Unravelling the Crustal Architecture of Cape Verde from the Seamount Xenolith Record
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Host Lavas
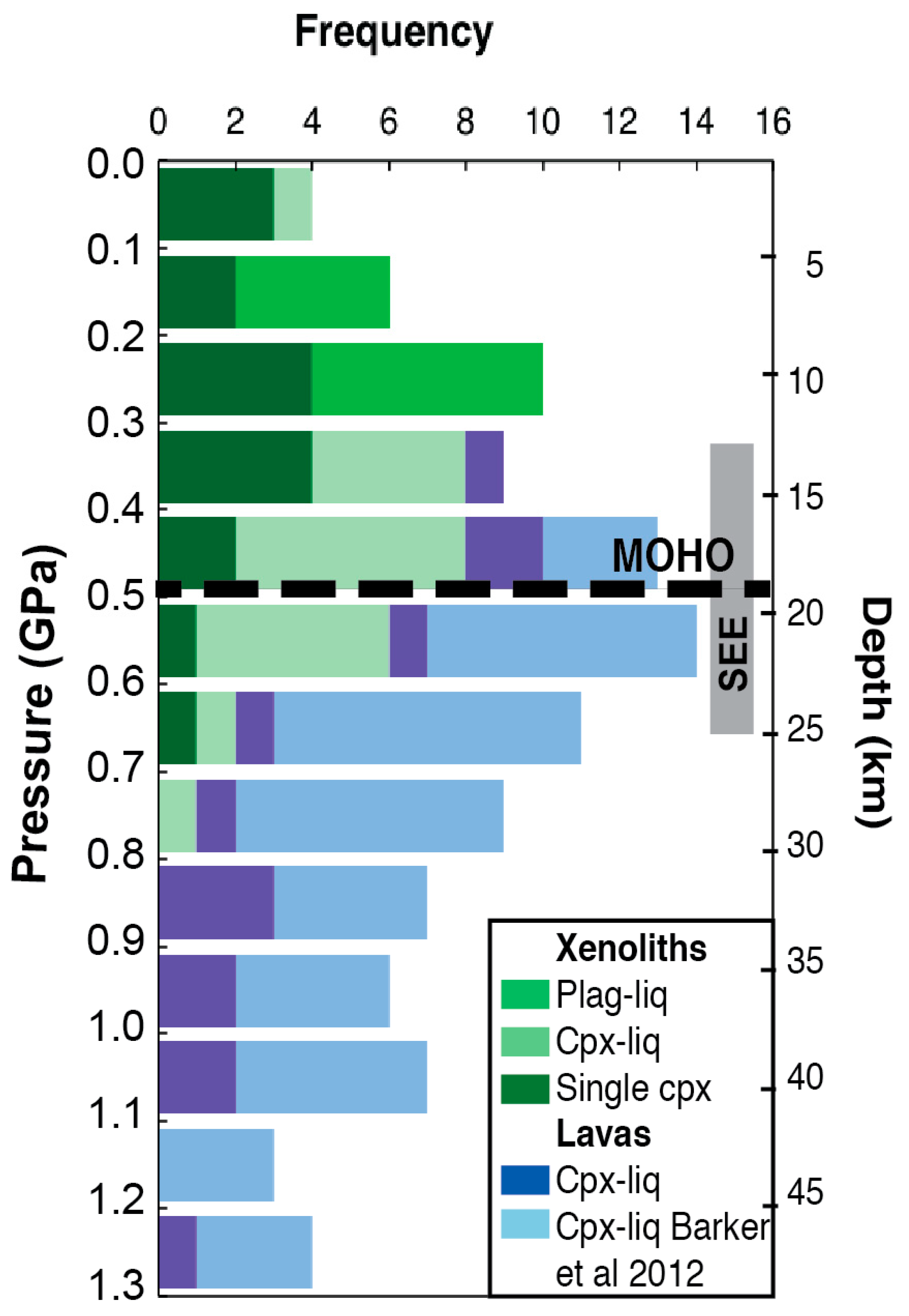
3.2. Xenoliths
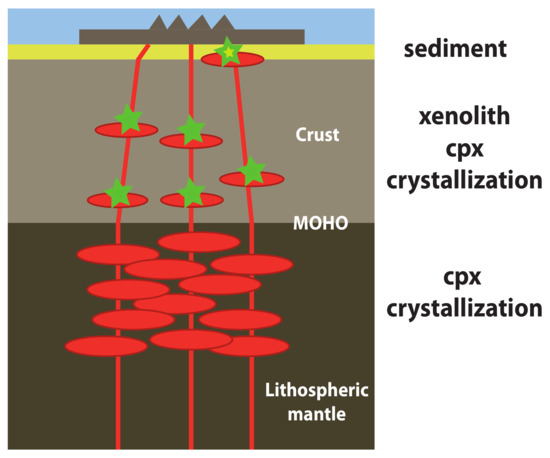
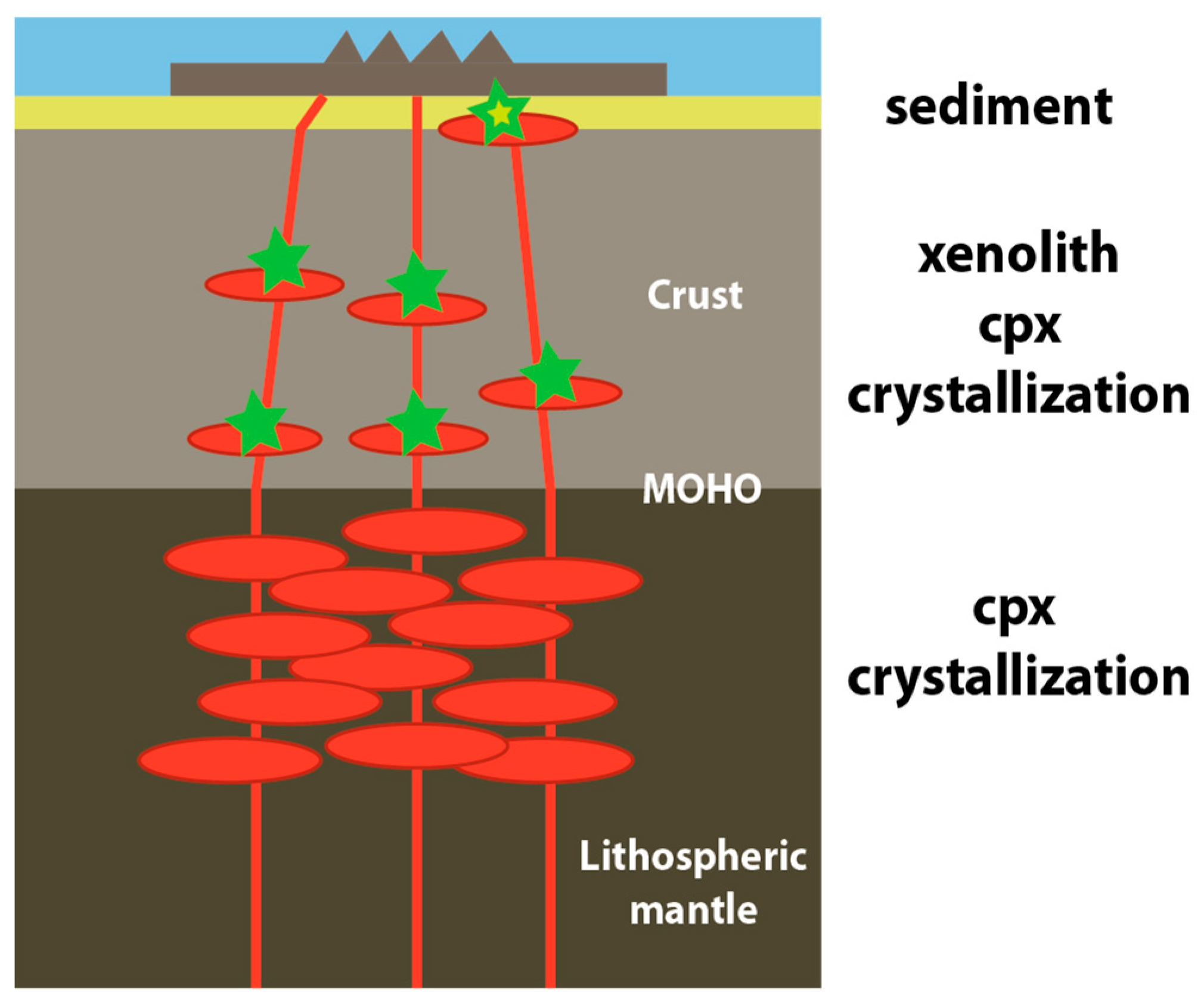
4. Discussion
4.1. Magma-Crust Interaction
4.2. Origin of Xenoliths
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gerlach, D.C.; Cliff, R.A.; Davies, G.R.; Norry, M.; Hodgson, N. Magma sources of the Cape Verdes archipelago: Isotopic and trace element constraints. Geochim. Cosmochim. Acta 1988, 52, 2979–2992. [Google Scholar] [CrossRef]
- Davies, G.R.; Norry, M.J.; Gerlach, D.C.; Cliff, R.A. A combined chemical and Pb-Sr-Nd isotope study of the Azores and Cape Verde hot-spots: The geodynamic implications. In Magmatism in Ocean Basins; Saunders, A.D., Norry, M.J., Eds.; Geological Society of London Special Publication: London, UK, 1989; Volume 42, pp. 231–255. [Google Scholar]
- Barker, A.K.; Holm, P.M.; Peate, D.W.; Baker, J.A. Geochemical stratigraphy of submarine lavas (3–5 Ma) from the Flamengos Valley, Santiago, Cape Verde. J. Petrol. 2009, 50, 169–193. [Google Scholar] [CrossRef]
- Barker, A.K.; Troll, V.R.; Ellam, R.M.; Hansteen, T.H.; Harris, C.; Stillman, C.J.; Andersson, A. Magmatic evolution of the Cadamosto Seamount, Cape Verde: Beyond the spatial extent of EM1. Contrib. Mineral. Petrol. 2012, 163, 949–965. [Google Scholar] [CrossRef]
- Bonadiman, C.; Beccaluva, L.; Coltorti, M.; Siena, F. Kimberlite-like metasomatism and ‘garnet signature’ in spinel-peridotite xenoliths from Sal, Cape Verde Archipelago: Relics of a subcontinental mantle domain within the Atlantic oceanic lithosphere? J. Petrol. 2005, 46, 2465–2493. [Google Scholar] [CrossRef]
- Klügel, A. Reactions between mantle xenoliths and host magma beneath La Palma (Canary Islands): Constraints on magma ascent rates and crustal reservoirs. Contrib. Mineral. Petrol. 1998, 131, 237–257. [Google Scholar] [CrossRef]
- Barker, A.K.; Troll, V.R.; Carracedo, J.C.; Nicholls, P. The magma plumbing system for the 1971 Tenguía eruption, La Palma, Canary Islands. Contrib. Mineral. Petrol. 2015, 170. [Google Scholar] [CrossRef]
- Araña, V.; Ibarrola, E. Rhyolitic pumice in the basaltic pyroclasts from the 1971 eruption of Teneguia volcano, Canary Islands. Lithos 1973, 6, 273–278. [Google Scholar] [CrossRef]
- Hansteen, T.H.; Troll, V.R. Oxygen isotope composition of xenoliths from the oceanic crust and volcanic edifice beneath Gran Canaria (Canary Islands): Consequences for crustal contamination of ascending magmas. Chem. Geol. 2003, 193, 181–193. [Google Scholar] [CrossRef]
- Troll, V.R.; Klügel, A.; Longpré, M.A.; Burchardt, S.; Deegan, F.M.; Carracedo, J.C.; Wiesmaier, S.; Kueppers, U.; Dahren, B.; Blythe, L.S.; et al. Floating sandstones off El Hierro (Canary Islands, Spain): The peculiar case of the October 2011 eruption. Solid Earth 2012, 3, 975–999. [Google Scholar] [CrossRef]
- Sigmarsson, O.; Laporte, D.; Carpentier, M.; Devouard, B.; Devidal, J.L.; Marti, J. Formation of U-depleted rhyolite from a basanite at El Hierro, Canary Islands. Contrib. Mineral. Petrol. 2013, 165, 601–622. [Google Scholar] [CrossRef]
- Zaczek, K.; Troll, V.R.; Cachao, M.; Ferreira, J.; Deegan, F.M.; Carracedo, J.C.; Soler, V.; Meade, F.C.; Burchardt, S. Nannofossils in 2011 El Hierro eruptive products reinstate plume model for Canary Islands. Sci. Rep. 2015, 5, 7945. [Google Scholar] [CrossRef] [PubMed]
- Putirka, K. Thermometers and barometers for volcanic systems. Rev. Mineral. Geochem. 2008, 69, 61–120. [Google Scholar] [CrossRef]
- Hildner, E.; Klügel, A.; Hauff, F. Magma storage and ascent during the 1995 eruption of Fogo, Cape Verde Archipelago. Contrib. Mineral. Petrol. 2011, 162, 751–772. [Google Scholar] [CrossRef]
- Hildner, E.; Klügel, A.; Hansteen, T.H. Barometry of lavas from the 1951 eruption of Fogo, Cape Verde Islands: Implications for historic and prehistoric magma plumbing systems. J. Volcanol. Geotherm. Res. 2012, 217, 73–90. [Google Scholar] [CrossRef]
- Ryan, W.B.; Carbotte, S.M.; Coplan, J.O.; O’Hara, S.; Melkonian, A.; Arko, R.; Weissel, R.A.; Ferrini, V.; Goodwillie, A.; Nitsche, F.; et al. Global multi-resolution topography synthesis. Geochem. Geophys. Geosyst. 2009, 10, 3. [Google Scholar] [CrossRef]
- Fietzke, J.; Frische, M. Experimental evaluation of elemental behavior during LA-ICP-MS: Influences of plasma conditions and limits of plasma robustness. J. Anal. At. Spectrom. 2016, 31, 234–244. [Google Scholar] [CrossRef]
- Jochum, K.P.; Stoll, B.; Herwig, K.; Willbold, M.; Hofmann, A.W.; Amini, M.; Aarburg, S.; Abouchami, W.; Hellebrand, E.; Mocek, B.; et al. MPI-DING reference glass for in situ microanalysis: New reference values for element concentration and isotope ratios. Geochem. Geophys. Geosyst. 2006, 7, Q02008. [Google Scholar] [CrossRef]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacon, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A.; et al. Determination of reference values for NIST SRM 610-617 Glasses following ISO Guidelines. Geostand. Geoanal. Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Ashchepkov, I.V.; André, L.; Downes, H.; Belyatsky, B.A. Pyroxenites and megacrysts from Vitim picrite-basalts (Russia): Polybaric fractionation of rising melts in the mantle? J. Asian Earth Sci. 2011, 42, 14–37. [Google Scholar] [CrossRef]
- Masotta, M.; Mollo, S.; Freda, C.; Gaeta, M.; Moore, G. Clinopyroxene–liquid thermometers and barometers specific to alkaline differentiated magmas. Contrib. Mineral. Petrol. 2013, 166, 1545–1561. [Google Scholar] [CrossRef]
- Putirka, K.; Ryerson, F.J.; Mikaelian, H. New igneous thermobarometers for mafic and evolved lava compositions, based on clinopyroxene + liquid equilibria. Am. Mineral. 2003, 88, 1542–1554. [Google Scholar] [CrossRef]
- Putirka, K. Igneous thermometers and barometers based on plagioclase + liquid equilibria: Tests of some existing models and new calibrations. Am. Mineral. 2005, 90, 336–346. [Google Scholar] [CrossRef]
- Baker, D.R. The fidelity of melt inclusions as records of melt composition. Contrib. Mineral. Petrol. 2008, 156, 377–395. [Google Scholar] [CrossRef]
- Holm, P.M.; Wilson, J.R.; Christensen, B.P.; Hansen, L.; Hansen, S.L.; Hein, K.H.; Mortensen, A.K.; Pedersen, R.; Plesner, S.; Runge, M.K. Sampling the Cape Verde mantle plume: Evolution of melt compositions on Santo Antão, Cape Verde Islands. J. Petrol. 2006, 47, 145–189. [Google Scholar] [CrossRef]
- Melson, W.G.; O’Hearn, T. Smithsonian Volcanic Glass File; Smithsonian Institution: Washington, DC, USA, 2003. [Google Scholar]
- Bougault, H.; Dmitriev, L.V.; Schilling, J.G.; Sobolev, A.V.; Joron, J.-L.; Needham, H.D. Mantle heterogeneity from trace elements: MAR triple junction near 14 N. Earth Planet. Sci. Lett. 1988, 88, 27–36. [Google Scholar] [CrossRef]
- Duprat, H.I.; Friis, J.; Holm, P.M.; Grandvuinet, T.; Sørensen, R.V. The volcanic and geochemical development of São Nicolau, Cape Verde Islands: Constraints from field and 40 Ar/39 Ar evidence. J. Volcanol. Geotherm. Res. 2007, 162, 1–19. [Google Scholar] [CrossRef]
- Ryabchikov, J.D.; Ntaflos, T.; Kurat, G.; Kogarko, L.N. Glass-bearing xenoliths from Cape Verde: Evidence for a hot rising mantle jet. Mineral. Petrol. 1995, 55, 217–237. [Google Scholar] [CrossRef]
- Herzberg, C.; Asimow, P.D. Petrology of some oceanic island basalts: PRIMELT2. XLS software for primary magma calculation. Geochem. Geophys. Geosyst. 2008, 9, Q09001. [Google Scholar] [CrossRef]
- Millet, M.-A.; Doucelance, R.; Schiano, P.; David, K.; Bosq, C. Mantle plume heterogeneity versus shallow-level interactions: A case study, the São Nicolau Island, Cape Verde archipelago. J. Volcanol. Geotherm. Res. 2008, 176, 265–276. [Google Scholar] [CrossRef]
- Barker, A.K.; Holm, P.M.; Peate, D.W.; Baker, J.A. A five million year record of compositional variations in mantle sources to magmatism on Santiago, southern Cape Verde archipelago. Contrib. Mineral. Petrol. 2010, 160, 133–154. [Google Scholar] [CrossRef]
- Elkins, L.T.; Grove, T.L. Ternary feldspar experiments and thermodynamic models. Am. Mineral. 1990, 75, 544–559. [Google Scholar]
- Klein, E.M. Geochemistry of the Igneous Oceanic Crust. Treatise Geochem. 2004, 3, 433–463. [Google Scholar]
- Schnetzler, C.C.; Philpotts, J.A. Partition coefficients of rare-earth elements between igneous matrix material and rock-forming mineral phenocrysts; II. Geochim. Cosmochim. Acta 1970, 34, 331–340. [Google Scholar] [CrossRef]
- Johnson, K.T.M. Experimental determination of partition coefficients for rare earth and high-field-strength elements between clinopyroxene, garnet, and basaltic melt at high pressures. Contrib. Mineral. Petrol. 1998, 133, 60–68. [Google Scholar] [CrossRef]
- Hansteen, T.H.; Klügel, A.; Schmincke, H.U. Multi-stage magma ascent beneath the Canary Islands: Evidence from fluid inclusions. Contrib. Mineral. Petrol. 1998, 132, 48–64. [Google Scholar] [CrossRef]
- Kerr, R.C. Convective crystal dissolution. Contrib. Mineral. Petrol. 1995, 121, 237–246. [Google Scholar] [CrossRef]
- Lofgren, G.E. Effect of heterogeneous nucleation on basaltic textures: A dynamic crystallization study. J. Petrol. 1983, 24, 229–255. [Google Scholar] [CrossRef]
- Tsuchiyama, A.; Takahashi, E. Melting kinetics of a plagioclase feldspar. Contrib. Mineral. Petrol. 1983, 84, 345–354. [Google Scholar] [CrossRef]
- Wilson, M. Igneous Petrogenesis a Global Tectonic Approach, 1st ed.; Chapman and Hall: London, UK, 1989. [Google Scholar]
- Doucelance, R.; Escrig, S.; Moriera, M.; Gariepy, C.; Kurz, M. Pb-Sr-He isotope and trace element geochemistry of the Cape Verde Archipelago. Geochim. Cosmochim. Acta 2003, 67, 3717–3733. [Google Scholar] [CrossRef]
- Lodge, A.; Helffrich, G. Depleted swell root beneath the Cape Verde Islands. Geology 2006, 34, 449–452. [Google Scholar] [CrossRef]
- Ali, M.Y.; Watts, A.B.; Hill, I.A. seismic reflection profile study of lithospheric flexure in the vicinity of the Cape Verde Islands. J. Geophys. Res. 2003, 108, 2239–2263. [Google Scholar] [CrossRef]
- Lancelot, Y.; Seibold, E.; Cepek, P.; Dean, W.E.; Eremeev, V.; Gardner, J.; Jansa, L.F.; Johnson, D.; Krasheninnikov, V.; Pflaumann, U.; et al. DSDP Site 367: Cape Verde Basin. DSDP Initial Rep. 1978, 151. [Google Scholar] [CrossRef]
- Hoernle, K.; Tilton, G.; Schminke, H.U. Sr-Nd-Pb isotopic evolution of Gran Canaria: Evidence for shallow enriched mantle beneath the Canary Islands. Earth Planet. Sci. Lett. 1991, 106, 44–63. [Google Scholar] [CrossRef]
- Grousett, F.E.; Parra, M.; Bory, A.; Martinez, P.; Bertrand, P.; Shimmield, G.; Ellam, R. Saharan wind regimes traced by the Sr-Nd isotopic composition of subtropical Atlantic sediements: Last Glacial maximum vs. today. Quat. Sci. Rev. 1998, 17, 395–409. [Google Scholar] [CrossRef]
- Abouchami, W.; Galer, S.J.G.; Koschinsky, A. Pb and Nd isotopes in NE Atlantic Fe-Mn crusts: Proxies for trace metal paleosources and paleocean circulation. Geochim. Cosmochim. Acta 1999, 63, 1489–1505. [Google Scholar] [CrossRef]
- Samrock, L.K.; Dullo, W.C.; Hansteen, T.H. Large-scale fossil dune on Maio, Cape Verdes. Int. J. Earth Sci. 2018, 107, 2931–2932. [Google Scholar] [CrossRef]
- Pim, J.; Peirce, C.; Watts, A.B.; Grevemeyer, L.; Krabbenhoeft, A. Crustal structure and origin of the Cape Verde Rise. Earth Planet. Sci. Lett. 2008, 272, 422–428. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barker, A.K.; Hansteen, T.H.; Nilsson, D. Unravelling the Crustal Architecture of Cape Verde from the Seamount Xenolith Record. Minerals 2019, 9, 90. https://doi.org/10.3390/min9020090
Barker AK, Hansteen TH, Nilsson D. Unravelling the Crustal Architecture of Cape Verde from the Seamount Xenolith Record. Minerals. 2019; 9(2):90. https://doi.org/10.3390/min9020090
Chicago/Turabian StyleBarker, Abigail K., Thor H. Hansteen, and David Nilsson. 2019. "Unravelling the Crustal Architecture of Cape Verde from the Seamount Xenolith Record" Minerals 9, no. 2: 90. https://doi.org/10.3390/min9020090
APA StyleBarker, A. K., Hansteen, T. H., & Nilsson, D. (2019). Unravelling the Crustal Architecture of Cape Verde from the Seamount Xenolith Record. Minerals, 9(2), 90. https://doi.org/10.3390/min9020090