Carbonate Precipitation in Mixed Cyanobacterial Biofilms Forming Freshwater Microbial Tufa
Abstract
:1. Introduction
2. Material and Methods
2.1. Site Description and Field Sampling
2.2. Enrichment using Cyanobacterial Biofilms
- (1)
- Culturing in liquid medium: the thin blue-green biofilms (1–3 mm thick) from the superficial green part of the Winogradsky column were transferred using sterile techniques into Petri dishes containing 20 mL of liquid culture medium BG-11, commonly used for cyanobacterial cultivation [41]. The pH was adjusted to 8 using sterile 0.1 N HCl to mimic the natural pH of the stream. The closed Petri dishes were incubated at 25°C for three weeks in 12 h light/12 h dark cycles (under ~2000 lux). This step produced the necessary biomass for a second stage of biofilm preparation;
- (2)
- Culturing in semi-liquid (soft-agar) medium: small (1 cm2) fragments of the biofilm grown in liquid (see 1) were transferred into a Petri dishes with sterile soft agar (0.75% w/v) containing half-strength BG-11 medium (i.e., 1:1 BG-11 medium and water) with a pH of circa 8. The soft agar was used to provide a substrate for biofilm growth [38]. In total, 18 Petri dishes were prepared: 12 with biofilms for biotic experiments and 6 with soft-agar BG-11 medium only as abiotic experimental controls. Petri dishes were kept for two weeks at 25 °C, in 2000 lux under 12 h light/12 h dark cycles (Figure 1f). The soft agar was used as a surrogate for gelatinous bacterial biofilms found in situ in the order to mimic the mineral precipitation processes.
2.3. Characterization of Cyanobacterial Communities
2.3.1. Light Microscopy and Scanning Electron Microscopy (SEM)
2.3.2. Isolation of Genomic DNA
2.4. Laboratory Experiments
2.5. Sampling and Analysis
2.5.1. Microscopic Observations and Spectroscopic Analysis of Biofilm
2.5.2. Geochemical Analysis
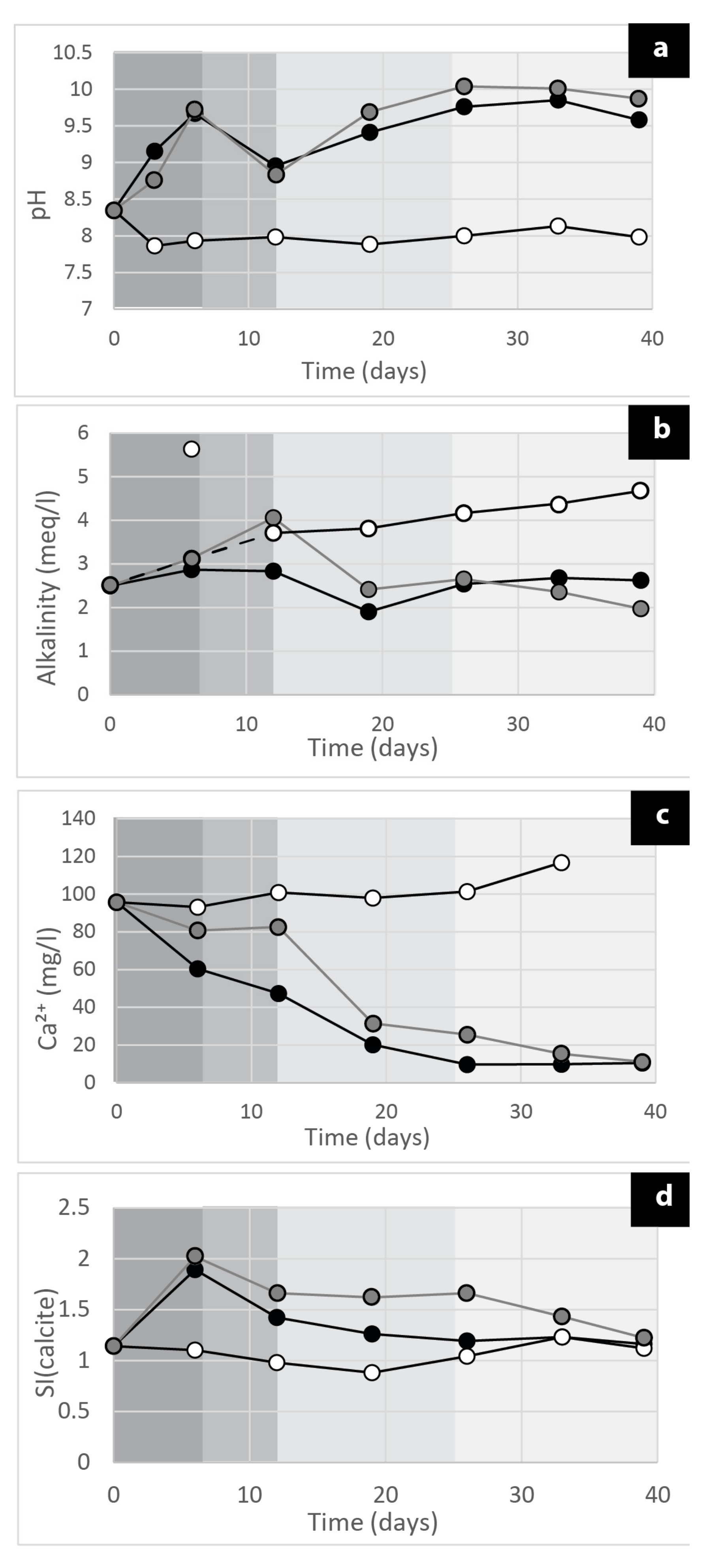
3. Results and Discussions
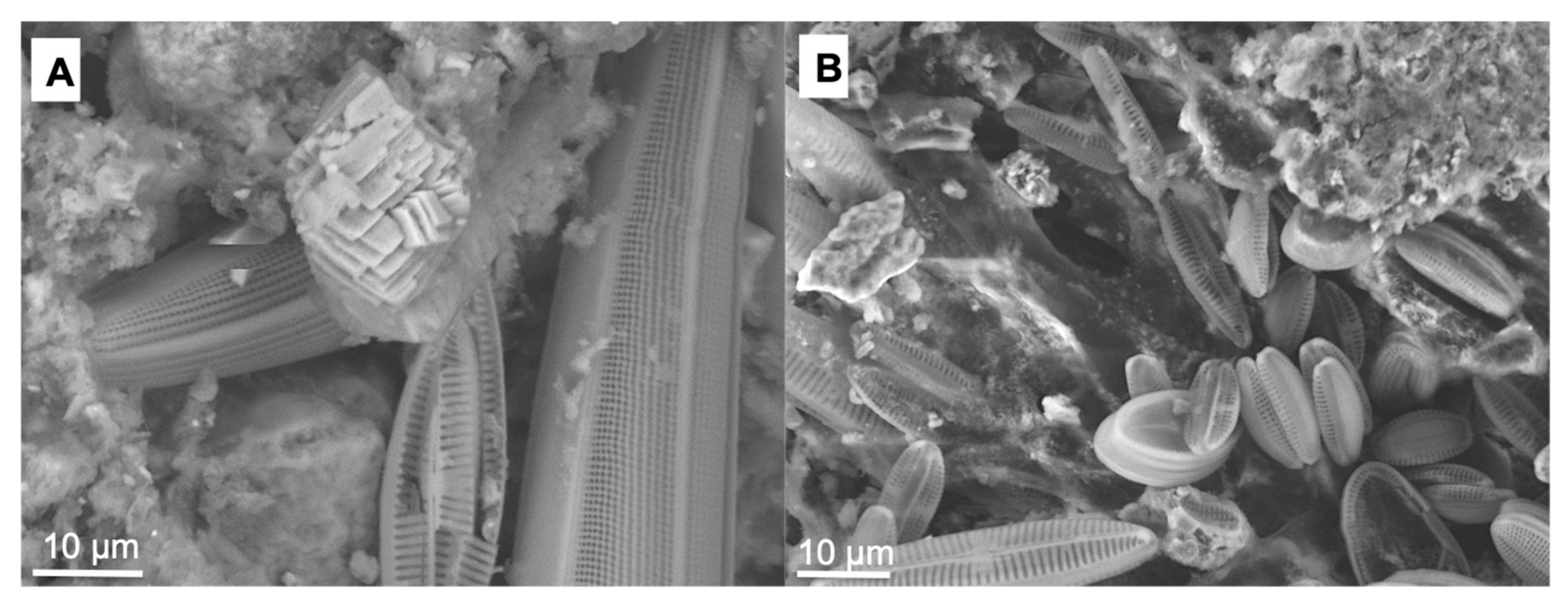
3.1. The Composition Cyanobacterial Biofilm Community
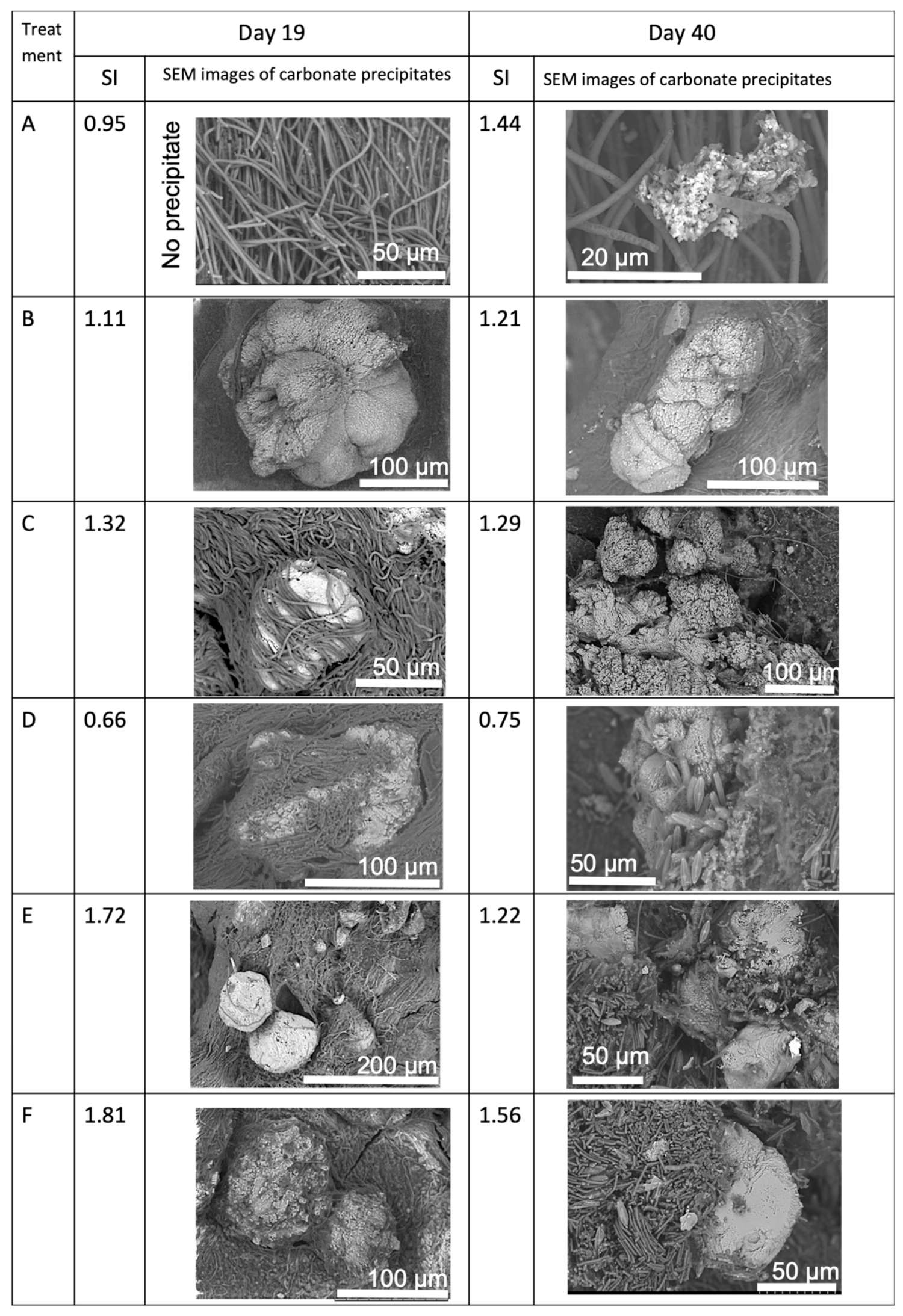
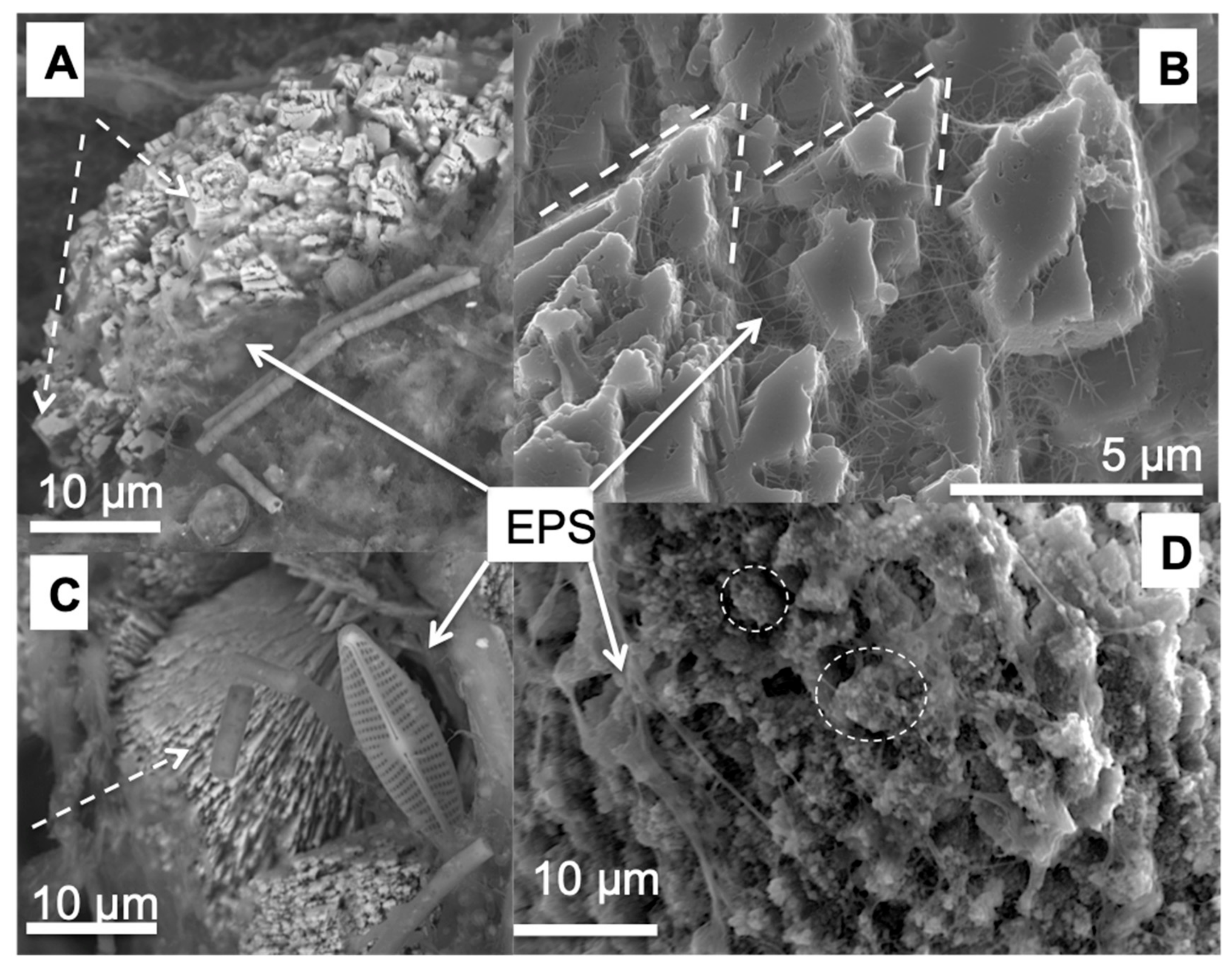
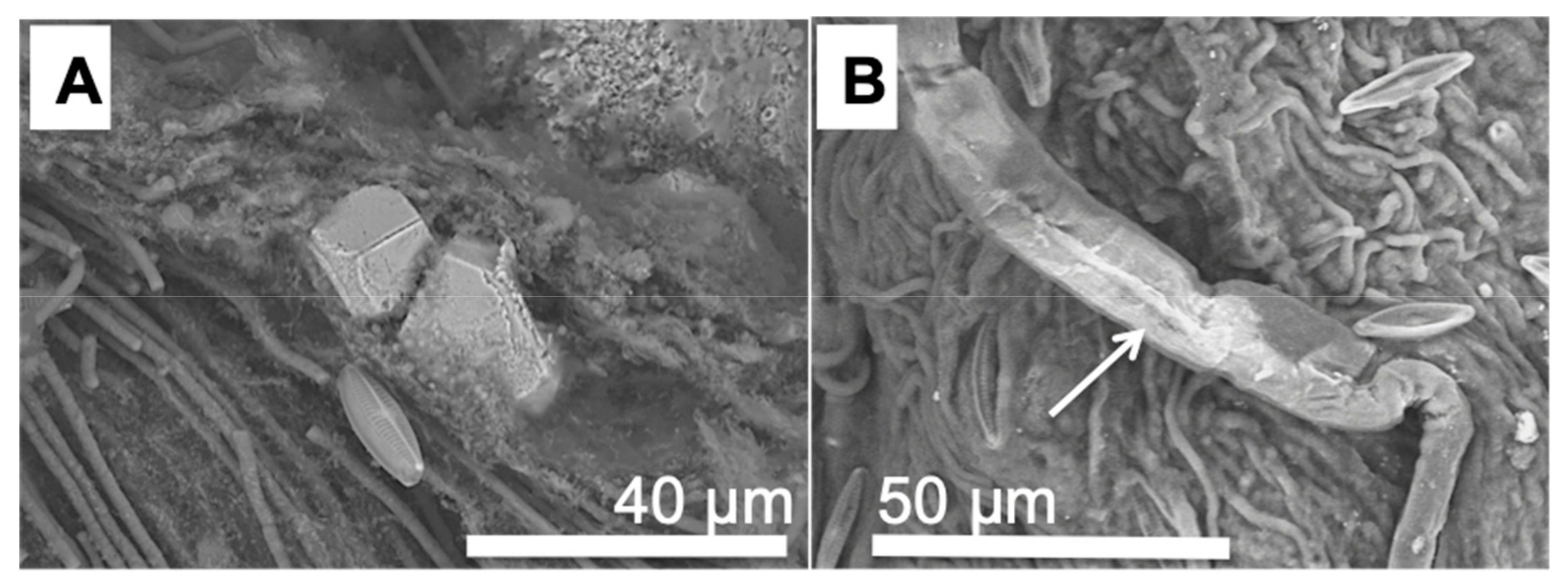
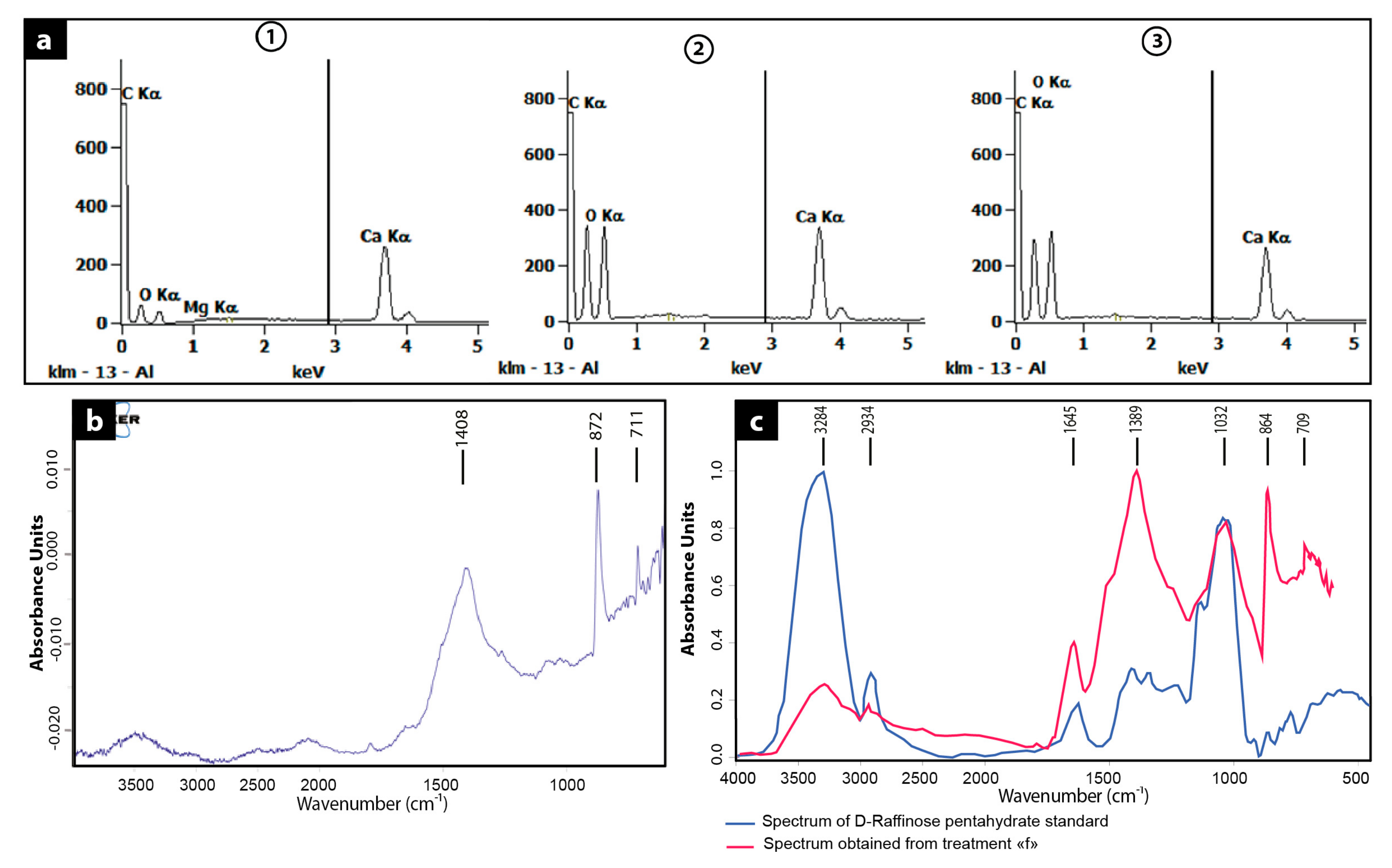
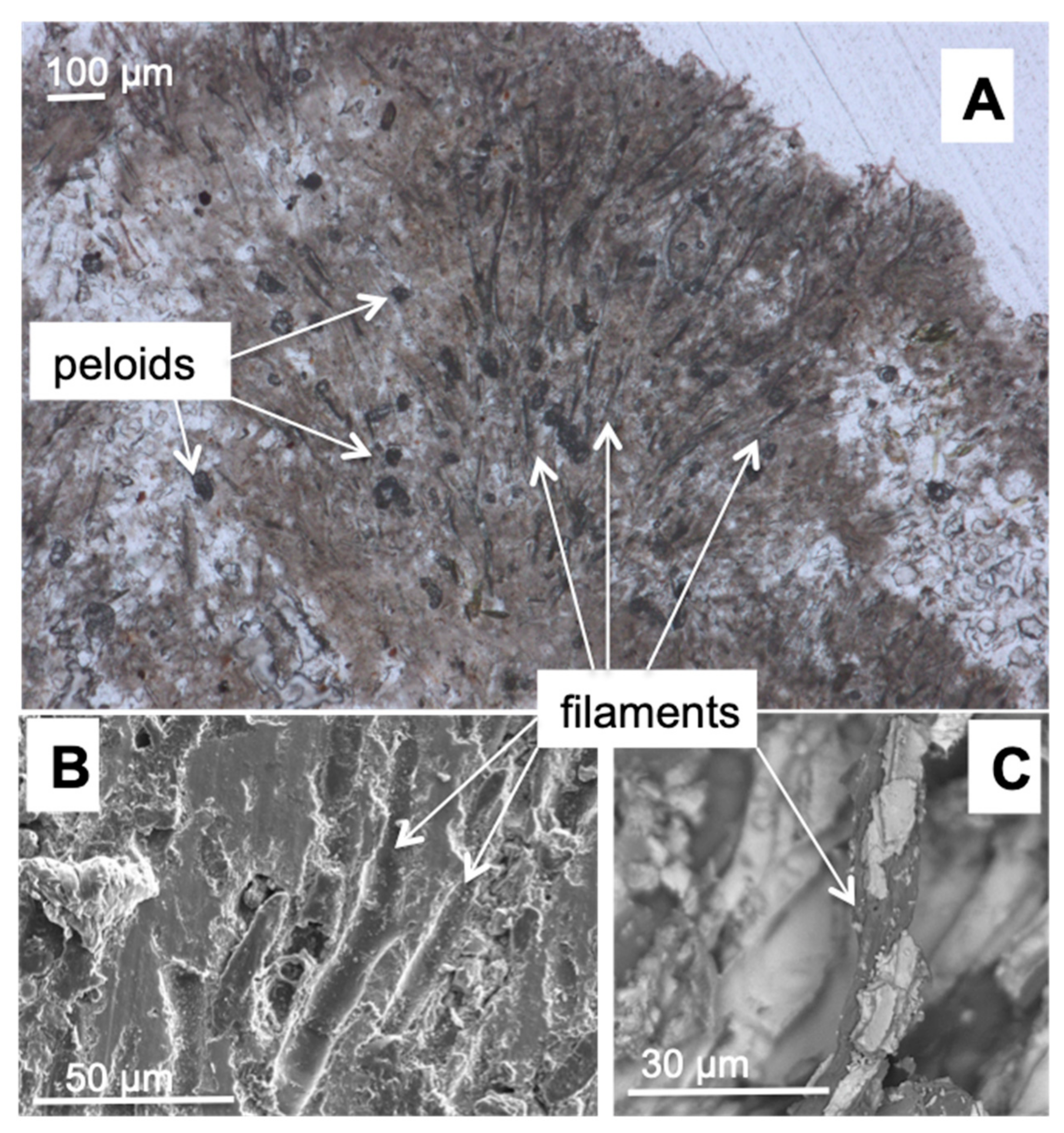
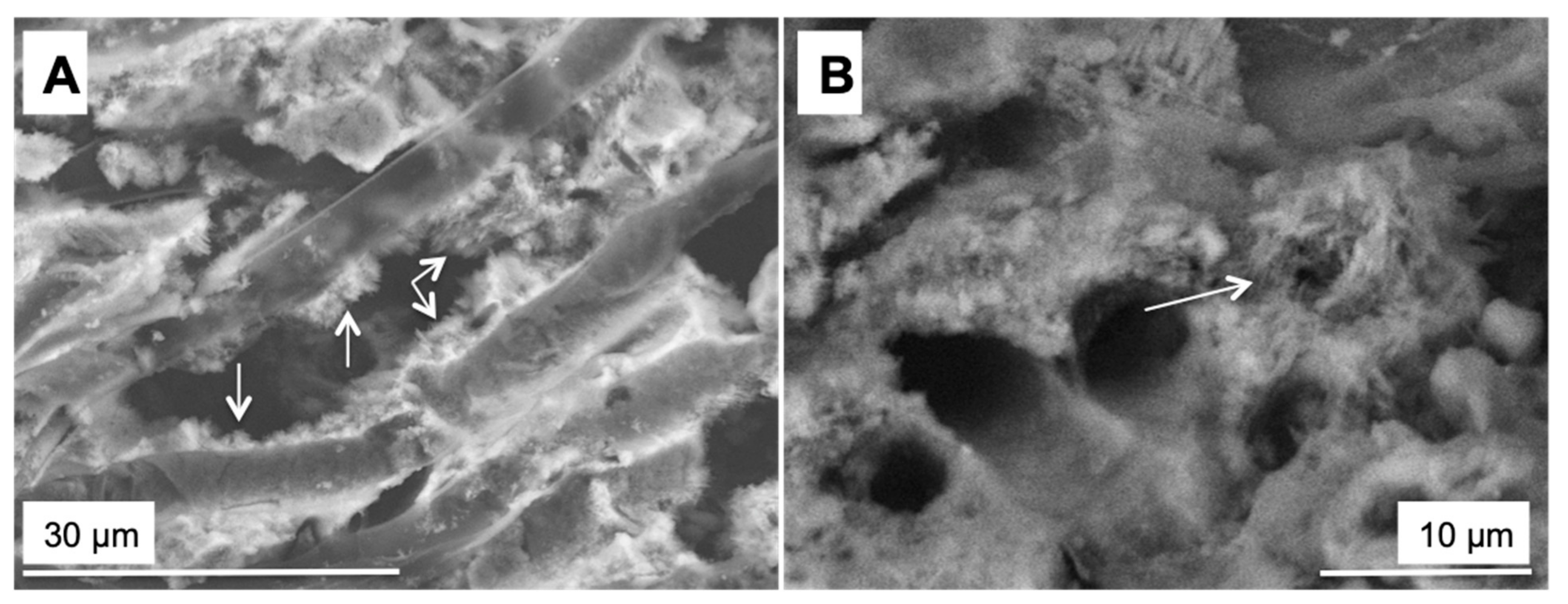
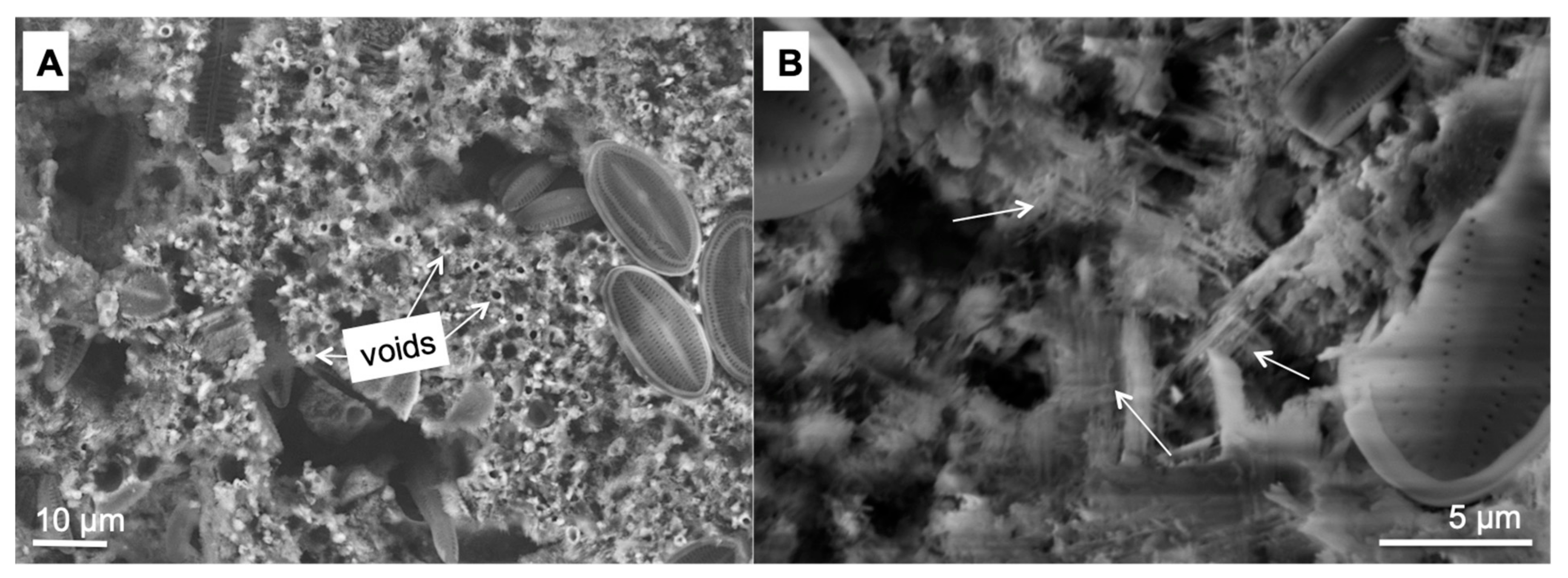
3.2. Morphology and Mineralogy of the Carbonate Precipitates
3.3. Comparison Carbonate Minerals Observed in the Natural Environment and in the Laboratory Experiment
3.4. The Carbonate Precipitation Process in the Cyanobacterial Biofilms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grotzinger, J.P.; Knoll, A.H. Stromatolites in Precambrian carbonates: Evolutionary Mileposts or Environmental Dipsticks? Annu. Rev. Earth Planet. Sci. 1999, 27, 313–358. [Google Scholar] [CrossRef] [PubMed]
- Burne, R.V.; Moore, L.S. Microbialites: organosedimentary deposits of benthic microbial communities. Palaios 1987, 2, 241–254. [Google Scholar] [CrossRef]
- Playford, P.E.; Cockbain, A.E. Chapter 8.2 Modern Algal Stromatolites at Hamelin Pool, A Hypersaline Barred Basin in Shark Bay, Western Australia. In Developments in Sedimentology; Walter, M.R., Ed.; Stromatolites; Elsevier: Canberra, Australia, 1976; Volume 20, pp. 389–411. [Google Scholar]
- Myshrall, K.L.; Dupraz, C.; Visscher, P.T. Patterns in Microbialites Throughout Geologic Time: Is the Present Really the Key to the Past? In Experimental Approaches to Understanding Fossil Organisms: Lessons from the Living; Hembree, D.I., Platt, B.F., Smith, J.J., Eds.; Topics in Geobiology; Springer: Dordrecht, The Netherlands, 2014; pp. 111–142. ISBN 978-94-017-8721-5. [Google Scholar]
- Reimer, A.; Landmann, G.; Kempe, S. Lake Van, Eastern Anatolia, Hydrochemistry and History. Aquat. Geochem. 2009, 15, 195–222. [Google Scholar] [CrossRef]
- Stal, L.J.; van Gemerden, H.; Krumbein, W.E. Structure and development of a benthic marine microbial mat. FEMS Microbiol. Ecol. 1985, 1, 111–125. [Google Scholar] [CrossRef]
- Ley, R.E.; Harris, J.K.; Wilcox, J.; Spear, J.R.; Miller, S.R.; Bebout, B.M.; Maresca, J.A.; Bryant, D.A.; Sogin, M.L.; Pace, N.R. Unexpected diversity and complexity of the Guerrero Negro hypersaline microbial mat. Appl. Environ. Microbiol. 2006, 72, 3685–3695. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, L.K.; Dupraz, C.; Buckley, D.H.; Spear, J.R.; Pace, N.R.; Visscher, P.T. Microbial species richness and metabolic activities in hypersaline microbial mats: insight into biosignature formation through lithification. Astrobiology 2009, 9, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.M. A macrobiological perspective on microbial species. Microbe Am. Soc. Microbiol. 2006, 1, 269–278. [Google Scholar] [CrossRef]
- Van Gemerden, H. Microbial mats: A joint venture. Mar. Geol. 1993, 113, 3–25. [Google Scholar] [CrossRef]
- Gallagher, K.L.; Dupraz, C.; Visscher, P.T. Two opposing effects of sulfate reduction on carbonate precipitation in normal marine, hypersaline, and alkaline environments: Comment. Geology 2014, 42, e313–e314. [Google Scholar] [CrossRef]
- Kempe, S.; Kazmierczak, J. The role of alkalinity in the evolution of ocean chemistry, organization of living systems, and biocalcification processes. Bull. Inst. Océan. Monaco 1994, 13, 61–117. [Google Scholar]
- Visscher, P.T.; Reid, R.P.; Bebout, B.M.; Hoeft, S.E.; Macintyre, I.G.; Thompson, J.A. Formation of lithified micritic laminae in modern marine stromatolites (Bahamas): The role of sulfur cycling. Am. Mineral. 1998, 83, 1482–1493. [Google Scholar] [CrossRef]
- Visscher, P.T.; Stolz, J.F. Microbial mats as bioreactors: Populations, processes, and products. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 219, 87–100. [Google Scholar] [CrossRef]
- Lyons, W.B.; Long, D.T.; Hines, M.E.; Gaudette, H.E.; Armstrong, P.B. Calcification of cyanobacterial mats in Solar Lake, Sinai. Geology 1984, 12, 623–626. [Google Scholar] [CrossRef]
- Gallagher, K.L.; Kading, T.J.; Braissant, O.; Dupraz, C.; Visscher, P.T. Inside the alkalinity engine: The role of electron donors in the organomineralization potential of sulfate-reducing bacteria. Geobiology 2012, 10, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Reid, P.; Dupraz, C.D.; Visscher, P.T.; Sumner, D.Y. Microbial Processes Forming Marine Stromatolites. In Fossil and Recent Biofilms: A Natural History of Life on Earth; Krumbein, W.E., Paterson, D.M., Zavarzin, G.A., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 103–118. ISBN 978-94-017-0193-8. [Google Scholar]
- Dupraz, C.; Visscher, P.T. Microbial lithification in marine stromatolites and hypersaline mats. Trends Microbiol. 2005, 13, 429–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decho, A.W. Microbial exopolymer secretions in ocean environments: Their role(s) in food webs and marine processes. Oceanogr. Mar. Biol. 1990, 28, 9–16. [Google Scholar]
- Braissant, O.; Cailleau, G.; Dupraz, C.; Verrecchia, E.P. Bacterially induced mineralization of calcium carbonate in terrestrial environments: the role of exopolysaccharides and amino acids. J. Sediment. Res. 2003, 73, 485–490. [Google Scholar] [CrossRef]
- Braissant, O.; Decho, A.W.; Przekop, K.M.; Gallagher, K.L.; Glunk, C.; Dupraz, C.; Visscher, P.T. Characteristics and turnover of exopolymeric substances in a hypersaline microbial mat. FEMS Microbiol. Ecol. 2009, 67, 293–307. [Google Scholar] [CrossRef] [Green Version]
- Reid, R.P.; Visscher, P.T.; Decho, A.W.; Stolz, J.F.; Bebout, B.M.; Dupraz, C.; Macintyre, I.G.; Paerl, H.W.; Pinckney, J.L.; Prufert-Bebout, L.; et al. The role of microbes in accretion, lamination and early lithification of modern marine stromatolites. Nature 2000, 406, 989–992. [Google Scholar] [CrossRef]
- Dupraz, C.; Visscher, P.T.; Baumgartner, L.K.; Reid, R.P. Microbe–mineral interactions: Early carbonate precipitation in a hypersaline lake (Eleuthera Island, Bahamas). Sedimentology 2004, 51, 745–765. [Google Scholar] [CrossRef]
- Bouton, A.; Vennin, E.; Pace, A.; Bourillot, R.; Dupraz, C.; Thomazo, C.; Brayard, A.; Désaubliaux, G.; Visscher, P.T. External controls on the distribution, fabrics and mineralization of modern microbial mats in a coastal hypersaline lagoon, Cayo Coco (Cuba). Sedimentology 2016, 63, 972–1016. [Google Scholar] [CrossRef]
- Pace, A.; Bourillot, R.; Bouton, A.; Vennin, E.; Braissant, O.; Dupraz, C.; Duteil, T.; Bundeleva, I.; Patrier, P.; Galaup, S.; et al. Formation of stromatolite lamina at the interface of oxygenic–anoxygenic photosynthesis. Geobiology 2018, 16, 378–398. [Google Scholar] [CrossRef] [PubMed]
- Merz-Preiß, M.; Riding, R. Cyanobacterial tufa calcification in two freshwater streams: Ambient environment, chemical thresholds and biological processes. Sediment. Geol. 1999, 126, 103–124. [Google Scholar] [CrossRef]
- Kremer, B.; Kazmierczak, J.; Stal, L.J. Calcium carbonate precipitation in cyanobacterial mats from sandy tidal flats of the North Sea. Geobiology 2008, 6, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak, J.; Fenchel, T.; Kühl, M.; Kempe, S.; Kremer, B.; Łącka, B.; Małkowski, K. CaCO3 precipitation in multilayered cyanobacterial mats: clues to explain the alternation of micrite and sparite layers in calcareous stromatolites. Life 2015, 5, 744–769. [Google Scholar] [CrossRef]
- Kühl, M.; Fenchel, T.; Kazmierczak, J. Growth, Structure and Calcification Potential of an Artificial Cyanobacterial Mat. In Fossil and Recent Biofilms: A Natural History of Life on Earth; Krumbein, W.E., Paterson, D.M., Zavarzin, G.A., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 77–102. ISBN 978-94-017-0193-8. [Google Scholar]
- Aloisi, G.; Gloter, A.; Krüger, M.; Wallmann, K.; Guyot, F.; Zuddas, P. Nucleation of calcium carbonate on bacterial nanoglobules. Geology 2006, 34, 1017–1020. [Google Scholar] [CrossRef]
- Bundeleva, I.A.; Shirokova, L.S.; Bénézeth, P.; Pokrovsky, O.S.; Kompantseva, E.I.; Balor, S. Calcium carbonate precipitation by anoxygenic phototrophic bacteria. Chem. Geol. 2012, 291, 116–131. [Google Scholar] [CrossRef]
- Liang, A.; Paulo, C.; Zhu, Y.; Dittrich, M. CaCO3 biomineralization on cyanobacterial surfaces: Insights from experiments with three Synechococcus strains. Colloids Surf. B Biointerfaces 2013, 111, 600–608. [Google Scholar] [CrossRef]
- Bundeleva, I.A.; Shirokova, L.S.; Pokrovsky, O.S.; Bénézeth, P.; Ménez, B.; Gérard, E.; Balor, S. Experimental modeling of calcium carbonate precipitation by cyanobacterium Gloeocapsa sp. Chem. Geol. 2014, 374–375, 44–60. [Google Scholar] [CrossRef]
- Balci, N.; Menekşe, M.; Karagüler, N.G.; Sönmez, M.Ş.; Meister, P. Reproducing authigenic carbonate precipitation in the hypersaline Lake Acıgöl (Turkey) with microbial cultures. Geomicrobiol. J. 2016, 33, 758–773. [Google Scholar] [CrossRef]
- Balci, N.; Demirel, C. Formation of carbonate nanoglobules by a mixed natural culture under hypersaline conditions. Minerals 2016, 6, 122. [Google Scholar] [CrossRef]
- Freytet, P. Les tufs de la vallée de la Mérantaise. Bull. Société Versaill. Sci. Nat. 1989, 16, 81–99. [Google Scholar]
- Roche, A.; Vennin, E.; Bundeleva, I.; Bouton, A.; Payandi-Rolland, D.; Amiotte-Suchet, P.; Gaucher, E.C.; Courvoisier, H.; Visscher, P.T. The role of the substrate on the mineralization potential of microbial mats in a modern freshwater river (Paris Basin, France). Minerals 2019, 9, 359. [Google Scholar] [CrossRef]
- Parks, S.T. Microbial Life in a Winogradsky Column: From Lab Course to Diverse Research Experience. J. Microbiol. Biol. Educ. 2015, 16, 82–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteban, D.J.; Hysa, B.; Bartow-McKenney, C. Temporal and spatial distribution of the microbial community of Winogradsky columns. PLoS ONE 2015, 10, e0134588. [Google Scholar] [CrossRef] [PubMed]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [PubMed]
- Wang, Y.; Qian, P.Y. Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PLoS ONE 2009, 4, e7401. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Aronesty, E. Ea-Utils: Command-Line Tools for Processing Biological Sequencing Data; Expression Analysis: Durham, NC, USA, 2011. [Google Scholar]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Gran, G. Equivalence volumes in potentiometric titrations. Anal. Chim. Acta 1988, 206, 111–123. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J. An Introduction Emphasizing Chemical Equilibria in Natural Waters, Aquatic Chemistry; John Wiley and Sons: New York, NY, USA, 1981. [Google Scholar]
- Cantrell, K.J.; Serkiz, S.M.; Perdue, E.M. Evaluation of acid neutralizing capacity data for solutions containing natural organic acids. Geochim. Cosmochim. Acta 1990, 54, 1247–1254. [Google Scholar] [CrossRef]
- Arp, G.; Reimer, A.; Reitner, J. Photosynthesis-induced biofilm calcification and calcium concentrations in Phanerozoic Oceans. Science 2001, 292, 1701–1704. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C. User’s Guide to PHREEQC (Version 2): A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Department of the Interior: Denver, CO, USA, 1999. [Google Scholar]
- The Proteobacteria. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Garrity, G. (Ed.) Springer: New York, NY, USA, 2005; Volume 2, ISBN 978-0-387-95040-2. [Google Scholar]
- Pedley, M.; Rogerson, M.; Middleton, R. Freshwater calcite precipitates from in vitro mesocosm flume experiments: A case for biomediation of tufas. Sedimentology 2009, 56, 511–527. [Google Scholar] [CrossRef]
- Braissant, O.; Cailleau, G.; Aragno, M.; Verrecchia, E.P. Biologically induced mineralization in the tree Milicia excelsa (Moraceae): Its causes and consequences to the environment. Geobiology 2004, 2, 59–66. [Google Scholar] [CrossRef]
- Jones, B. Review of aragonite and calcite crystal morphogenesis in thermal spring systems. Sediment. Geol. 2017, 354, 9–23. [Google Scholar] [CrossRef]
- Decho, A.W. Exopolymer microdomains as a structuring agent for heterogeneity within Microbial biofilms. In Microbial Sediments; Riding, R.E., Awramik, S.M., Eds.; Springer: Berlin, Germany; Heidelberg, Germany, 2000; pp. 9–15. ISBN 978-3-662-04036-2. [Google Scholar]
- Marvasi, M.; Visscher, P.T.; Casillas Martinez, L. Exopolymeric substances (EPS) from Bacillus subtilis: Polymers and genes encoding their synthesis. FEMS Microbiol. Lett. 2010, 313, 1–9. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.P.; Visscher, P.T. Microbialites, Modern. In Encyclopedia of Geobiology; Reitner, J., Thiel, V., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 617–635. ISBN 978-1-4020-9211-4. [Google Scholar]
- Tsuneda, S.; Aikawa, H.; Hayashi, H.; Yuasa, A.; Hirata, A. Extracellular polymeric substances responsible for bacterial adhesion onto solid surface. FEMS Microbiol. Lett. 2003, 223, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Turner, E.C.; Jones, B. Microscopic calcite dendrites in cold-water tufa: Implications for nucleation of micrite and cement. Sedimentology 2005, 52, 1043–1066. [Google Scholar] [CrossRef]
- Gradziński, M. Factors controlling growth of modern tufa: Results of a field experiment. Geol. Soc. Lond. Spec. Publ. 2010, 336, 143–191. [Google Scholar] [CrossRef]
- Tourney, J.; Ngwenya, B.T. Bacterial extracellular polymeric substances (EPS) mediate CaCO3 morphology and polymorphism. Chem. Geol. 2009, 262, 138–146. [Google Scholar] [CrossRef]
- Prasanna, R.; Pattnaik, S.; Sugitha, T.C.K.; Nain, L.; Saxena, A.K. Development of cyanobacterium-based biofilms and their in vitro evaluation for agriculturally useful traits. Folia Microbiol. 2011, 56, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Fenchel, T.; Kühl, M. Artificial Cyanobacterial Mats: Growth, Structure, and Vertical Zonation Patterns. Microb. Ecol. 2000, 40, 85–93. [Google Scholar] [Green Version]
- Visscher, P.T.; van Gemerden, H. Production and consumption of dimethylsulfoniopropionate in marine microbial mats. Appl. Environ. Microbiol. 1991, 57, 3237–3242. [Google Scholar] [PubMed]
- Stal, L.J.; Moezelaar, R. Fermentation in cyanobacteria. FEMS Microbiol. Rev. 1997, 21, 179–211. [Google Scholar] [CrossRef] [Green Version]
- Decho, A.W.; Visscher, P.T.; Reid, R.P. Production and cycling of natural microbial exopolymers (EPS) within a marine stromatolite. In Geobiology: Objectives, Concepts, Perspectives; Noffke, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 71–86. ISBN 978-0-444-52019-7. [Google Scholar]
- Bateson, M.M.; Ward, D.M. Photoexcretion and fate of glycolate in a hot spring cyanobacterial mat. Appl. Environ. Microbiol. 1988, 54, 1738–1743. [Google Scholar]



| Physicochemical Attribute | 2016 (Autumn/Winter) | 2017 (Spring) | ||
|---|---|---|---|---|
| October | November | December | March | |
| Water temperature (°C) | 10.8 | 7.2 | 6.5 | 8.2 |
| pH | 8.31 | 8.24 | 8.60 | 8.03 |
| Alkalinity (meq/L) | 5.56 | 5.34 | 3.50 | 6.61 |
| Conductivity (μS/cm) | 712 | 704 | 704 | 667 |
| Dissolved O2 (mg/L) | 10.8 | 14.2 | - | 10.2 |
| Ca2+ (mg/L) | 142.2 | 149.7 | 104.9 | 88.1 |
| K+ (mg/L) | 3.5 | 3.6 | 2.8 | 2.8 |
| Mg2+ (mg/L) | 13.8 | 14.2 | 9.9 | 10.8 |
| Na+ (mg/L) | 19.3 | 19.7 | 13.5 | 13.8 |
| Cl− (mg/L) | 36.0 | 34.0 | 24.7 | 23.4 |
| NO3− (mg/L) | 90.1 | 90.9 | 12.9 | 17.3 |
| SO42− (mg/L) | 22.8 | 18.3 | 68.5 | 61.4 |
| SI calcite | 1.30 ± 0.33 | 1.19 ± 0.25 | 1.23 ± 0.06 | 0.90 ± 0.30 |
| SI aragonite | 1.16 ± 0.30 | 1.04 ± 0.30 | 1.08 ± 0.05 | 0.75 ± 0.25 |
| Treatment * | pH | Ca2+, mg/L | Alkalinity, meq/L | SIcalcite |
|---|---|---|---|---|
| A | 8.76 | 0 | 0.93 | 0 |
| B | 8.32 | 50 | 1.34 | 0.63 |
| C | 8.60 | 50 | 2.87 | 1.17 |
| D | 8.65 | 50 | 4.62 | 1.38 |
| E | 8.35 | 100 | 2.49 | 1.15 |
| F | 8.40 | 150 | 2.66 | 1.36 |
| Cyanobacterial Metabolism | Example Reaction Equation(s) | Light | pH | Alkalinity | [Ca2+] | Precipitation Potential |
|---|---|---|---|---|---|---|
| 1. Carbon dioxide fixation | CO2 + RuBP → 2PGA (→ → glycogen) HCO3− → CO2 + OH− | ✓ | ↑ | ↑ | - | ↑ |
| 2. Photorespiration | O2 + RuBP → PGA + glycolate | ✓ | ↓ | ↑/? | ↓ | ↓ |
| 3. EPS production | CO2 + RuBP → 2PGA → →(glycogen) → → EPS | ✓ | -/↑ | - | ↓ | ↓(fresh)/↑(degraded) |
| 4. Fermentation | Trehalose → → glucose → →acetate | ✗ | ↓ | ↑/- | ↓ | ↓ |
| Glycogen → → formate | ||||||
| → → acetate | ↓ | ↑/- | ↓ | |||
| → → lactate | ||||||
| → → ethanol | - | - | - | |||
| → → H2 | ||||||
| and | ||||||
| So → H2S | - | ↑ | - | |||
| (5. Induction of mineral precipitation) | CO2 + RuBP → 2PGA HCO3− → CO2 + OH− HCO3− + Ca2+ →CaCO3 + H+ H+ + OH− → H2O | ✓ | - | ↓ | ↓ | ↑ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payandi-Rolland, D.; Roche, A.; Vennin, E.; Visscher, P.T.; Amiotte-Suchet, P.; Thomas, C.; Bundeleva, I.A. Carbonate Precipitation in Mixed Cyanobacterial Biofilms Forming Freshwater Microbial Tufa. Minerals 2019, 9, 409. https://doi.org/10.3390/min9070409
Payandi-Rolland D, Roche A, Vennin E, Visscher PT, Amiotte-Suchet P, Thomas C, Bundeleva IA. Carbonate Precipitation in Mixed Cyanobacterial Biofilms Forming Freshwater Microbial Tufa. Minerals. 2019; 9(7):409. https://doi.org/10.3390/min9070409
Chicago/Turabian StylePayandi-Rolland, Dahédrey, Adeline Roche, Emmanuelle Vennin, Pieter T. Visscher, Philippe Amiotte-Suchet, Camille Thomas, and Irina A. Bundeleva. 2019. "Carbonate Precipitation in Mixed Cyanobacterial Biofilms Forming Freshwater Microbial Tufa" Minerals 9, no. 7: 409. https://doi.org/10.3390/min9070409
APA StylePayandi-Rolland, D., Roche, A., Vennin, E., Visscher, P. T., Amiotte-Suchet, P., Thomas, C., & Bundeleva, I. A. (2019). Carbonate Precipitation in Mixed Cyanobacterial Biofilms Forming Freshwater Microbial Tufa. Minerals, 9(7), 409. https://doi.org/10.3390/min9070409






