Geochemistry of Brine and Paleoclimate Reconstruction during Sedimentation of Messinian Salt in the Tuz Gölü Basin (Türkiye): Insights from the Study of Fluid Inclusions
Abstract
1. Introduction
- (1)
- the direction and causes of changes in the chemical composition of brines of the Tuz Gölü Basin,
- (2)
- the stability of the vertical zoning of the water column of the salt basin depending on climatic, geological, and hydrographic conditions,
- (3)
- features of the paleoclimate of the region.
2. Geological Setting

3. Materials and Methods
4. Results
4.1. Sedimentary Textures of Halite
4.2. Chemical Composition of Primary Fluid Inclusions in Halite
| Sample | Location, Well-Depth of Sampling, m | Content, g/L | rNa/rCI | |||
|---|---|---|---|---|---|---|
| NaCl | KCl | MgSO4 | MgCl2 | |||
| 14 | TG5-677.9 | 270.4 (106.4 + 164.0) | 0.2 (0.1 + 0.1) | 9.9 (2.0 + 7.9) | 29.4 (7.5 + 21.9) | 0.88 |
| 13 | TG5-889.8 | Fluid inclusions are smaller than 40 µm | ||||
| 12 | TG6-484.9 | 266.9 (105.0 + 161.9) | 2.7 (1.4 + 1.3) | 13.8 (2.8 + 11.0) | 30.6 (7.8 + 22.8) | 0.87 |
| 11 | TG6-618.4 | 289.9 (114.1 + 175.8) | 2.5 (1.3 + 1.2) | 22.8 (4.6 + 18.2) | 12.1 (3.1 + 9.0) | 0.95 |
| 10 | TG6-679.5 | Sample without primary fluid inclusions | ||||
| 9 | TG6-728.5 | 276.8 (108.9 + 167.9) | 1.9 (1.0 + 0.9) | 5.6 (1.1 + 4.5) | 23.1 (5.9 + 17.2) | 0.90 |
| 8 | TG6-730.0 | 295.8 (116.4 + 179.4) | 2.4 (1.25 + 1.1) | 22.4 (4.5 + 17.9) | 7.4 (1.9 + 5.5) | 0.97 |
| 7 | TG6-762.7 | 258.7 (101.8 + 156.9) | 1.7 (0.9 + 0.8) | 10.6 (2.1 + 8.45) | 38.0 (9.7 + 28.3) | 0.85 |
| 6 | TG7-360.3 | 283.4 (111.5 + 171.9) | 3.4 (1.75 + 1.6) | 9.1 (1.8 + 7.3) | 16.8 (4.3 + 12.5) | 0.92 |
| 5 | TG7-422.0 | 270.6 (106.5 + 164.1) | 2.7 (1.4 + 1.3) | 13.0 (2.6 + 10.35) | 27.7 (7.05 + 20.6) | 0.89 |
| 4 | TG7-450.0 | 279.1 (109.8 + 169.25) | 1.8 (0.95 + 0.85) | 8.4 (1.7 + 6.7) | 21.4 (5.45 + 15.9) | 0.91 |
| 3 | TG7-455.0 | 275.3 (108.3 + 167.0) | 1.7 (0.9 + 0.8) | 20.3 (4.1 + 16.2) | 24.5 (6.25 + 18.25) | 0.90 |
| 2 | TG7-518.6 | 280.1 (110.2 + 169.9) | 0.3 (0.15 + 0.1) | 5.9 (1.2 + 4.7) | 21.5 (5.5 + 16.0) | 0.91 |
| 1 | TG7-562.9 | 245.5 (96.6 + 148.9) | 4.5 (2.35 + 2.1) | 10.0 (2.0 + 8.0) | 47.0 (12.0 + 35.0) | 0.80 |
| Eocene seawater at the stage of halite crystallization, after [37] | ||||||
| IX | Navarra, Spain | 122.8 (48.3 + 74.5) | 31.3 (16.4 + 14.9) | 15.7 (3.2 + 12.5) | 129.7 (33.1 + 96.6) | 0.40 |
| Badenian seawater at the stage of halite crystallization, after [38] | ||||||
| VIII | Carpathian area | 211.9 (83.4 + 128.5) | 15.4 (8.1 + 7.3) | 22.1 (4.5 + 17.6) | 67.4 (17.2 + 50.2) | 0.73 |
| Messinian seawater at the stage of halite crystallization, after [37,39,40,41] | ||||||
| VII | Red Sea Basin | 167.3 (63.8 + 98.3) | 29.2 (15.3 + 13.9) | 57.5 (11.6 + 45.9) | 99.1 (25.3 + 73.8) | 0.60 |
| VI | 212.6 (81.6 + 125.7) | 15.3 (8.0 + 7.3) | 31.3 (6.3 + 25.0) | 71.3 (18.2 + 53.1) | 0.71 | |
| V | Caltanissetta, Sicily | 101.0 (48.3 + 74.4) | 34.1 (17.9 + 16.2) | 65.2 (13.2 + 52.0) | 171.6 (43.8 + 127.8) | 0.49 |
| IV | Lorca, Spain | 186.4 (71.0 + 109.4) | 25.2 (13.2 + 12.0) | 50.9 (10.3 + 40.6) | 86,7 (22.1 + 64.6) | 0.65 |
| Modern seawater at the stage of halite crystallization, after [35,36] | ||||||
| III | Bahamian Islands (W46) | 255.9 (103.0 + 152.9) | 7.4 (3.9 + 3.5) | 22.1 (4.5 + 17.6) | 31.7 (8.1 + 23.6) | 0.90 |
| II | Bahamian Islands (W33) | 215.2 (84.2 + 131.0) | 16.6 (8.7 + 7.9) | 47.6 (9.7 + 38.2) | 71.3 (18.2 + 53.1) | 0.72 |
| I | Black Sea | 262.5 (104.1 + 158.4) | 6.3 (3.3 + 3.0) | 26.3 (5.3 + 21.0) | 40.0 (10.2 + 29.8) | 0.90 |
| Sample | Location, Well-Depth of Sampling, m | Janecke Unit, mol % (for Figure 7) | Janecke Unit, mol % (for Figures 8 and 11) | |||||
|---|---|---|---|---|---|---|---|---|
| 2K | Mg | SO4 | Mg | 2Na | SO4 | 2Cl | ||
| 14 | TG5-677.9 | 0.3 | 82.4 | 17.3 | 70.3 | 29.7 | 9.5 | 90.5 |
| 13 | TG5-889.8 | Fluid inclusions smaller than 40 µm | ||||||
| 12 | TG6-484.9 | 3.1 | 76.7 | 20.2 | 65.3 | 34.7 | 11.6 | 88.4 |
| 11 | TG6-618.4 | 3.2 | 60.6 | 36.2 | 45.5 | 54.5 | 23.0 | 77.0 |
| 10 | TG6-679.5 | Samples without primary fluid inclusions | ||||||
| 9 | TG6-728.5 | 3.7 | 82.8 | 13.5 | 75.1 | 24.9 | 7.6 | 92.4 |
| 8 | TG6-730.0 | 3.4 | 56.6 | 40.0 | 41.3 | 58.7 | 26.1 | 73.9 |
| 7 | TG6-762.7 | 2.0 | 83.0 | 15.0 | 73.7 | 26.3 | 8.3 | 91.7 |
| 6 | TG6-360.3 | 6.4 | 71.8 | 21.8 | 62.3 | 37.7 | 13.1 | 86.9 |
| 5 | TG7-422.0 | 3.4 | 76.0 | 20.6 | 64.7 | 35.3 | 11.9 | 88.1 |
| 4 | TG7-450.0 | 3.2 | 78.2 | 18.6 | 67.9 | 32.1 | 10.6 | 89.4 |
| 3 | TG7-455.0 | 1.9 | 70.3 | 27.8 | 60.9 | 39.1 | 14.0 | 86.0 |
| 2 | TG7-518.6 | 0.4 | 84.6 | 15.0 | 73.3 | 26.7 | 8.1 | 91.9 |
| 1 | TG7-562.9 | 4.4 | 83.6 | 12.1 | 77.7 | 22.3 | 6.7 | 93.3 |
| Eocene seawater at the stage of halite crystallization, after [37] | ||||||||
| IX | Navarra, Spain (37 Ma) | 11.4 | 81.5 | 7.1 | 85.0 | 15.0 | 4.2 | 95.8 |
| Badenian seawater at the stage of halite crystallization, after [38] | ||||||||
| VIII | Carpathian area (13.8 Ma) | 8.8 | 75.7 | 15.5 | 71.0 | 29.0 | 9.3 | 90.7 |
| Messinian seawater at the stage of halite crystallization, after [37,39,40,41] | ||||||||
| VII | Red Sea Basin (5.0–6.0 Ma) | 8.9 | 69.3 | 21.8 | 61.3 | 38.7 | 13.6 | 50.7 |
| VI | 7.5 | 73.5 | 19.0 | 65.9 | 34.1 | 11.4 | 88.6 | |
| V | Caltanissetta, Sicily (5.6–6.0 Ma) | 7.5 | 74.8 | 17.7 | 68.4 | 31.6 | 10.3 | 89.7 |
| IV | Lorca, Spain (7.6 Ma) | 8.7 | 69.3 | 22.0 | 61.1 | 38.9 | 13.7 | 86.3 |
| Modern seawater at the stage of halite crystallization, after [35,36] | ||||||||
| III | Bahamian Islands (W46) | 6.6 | 69.0 | 24.4 | 58.7 | 41.3 | 15.1 | 84.9 |
| II | Bahamian Islands (W33) | 6.7 | 69.3 | 24.0 | 59.2 | 40.8 | 14.8 | 85.2 |
| I | Black Sea | 4.7 | 71.0 | 24.3 | 59.2 | 40.8 | 14.6 | 85.4 |
4.3. Sulfate Minerals in Rock Salt
4.4. Homogenization Temperature of Liquid Inclusions
4.5. Bromine Content in Halite
5. Interpretation and Discussion
5.1. The Chemical Composition of Messinian Sedimentary Brines from the Tuz Gölü Basin
5.2. Peculiarities of the Formation of Sulfate Minerals in the Studied Samples
5.2.1. Calcium Sulfate Minerals
5.2.2. Sodium Calcium Sulfate
CaSO4 + 2H2O xMg(OH)2 × yMgCO3
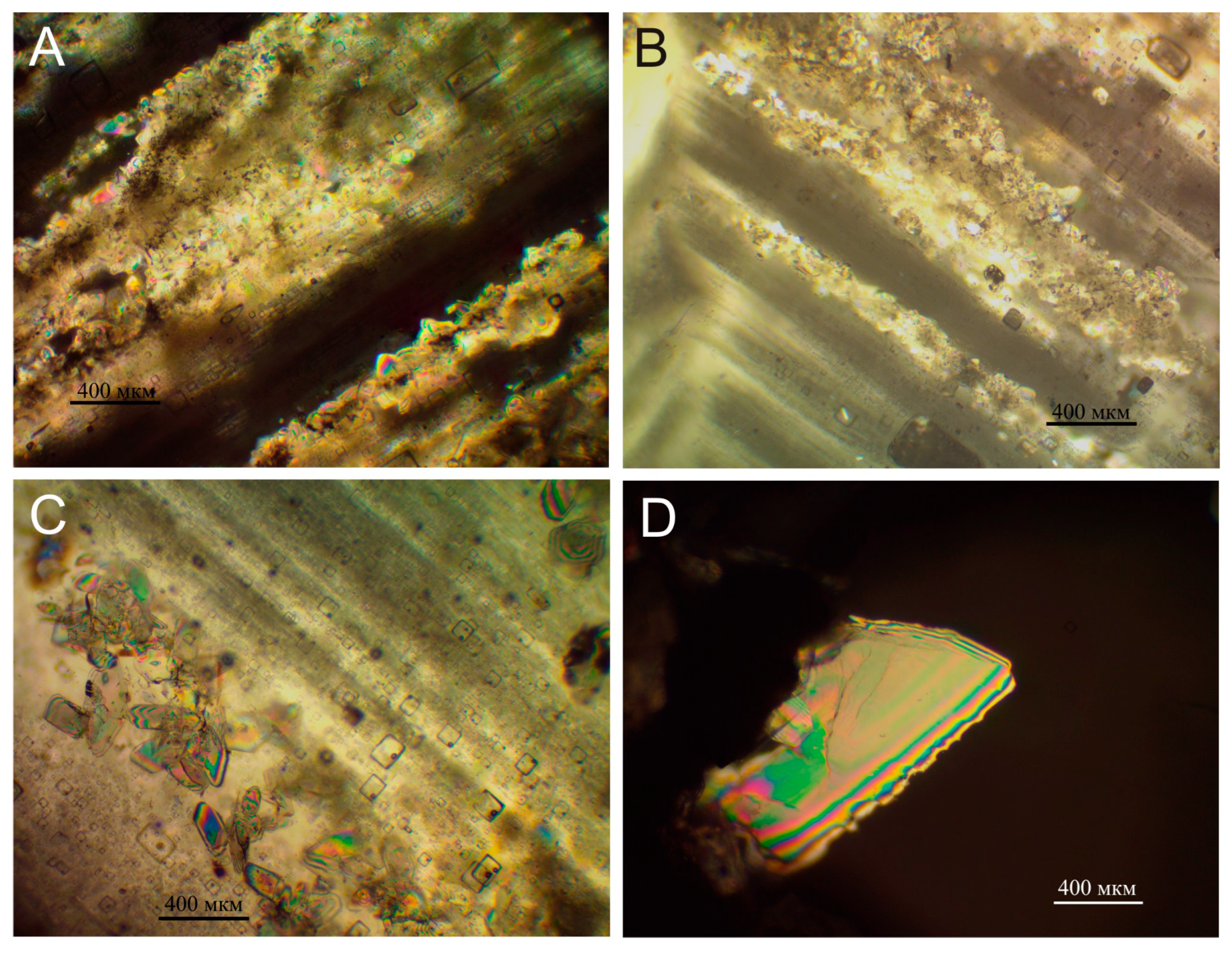

5.2.3. Sodium Sulfate
5.3. Paleoclimatic Characteristics
5.4. Peculiarities of Bromine Content in Tuz Gölü Halite
6. Conclusions
- Using an ultramicrochemical method, it was determined that the halogenesis in the Tuz Gölü Basin in Messinian corresponds to the sulfate type and the magnesium sulfate subtype. Compared to the Na–K–Mg–Cl–SO4 marine brines from the halite deposition stage, the Tuz Gölü has slightly higher [Na+] and lower [K+] concentrations. The basin brine salinity during salt accumulation changed twice based on the [Mg2+] and [K+] concentration data. The concentration of [SO42−] varies over a wider range than other ions. Similar to the concentration of [Mg2+], it sometimes reaches typical values of sea brines at the beginning of halite precipitation. A significant decrease in the concentration of [SO42−] in sedimentation brines from 18.2 to 4.5 g/L is caused by physicochemical processes in the near-surface and bottom layers of the basin water during the suspension of halite precipitation. During these periods, an intensive inflow of Ca(HCO3)2 into the sedimentary basin occurred, and glauberite layers formed. Under certain physicochemical conditions, crystallization of glauberite from newly deposited finely dispersed gypsum co-occurred with halite sedimentation.
- It was established via XRD and immersion methods that within fluid inclusions in halite, allogeneic calcium sulfate crystals are represented by gypsum, bassanite, and anhydrite. Sulfate minerals in halite crystals are glauberite, anhydrite, and thenardite. During halite deposition at reduced concentrations of [SO42−], the newly formed gypsum did not transform into glauberite in zoned halite and thin layers with terrigenous material. However, the high content of sulfate ions was also not a sufficient condition for such a transformation.
- By thermometric studies, fluctuations in daily and seasonal air temperatures in the Tuz Gölü basin were determined. Climatic indicators during the Messinian halite forming/precipitation in the region revealed rapid changes in daytime air temperature from 15.7–22.1 °C in spring or cool summer–autumn, 20.6–35.0 °C in late spring–early summer, and up to 15.6–49.1 °C in summer. During some periods, Tuz Gölü halite crystallized at extremely high temperatures of 61.8–73.5 °C. The “greenhouse effect” in the basin was created for a short time but periodically renewed due to the influx of “fresh” waters.
- The study of halite homogenization temperatures indicates that the depth of the basin was shallow during sedimentation, and the study of bromine in halite reveals the variation of the basin’s paleotectonic conditions and the partial or temporary separation of different sections of the broad basin from each other.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petrichenko, O.I. Physico-Chemical Conditions of Sedimentation in Ancient Salt-Bearing Basins; Naukova Dumka: Kyiv, Ukraine, 1988; 128p. (In Russian) [Google Scholar]
- Galamay, A.R.; Meng, F.; Bukowski, K.; Lyubchak, A.; Zhang, Y.; Ni, P. Calculation of salt basin depth using fluid inclusions in halite from the Ordovician Ordos Basin in China. Geol. Q. 2019, 63, 619–628. [Google Scholar] [CrossRef]
- Galamay, A.R.; Bukowski, K.; Sydor, D.V.; Meng, F. The Ultramicrochemical Analyses (UMCA) of Fluid Inclusions in Halite and Experimental Research to Improve the Accuracy of Measurement. Minerals 2020, 10, 823. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Y.; Wang, M.; Hu, Y.; Liu, C.; Zhang, H. Estimation of the Ambient Temperatures during the Crystallization of Halite in the Oligocene Salt Deposit in the Shulu Sag, Bohaiwan Basin, China. Minerals 2022, 12, 410. [Google Scholar] [CrossRef]
- Karakaya, M.C.; Bozdağ, A.; Karakaya, N. Elemental and C, O and Mg isotope geochemistry of middle-late Miocene carbonates from the Tuz Gölü Basin (Central Anatolia, Turkey): Evidence for Mediterranean incursions. J. Asian Earth Sci. 2021, 221, 1–15. [Google Scholar] [CrossRef]
- Palmer, M.R.; Helvacı, C.; Fallick, A.E. Sulphur, sulfate oxygen and strontium isotope composition of Cenozoic Turkish evaporites. Chem. Geol. 2004, 209, 341–356. [Google Scholar] [CrossRef]
- Karakaya, M.C.; Bozdağ, A.; Ercan, H.Ü.; Karakaya, N. The origin of Miocene evaporites in the Tuz Gölü basin (Central Anatolia, Turkey): Implications from strontium, sulfur and oxygen isotopic compositions of the Ca-Sulfate minerals. Appl. Geochem. 2020, 120, 1–14. [Google Scholar] [CrossRef]
- Ercan, H.Ü.; Karakaya, M.C.; Bozdağ, A.; Karakaya, N.; Delikan, A. Origin and evolution of halite based on stable isotopes (δ37Cl, δ81Br, δ11B and δ7Li) and trace elements in Tuz Gölü Basin, Turkey. Appl. Geochem. 2019, 105, 17–30. [Google Scholar] [CrossRef]
- Karakaya, M.C.; Bozdağ, A.; Ercan, H.Ü.; Karakaya, N.; Delikan, A. Origin of Miocene halite from Tuz Gölü basin in Central Anatolia, Turkey: Evidences from the pure halite and fluid inclusion geochemistry. J. Geochem. Explor. 2019, 202, 1–12. [Google Scholar] [CrossRef]
- Görür, N.; Tüysüz, O.; Şengör, A.M.C. Tectonic evolution of the Central Anatolian basins. Int. Geol. Rev. 1998, 40, 831–850. [Google Scholar] [CrossRef]
- Clark, M.; Robertson, A. Uppermost Cretaceous–Lower Tertiary Ulukışla Basin, South-Central Turkey: Sedimentary evolution of part of a unified basin complex within an evolving Neotethyan suture zone. Sediment. Geol. 2005, 173, 15–51. [Google Scholar] [CrossRef]
- Görür, N.; Derman, A.S. Stratigraphic and Tectonic Analysis of the Tuz Gölü-Haymana Basin; Turkish Petroleum Corporation Report 1514; TPAO: Ankara, Turkey, 1978. [Google Scholar]
- Demir, E.; Varol, E. Origin and palaeodepositional environment of evaporites in the Bala sub-basin, Central Anatolia, Turkiye. Int. Geol. Rev. 2022. [Google Scholar] [CrossRef]
- Görür, N.; Oktay, F.Y.; Seymen, İ.; Şengör, A.M.C. Paleotectonic evolution of the Tuzgölü basin complex, Central Turkey: Sedimentary record of a Neo-Tethyan closure. J. Geol. Soc. Lond. Spec. Publ. 1984, 17, 467–482. [Google Scholar] [CrossRef]
- Dönmez, M.; Bilgin, Z.R.; Akçay, A.E.; Kara, H.; Yergök, A.F.; Esentürk, K. 1:100 000 Ölçekli Türkiye Jeoloji Haritaları Kırşehir- İ30 Paftası; Geological Maps of Turkey; no. 90, scale 1:100.000; MTA Publications: Kırşehir, Turkey, 2008; 30p. [Google Scholar]
- Oktay, F.Y. Stratigraphy and geological evolution of Ulukışla and its surroundings. Bull. Geol. Soc. Turk. 1982, 25, 15–23. [Google Scholar]
- Kadınkız, G.; Pekgöz, M.; Karakaş, M.; Murat, A. Sodium sulfate (Glauberite-Bloedite)-Halite association in the tertiary (upper Miocene-Pliocene Katrandedetepe formation, Ereğli-Bor basin, Turkey. Bull. Mineral Res. Explorat. 2017, 154, 135–156. [Google Scholar]
- Gürbüz, E.; Seyitoğlu, G.; Güney, A. Late Cenozoic tectono-sedimentary evolution of the Ulukıs¸la Basin: Progressive basin development in sought-central Turkey. Int. J. Earth Sci. 2020, 109, 345–371. [Google Scholar] [CrossRef]
- Akgün, F.; Kayseri-Özer, M.S.; Tekin, E.; Varol, B.; Şen, Ş.; Herece, E.; Gündoğan, İ.; Sözeri, K.; Us, M.S. Late Eocene to Late Miocene palaeoecological and palaeoenvironmental dynamics of the Ereğli–Ulukışla Basin (Southern Central Anatolia). Geol. J. 2021, 56, 673–703. [Google Scholar] [CrossRef]
- Bassant, P.; Van Buchem, F.S.P.; Strasser, A.; Gorür, N. The stratigraphic architecture and evolution of the Burdigalian carbonate-siliciclastic sedimentary systems of the Mut Basin, Turkey. Sed. Geol. 2005, 173, 187–232. [Google Scholar] [CrossRef]
- Koç, A.; Kaymakci, N.; Van Hinsbergen, D.J.J.; Kuiper, K.F. Miocene tectonic history of the Central Tauride intermontane basins and the paleogeographic evolution of the Central Anatolian Plateau. Glob. Planet. Change 2017, 158, 83–102. [Google Scholar] [CrossRef]
- Schildgen, T.F.; Cosentino, D.; Bookhagen, B.; Niedermann, S.; Yildirim, C.; Echtler, H.P.; Wittmann, H.; Strecker, M.R. Multi-phase uplift of the southern margin of the Central Anatolian plateau: A record of tectonic and upper mantle processes. Earth Planet. Sci. Lett. 2012, 317–318, 85–95. [Google Scholar] [CrossRef]
- Schildgen, T.F.; Cosentino, D.; Caruso, A.; Buchwaldt, R.; Yıldırım, C.; Bowring, S.A.; Rojay, B.; Echtler, H.; Strecker, M.R. Surface expression of eastern Mediterranean slab dynamics: Neogene topographic and structural evolution of the southwest margin of the Central Anatolian Plateau, Turkey. Tectonics 2012, 31, TC2005. [Google Scholar] [CrossRef]
- Cipollari, P.; Halàsovà, E.; Gürbüz, K.; Cosentino, D. Middle-Upper Miocene palaeogeography of southern Turkey: Insights from stratigraphy and calcareous nannofossil biochronology of the Olukpınar and Bas¸yayla sections (Mut-Ermenek Basin). Turk. J. Earth Sci. 2013, 22, 820–838. [Google Scholar] [CrossRef]
- McNab, F.; Ball, P.W.; Hoggard, M.J.; White, N.J. Neogene uplift and magmatism of Anatolia: Insights from drainage analysis and basaltic geochemistry. Geochem. Geophys. Geosyst. 2018, 19, 175–213. [Google Scholar] [CrossRef]
- Şafak, Ü.; Kelling, G.; Gökçen, N.S.; Gürbüz, K. The mid-Cenozoic succession and evolution of the Mut basin, southern Turkey, and its regional significance. Sediment. Geol. 2005, 173, 121–150. [Google Scholar] [CrossRef]
- Görür, N.; Okay, A.; Şengör, A.M.C.; Tüysüz, O.; Sakınç, M.; Yiğitbaş, E.; Akkök, R.; Barka, A.; Oktay, F.Y.; Sarıca, N.; et al. Triassic to Miocene Paleogeographic Atlas of Turkey; General Directorate of Mineral Research and Exploration: Ankara, Turkey, 1998; 43p.
- Petrichenko, O.I. Methods of Study of Inclusions in Minerals of Saline Deposits; Naukova Dumka: Kyiv, Ukraine, 1973; 90p. (In Russian) [Google Scholar]
- Emmons, R.C.; Gates, R.M. The use of Becke line colors in refractive index determination. Amer. Mineral. 1948, 33, 9–10, 612. [Google Scholar]
- Winchell, A.N.; Emmons, R.C. Some Methods for Determining Refractive Indices. Amer. Mineral. 1926, 11, 115–118. [Google Scholar]
- Saylor, C.P. Accuracy of microscopical methods for determining refractive index by immersion. J. Res. Nat. Bur. Stand. 1935, 15, 277–294. [Google Scholar] [CrossRef]
- Döbelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Galamay, A.R.; Bukowski, K.; Zinchuk, I.M.; Meng, F. The Temperature of Halite Crystallization in the Badenian Saline Basins, in the Context of Paleoclimate Reconstruction of the Carpathian Area. Minerals 2021, 11, 831. [Google Scholar] [CrossRef]
- Galamay, A.R.; Sidor, D.; Lyubchak, O. Peculiarities of the appearance of the gas phase in single-phase liquid inclusions in halite (to determine the temperature of its crystallization). VIII Sciences. In Proceedings of the Mineralogy: Present and Future, Lviv-Chinadiyeve, Lviv, Ukraine, 11–14 September 2014; pp. 34–36. (In Ukrainian). [Google Scholar]
- McCaffrey, M.A.; Lazar, B.; Holland, H.D. The evaporation path of seawater and the coprecipitation of Br and K with halite. J. Sed. Petrol. 1987, 5, 928–937. [Google Scholar]
- Valiashko, M.G. The Principle of Forming of Salt Deposits; M.G.U.: Moscow, Russia, 1962; 396p. (In Russian) [Google Scholar]
- Ayora, C.; Garcia-Veigas, J.; Pueyo, J.J. The chemical and hydrological evolution of an ancient potash-forming evaporite basin as constrained by mineral sequence, fluid inclusion composition, and numerical simulation. Geochim. Cosmochim. Acta 1994, 58, 3379–3394. [Google Scholar] [CrossRef]
- Galamay, A.R.; Bukowski, K. Chemical composition of Badenian brines from primary fluid inclusions in halite (Transcarpathian Basin, Ukraine). Geologia Kwart. A.G.H. 2011, 37, 245–267, (In Polish with English Summary). [Google Scholar]
- Kovalevych, V.M.; Jarmołowicz-Szulc, K.; Peryt, T.M.; Poberegski, A.V. Messinian chevron halite from the Red Sea (DSDP Sites 225 and 227): Fluid inclusion study. Neues Jahrbuch für Mineralogie-Monatshefte 1997, 10, 433–450. [Google Scholar] [CrossRef]
- Zimmermann, H. Tertiary seawater chemistry—Implications from inclusions in marine halite. Am. J. Sci. 2000, 300, 723–767. [Google Scholar] [CrossRef]
- Garcia-Veigas, J.; Orti, F.; Rosell, L.; Ayora, C.; Rouchy, J.M.; Lugli, S. The Messinian salt of the Mediterranean: Geochemical study of the salt from the Central Sicily Basin and comparison with the Lorca Basin (Spain). Bull. Soc. Geol. Fr. 1995, 166, 699–710. [Google Scholar]
- Dellwig, L.F. Origin of the salina salt of Michigan. Sediment. Res. 1955, 25, 83–110. [Google Scholar]
- Kovalevich, V.M. On genetic informativity of inclusions in halite. Lithol. Miner. 1980, 1, 147–152. (In Russian) [Google Scholar]
- Sulin, V.A. Hydrogeology of Oil Fields; Gostoptekhizdat: Leningrad, Russian, 1948; 480p. (In Russian) [Google Scholar]
- Stankevich, E.F.; Batalin, Y.V.; Chaikin, V.G. On the differences between marine and continental halogen deposits. In Problems of Marine and Continental Halogenesis; Nauka: Novosibirsk, Russia, 1991. (In Russian) [Google Scholar]
- Sonnenfeld, P. Brines and Evaporites; Academic Press Inc.: Orlando, FL, USA, 1984; 613p. [Google Scholar]
- Buneev, A.N. Fundamentals of Hydrochemistry of Mineral Waters of Sedimentary Deposits; Gosgeoltekhizdat: Moscow, Russian, 1956; 226p. (In Russian) [Google Scholar]
- Lebedev, V.I. On the reasons for the absence of potassium salts among the halogen sediments of continental lakes. In Proceedings of the VNIIG Galurgii; Goskhimizdat: Leningrad, Russian, 1959; Volume 35, pp. 292–295. (In Russian) [Google Scholar]
- Timofeeff, M.N.; Lowenstein, T.K.; Martins da Silva, M.A.; Harris, N.B. Secular variation in the major-ion chemistry of seawater: Evidence from fluid inclusions in Cretaceous halites. Geochim. Cosmochim. Acta 2006, 70, 1977–1994. [Google Scholar] [CrossRef]
- Galamay, A.R.; Meng, F. Chemical composition of the southeastern part of the Cretaceous Sakon Nakhon salt-bearing basin of Laos in the context of the evolution of the composition of oceanic water. In Proceedings of the MinGeoIntegration XXI, Kyiv, Ukraine, 23–25 September 2020; pp. 20–24. (In Ukrainian). [Google Scholar]
- Kovalevich, V.M. Halogenesis and Chemical Evolution of Ocean in the Phanerozoic; Naukova Dumka: Kyiv, Ukraine, 1990; p. 154. (In Russian) [Google Scholar]
- Galamay, A.R.; Meng, F.; Bukowski, K. Sulphur isotopes in anhydrite from Badenian (Middle Miocene) salts of the Hrynivka area (Ukrainian Carpathian Foredeep). Geol. Q. 2014, 58, 429–438. [Google Scholar]
- Gündogan, I.; Helvaci, C. Geology, hydrochemistry, mineralogy and economic potential of the Bolluk lake (Cihanbeyli-Konya) and the adjacent area. J. Earth Sci. 1996, 5, 91–104. [Google Scholar] [CrossRef]
- Salvany, J.M.; Veigas, J.G.; Orti, F. Glauberite-halite association of the Zaragoza Gypsum Formation (Lower Miocene, Ebro Basin, NE Spain). Sedimentology 2007, 54, 443–467. [Google Scholar] [CrossRef]
- Kashkarov, O.D. Salt planting in salt lakes. In Materials for the Study of Areas of Modern and Fossil Salt Accumulation, Proceedings of the VNIIG Galurgii; Goskhimizdat: Leningrad, Russian, 1956; Volume 32, pp. 3–33. (In Russian) [Google Scholar]
- Zen, E.A. Solubility measurements in the system CaSO4–NaCl–H2O at 35 °C, 50 °C and 70 °C and one atmospheric pressure. J. Petrol. 1965, 6, 124–164. [Google Scholar] [CrossRef]
- Petrichenko, O.I. Epigenesis of Evaporites; Naukova Dumka: Kyiv, Ukraine, 1989; 63p. (In Ukrainian) [Google Scholar]
- Galamay, A.R. Sulfate minerals in fluid inclusions in halite as indicators of conditions of saline sedimentation. Geol. Geochem. Combust. Miner. 2002, 4, 77–82, (In Ukrainian with English Summary). [Google Scholar]
- Vakhrameeva, V.A. On the mineralogy and petrography of salt deposits in the Karabogaz-Gol Bay. In Materials for the STUDY of Areas of Modern and Fossil Salt Accumulation, Proceedings of the VNIIG Galurgii; Goskhimizdat: Leningrad, Russian, 1956; Volume 32, pp. 67–86. (In Russian) [Google Scholar]
- Fridman, Y.D.; Zinoviev, A.A.; Bogdanovskaya, R.Z. On double sulfates of calcium and sodium. Tr. Inst. Chemistry. Kirg. FAN USSR 1953, 5, 35–48. (In Russian) [Google Scholar]
- Galamay, A.R. Influence of continental run-off on the composition of marine brines of Badenian salt basin central part (Ukrainian Carpathian Foredeep). Mineral. Zbirnyk 2012, 62, 228–235, (In Ukrainian with English summary). [Google Scholar]
- Valyashko, M.G.; Pelsh, G.K. Metamorphization of saturated sulfate solutions by calcium bicarbonate. Tr. VNIIG 1952, 23, 177–200. [Google Scholar]
- Charykova, M.V.; Kurilenko, W.; Charykov, N.A. Temperatures of formation of certain salts in sulfate-type brines. J. Appl. Chem. USSR 1992, 65, 1037–1040. [Google Scholar]
- Pavlyshyn, V.I.; Dyakiv, V.O.; Tsar, H.M.; Kitsmur, I.I. Ontogenic regularities of crystallization of mirabilite-thenardite aggregates from brine of the Forecarpathian potash deposits. Mineral. J. 2012, 2, 17–25. (In Ukrainian) [Google Scholar]
- Benison, K.C.; Goldstein, R.H. Permian paleoclimate data from fluid inclusions in halite. Chem. Geol. 1999, 154, 113–132. [Google Scholar] [CrossRef]
- Kovalevych, V.M. Physicochemical Conditions of Salt Formation in the Stebnik Potash Deposit; Naukova Dumka: Kyiv, Ukraine, 1978; pp. 1–100. (In Russian) [Google Scholar]
- Acros, D.; Ayora, C. The use of fluid inclusions in halite as environmental thermometer: An experimental study. In Proceedings of the European Current Research on Fluid Inclusions, Nancy, France, 1–4 July 1997; Boiron, M.C., Pironon, J., Eds.; Université de Lorraine—CNRS: Nancy, France, 1997; pp. 10–11. [Google Scholar]
- Roberts, S.M.; Spencer, R.J. Paleotemperatures preserved in fluid inclusions in halite. Geochim. Cosmochim. Acta 1995, 59, 3929–3942. [Google Scholar] [CrossRef]
- Satterfield, C.L.; Lowenstein, T.K.; Russell, H.V.; Rosenzweig, W.D. Paleobrine temperatures, chemistries, and paleoenvironments of Silurian Salina Formation F-1 Salt, Michigan Basin, U.S.A., from petrography and fluid inclusions in halite. J. Sediment. Res. 2005, 75, 534–546. [Google Scholar] [CrossRef]
- Lowenstein, T.; Li, J.; Brownet, C. Paleotemperatures from fluid inclusions in halite: Method verification and a 100,000-year paleotemperature record, Death Valley, CA. Chem. Geol. 1998, 150, 223–245. [Google Scholar] [CrossRef]
- Egorov, A.N.; Zilintikevich, S.S. Thermodynamic structure of a salt lake with a greenhouse effect. Meteorol. Hydrol. 1999, 4, 98–105. (In Russian) [Google Scholar]
- Galamay, A.R.; Bukowski, K.; Poberezhskyy, A.V.; Karoli, S.; Kovalevich, V.M. Origin of the Badenian salt from East Slovakian basin indicated by based on the analysis of fluid inclusions. Ann. Soc. Geol. Pol. 2004, 74, 267–276. [Google Scholar]
- Wang, J.; Lowenstein, T.K. Anomalously high cretaceous paleobrine temperatures: Hothouse, hydrothermal or solar heating? Minerals 2017, 7, 245. [Google Scholar] [CrossRef]
- Egorov, A.N. Greenhouse effect in saline lakes. Water Resour. 1991, 6, 31–37. (In Russian) [Google Scholar]
- Holser, W.T. Trace elements and isotopes in evaporites. Marine Minerals. Mineral. Soc. Am. Rev. Miner. 1979, 6, 295–346. [Google Scholar]
- Braitsch, O.; Herrmann, A.G. Zur Bromverteilung in salinaren Salzsystemen bei 25 °C. Die Naturwissenschaften 1962, 49, 346. [Google Scholar] [CrossRef]
- Holser, W.T.; Wardlaw, N.S.; Watson, D.W. Bromine in salt rocks: Extraordinarily low content in the Lower Elk Point salt, Canada. Geology of saline deposits. Proc. Hanover Symp. 1968, 69–75. [Google Scholar]
- Holser, W.T. Bromine geochemistry of some non-marine salt deposits in the southern Great Basin. Mineral. Soc. Am. Spec. Paper 1970, 3, 307–319. [Google Scholar]
- Galamay, A.R. Bromine content in the Badenian halite deposits of the Carpathian region as an indicator of their genesis and conditions of formation. Geol. Geochem. Combust. Miner. 2003, 3–4, 102–111, (In Ukrainian with English summary). [Google Scholar]
- Bukowski, K.; Czapowski, G.; Karoli, S.; Babel, M. Sedimentology and geochemistry of the Middle Miocene (Badenian) salt-bearing succession from East Slovakian Basin (Zbudza Formation). In Evaporites through Space and Time; Special Publications; Schreiber, B.C., Lugli, S., Babel, M., Eds.; Geological Society: London, UK, 2007; Volume 285, pp. 247–264. [Google Scholar]
- Galamay, A.R.; Sydor, D.V. Sources of salts of ancient evaporite basins (study of bromine content in halite of the Fore-Carpathian and Fore-Urals regions). In Proceedings of the Institute of Soil Science Conference Achievements and Prospects for the Development of Geological Science in Ukraine, Kyiv, Ukraine, 14–16 May 2019; Volume T.2, pp. 35–36. (In Ukrainian). [Google Scholar]

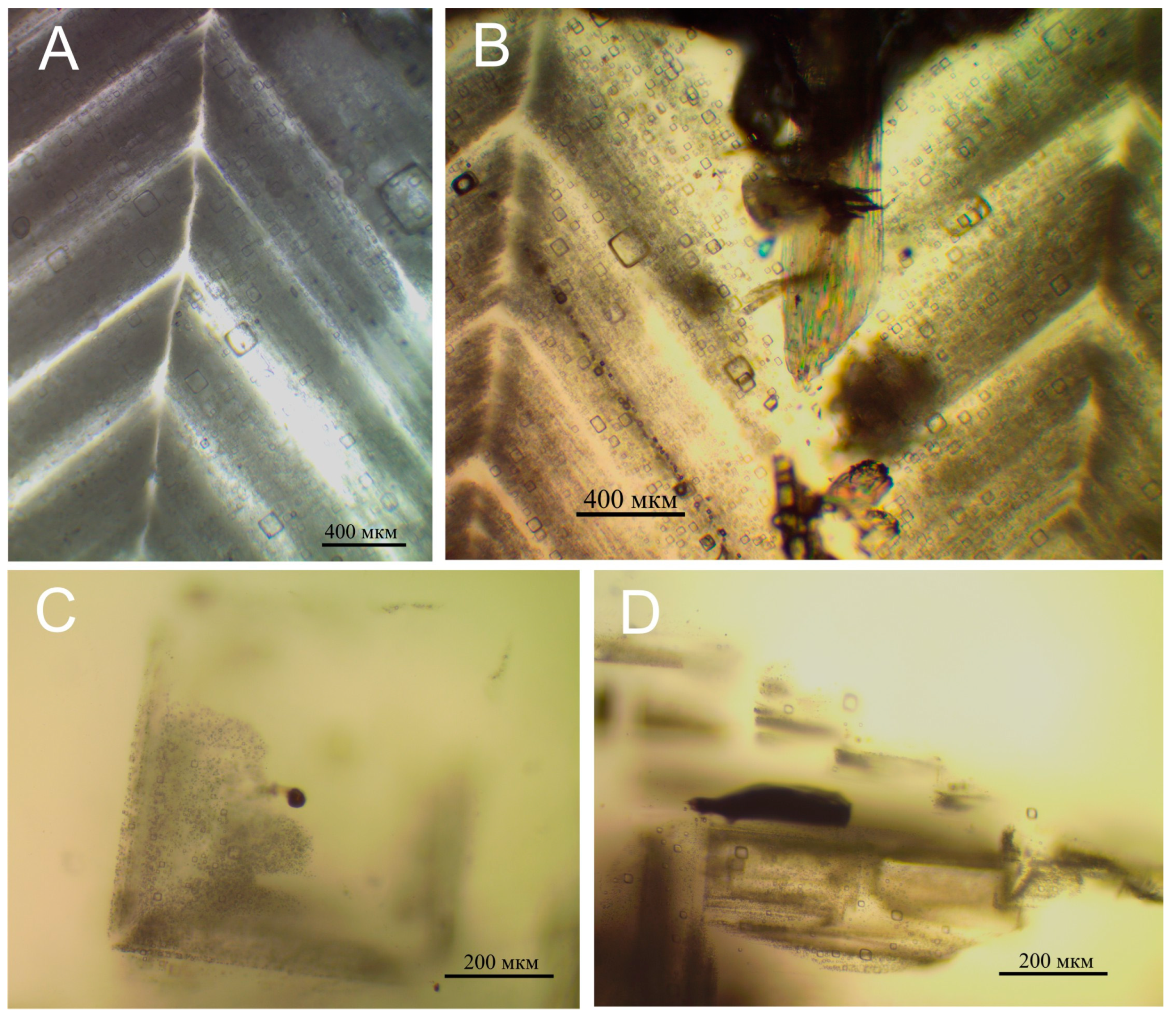






| Sample | The Number of Investigated Bands of Inclusions from Different Growth Zones of Halite | Homogenization Temperature, °C | Crystallization Temperature, °C | Remarks |
|---|---|---|---|---|
| 12 | 4 | 14.5; 16.0; 16.8; 16.9; 18.5 15.7; 15.7; 16.6; 18.0; 18.6 14.3; 14.5; 17.8 13.3; 13.6; 14.0; 15.7 | 15.7–18.6 | The sample contains sedimentary textures with fluid inclusions of different phase compositions: 1. All fluid inclusions are gas–liquid 2. Gas–liquid fluid inclusions are only in certain zones |
| 3 | 17.3; 17.4; 21.3; 21.5 19.5; 21.5; 21.9 21.3; 21.5; 22.0; 22.1 | 21.5–22.1 | ||
| 1 | 53.6; 55.4; 64.3; 68.7; 72.4 | 72.4 * | ||
| 11 | 1 | 19.2; 20.0; 20.0; 20.6 | 20.6 | All sedimentary textures consist of single-phase fluid inclusions. Large gas–liquid inclusions occur in separate zones. |
| 1 | 20.0; 20.3; 23.9; 24.0; 24.3 | 24.3 | ||
| 1 | 24.2; 27.5; 28.0; 28.6; 28.6 | 28.6 | ||
| 2 | 30.9; 33.6; 33.9 30.6; 33.3; 35.0 | 33.9–35.0 * | ||
| 9 | 3 | 15.0; 15.1; 15.5; 16.7 14.1; 15.1; 15.5; 15.6 14.3; 14.5; 14.7; 17.6 | 15.6–17.6 | There are gas–liquid inclusions in the upper parts of sedimentary textures |
| 1 | 18.6; 19.5; 21.0; 21.1 | 21.1 | ||
| 1 | 22.5; 23.7; 25.0 | 25.0 | ||
| 1 | 24.3; 25.0; 25.5; 25.5 | 25.5 | ||
| 1 | 40.2; 46.9; 47.9; 49.0; 49.1 | 49.1 * | ||
| 1 | 68.1; 69.9; 70.4; 72.0; 73.0 | 73.0 * | ||
| 8 | 2 | 15.1; 17.3; 18.6 14.7; 15.0; 16.0; 17.0; 17.9 | 17.9–18.6 | The sample contains sedimentary textures with fluid inclusions of different phase compositions: 1. All fluid inclusions are gas–liquid 2. Gas–liquid fluid inclusions are only in certain zones 3. Large gas–liquid inclusions occur in separate zones 4. All fluid inclusions are single-phase |
| 3 | 18.4; 18.9; 18.9; 19.1; 19.6 19.7; 20.6; 20.7 19.9; 20.4; 20.4; 21.6 | 19.6–21.6 | ||
| 2 | 21.0; 21.7; 23.1 20.1; 20.9; 23.0; 23.3 | 21.3–23.3 | ||
| 1 | 26.3; 26.5; 26.6; 27.0; 27.0 | 27.0 | ||
| 1 | 33.5; 33.5; 33.7; 33.9 | 33.9 * | ||
| 1 | 40.8; 41.0; 42.0 | 42.0 * | ||
| 2 | 59.4; 60.4; 61.8; 62.7; 62.9 53.8; 54.2; 56.8; 57.7; 61.8 | 61.8–62.9 * | ||
| 3 | 60.0; 65.1; 65.7; 66.2; 66.3; 71.0 59.0; 70.0; 73.0; 73.5 69.7; 70.4; 70.6; 71.7 | 71.7–73.5 * | ||
| 7 | 1 | 12.7; 12.8; 14.0; 14.8; 16.6 | 16.6 | There are large gas–liquid fluid inclusions in the lower parts of sedimentation textures |
| 2 | 17.4; 17.5; 18.1; 20.5 17.0; 19.3; 19.7; 19.9; 20.3; 20.3 | 20.3–20.5 | ||
| 1 | 39.2; 40.8; 40.8; 42.3; 44.1 | 44.1 * |
| Sample | Well Depth of Sampling, m | Br Content, ppm |
|---|---|---|
| 14 | TG5-677.9 | 47.17 |
| 11 | TG6-618.4 | 19.00 |
| 9 | TG6-728.5 | 36.00 |
| 8 | TG6-730.0 | 15.07 |
| 7 | TG6-762.7 | 6.15 |
| 5 | TG7-422.0 | 15.10 |
| 4 | TG7-450.0 | 6.32 |
| 3 | TG7-455.0 | 2.92 |
| 2 | TG7-518.6 | 8.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galamay, A.R.; Karakaya, M.Ç.; Bukowski, K.; Karakaya, N.; Yaremchuk, Y. Geochemistry of Brine and Paleoclimate Reconstruction during Sedimentation of Messinian Salt in the Tuz Gölü Basin (Türkiye): Insights from the Study of Fluid Inclusions. Minerals 2023, 13, 171. https://doi.org/10.3390/min13020171
Galamay AR, Karakaya MÇ, Bukowski K, Karakaya N, Yaremchuk Y. Geochemistry of Brine and Paleoclimate Reconstruction during Sedimentation of Messinian Salt in the Tuz Gölü Basin (Türkiye): Insights from the Study of Fluid Inclusions. Minerals. 2023; 13(2):171. https://doi.org/10.3390/min13020171
Chicago/Turabian StyleGalamay, Anatoliy R., Muazzez Çelik Karakaya, Krzysztof Bukowski, Necati Karakaya, and Yaroslava Yaremchuk. 2023. "Geochemistry of Brine and Paleoclimate Reconstruction during Sedimentation of Messinian Salt in the Tuz Gölü Basin (Türkiye): Insights from the Study of Fluid Inclusions" Minerals 13, no. 2: 171. https://doi.org/10.3390/min13020171
APA StyleGalamay, A. R., Karakaya, M. Ç., Bukowski, K., Karakaya, N., & Yaremchuk, Y. (2023). Geochemistry of Brine and Paleoclimate Reconstruction during Sedimentation of Messinian Salt in the Tuz Gölü Basin (Türkiye): Insights from the Study of Fluid Inclusions. Minerals, 13(2), 171. https://doi.org/10.3390/min13020171









