Microstructure and Minerals Evolution of Iron Ore Sinter: Influence of SiO2 and Al2O3
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
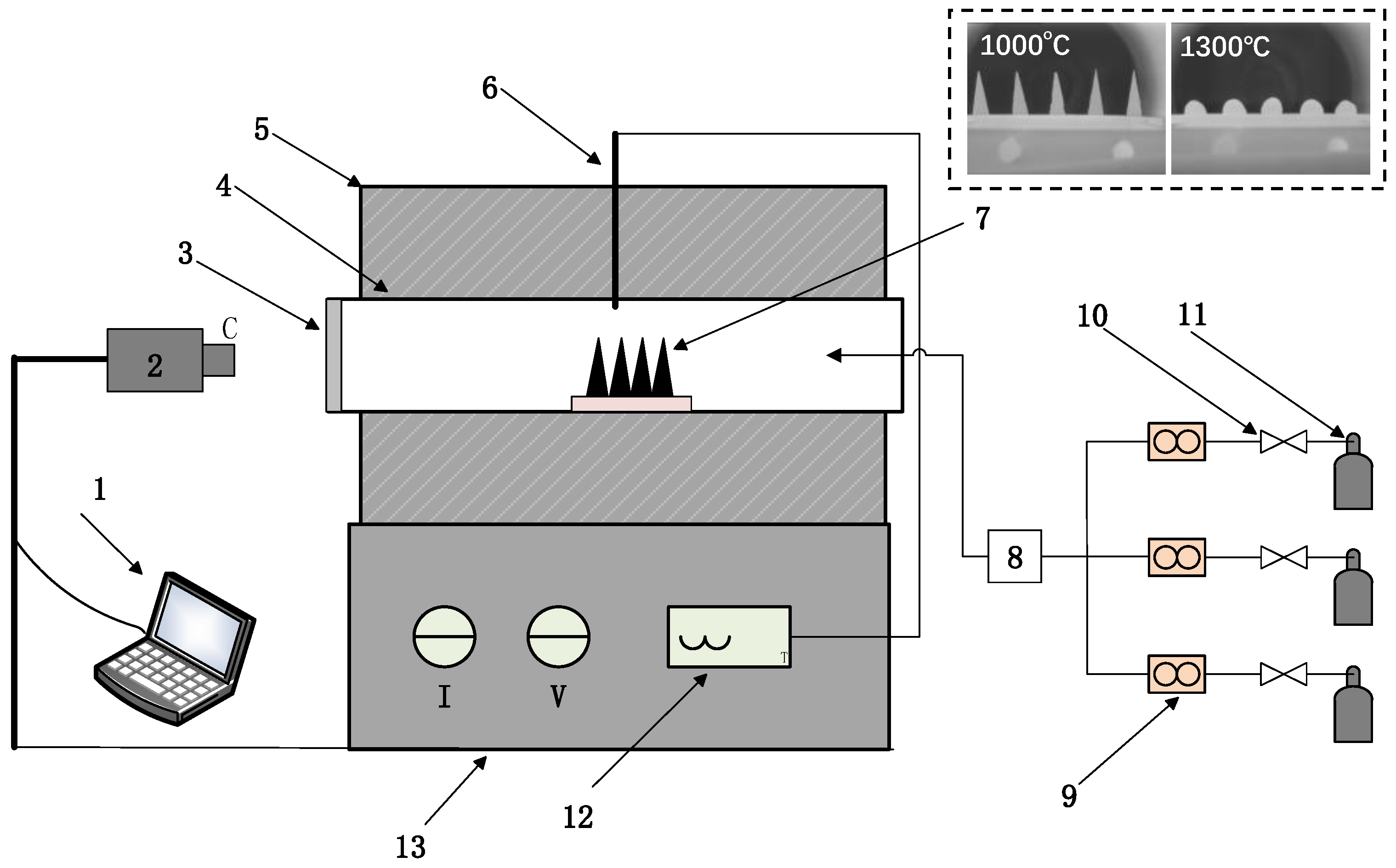
2.2.1. Sinter Pot Trials
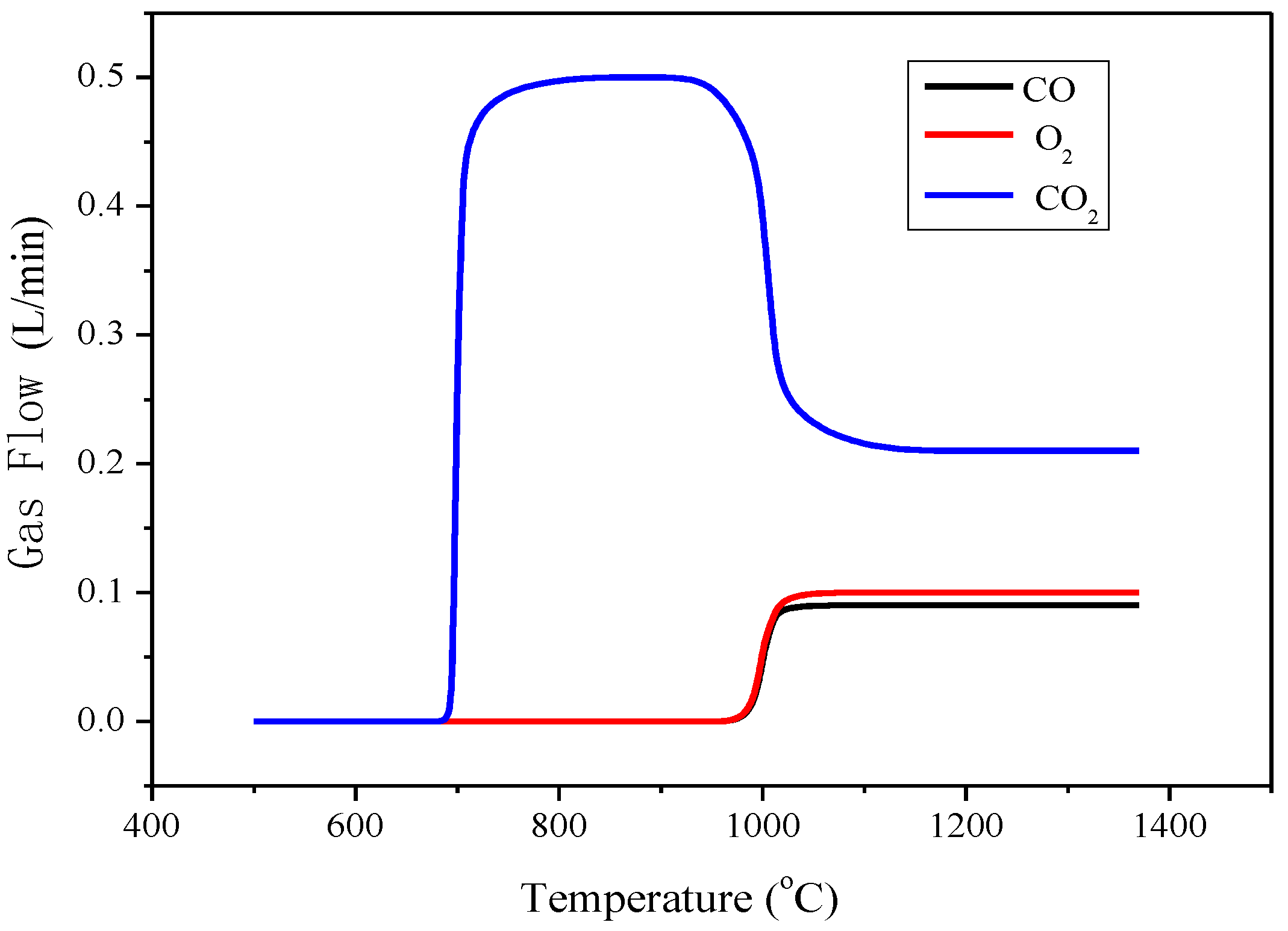
2.2.2. Minerals Analysis and Mechanical Strength Examination
3. Results and Discussion
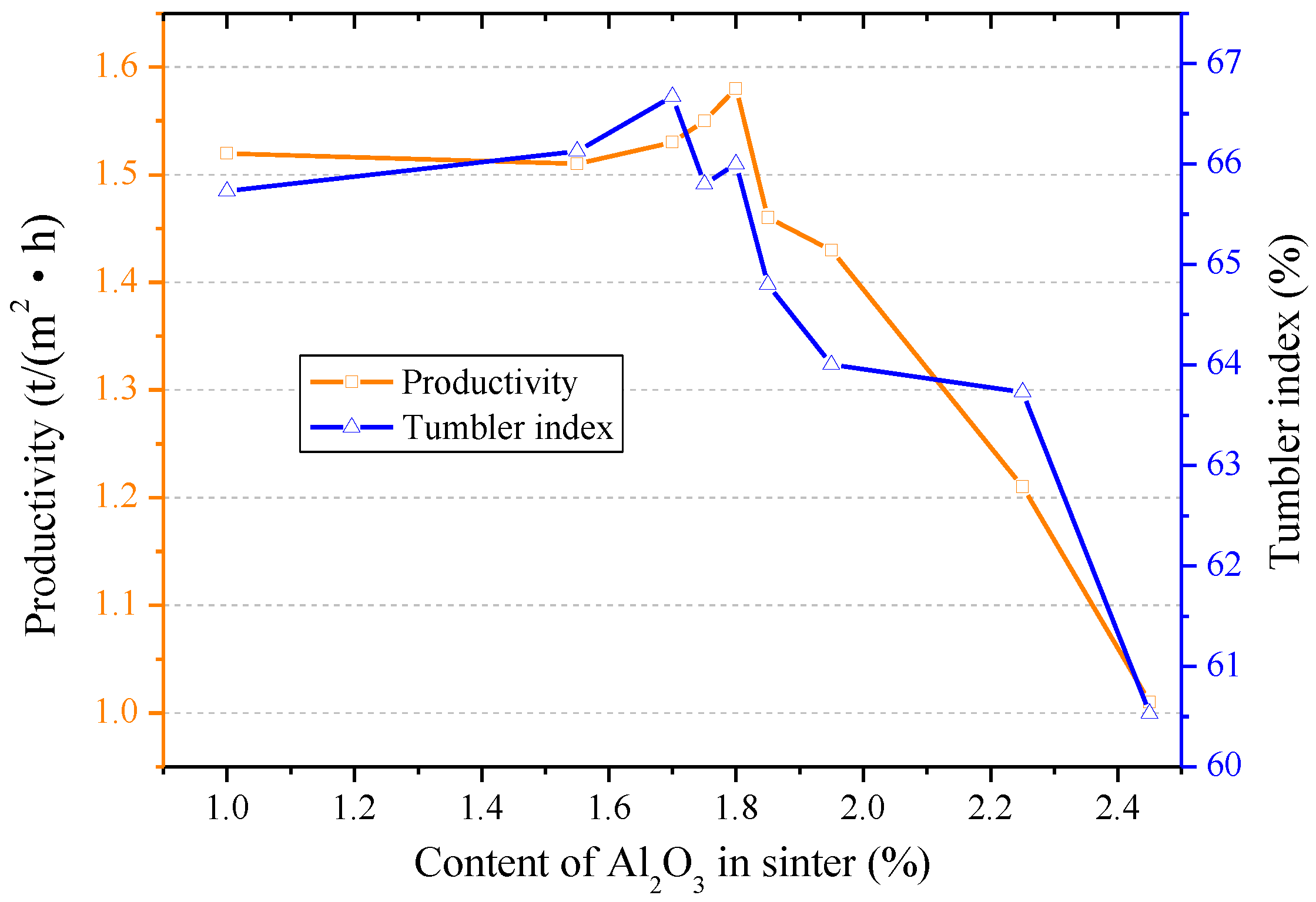
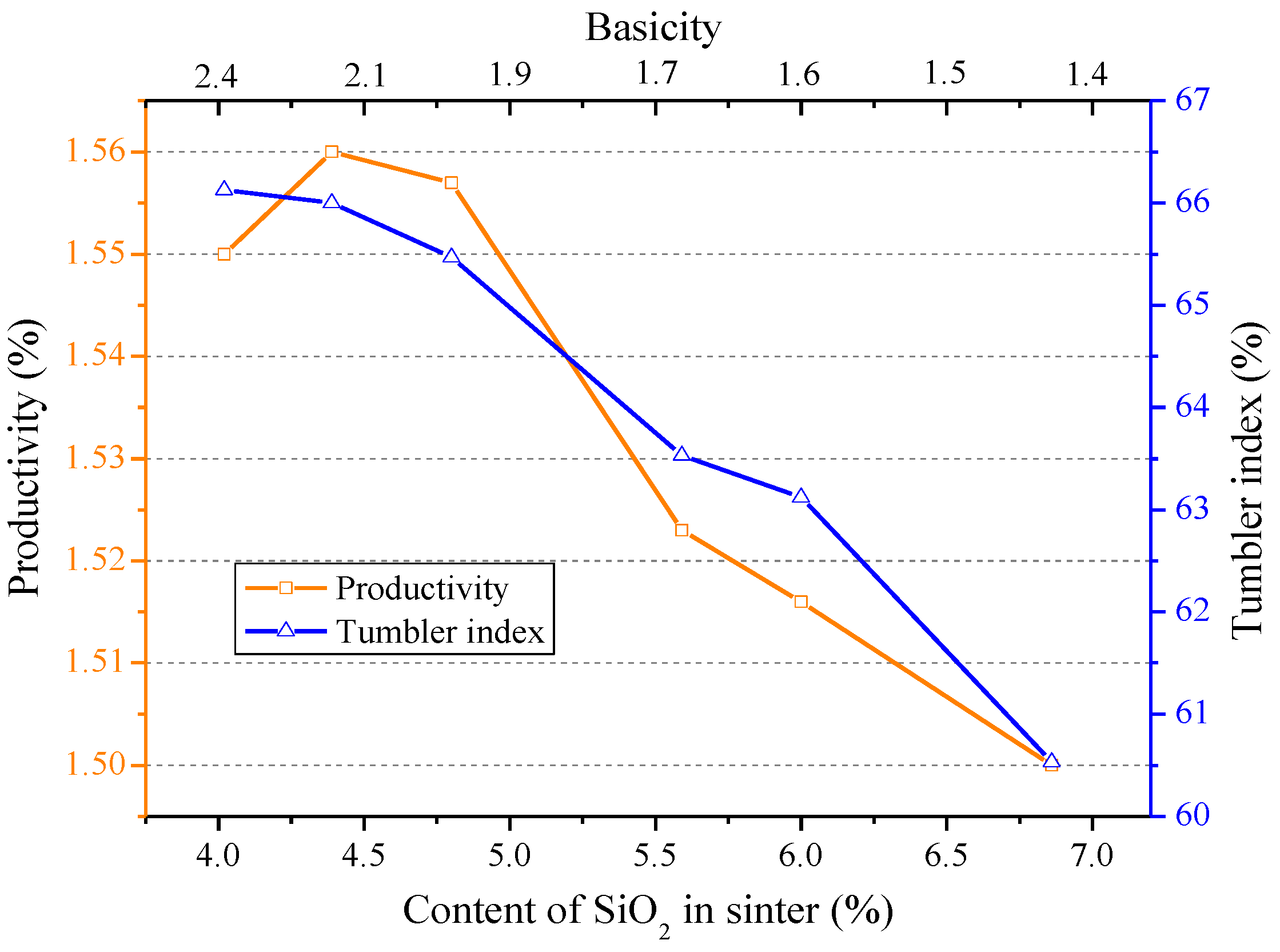
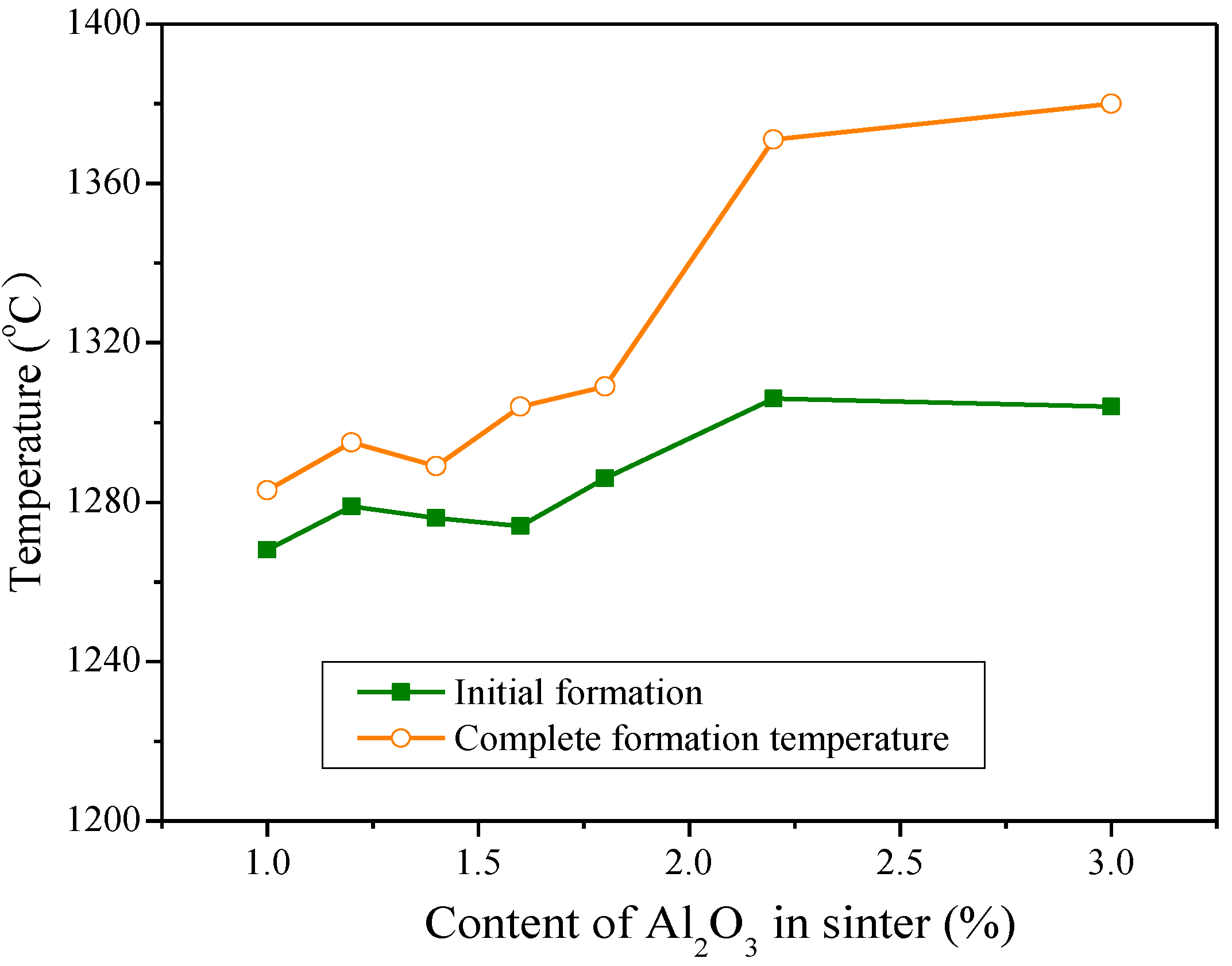
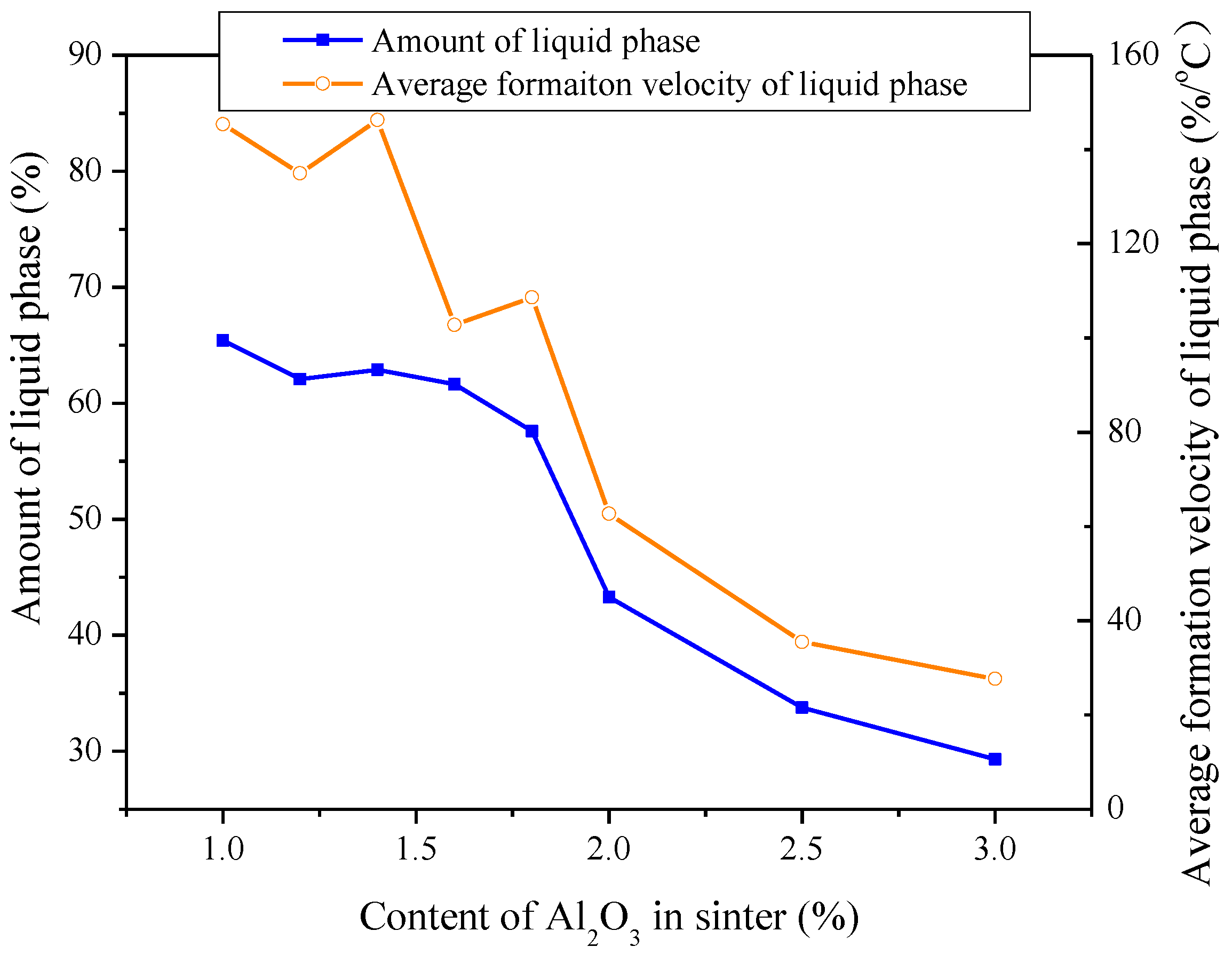
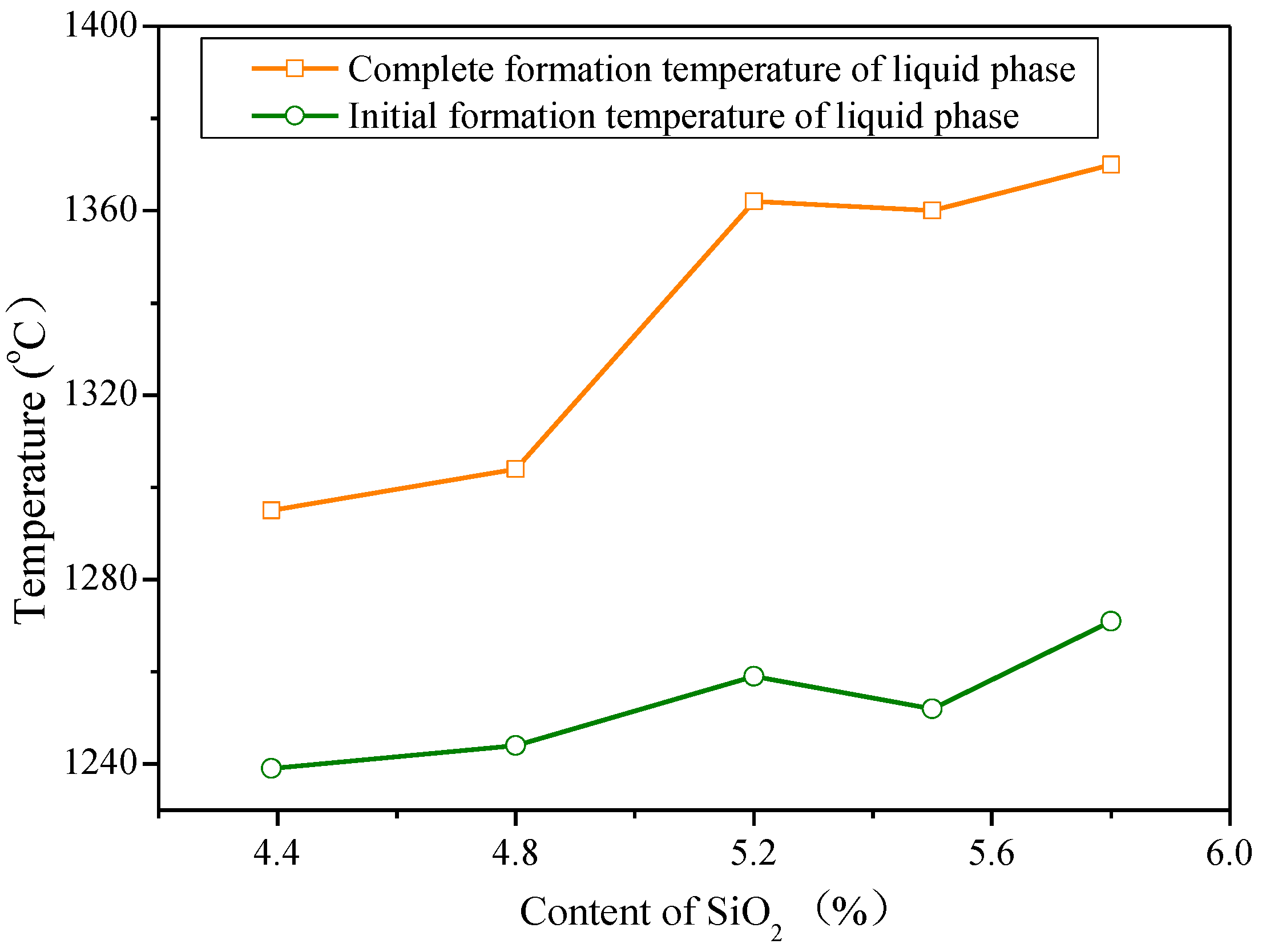
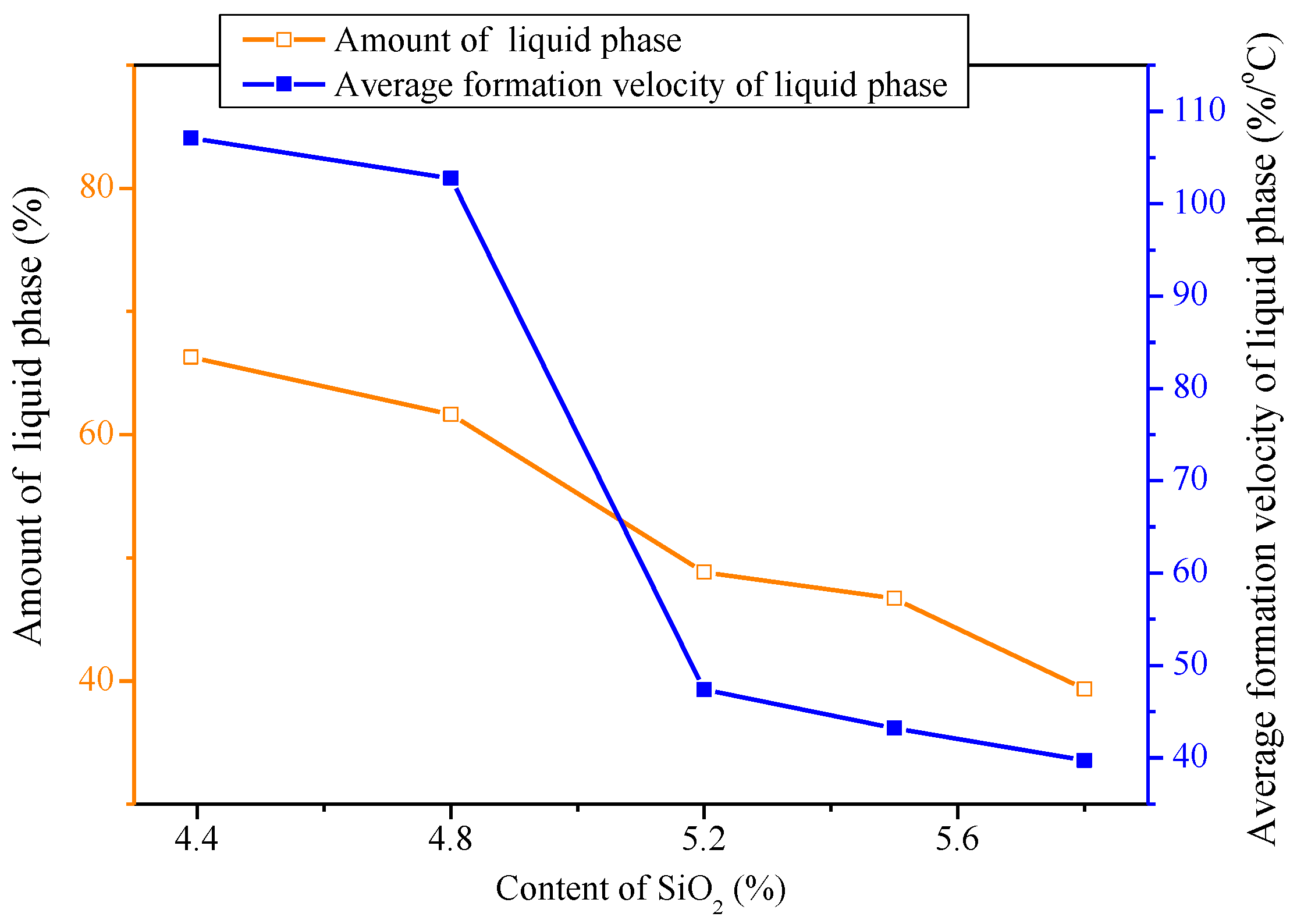
3.1. Influences of Al2O3 and SiO2 on Sintering Performance
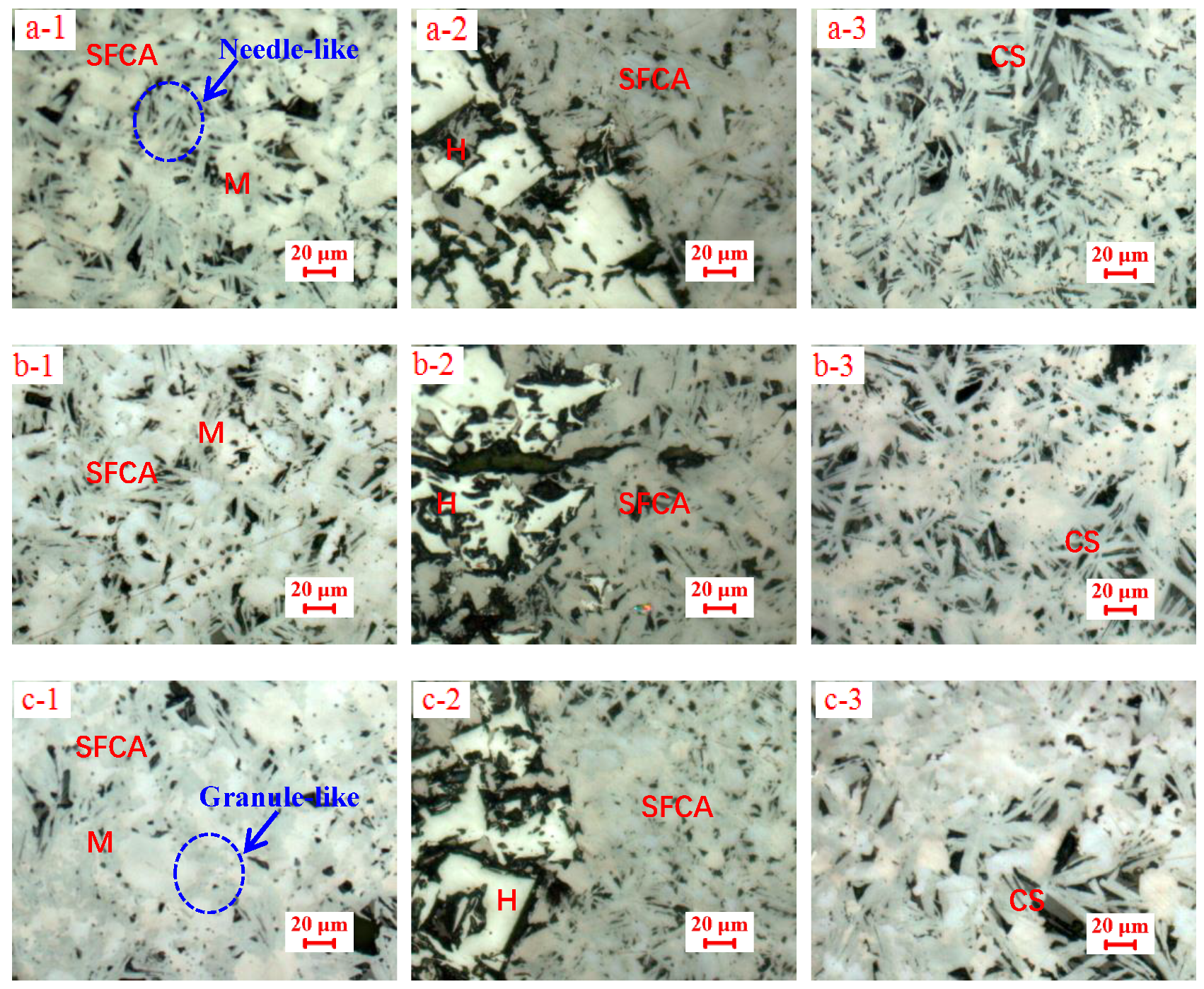
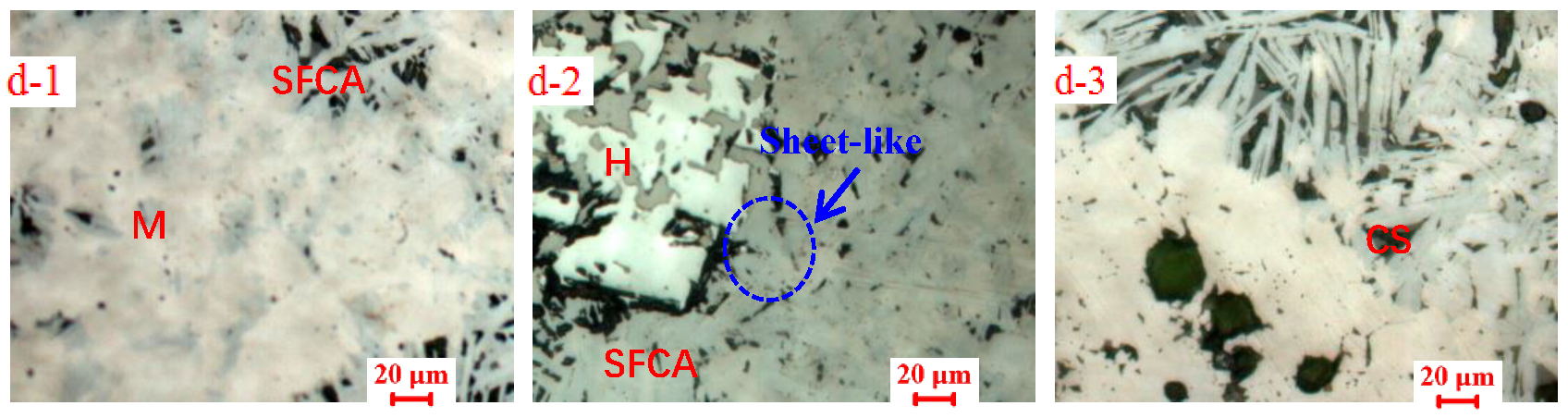
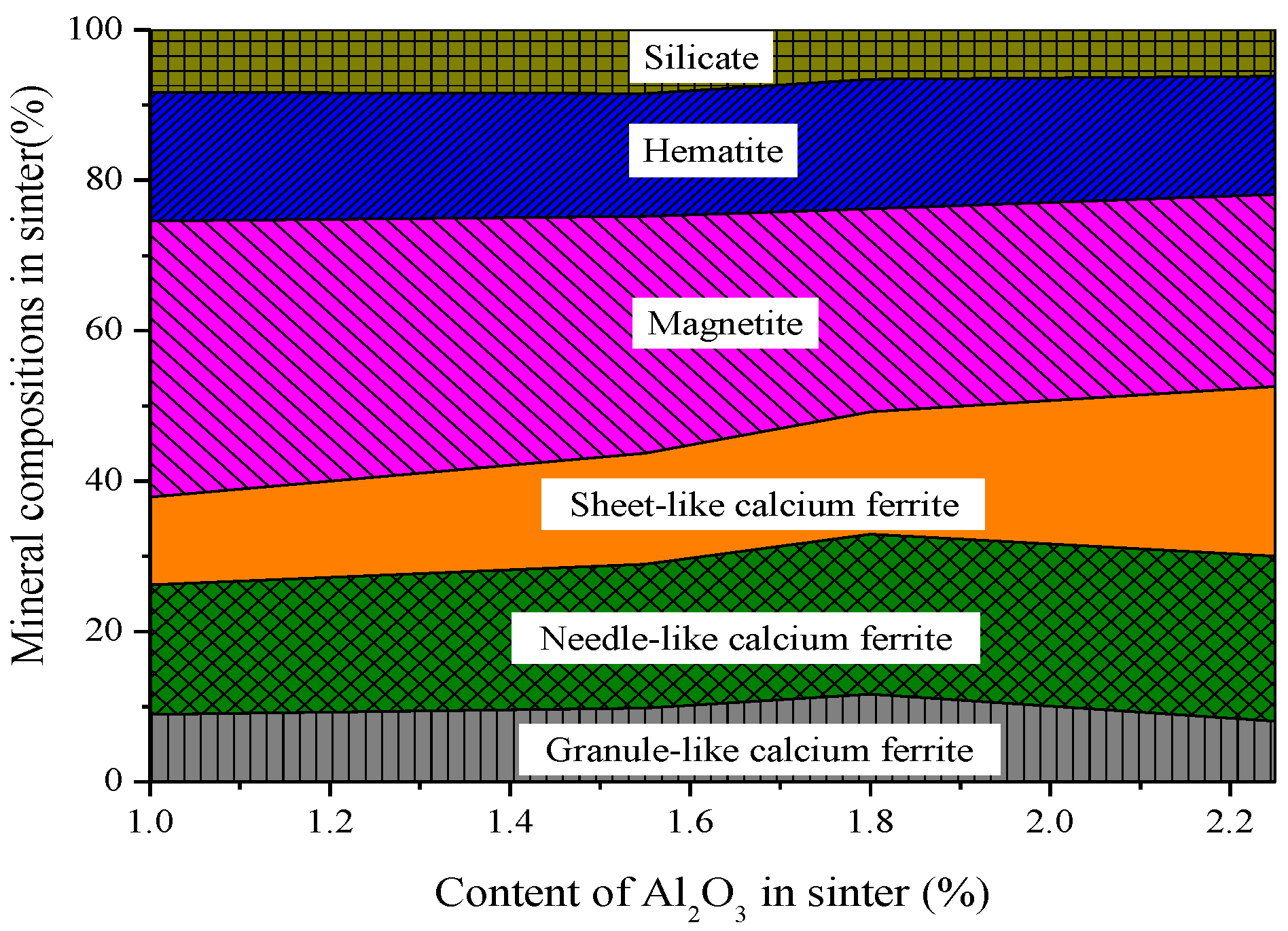
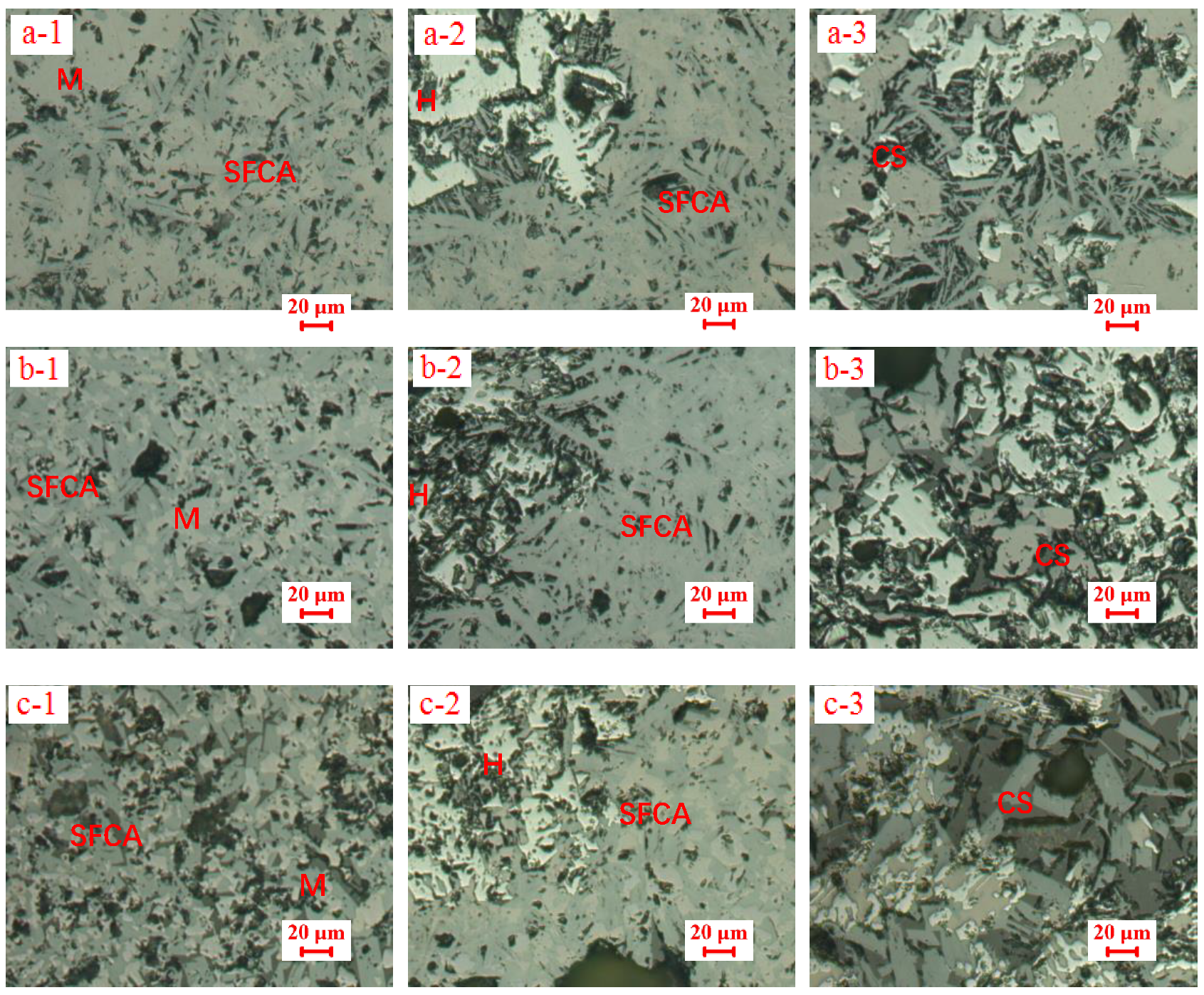
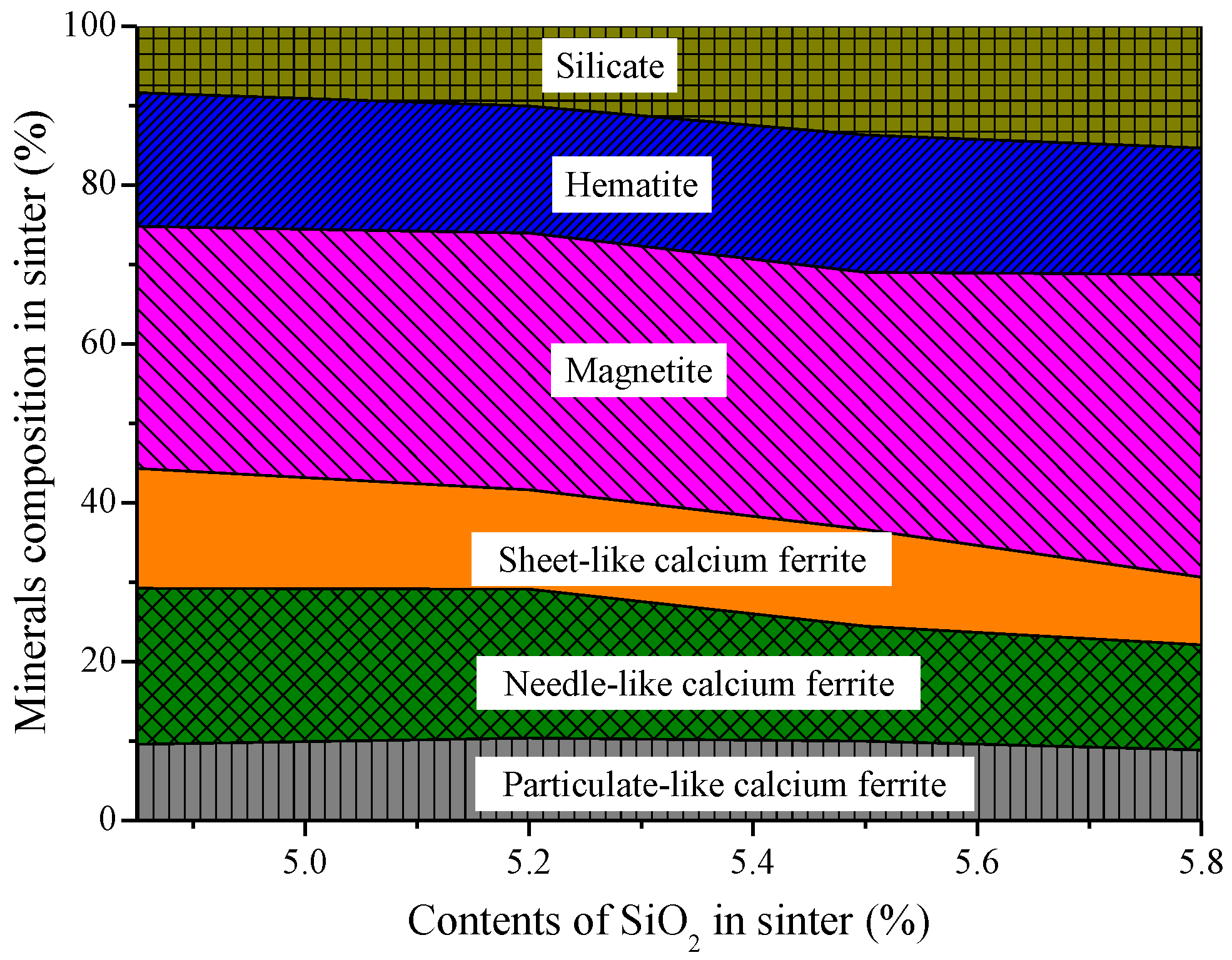
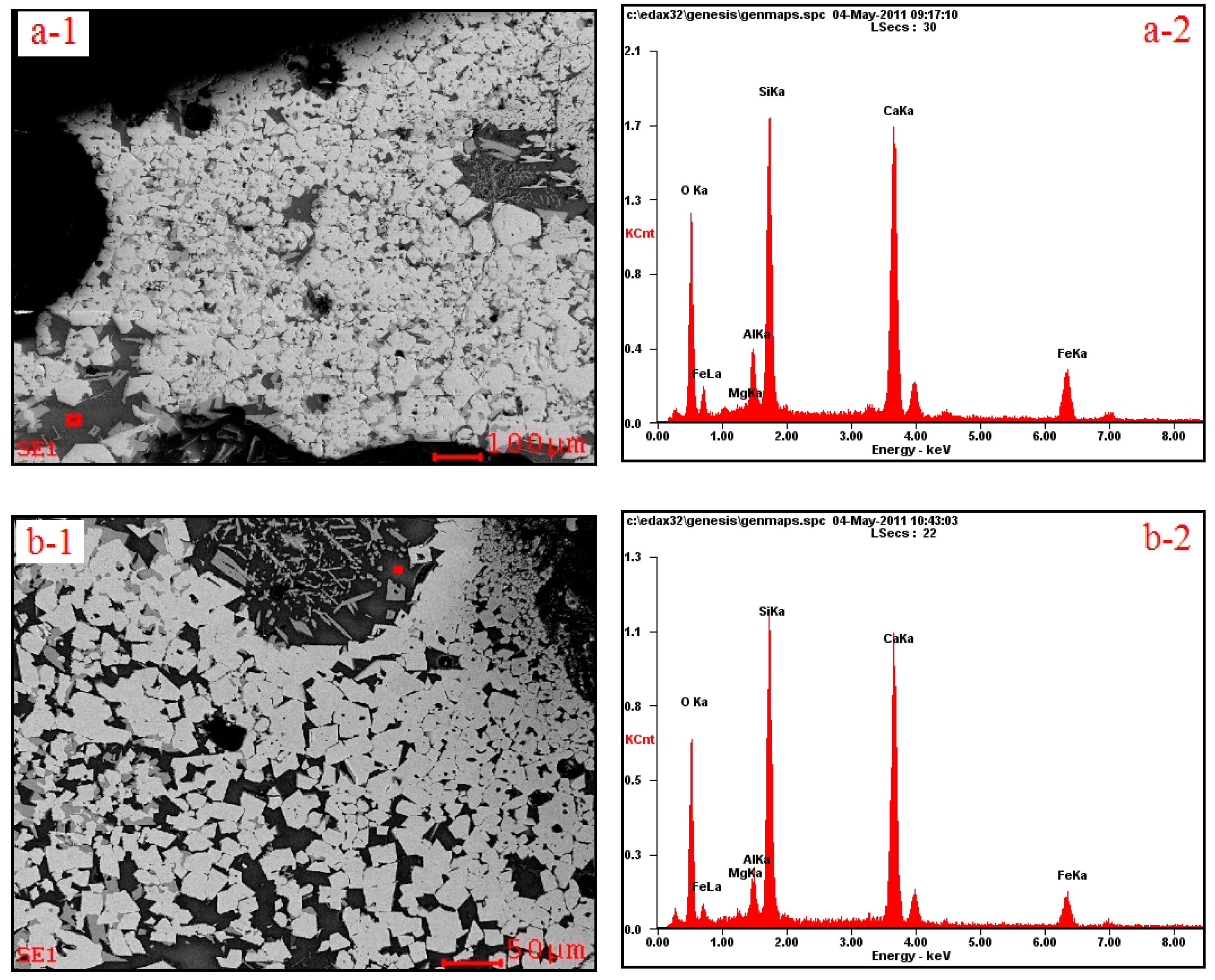
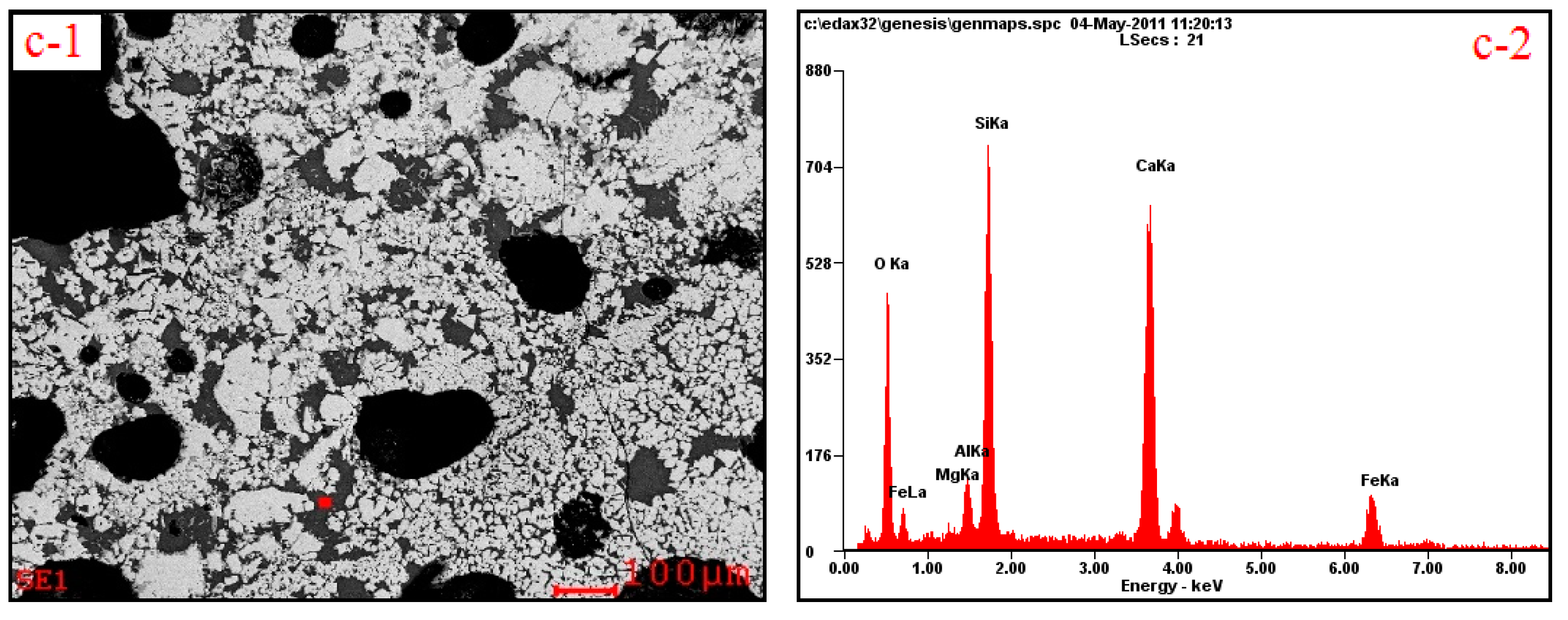
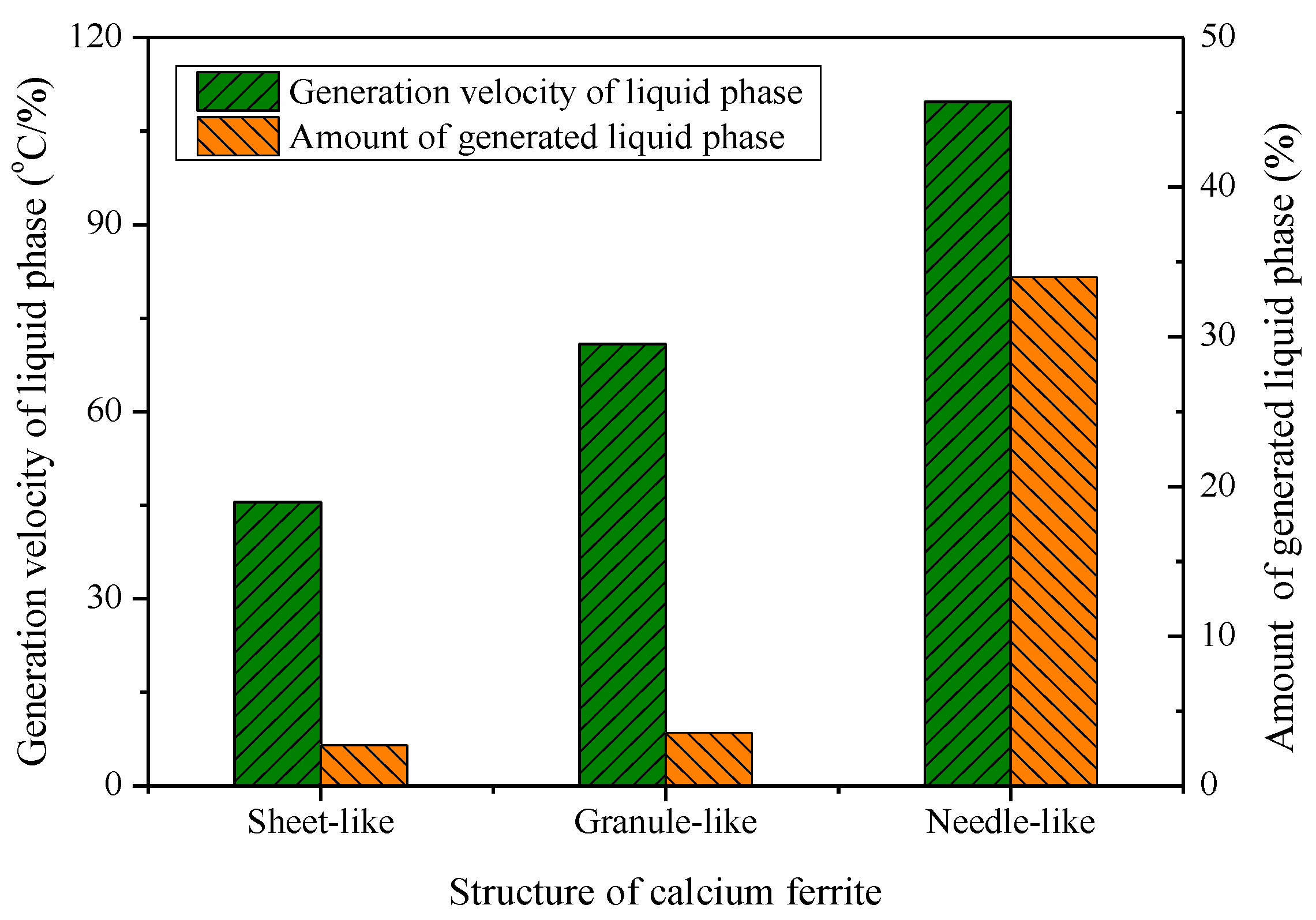
3.2. Influences of Al2O3 and SiO2 on Microcharacteristics of Sinter
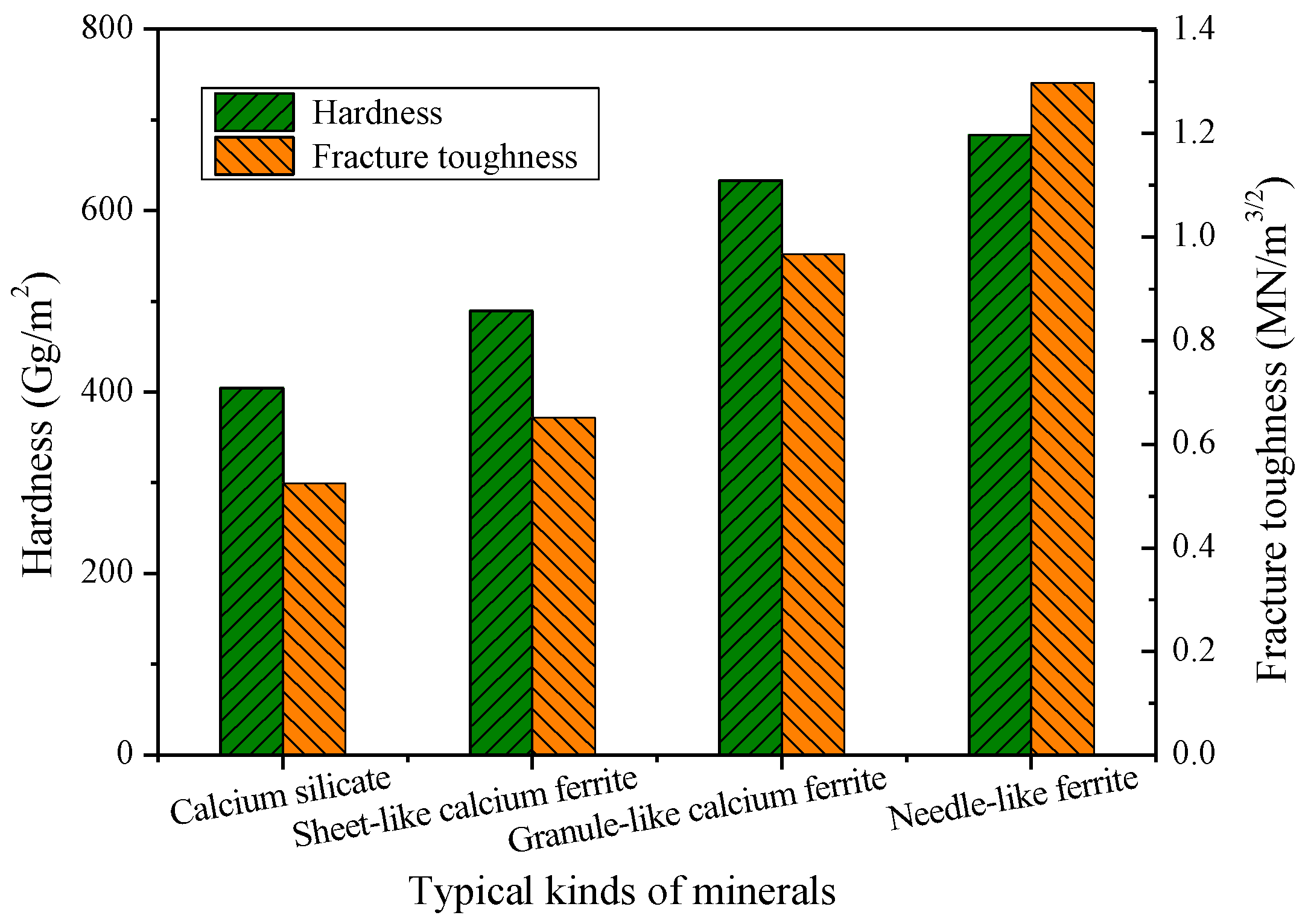
3.3. Function Mechanisms of Al2O3 and SiO2 on Sinter Quality
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Q.; Zhang, W.; Wang, Y.J.; Xu, J.; Cao, X.C. Potential of energy saving and emission reduction and energy efficiency improvement of China’s steel industry. Iron Steel 2019, 54, 7–14. [Google Scholar]
- Li, X.C. Road map to high-quality development of iron and steel industry in new age. Iron Steel 2019, 54, 1–7. [Google Scholar]
- Hessien, M.M.; Kashiwaya, Y.; Ishii, K.; Nasr, M.I.; El-Geassy, A.A. Sintering and heating reduction processes of alumina containing iron ore samples. Ironmak. Steelmak. 2008, 35, 191–204. [Google Scholar] [CrossRef]
- Umadevi, T.; Prakash, S.; Bandopadhyay, U.K. Influence of alumina on iron ore sinter quality and productivity. World Iron Steel 2010, 4, 12–18. [Google Scholar]
- Clout, J.; Manuel, J. Fundamental investigations of differences in bonding mechanisms in iron ore sinter formed from magnetite concentrates and hematite ores. Powder Technol. 2003, 130, 393–399. [Google Scholar] [CrossRef]
- Chen, C.L.; Lu, L.M.; Jiao, K.X. Thermodynamic modelling of iron ore sintering reactions. Minerals 2019, 9, 361. [Google Scholar] [CrossRef]
- Park, J.H.; Cho, Y.J.; Yoon, S.S. Effect of Al2O3, SiO2 and MgO on the formation of SFCAs in sinter using X-ray diffraction method. J. Korean Inst. Met. Mater. 2002, 40, 811–817. [Google Scholar]
- Liu, J.B.; Li, L.S. Influence of Al2O3 on sinter for its phase compositions and properties in equilibrium. J. Anhui Univ. Technol. 2009, 26, 333–337. [Google Scholar]
- Lu, L.; Holmes, R.J.; Manuel, J.R. Effect of alumina on sinter performance of hematite iron ores. ISIJ Int. 2007, 47, 349–358. [Google Scholar] [CrossRef]
- Webster, N.A.S.; Odea, D.P.; Ellis, B.G.; Pownceby, M.I. Effects of gibbsite, kaolinite and Al-rich goethite as alumina sources on silico-ferrite of calcium and aluminum (SFCA) and SFCA-I iron ore sinter bonding phase formation. ISIJ Int. 2017, 57, 41–47. [Google Scholar] [CrossRef]
- Webster, N.A.S.; Pownceby, M.I.; Madsen, I.C.; Studer, A.J.; Manuel, J.R.; Kimpton, J.A. Fundamentals of silico-ferrite of calcium and aluminum (SFCA) and SFCA-I iron ore sinter bonding phase formation: Effects of CaO:SiO2 ratio. Metall. Mater. Trans. B 2014, 45, 2097–2105. [Google Scholar] [CrossRef]
- Feng, X.P.; Zhang, Y.Z. Influence of basicity on the low silicon sinter strength. Sinter. Pelletizing 2004, 29, 41–47. [Google Scholar]
- Zhang, Y.Z.; Feng, X.P.; Li, Z.G.; Yin, H.S. Study and practice of improving the metallurgical property of low silicon sinter. Sinter. Pelletizing 2004, 29, 4–7. [Google Scholar]
- Wang, R.C.; Fu, J.Y. Study on production of high Fe and Low SiO2 Sinter. Iron Steel 2007, 42, 18–23. [Google Scholar]
- Fan, X.H.; Ji, Z.Y.; Gan, M.; Chen, X.L.; Jiang, T. Integrated assessment on the characteristics of straw-based fuels and their effects on iron ore sintering performance. Fuel Process. Technol. 2016, 150, 1–9. [Google Scholar] [CrossRef]
- Fan, X.H.; Jiang, T.; Gan, M. A Method for Detecting the Liquid Phase Formation Characteristics of Sintered Iron Ore. Chinese Patent ZL200910307772.9, 22 June 2011. [Google Scholar]
- Gan, M.; Fan, X.H.; Chen, X.L.; Ji, Z.Y. High temperature mineralization behavior of mixtures during iron ore sintering and optimizing methods. ISIJ Int. 2015, 55, 742–750. [Google Scholar] [CrossRef]
- Loo, C.E.; Wan, K.T.; Howes, V.R. Mechanical properties of natural and synthetic mineral phases in sinters having varying reduction degradation indices. Ironmak. Steelmak. 1998, 15, 279–285. [Google Scholar]
- Ying, Z.W.; Jiang, M.F.; Xu, L.X. Effects of Mineral Composition and Microstructure on Crack Resistance of Sintered Ore. J. Iron Steel Res. Int. 2006, 13, 9–12. [Google Scholar] [CrossRef]



| Iron Ore Types | Number | Chemical Compositions/% | bLOI/% | |||||
|---|---|---|---|---|---|---|---|---|
| aTFe | FeO | CaO | MgO | SiO2 | Al2O3 | |||
| Typical Brazilian iron ores | 1# | 63.86 | 5.02 | 0.10 | 0.11 | 4.59 | 1.14 | 2.79 |
| 2# | 65.69 | 1.01 | 0.00 | 0.11 | 3.91 | 0.69 | 1.72 | |
| 3# | 67.22 | 0.14 | 0.07 | 0.27 | 1.04 | 1.00 | 1.78 | |
| 4# | 68.09 | 0.22 | 0.02 | 0.26 | 1.38 | 0.39 | 1.07 | |
| 5# | 66.08 | 0.33 | 0.16 | 0.16 | 1.75 | 1.21 | 1.25 | |
| 6# | 60.85 | 0.51 | 0.12 | 0.07 | 8.42 | 1.82 | 2.08 | |
| Typical Australian iron ores | 7# | 62.13 | 2.59 | 0.33 | 0.26 | 3.80 | 1.93 | 4.81 |
| 8# | 61.79 | 1.15 | 0.08 | 0.26 | 4.22 | 1.98 | 4.88 | |
| 9# | 61.93 | 0.86 | 0.04 | 0.18 | 2.93 | 1.77 | 6.32 | |
| 10# | 61.20 | 0.41 | 0.08 | 0.09 | 3.22 | 2.51 | 6.30 | |
| 11# | 60.19 | 0.29 | 0.48 | 0.20 | 2.67 | 1.14 | 9.08 | |
| 12# | 57.21 | 1.29 | 0.00 | 0.10 | 5.62 | 1.37 | 11.21 | |
| Typical Indian iron ores | 13# | 63.32 | 1.72 | 0.05 | 0.16 | 2.84 | 2.32 | 3.71 |
| 14# | 58.21 | 0.27 | 0.09 | 0.09 | 5.51 | 3.49 | 5.93 | |
| 15# | 62.80 | 1.54 | 0.29 | 0.34 | 4.79 | 2.64 | 2.19 | |
| Typical South Africa iron ores | 16# | 64.33 | 0.29 | 0.19 | 0.24 | 4.84 | 1.60 | 0.90 |
| 17# | 63.06 | 0.24 | 0.10 | 0.04 | 6.02 | 2.04 | 1.38 | |
| 18# | 65.17 | 0.29 | 0.15 | 0.04 | 4.26 | 1.15 | 0.63 | |
| Typical Canadian iron ores | 19# | 66.8 | 12.54 | 0.53 | 0.80 | 4.02 | 0.10 | 0.34 |
| Typical domestic iron ores | 20# | 54.84 | 2.45 | 0.47 | 0.31 | 13.64 | 1.68 | 1.05 |
| 21# | 63.46 | 22.42 | 1.19 | 0.82 | 5.97 | 0.89 | 1.61 | |
| 22# | 60.07 | 18.11 | 1.33 | 0.93 | 7.00 | 2.16 | 3.02 | |
| 23# | 62.89 | 21.27 | 0.09 | 0.38 | 9.68 | 0.84 | 1.40 | |
| Material Types | Chemical Composition | LOI/% | ||||||
|---|---|---|---|---|---|---|---|---|
| TFe | FeO | CaO | MgO | SiO2 | Al2O3 | S | ||
| Limestone | - | - | 50.66 | 2.28 | 1.49 | - | - | 40.72 |
| Dolomite | - | - | 32.64 | 19.83 | 0.71 | - | - | 46.47 |
| Quicklime | - | - | 75.33 | 3.60 | 3.58 | - | 0.17 | 17.03 |
| Coke breeze | 4.11 | - | 1.54 | 0.28 | 2.63 | 1.88 | - | 89.16 |
| Return fines | 55.46 | 10.21 | 9.42 | 2.02 | 5.03 | 4.30 | 0.58 | 0.21 |
| SiO2/% | Ca/% | Si/% | Al/% | Fe/% |
|---|---|---|---|---|
| 4.00 | 23.44 | 16.64 | 2.89 | 11.39 |
| 5.32 | 26.71 | 18.15 | 3.24 | 9.46 |
| 6.00 | 27.06 | 18.80 | 2.52 | 11.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Z.; Zhao, Y.; Gan, M.; Fan, X.; Chen, X.; Hu, L. Microstructure and Minerals Evolution of Iron Ore Sinter: Influence of SiO2 and Al2O3. Minerals 2019, 9, 449. https://doi.org/10.3390/min9070449
Ji Z, Zhao Y, Gan M, Fan X, Chen X, Hu L. Microstructure and Minerals Evolution of Iron Ore Sinter: Influence of SiO2 and Al2O3. Minerals. 2019; 9(7):449. https://doi.org/10.3390/min9070449
Chicago/Turabian StyleJi, Zhiyun, Yuanjie Zhao, Min Gan, Xiaohui Fan, Xuling Chen, and Lin Hu. 2019. "Microstructure and Minerals Evolution of Iron Ore Sinter: Influence of SiO2 and Al2O3" Minerals 9, no. 7: 449. https://doi.org/10.3390/min9070449




