Electrochemical, Spectroscopic, and Computational Investigations on Redox Reactions of Selenium Species on Galena Surfaces
Abstract
1. Introduction
2. Methods
2.1. Sample Characterization
2.2. Voltammetry
2.3. X-Ray Photoelectron Spectroscopy (XPS)
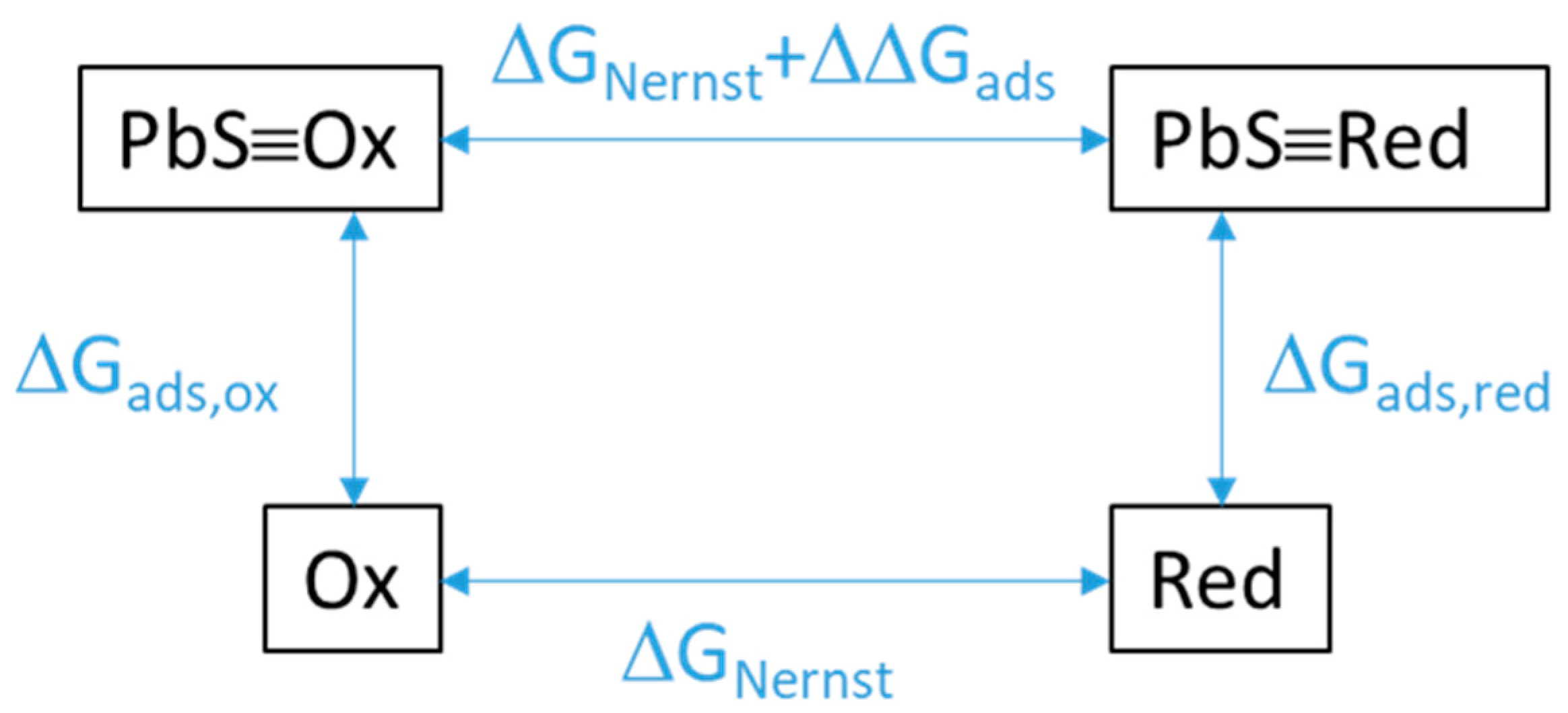
2.4. Calculation of Adsorption
3. Results
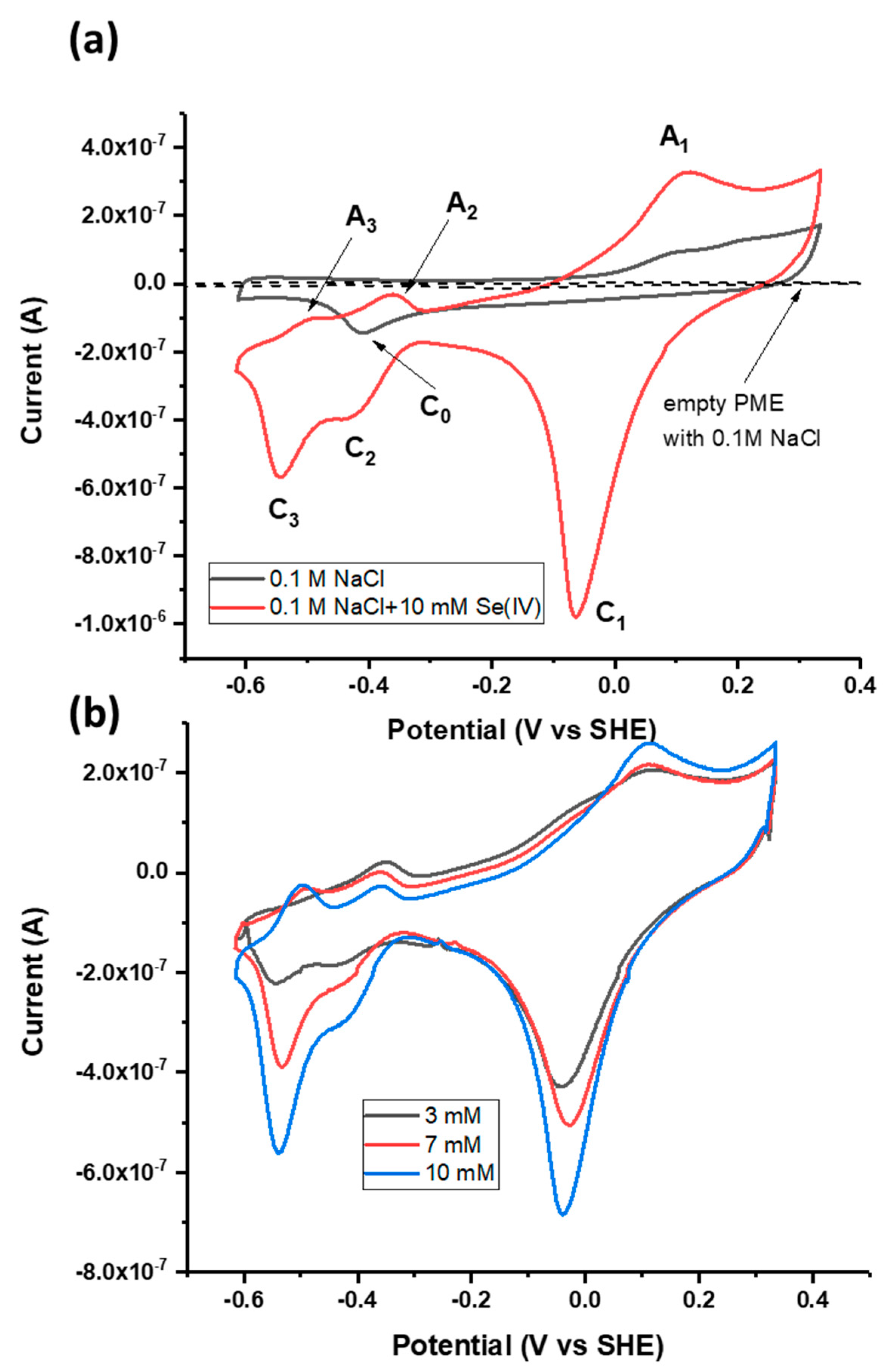
3.1. Cyclic Voltammetry of Galena with and without Se(IV)
3.2. Peak Pairing and Assignment to Se Redox Transformations
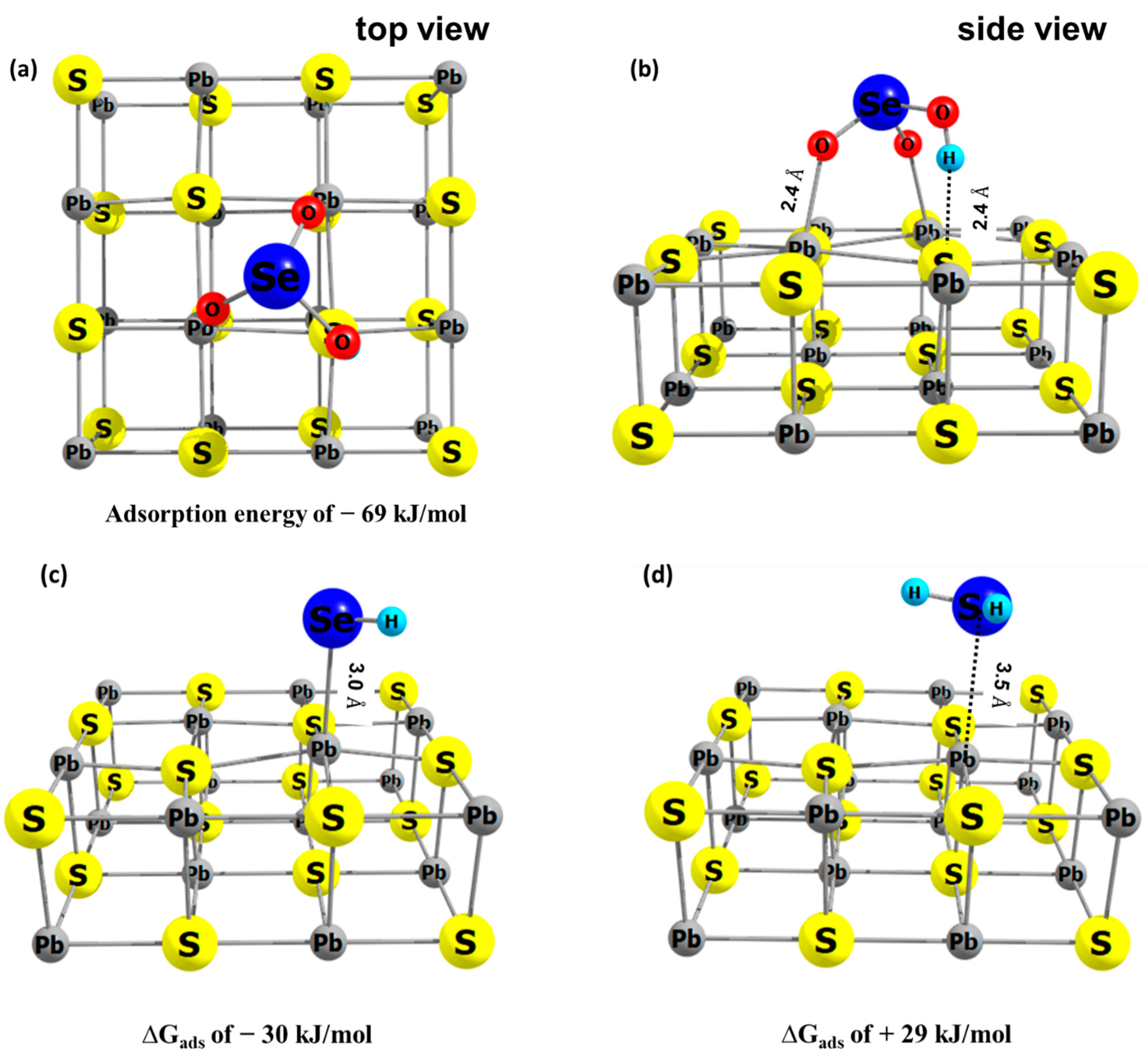
3.3. Adsorption of Se Species on Galena
3.4. XPS Measurements
4. Discussion
4.1. Possible Forms and Behavior of Products of Se Reduction Mediated by Galena
4.2. Geochemical Implications
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dubrovsky, N.M.; Neil, J.M.; Fujii, R.; Oremland, R.; Hollibaugh, J. Influence of Redox potential on selenium distribution in ground-water, Mendota, western San-Joaquin Valley, California. US Geol. Surv. Open File Rep. 1990, 90138, 24. [Google Scholar]
- Wu, L. Review of 15 years of research on ecotoxicology and remediation of land contaminated by agricultural drainage sediment rich in selenium. Ecotoxicol. Environ. Saf. 2004, 57, 257–269. [Google Scholar] [CrossRef]
- Naftz, D.L.; Rice, J.A. Geochemical processes controlling selenium in ground water after mining, Powder River Basin, Wyoming, USA. Appl. Geochem. 1989, 4, 565–575. [Google Scholar] [CrossRef]
- Clark, S.K.; Johnson, T.M. Selenium stable isotope investigation into selenium biogeochemical cycling in a lacustrine environment: Sweitzer Lake, Colorado. J. Environ. Qual. 2010, 39, 2200–2210. [Google Scholar] [CrossRef] [PubMed]
- Yudovich, Y.E.; Ketris, M. Selenium in coal: A review. Int. J. Coal Geol. 2006, 67, 112–126. [Google Scholar] [CrossRef]
- Martínez-Torrents, A.; Giménez, J.; Martínez-Lladó, X.; de Pablo, J.; Casas, I. Incorporation of selenium (IV) and selenium (VI) on uranyl peroxide. J. Radioanal. Nucl. Chem. 2015, 303, 153–159. [Google Scholar] [CrossRef]
- Bott, A.W. Electrochemistry of semiconductors. Curr. Sep. 1998, 17, 87–92. [Google Scholar]
- Rosso, K.M.; Becker, U. Proximity effects on semiconducting mineral surfaces II: Distance dependence of indirect interactions. Geochim. Cosmochim. Acta 2003, 67, 941–953. [Google Scholar] [CrossRef]
- Arumugam, K.; Renock, D.; Becker, U. The basis for reevaluating the reactivity of pyrite surfaces: Spin states and crystal field d- orbital splitting energies of bulk, terrace, edge, and corner Fe( II) ions. PCCP 2019, 21, 6415–6431. [Google Scholar] [CrossRef]
- Renock, D.; Becker, U. A first principles study of the oxidation energetics and kinetics of realgar. Geochim. Cosmochim. Acta 2010, 74, 4266–4284. [Google Scholar] [CrossRef]
- Becker, U.; Rosso, K.M.; Hochella, M.F. The proximity effect on semiconducting mineral surfaces: A new aspect of mineral surface reactivity and surface complexation theory? Geochim. Cosmochim. Acta 2001, 65, 2641–2649. [Google Scholar] [CrossRef]
- Skomurski, F.N.; Shuller, L.C.; Ewing, R.C.; Becker, U. Corrosion of UO2 and ThO2: A quantum-mechanical investigation. J. Nucl. Mater. 2008, 375, 290–310. [Google Scholar] [CrossRef]
- Taylor, S.D.; Becker, U.; Rosso, K.M. Electron Transfer Pathways Facilitating U(VI) Reduction by Fe(II) on Al- vs Fe-Oxides. J. Phys. Chem. C 2017, 121, 19887–19903. [Google Scholar] [CrossRef]
- Taylor, S.D.; Marcano, M.C.; Rosso, K.M.; Becker, U. An experimental and ab initio study on the abiotic reduction of uranyl by ferrous iron. Geochim. Cosmochim. Acta 2015, 156, 154–172. [Google Scholar] [CrossRef][Green Version]
- Grundl, T.J.; Haderlein, S.; Nurmi, J.T.; Tratnyek, P.G. Introduction to Aquatic Redox Chemistry. In Aquatic Redox Chemistry; Tratnyek, P.G., Grundl, T.J., Haderlein, S.B., Eds.; American Chemical Society: Columbus, OH, USA, 2011; Volume 1071, pp. 1–632. [Google Scholar]
- Gorski, C.A.; Nurmi, J.T.; Tratnyek, P.G.; Hofstetter, T.B.; Scherer, M.M. Redox behavior of magnetite: Implications for contaminant reduction. Environ. Sci. Technol. 2009, 44, 55–60. [Google Scholar] [CrossRef]
- Pridmore, D.; Shuey, R. The electrical resistivity of galena, pyrite, and chalcopyrite. Am. Mineral. 1976, 61, 248–259. [Google Scholar]
- Renock, D.; Becker, U. A first principles study of coupled substitution in galena. Ore Geol. Rev. 2011, 42, 71–83. [Google Scholar] [CrossRef]
- Bougouma, M.; Van Elewyck, A.; Steichen, M.; Buess-Herman, C.; Doneux, T. Selenium electrochemistry in choline chloride–urea deep eutectic electrolyte. J. Solid State Electrochem. 2013, 17, 527–536. [Google Scholar] [CrossRef]
- Cabral, M.F.; Pedrosa, V.A.; Machado, S.A.S. Deposition of selenium thin layers on gold surfaces from sulphuric acid media: Studies using electrochemical quartz crystal microbalance, cyclic voltammetry and AFM. Electrochim. Acta 2010, 55, 1184–1192. [Google Scholar] [CrossRef]
- Ivandini, T.A.; Einaga, Y. Electrochemical detection of selenium (IV) and (VI) at gold-modified diamond electrodes. Electrocatalysis 2013, 4, 367–374. [Google Scholar] [CrossRef]
- Kowalik, R. Microgravimetric studies of selenium electrodeposition onto different substrates. Arch. Metall. Mater. 2014, 59, 871–877. [Google Scholar] [CrossRef]
- Maranowski, B.; Strawski, M.; Osowiecki, W.; Szklarczyk, M. Study of selenium electrodeposition at gold electrode by voltammetric and rotating disc electrode techniques. J. Electroanal. Chem. 2015, 752, 54–59. [Google Scholar] [CrossRef]
- Solaliendres, M.; Manzoli, A.; Salazar-Banda, G.; Eguiluz, K.; Tanimoto, S.; Machado, S. The processes involved in the Se electrodeposition and dissolution on Au electrode: The H2Se formation. J. Solid State Electrochem. 2008, 12, 679–686. [Google Scholar] [CrossRef]
- Wei, C.; Myung, N.; Rajeshwar, K. A combined voltammetry and electrochemical quartz crystal microgravimetry study of the reduction of aqueous Se (IV) at gold. J. Electroanal. Chem. 1994, 375, 109–115. [Google Scholar] [CrossRef]
- Feliu, J.; Gómez, R.; Llorca, M.; Aldaz, A. Electrochemical behavior of irreversibly adsorbed selenium dosed from solution on Pt (h, k, l) single crystal electrodes in sulphuric and perchloric acid media. Surf. Sci. 1993, 289, 152–162. [Google Scholar] [CrossRef]
- Santos, M.C.; Machado, S.A. Microgravimetric, rotating ring-disc and voltammetric studies of the underpotential deposition of selenium on polycrystalline platinum electrodes. J. Electroanal. Chem. 2004, 567, 203–210. [Google Scholar] [CrossRef]
- Kazacos, M.S.; Miller, B. Studies in selenious acid reduction and CdSe film deposition. J. Electrochem. Soc. 1980, 127, 869–873. [Google Scholar] [CrossRef]
- Curti, E.; Aimoz, L.; Kitamura, A. Selenium uptake onto natural pyrite. J. Radioanal. Nucl. Chem. 2013, 295, 1655–1665. [Google Scholar] [CrossRef]
- Han, D.S.; Batchelor, B.; Abdel-Wahab, A. Sorption of selenium (IV) and selenium (VI) onto synthetic pyrite (FeS2): Spectroscopic and microscopic analyses. J. Colloid Interface Sci. 2012, 368, 496–504. [Google Scholar] [CrossRef]
- Martinez, M.; Gimenez, J.; De Pablo, J.; Rovira, M.; Duro, L. Sorption of selenium (IV) and selenium (VI) onto magnetite. Appl. Surf. Sci. 2006, 252, 3767–3773. [Google Scholar] [CrossRef]
- Naveau, A.; Monteil-Rivera, F.; Guillon, E.; Dumonceau, J. Interactions of aqueous selenium (− II) and (IV) with metallic sulfide surfaces. Environ. Sci. Technol. 2007, 41, 5376–5382. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Hayes, R.; Prestidge, C.; Ralston, J.; Smart, R.S.C. Scanning tunnelling microscopy studies of galena: The mechanism of oxidation in air. Appl. Surf. Sci. 1994, 78, 385–397. [Google Scholar] [CrossRef]
- Laajalehto, K.; Kartio, I.; Heinonen, M.; Laiho, T. Temperature Controled Photoelectron Spectroscopic Investigation of Volatile Species on PbS (100) Surface. Jpn. J. Appl. Phys. 1999, 38, 265. [Google Scholar] [CrossRef]
- Wittstock, G.; Kartio, I.; Hirsch, D.; Kunze, S.; Szargan, R. Oxidation of galena in acetate buffer investigated by atomic force microscopy and photoelectron spectroscopy. Langmuir 1996, 12, 5709–5721. [Google Scholar] [CrossRef]
- Cha, C.S.; Li, C.M.; Yang, H.; Liu, P. Powder microelectrodes. J. Electroanal. Chem. 1994, 368, 47–54. [Google Scholar] [CrossRef]
- Renock, D.; Mueller, M.; Yuan, K.; Ewing, R.C.; Becker, U. The energetics and kinetics of uranyl reduction on pyrite, hematite, and magnetite surfaces: A powder microelectrode study. Geochim. Cosmochim. Acta 2013, 118, 56–71. [Google Scholar] [CrossRef]
- Moulder, J.F. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics Division, Perkin-Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef]
- Dunning Jr, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Dunning, T.H.; Hay, P.J. Gaussian basis sets for molecular calculations. In Methods of Electronic Structure Theory; Springer: Berlin/Heidelberg, Germany, 1977; pp. 1–27. [Google Scholar]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Urban, D.R.; Wilcox, J. A theoretical study of properties and reactions involving arsenic and selenium compounds present in coal combustion flue gases. J. Phys. Chem. A 2006, 110, 5847–5852. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.; Becker, U.; Helean, K.; Ewing, R. Perrhenate and pertechnetate behavior on iron and sulfur-bearing compounds. In Proceedings of the symposium on Scientific Basis for Nuclear Waste Management XXX, Boston, MA, USA, 27 November 2006. [Google Scholar]
- Becker, U.; Hochella Jr, M.F.; Vaughan, D.J. The adsorption of gold to galena surfaces: Calculation of adsorption/reduction energies, reaction mechanisms, XPS spectra, and STM images. Geochim. Cosmochim. Acta 1997, 61, 3565–3585. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.; Woods, R. A study of the surface oxidation of galena using cyclic voltammetry. J. Electroanal. Chem. Interfacial Electrochem. 1979, 100, 447–459. [Google Scholar] [CrossRef]
- Higgins, S.; Hamers, R. Spatially-resolved electrochemistry of the lead sulfide (galena)(001) surface by electrochemical scanning tunneling microscopy. Surf. Sci. 1995, 324, 263–281. [Google Scholar] [CrossRef]
- Pritzker, M.; Yoon, R. A voltammetric study of galena immersed in acetate solution at pH 4.6. J. Appl. Electrochem. 1988, 18, 323–332. [Google Scholar] [CrossRef]
- Huang, B.M.; Lister, T.E.; Stickney, J.L. Se adlattices formed on Au (100), studies by LEED, AES, STM and electrochemistry. Surf. Sci. 1997, 392, 27–43. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; Leddy, J.; Zoski, C.G. Electrochemical methods: Fundamentals and applications; Wiley: New York, NY, USA, 1980; Volume 2. [Google Scholar]
- Bouroushian, M. Electrochemistry of the Chalcogens. In Electrochemistry of Metal Chalcogenides; Springer: Berlin/Heidelberg, Germany, 2010; pp. 57–75. [Google Scholar]
- Bylaska, E.J.; Salter-Blanc, A.J.; Tratnyek, P.G. One-electron reduction potentials from chemical structure theory calculations. In Aquatic Redox Chemistry; ACS Publications: Washington, DC, USA, 2011; pp. 37–64. [Google Scholar]
- Pavitt, A.S.; Bylaska, E.J.; Tratnyek, P.G. Oxidation potentials of phenols and anilines: Correlation analysis of electrochemical and theoretical values. Environ. Sci. Process. Impacts 2017, 19, 339–349. [Google Scholar] [CrossRef]
- Salter-Blanc, A.J.; Bylaska, E.J.; Johnston, H.J.; Tratnyek, P.G. Predicting reduction rates of energetic nitroaromatic compounds using calculated one-electron reduction potentials. Environ. Sci. Technol. 2015, 49, 3778–3786. [Google Scholar] [CrossRef]
- Rupp, H.; Weser, U. X-ray photoelectron spectroscopy of some selenium containing amino acids. Bioinorg. Chem. 1975, 5, 21–32. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Li, L.; D’Ulivo, A.; Belzile, N. Extraction and determination of elemental selenium in sediments—A comparative study. Anal. Chim. Acta 2006, 577, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, K.L.; Allen, F.S. Hydrogen selenide ion adsorption to colloidal elemental selenium. Inorg. Chim. Acta 1984, 89, 199–201. [Google Scholar] [CrossRef]
- Scheinost, A.C.; Kirsch, R.; Banerjee, D.; Fernandez-Martinez, A.; Zaenker, H.; Funke, H.; Charlet, L. X-ray absorption and photoelectron spectroscopy investigation of selenite reduction by FeII-bearing minerals. J. Contam. Hydrol. 2008, 102, 228–245. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Tascón, M.; Vázquez, M.; Batanero, P.S. Electroanalytical study of selenium (+ IV) at a carbon paste electrode with electrolytic binder and electroactive compound incorporated. Electrochim. Acta 1992, 37, 1165–1172. [Google Scholar] [CrossRef]
- Scheinost, A.C.; Charlet, L. Selenite reduction by mackinawite, magnetite and siderite: XAS characterization of nanosized redox products. Environ. Sci. Technol. 2008, 42, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Renock, D.; Ewing, R.C.; Becker, U. Uranium reduction on magnetite: Probing for pentavalent uranium using electrochemical methods. Geochim. Cosmochim. Acta 2015, 156, 194–206. [Google Scholar] [CrossRef]
- Kim, Y.; Yuan, K.; Ellis, B.R.; Becker, U. Redox reactions of selenium as catalyzed by magnetite: Lessons learned from using electrochemistry and spectroscopic methods. Geochim. Cosmochim. Acta 2017, 199, 304–323. [Google Scholar] [CrossRef]
- Kang, M.; Chen, F.; Wu, S.; Yang, Y.; Bruggeman, C.; Charlet, L. Effect of pH on aqueous Se (IV) reduction by pyrite. Environ. Sci. Technol. 2011, 45, 2704–2710. [Google Scholar] [CrossRef]





| Redox Reaction | Standard Redox Potential * (vs. SHE) |
|---|---|
| (I) HSeO3− + 5H+ + 4e− = Se(s) + 3H2O | E0 = 0.78 V |
| E = 0.78 – 0.074 pH + 0.015 log[HSeO3−] | |
| (II) HSeO3− + 6H+ + 6e− = H2Se + 3H2O E = 0.386 – 0.059pH + 0.010 log[HSeO3−]/[H2Se] | E0 = 0.386 |
| (III) HSeO3− + 6H+ + 6e− = HSe− + 3H2O E = 0.35 – 0.059pH + 0.010 log[HSeO3−]/[HSe−] | E0 = 0.35 |
| (IV) Se(s) + 2H+ + 2e− = H2Se E = −0.4 – 0.059 pH − 0.030 log[H2Se] | E0 = −0.4 |
| (V) Se(s) + H+ + 2e− = HSe− E = −0.51 – 0.030 pH − 0.030 log[HSe−] | E0 = −0.51 |
| (IV) SeO42− + 3H+ + 2e− = HSeO3− + H2O E = 1.08 – 0.089 pH + 0.030 log[SeO42−]/[HSeO3−] | E0 = 1.08 |
| (VII) SeO42− + 8H+ + 6e− = Se(s) + 4H2O E = 1.86 – 0.079 pH + 0.010 log[SeO42−] | E0 = 1.86 |
| (VIII) SeO42− + 9H+ + 8e− = HSe− + 4H2O E = 1.43 – 0.066 pH + 0.007 log[SeO42−]/[HSe−] | E0 = 1.43 |
| Oxidant/Reductant Pair | Equilibrium Pot. @ pH 4.5 * (V) | ∆∆Gads Per e− Transferred (V) | Reduction Potential with Correction of ∆∆Gads (V) | CV Peak Assign-ment | Mid-Point Potential (V) |
|---|---|---|---|---|---|
| HSeO3−/Se(0) | 0.32 | −0.18 | 0.14 | ||
| HSeO3−/H2Se | 0.15 | −0.17 | −0.01 | C1/A1 | 0.03 |
| HSeO3−/HSe− | 0.11 | −0.07 | 0.04 | C1/A1 | 0.03 |
| Se(0)/H2Se | −0.49 | −0.15 | −0.64 | C3/A3 | −0.52 |
| Se(0)/HSe− | −0.49 | 0.15 | −0.34 | C2/A2 | −0.40 |
| SeO42−/HSeO3− | 0.55 | −0.02 | 0.54 | ||
| SeO42−/Se(0) | 1.44 | −0.17 | 1.27 | ||
| SeO42−/HSe− | 1.12 | −0.05 | 1.06 |
| XPS Binding Energy of Se Species | Se(IV) | Se(0) | Se(−II) | |||
|---|---|---|---|---|---|---|
| 3d 5/2 (eV) | 3d 3/2 (eV) | 3d 5/2 (eV) | 3d 3/2 (eV) | 3d 5/2 (eV) | 3d 3/2 (eV) | |
| Polarization @ −125 mV | 58.5 | 59.2 | 55 | 55.9 | - | - |
| Relative proportion | 49% | 51% | - | |||
| Polarization @ −490 mV | 58.2 | 59.1 | 54.4 | 55.3 | 52.5 | 53.3 |
| Relative proportion | 39% | 32% | 29% | |||
| Black selenium | - | - | 55.1 | 55.9 | - | - |
| Na2SeO3 | 58.3 | 59.4 | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cook, P.; Kim, Y.; Yuan, K.; Marcano, M.C.; Becker, U. Electrochemical, Spectroscopic, and Computational Investigations on Redox Reactions of Selenium Species on Galena Surfaces. Minerals 2019, 9, 437. https://doi.org/10.3390/min9070437
Cook P, Kim Y, Yuan K, Marcano MC, Becker U. Electrochemical, Spectroscopic, and Computational Investigations on Redox Reactions of Selenium Species on Galena Surfaces. Minerals. 2019; 9(7):437. https://doi.org/10.3390/min9070437
Chicago/Turabian StyleCook, Peter, YoungJae Kim, Ke Yuan, Maria C. Marcano, and Udo Becker. 2019. "Electrochemical, Spectroscopic, and Computational Investigations on Redox Reactions of Selenium Species on Galena Surfaces" Minerals 9, no. 7: 437. https://doi.org/10.3390/min9070437
APA StyleCook, P., Kim, Y., Yuan, K., Marcano, M. C., & Becker, U. (2019). Electrochemical, Spectroscopic, and Computational Investigations on Redox Reactions of Selenium Species on Galena Surfaces. Minerals, 9(7), 437. https://doi.org/10.3390/min9070437





