
| TOTAL | 100.00 | 100.22 | 99.45 | 100.99 | 100.00 | 100.00 | 100.00 | 100.00 |
| Mineral | Danburite | Danburite | Datolite | Dumort. | Dumort. | Prismatine | Serendibite | Vonsenite |
|---|---|---|---|---|---|---|---|---|
| Map ID | 1 | 2 | 4 | 8 | 10 | 16 | 11 | 18 |
| Locality | Russell | Macomb | Harrisville | Batchellerville | Benson | Moose R. | Johnsburg | Jayville |
| Method | LA-ICP-MS | LA-ICP-MS | LA-ICP-MS | LA-ICP-MS | LA-ICP-MS | LA-ICP-MS | LA-ICP-MS | LA-ICP-MS |
| Sc | 0.48 | 0.46 | 0.30 | 9.30 | 4.5 | 121 | 4.5 | 1.8 |
| V | 0.62 | 0.68 | 0.59 | nd | 7.6 | 538 | 7 | 16 |
| Cr | nd | nd | nd | nd | 0.1 | 247 | nd | 2.7 |
| Co | nd | nd | nd | nd | 39.7 | 3.7 | 14.0 | |
| Ni | nd | nd | 0.33 | nd | 24.6 | 3.8 | 5.4 | |
| Zn | 0.28 | nd | nd | 18.0 | 753 | 30 | 436 | |
| Ga | nd | 0.21 | 1.24 | 127 | 85 | 99 | 10.5 | |
| Ge | 0.9 | 1.6 | 1.8 | 19 | 2.4 | 2.3 | 0.6 | |
| As | nd | 0.4 | nd | 346 | 2363 | nd | 1.8 | nd |
| Sb | nd | 0.05 | nd | 31 | 4070 | nd | nd | nd |
| Bi | nd | 0.02 | nd | 25.5 | 133 | nd | nd | nd |
| Sr | 826 | 713 | 11 | 0.04 | 0.11 | 4.5 | 0.04 | |
| Y | 0.49 | 0.24 | 0.92 | nd | 10 | 59 | 0.014 | |
| Zr | 0.008 | nd | 0.013 | 16 | 45 | 21 | 9 | 52.1 |
| Nb | 0.001 | 0.005 | 0.11 | 206 | 73.9 | 0.18 | 0.04 | 2.7 |
| Mo | 0.026 | 0.004 | nd | 7.7 | 0.011 | 0.014 | 3.4 | |
| Cd | nd | nd | nd | 0.6 | 0.42 | 0.32 | 93 | |
| In | nd | nd | nd | nd | 0.54 | 0.03 | 18 | |
| Sn | 0.18 | 0.11 | 0.23 | 7.0 | 2.38 | 2.10 | 1827 | |
| Ba | 0.32 | 0.10 | 0.02 | nd | 1.21 | 1.11 | 0.17 | |
| La | 21 | 16 | 15 | 0.3 | 0.002 | 0.646 | 0.004 | |
| Ce | 37 | 30 | 18 | 0.267 | 0.006 | 3.422 | 0.013 | |
| Pr | 3.36 | 2.62 | 1.21 | nd | 0.001 | 0.864 | 0.005 | |
| Nd | 9.3 | 6.7 | 3.1 | nd | 0.012 | 5.57 | 0.013 | |
| Sm | 0.77 | 0.61 | 0.30 | nd | nd | 3.748 | nd | |
| Eu | 0.194 | 0.170 | 0.067 | 0.031 | nd | 0.114 | 0.004 | |
| Gd | 0.20 | 0.17 | 0.24 | 0.110 | 0.131 | 6.58 | 0.003 | |
| Tb | 0.034 | 0.025 | 0.028 | 0.029 | 0.082 | 1.375 | 0.002 | |
| Dy | 0.134 | 0.082 | 0.148 | 0.054 | 0.943 | 8.80 | 0.004 | |
| Ho | 0.0150 | 0.0138 | 0.0298 | nd | 0.354 | 1.783 | 0.003 | |
| Er | 0.041 | 0.022 | 0.060 | 0.034 | 1.678 | 5.00 | 0.003 | |
| Tm | 0.0042 | 0.0057 | 0.0044 | nd | 0.406 | 0.688 | 0.001 | |
| Yb | 0.0120 | 0.0210 | 0.0214 | nd | 3.933 | 4.660 | 0.045 | |
| Lu | 0.0018 | 0.0041 | 0.0046 | nd | 0.800 | 0.694 | 0.023 | |
| Hf | 0.0023 | 0.0021 | 0.0038 | 2.1 | 0.515 | 0.979 | 1.90 | |
| Ta | 0.0025 | 0.0017 | 0.0082 | 53.1 | 10.8 | 0.007 | 0.024 | 0.433 |
| W | 0.0046 | 0.0106 | 0.70 | 5.4 | 0.019 | 0.020 | 0.003 | |
| Pb | 0.22 | 0.16 | 0.38 | 4.5 | 0.464 | 0.667 | 0.053 | |
| Th | 0.003 | 0.014 | 0.74 | 0.5 | nd | 5.4 | 0.005 | |
| U | 0.0003 | 0.0022 | 4.5 | 0.5 | 0.036 | 2.5 | 0.009 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailey, D.G.; Lupulescu, M.V.; Darling, R.S.; Singer, J.W.; Chamberlain, S.C. A Review of Boron-Bearing Minerals (Excluding Tourmaline) in the Adirondack Region of New York State. Minerals 2019, 9, 644. https://doi.org/10.3390/min9100644
Bailey DG, Lupulescu MV, Darling RS, Singer JW, Chamberlain SC. A Review of Boron-Bearing Minerals (Excluding Tourmaline) in the Adirondack Region of New York State. Minerals. 2019; 9(10):644. https://doi.org/10.3390/min9100644
Chicago/Turabian StyleBailey, David G., Marian V. Lupulescu, Robert S. Darling, Jared W. Singer, and Steven C. Chamberlain. 2019. "A Review of Boron-Bearing Minerals (Excluding Tourmaline) in the Adirondack Region of New York State" Minerals 9, no. 10: 644. https://doi.org/10.3390/min9100644
APA StyleBailey, D. G., Lupulescu, M. V., Darling, R. S., Singer, J. W., & Chamberlain, S. C. (2019). A Review of Boron-Bearing Minerals (Excluding Tourmaline) in the Adirondack Region of New York State. Minerals, 9(10), 644. https://doi.org/10.3390/min9100644




