Cryogenic Raman Spectroscopic Studies on Common Ore-forming Fluid Systems
Abstract
:1. Introduction
2. Experimental Methodology
3. Results and Discussion
3.1. Determining Optimal Experimental Conditions for Hydrate Generation from NaCl–H2O and MgCl2–H2O Solutions
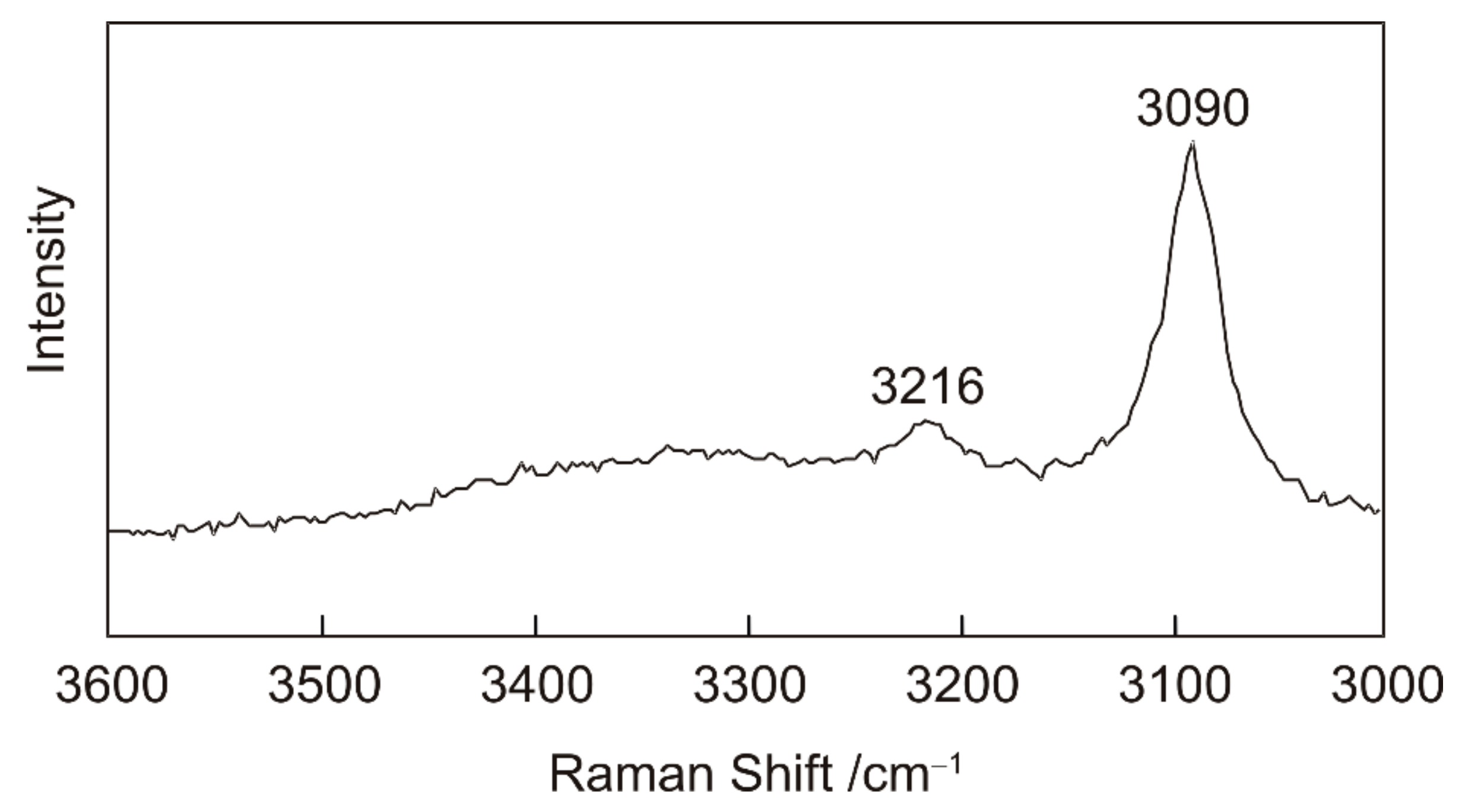
3.1.1. Cryogenic Raman Spectroscopy of H2O
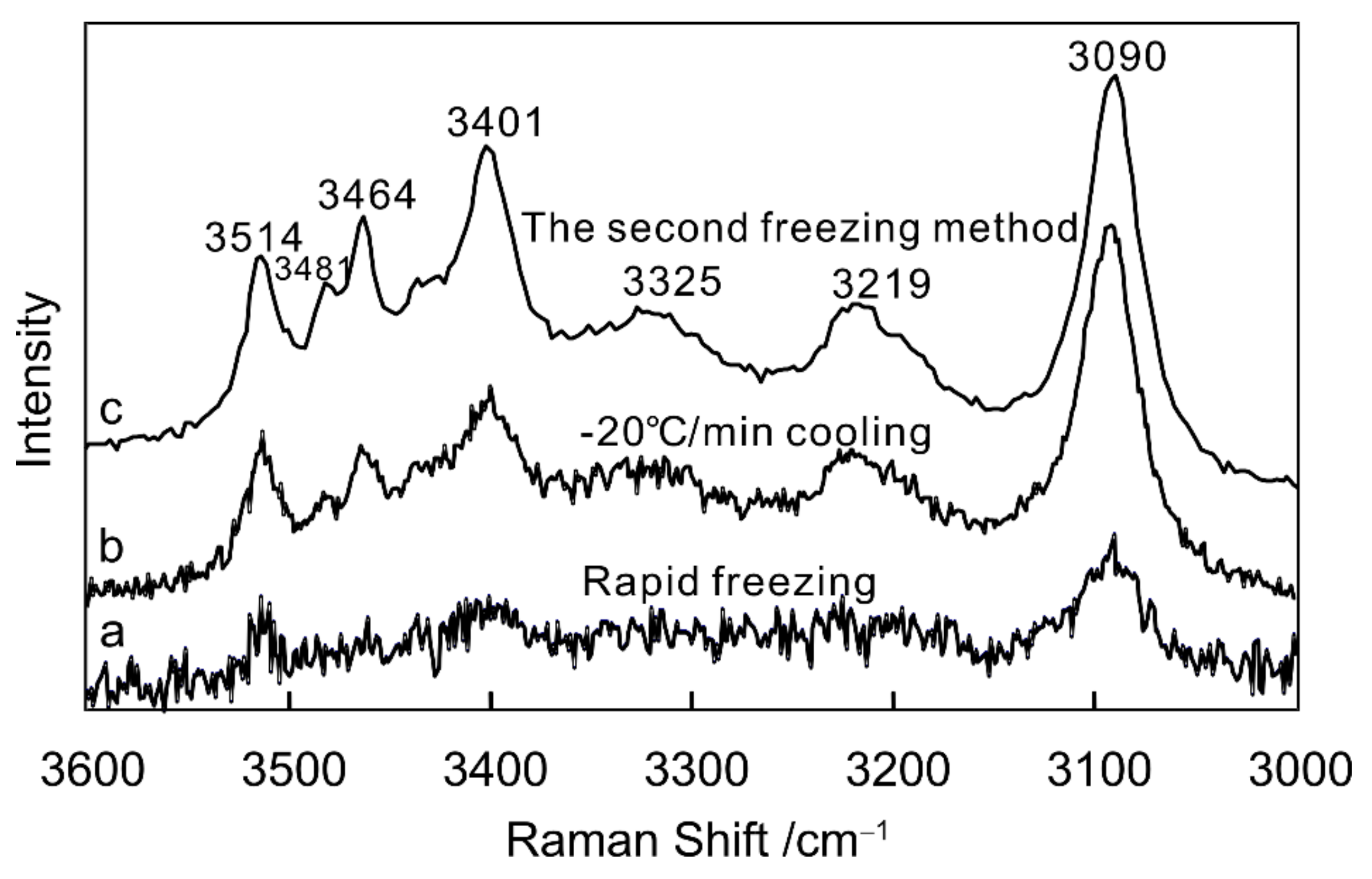
3.1.2. Cryogenic Raman Spectroscopy of NaCl–H2O Solution
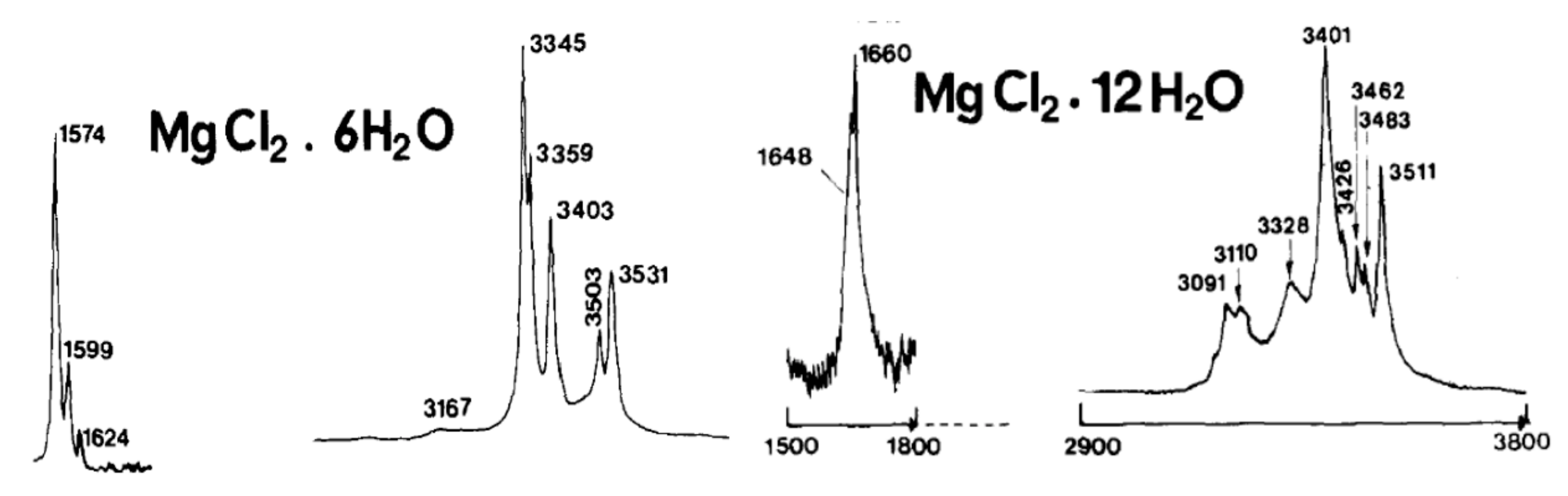
3.1.3. Cryogenic Raman Spectroscopy of MgCl2–H2O Solution
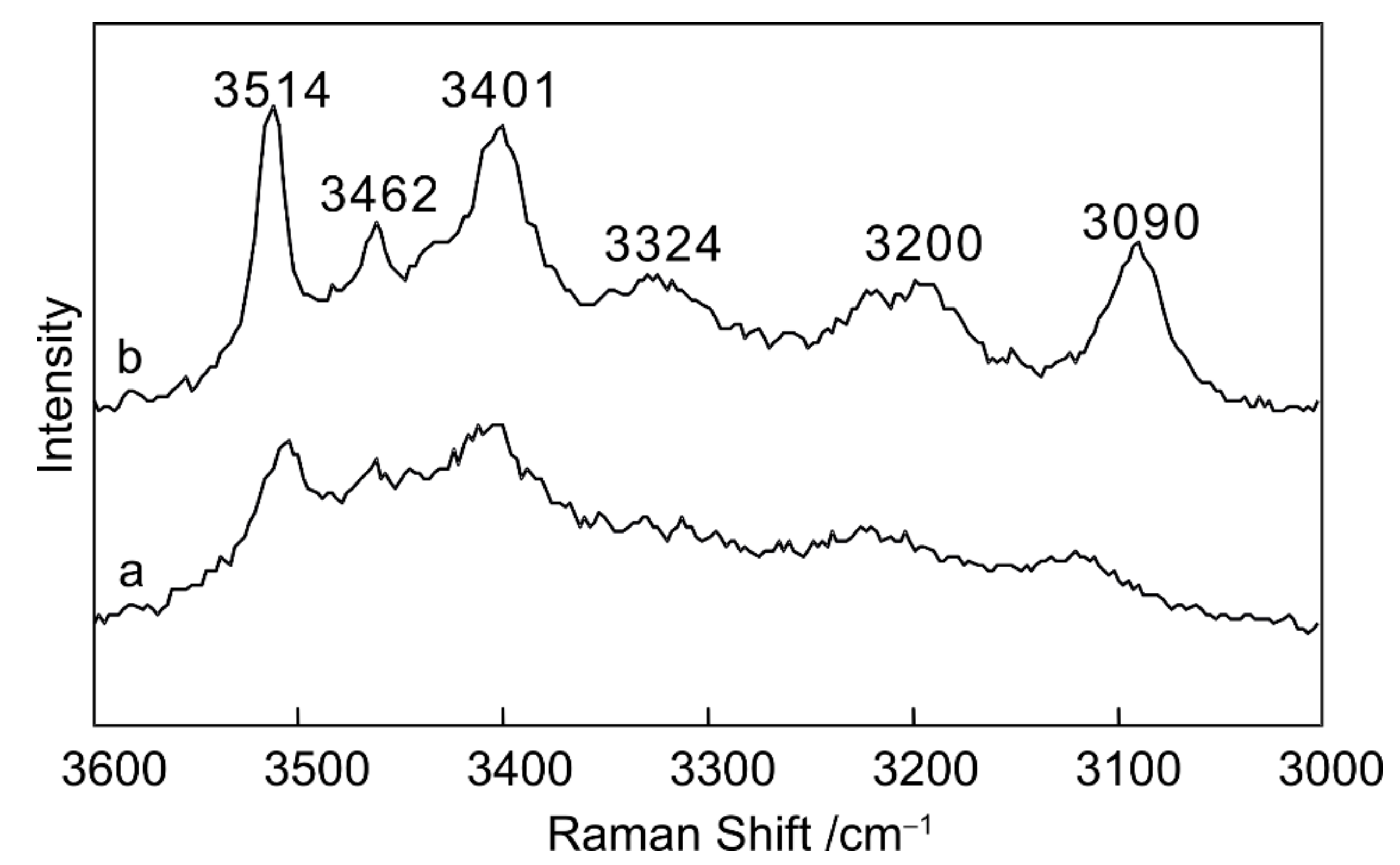
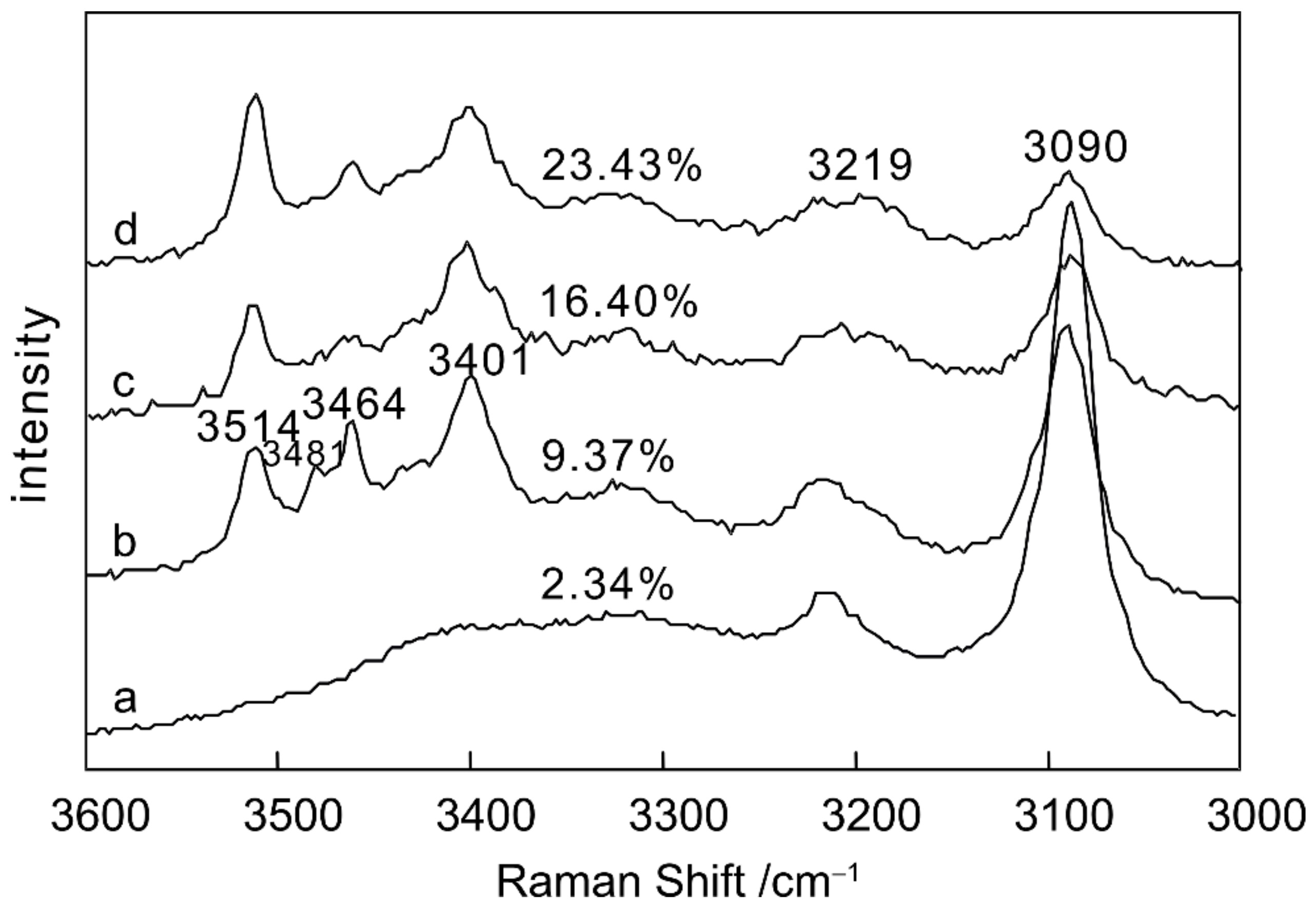
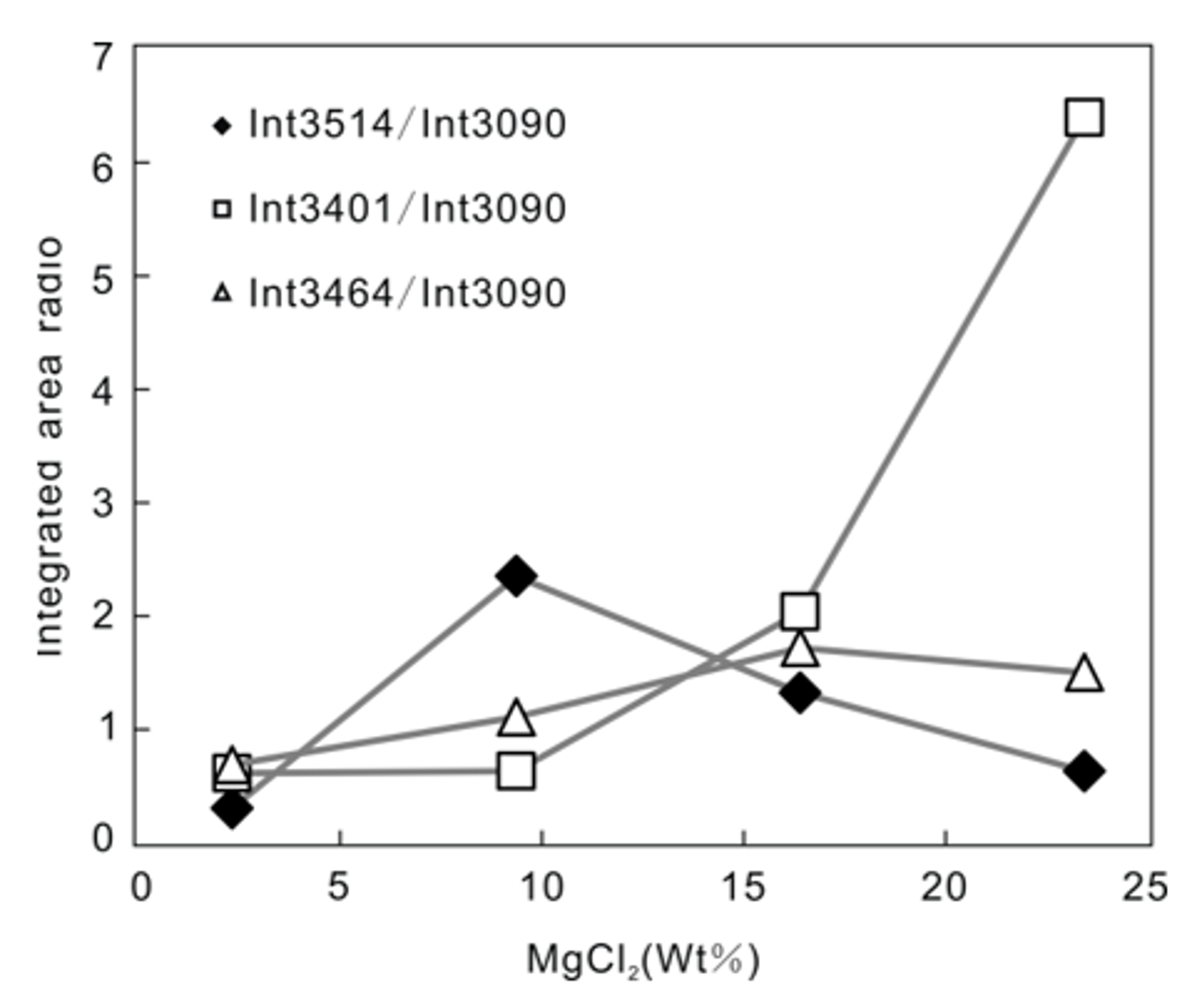
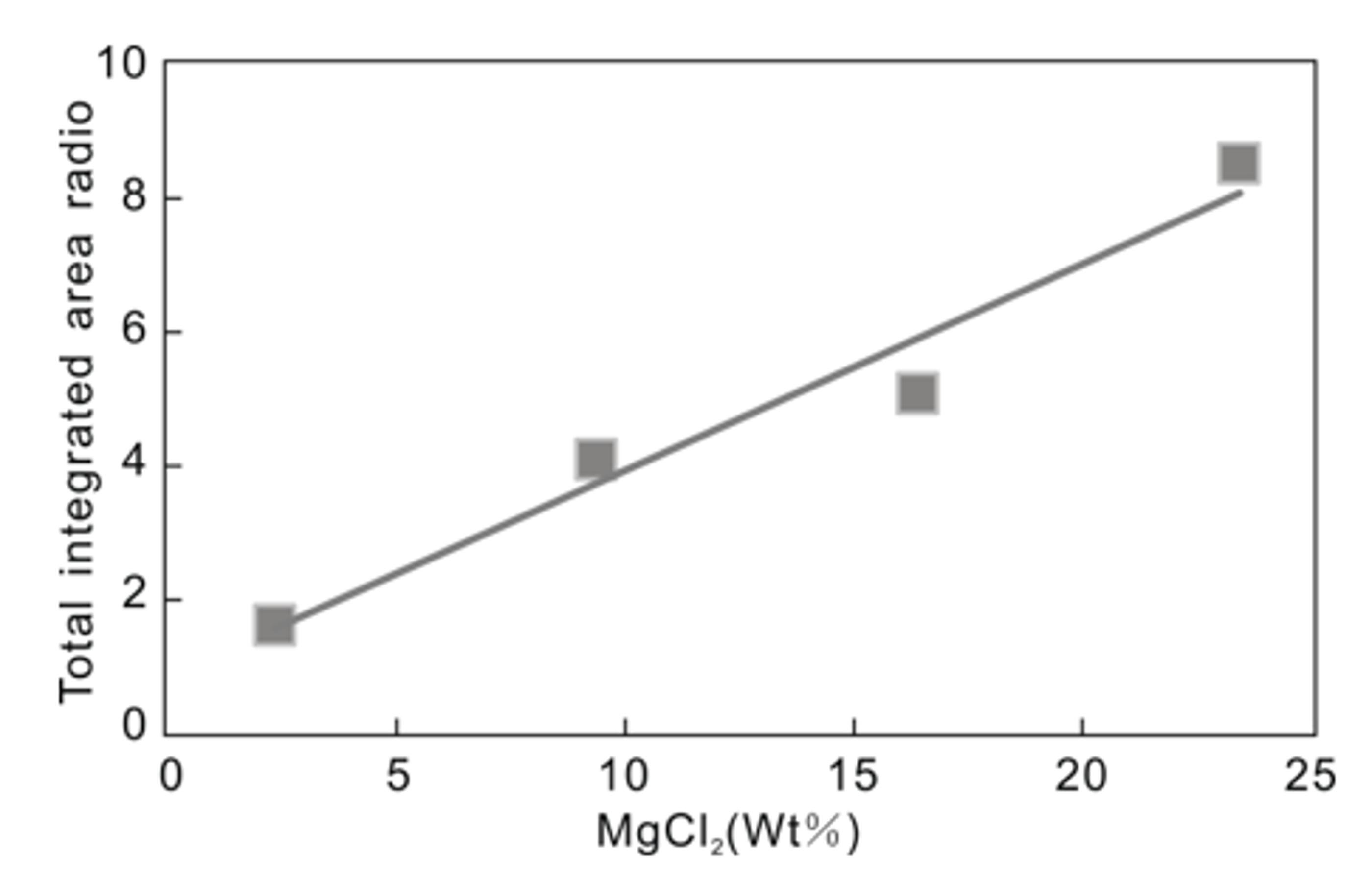
3.2. Quantitative Analysis of MgCl2–H2O
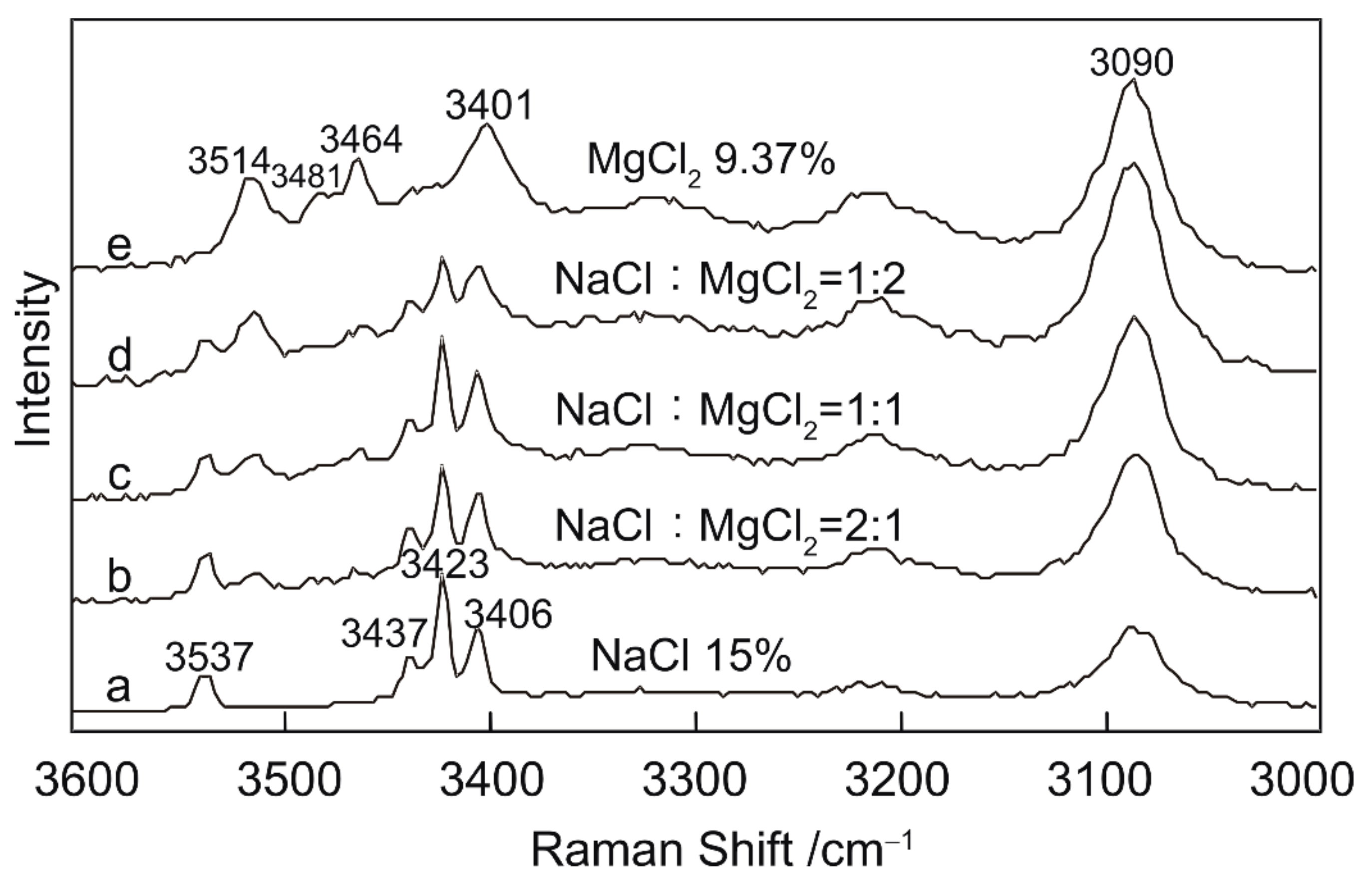
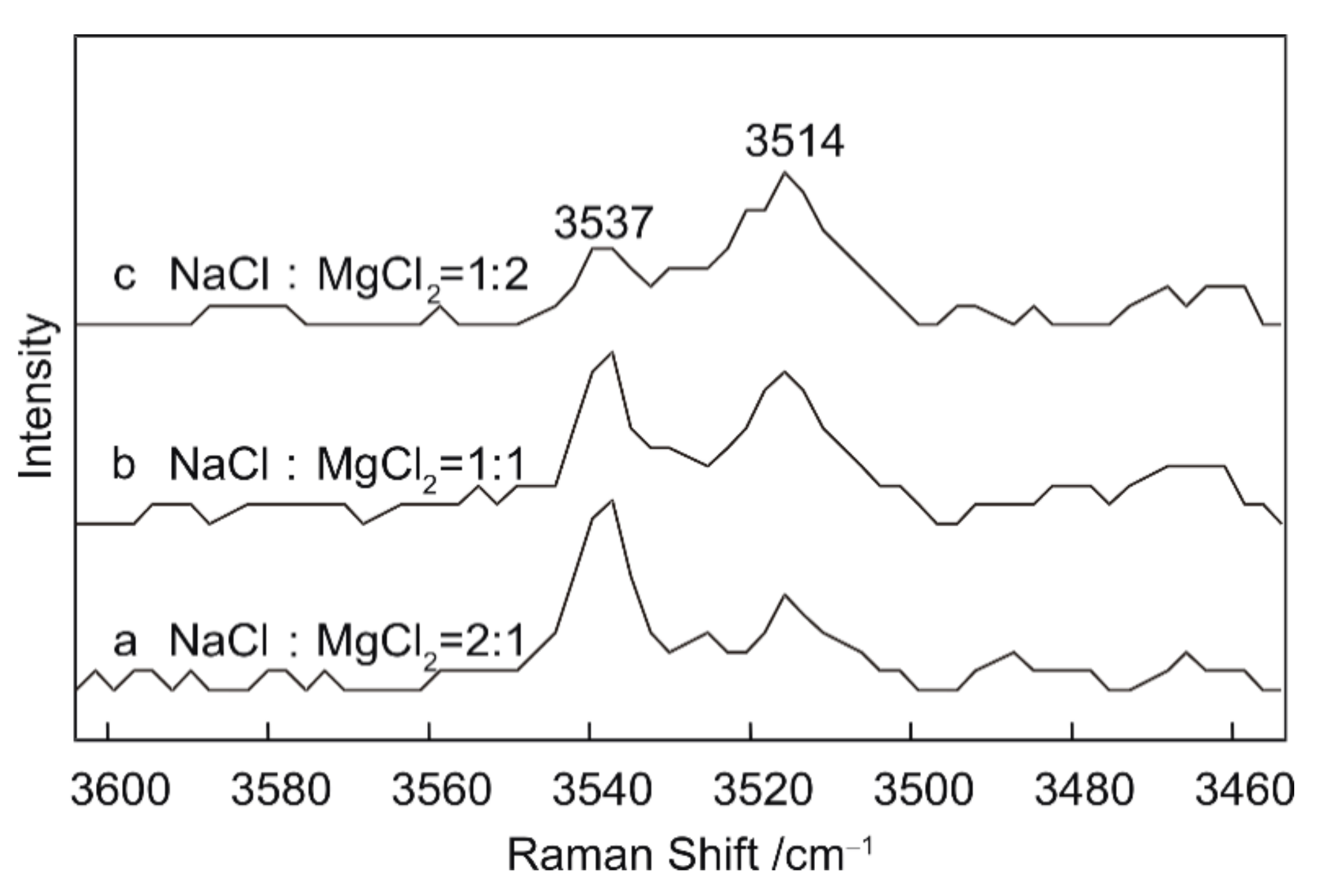
3.3. Semi-quantitative Research on NaCl–MgCl2–H2O system
Experimental Results and Spectral Analysis
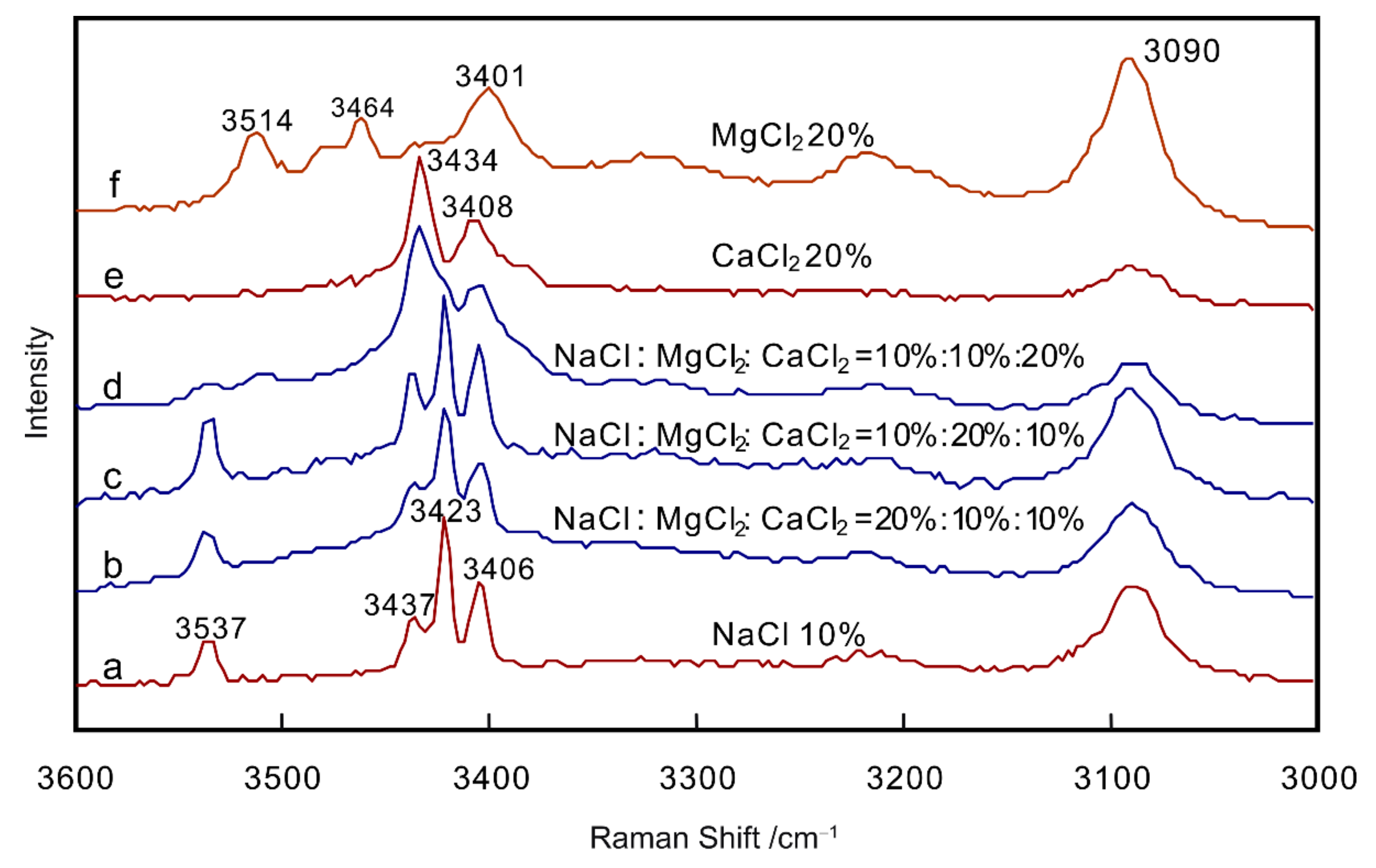
3.4. Attempt to Quantify NaCl–MgCl2–CaCl2–H2O System
3.5. Discussion on the Mode of Cryogenic Hydrate Formation
4. Conclusions
- (1)
- Optimal experimental conditions for the formation of hydrates in the NaCl–H2O and MgCl2–H2O systems were determined. Secondary freezing is the most effective way of producing hydrates in both systems.
- (2)
- The quantitative determination of the concentration of salt in solution was attempted for a solution containing MgCl2–H2O. The intensity ratio and salt concentration show a positive correlation, as does the relationship between the concentration and the ratio of the total integrated area of MgCl2 hydrate peaks to the 3090 cm−1 peak. The correlation coefficient is >0.90.
- (3)
- The peak observed at 3514 cm−1 is a feature of MgCl2 hydrate that distinguishes the Raman spectrum of this hydrate from that of hydrohalite and antarcticite. The ratio of the intensity of the peak at 3514 cm−1 to that at 3090 cm−1 shows a positive correlation when plotted against salt concentration.
- (4)
- Semi-quantitative analysis of NaCl–MgCl2–H2O solutions indicates that peaks at 3437 and 3537 cm−1 reflect the presence of NaCl in the solution. Similarly, a peak at 3514 cm−1 is indicative of the presence of MgCl2. The relative intensities of these peaks may be related to the relative abundances of NaCl and MgCl2.
- (5)
- A quantitative attempt was made on NaCl–MgCl2–CaCl2–H2O system, and it was found that quantifying NaCl, MgCl2 and CaCl2 separately in NaCl–MgCl2–CaCl2–H2O system by the secondary freezing method is difficult.
- (6)
- Phase changes observed during secondary freezing method can be caused by the formation of hydrates from the crystallization of a hypersaline liquid or the re-crystallization of a vitreous salt, rather than melting processes.
Author Contributions
Funding
Conflicts of Interest
References
- Audétat, A.; Günther, D.; Heinrich, C.A. Formation of a Magmatic-Hydrothermal Ore Deposit: Insights with LA-ICP-MS Analysis of Fluid Inclusions. Science 1998, 279, 2091–2094. [Google Scholar] [PubMed] [Green Version]
- Audétat, A. Quantitative analysis of melt and fluid inclusions by LA-ICP-MS: Practical aspects and selected results. Acta Petrol. Sin. 2000, 16, 715–716. [Google Scholar]
- Guillong, M.; Latkoczy, C.; Seo, J.H.; Günther, D.; Heinrich, C.A. Determination of sulfur in fluid inclusions by laser ablation ICP-MS. J. Anal. Atom. Spectrom. 2008, 23, 1581–1589. [Google Scholar] [CrossRef]
- Seo, J.H.; Guillong, M.; Aerts, M.; Zajacz, Z.; Heinrich, C.A. Microanalysis of S, Cl, and Br in fluid inclusions by LA–ICP-MS. Chem. Geol. 2011, 284, 35–44. [Google Scholar] [CrossRef]
- Pettke, T.; Oberli, F.; Audétat, A.; Guillong, M.; Simon, A.C.; Hanley, J.J.; Klemm, L.M. Recent developments in element concentration and isotope ratio analysis of individual fluid inclusions by laser ablation single and multiple collector ICP-MS. Ore Geol. Rev. 2012, 44, 10–38. [Google Scholar] [CrossRef] [Green Version]
- Siljeström, S.; Volk, H.; George, S.C.; Lausmaa, J.; Sjövall, P.; Dutkiewicz, A.; Hode, T. Analysis of single oil-bearing fluid inclusions in mid-Proterozoic sandstones (Roper Group, Australia). Geochim. Cosmochim. Acta 2013, 122, 448–463. [Google Scholar] [CrossRef]
- Heinrich, C.A.; Ryan, C.G.; Mernagh, T.P.; Eadington, P.J. Segregation of Ore Metals between Magmatic Brine and Vapor: A Fluid Inclusion Study using PIXE Microanalysis. Econ. Geol. 1992, 87, 1566–1583. [Google Scholar] [CrossRef]
- Heinrich, C.A.; Günther, D.; Audétat, A.; Ulrich, T.; Frischknecht, R. Metal fractionation between magmatic brine and vapor, determined by microanalysis of fluid inclusions. Geology 1999, 27, 755–758. [Google Scholar] [CrossRef]
- Vanko, D.A.; Bonnin-Mosbah, M.; Philippot, P.; Roedder, E.; Sutton, S.R. Fluid inclusions in quartz from oceanic hydrothermal specimens and the Bingham, Utah porphyry-Cu deposit: A study with PIXE and SXRF. Chem. Geol. 2001, 173, 227–238. [Google Scholar] [CrossRef]
- Ménez, B.; Philippot, P.; Bonnin-Mosbah, M.; Simionovici, A.; Gibert, F. Analysis of individual fluid inclusions using synchrotron X-ray fluorescence microprobe: Progress toward calibration for trace elements. Geochim. Cosmochim. Acta 2002, 66, 561–576. [Google Scholar] [CrossRef]
- Cauzid, J.; Philippot, P.; Somogyi, A.; Simionovici, A.; Bleuet, P. Quantification of single fluid inclusions by combining synchrotron radiation-induced mu-X-ray fluorescence and transmission. Anal. Chem. 2004, 76, 3988–3994. [Google Scholar] [CrossRef] [PubMed]
- Cauzid, J.; Philippot, P.; Somogyi, A.; Ménez, B.; Simionovici, A.; Bleuet, P. Standardless quantification of single fluid inclusions using synchrotron radiation induced X-ray fluorescence. Chem. Geol. 2006, 227, 165–183. [Google Scholar] [CrossRef]
- Cauzid, J.; Philippot, P.; Martinez-Criado, G.; Ménez, B.; Labouré, S. Contrasting Cu-complexing behaviour in vapour and liquid fluid inclusions from the Yankee Lode tin deposit, Mole Granite, Australia. Geochim. Cosmochim. Acta 2007, 246, 39–54. [Google Scholar] [CrossRef]
- Foriel, J.; Philippot, P.; Rey, P.; Somogyi, A.; Banks, D.; Ménez, B. Biological control of Cl/Br and low sulfate concentration in a 3.5-Gyr-old seawater from North Pole, Western Australia. Earth Planet. Sci. Lett. 2004, 228, 451–463. [Google Scholar] [CrossRef]
- Pasteris, J.D.; Wopenka, B.; Seitz, J.C. Practical aspects of quantitative laser Raman microprobe spectroscopy for the study of fluid inclusions. Geochim. Cosmochim. Acta 1988, 52, 979–988. [Google Scholar] [CrossRef]
- Bakker, R.J. Raman spectra of fluid and crystal mixtures in the systems H2O, H2O-NaCl and H2O-MgCl2 at low temperatures: Applications to fluid-inclusion research. Can. Mineral. 2004, 42, 1283–1314. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J.D. Limitations to quantitative analysis in of fluid inclusions in geological samples by laser Raman microprobe spectroscopy. Appl. Spectrosc. 1986, 40, 144–151. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J.D. Raman intensities and detections limits of geochemically relevant gas mixtures for a laser Raman microprobe. Anal. Chem. 1987, 59, 2165–2170. [Google Scholar] [CrossRef]
- Seitz, J.C.; Pasteris, J.D.; Wopenka, B. Characterization of CO2–CH4–H2O fluid inclusions by microthermometry and laser Raman microprobe spectroscopy: Inferences for clathrate and fluid equilibria. Geochim. Cosmochim. Acta 1987, 51, 1651–1664. [Google Scholar] [CrossRef]
- Murphy, P.J.; Roberts, S. Micro-Raman spectroscopy of gas portioning during clathrate formation: Implications for micro-thermaometric analysis. Arch Miner. 1993, 49, 152. [Google Scholar]
- Mernagh, T.P.; Wilde, A.R. The use of the laser Raman microprobe for the determination of salinity in fluid inclusions. Geochim. Cosmochim. Acta 1989, 53, 765–771. [Google Scholar] [CrossRef]
- Dubessy, J.; Audeoud, D.; Wilkins, R.; Kosztolanyi, C. The use of the Raman microprobe MOLE in the determination of the electrolytes dissolved in the aqueous phase of fluid inclusions. Chem. Geol. 1982, 37, 137–150. [Google Scholar] [CrossRef]
- Winter, C.J.; Roberts, S. Laser Raman studies of low temperature hydrates produced in the highly saline fluids. Arch Miner. 1993, 49, 246. [Google Scholar]
- Grishina, S.; Dubessy, J.; Kontorovich, A.; Pironon, J. Inclusions in salt beds resulting from thermal metamorphism by dolerite sills (eastern Siberia, Russia). Eur. J. Mineral. 1992, 4, 1187–1202. [Google Scholar] [CrossRef]
- Samson, I.M.; Walker, R.T. Cryogenic Raman spectroscopoic studies in the system NaCl–CaCl2–H2O and implications for low-temperaturephase behavior in aqueous fluid inclusions. Can. Mineral. 2000, 38, 35–43. [Google Scholar] [CrossRef]
- Ni, P.; Ding, J.Y.; Rao, B. Application of in situ cryogenic Raman spectroscopy to analyze synthetic fluid inclusions in systems H2O, NaCl–H2O. Chin. Sci. Bull. 2006, 51, 1073–1078. [Google Scholar] [CrossRef]
- Ni, P.; Ding, J.Y.; Dubeesy, J.; Zhang, T. Application of in situ ryogenic Raman spectroscopy to analyze synthetic fluid inclusions in the systems CaCl2–H2O and MgCl2–H2O II: Phase transformation behavior at lower temperatures. Acta Petrol. Sinica 2008, 24, 1968–1974. [Google Scholar]
- Ni, P.; Ding, J.Y.; Dubeesy, J.; Zhang, T. Application of in situ ryogenic Raman spectroscopy to analyze synthetic fluid inclusions in the systems CaCl2- H2O and MgCl2- H2O I: Cryogenic Raman spectra. Acta Petrol. Sinica 2008, 24, 1961–1967. [Google Scholar]
- Mao, C.; Chen, Y.; Zhou, Y.Q.; Ge, Y.J.; Zhou, Z.Z.; Wang, Y.Z. Cryogenic Raman spectroscopic characteristics of NaCl–H2O, CaCl2–H2O, and NaCl–CaCl2–H2O:application to analysis of fluid inclusions. Spectrosc. Spectr. Anal. 2010, 30, 3258–3263. [Google Scholar]
- Chi, G.X.; Chu, H.X.; Ryan, S.; Chou, I.M. A New Method for Determining Fluid Compositions in the H2O-NaCl-CaCl2 System with Cryogenic Raman Spectroscopy. Acta Geol. Sinica 2014, 88, 1169–1182. [Google Scholar] [CrossRef]
- Chu, H.; Chi, G. Determining Fluid Compositions in the H2O-NaCl-CaCl2 System with Cryogenic Raman Spectroscopy: Application to Natural Fluid Inclusions. Acta Geol. Sinica 2015, 89, 894–901. [Google Scholar]
- Taylor, M.J.; Whalley, E. Raman Spectra of Ices Ih, Ic, II, III, and V. J. Chem. Phys. 1964, 40, 1660–1664. [Google Scholar] [CrossRef]
- Whalley, E. A detailed assignment of the O-H stretching bands of ice I. Can. J. Chem. 1977, 55, 3429–3441. [Google Scholar] [CrossRef]
- Bertie, J.E.; Calvert, L.D.; Whalley, E. Transformations of Ice II, Ice III, and Ice V at Atmospheric Pressure. J. Chem. Phys. 1963, 38, 840–846. [Google Scholar] [CrossRef]
- Franks, F. The properties of ice. In The Physics and Physical Chemistry of Water; Water, A Comprehensive Treatise.1; Plenum Press: New York, NY, USA, 1972; pp. 115–149. [Google Scholar]
- Davis, D.W.; Lowenstein, T.K.; Spencer, R.J. Melting behavior of fluid inclusions in laboratory-grown halite crystals in the systems NaCl–H2O, NaCl–KCl–H2O, NaCl–MgCl2-H2O, and NaCl–CaCl2–H2O. Geochim. Cosmochim. Acta 1990, 54, 591–601. [Google Scholar] [CrossRef]
- Vanko, D.A.; Bodnar, R.J.; Sterner, S.M. Synthetic fluid inclusions: VIII. Vapor-saturated halite solubility in part of the system NaCl–CaCl2–H2O, with application to fluid inclusions from oceanic hydrothermal systems. Geochim. Cosmochim. Acta 1988, 52, 2451–2456. [Google Scholar] [CrossRef]




| System | Components | Proportion |
|---|---|---|
| NaCl–H2O | NaCl | 15 (wt.%) |
| MgCl2–H2O | MgCl2 | 2.34, 9.37, 16.40, 23.43 (wt.%) |
| NaCl–MgCl2–H2O | NaCl, MgCl2 | NaCl: MgCl2 = 1:2, 1:1, 2:1 (NaCl: 15 wt.%, MgCl2: 9.37 wt.%) |
| NaCl–MgCl2–CaCl2–H2O | NaCl, MgCl2, CaCl2 | NaCl:MgCl2:CaCl2 = 10 wt.%:10 wt.%:20 wt.% = 10 wt.%:20 wt.%:10 wt.% = 20 wt.%:10 wt.%:10 wt.% |
| Parameter | MgCl2 (wt.%) | 3090 cm−1 | 3401 cm−1 | 3464 cm−1 | 3514 cm−1 | 3401 cm−1/3090 cm−1 | 3464 cm−1/3090 cm−1 | 3514 cm−1/3090 cm−1 |
|---|---|---|---|---|---|---|---|---|
| intensity | 2.34 | 11,843.270 | 1480.400 | 2328.384 | 666.416 | 0.1250 | 0.1966 | 0.0563 |
| 9.37 | 5869.389 | 3558.564 | 2584.893 | 952.628 | 0.6063 | 0.4404 | 0.1623 | |
| 16.40 | 1678.401 | 1385.837 | 744.722 | 953.511 | 0.8257 | 0.4437 | 0.5681 | |
| 23.43 | 3341.884 | 3333.539 | 2200.561 | 4039.088 | 0.9975 | 0.6585 | 1.2086 | |
| FWHM | 2.34 | 29.714 | 145.077 | 103.595 | 177.252 | 4.8824 | 3.4864 | 5.9653 |
| 9.37 | 31.572 | 30.54827 | 109.078 | 626.886 | 0.9676 | 3.4549 | 19.8558 | |
| 16.40 | 34.121 | 38.19824 | 132.044 | 12.552 | 1.1195 | 3.8698 | 0.36787 | |
| 23.43 | 32.513 | 195.3602 | 80.369 | 17.858 | 6.0086 | 2.4719 | 0.5493 | |
| integrated area | 2.34 | 552,780.900 | 337,363.114 | 378,889.813 | 167,682.910 | 0.6103 | 0.6854 | 0.3033 |
| 9.37 | 271,251.500 | 170,758.141 | 300,133.212 | 637,126.402 | 0.6295 | 1.1065 | 2.3488 | |
| 16.40 | 899,57.820 | 183,152.514 | 154,465.901 | 118,800.031 | 2.0360 | 1.7171 | 1.3206 | |
| 23.43 | 159,941.112 | 1,022,967.023 | 239,932.803 | 100,747.502 | 6.3959 | 1.5001 | 0.6299 | |
| Total integrated area of MgCl2 hydrate peaks | Total integrated area of MgCl2 hydrate peaks/3090 cm−1 | |||||||
| 2.34 | 883,935.682 | 1.5991 | ||||||
| 9.37 | 1,108,017.733 | 4.0848 | ||||||
| 16.40 | 456,418.382 | 5.0737 | ||||||
| 23.43 | 1,363,647.301 | 8.5259 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Xiong, X.; Chen, W. Cryogenic Raman Spectroscopic Studies on Common Ore-forming Fluid Systems. Minerals 2019, 9, 363. https://doi.org/10.3390/min9060363
Yang D, Xiong X, Chen W. Cryogenic Raman Spectroscopic Studies on Common Ore-forming Fluid Systems. Minerals. 2019; 9(6):363. https://doi.org/10.3390/min9060363
Chicago/Turabian StyleYang, Dan, Xin Xiong, and Weishi Chen. 2019. "Cryogenic Raman Spectroscopic Studies on Common Ore-forming Fluid Systems" Minerals 9, no. 6: 363. https://doi.org/10.3390/min9060363
APA StyleYang, D., Xiong, X., & Chen, W. (2019). Cryogenic Raman Spectroscopic Studies on Common Ore-forming Fluid Systems. Minerals, 9(6), 363. https://doi.org/10.3390/min9060363




