2.1. Device Description
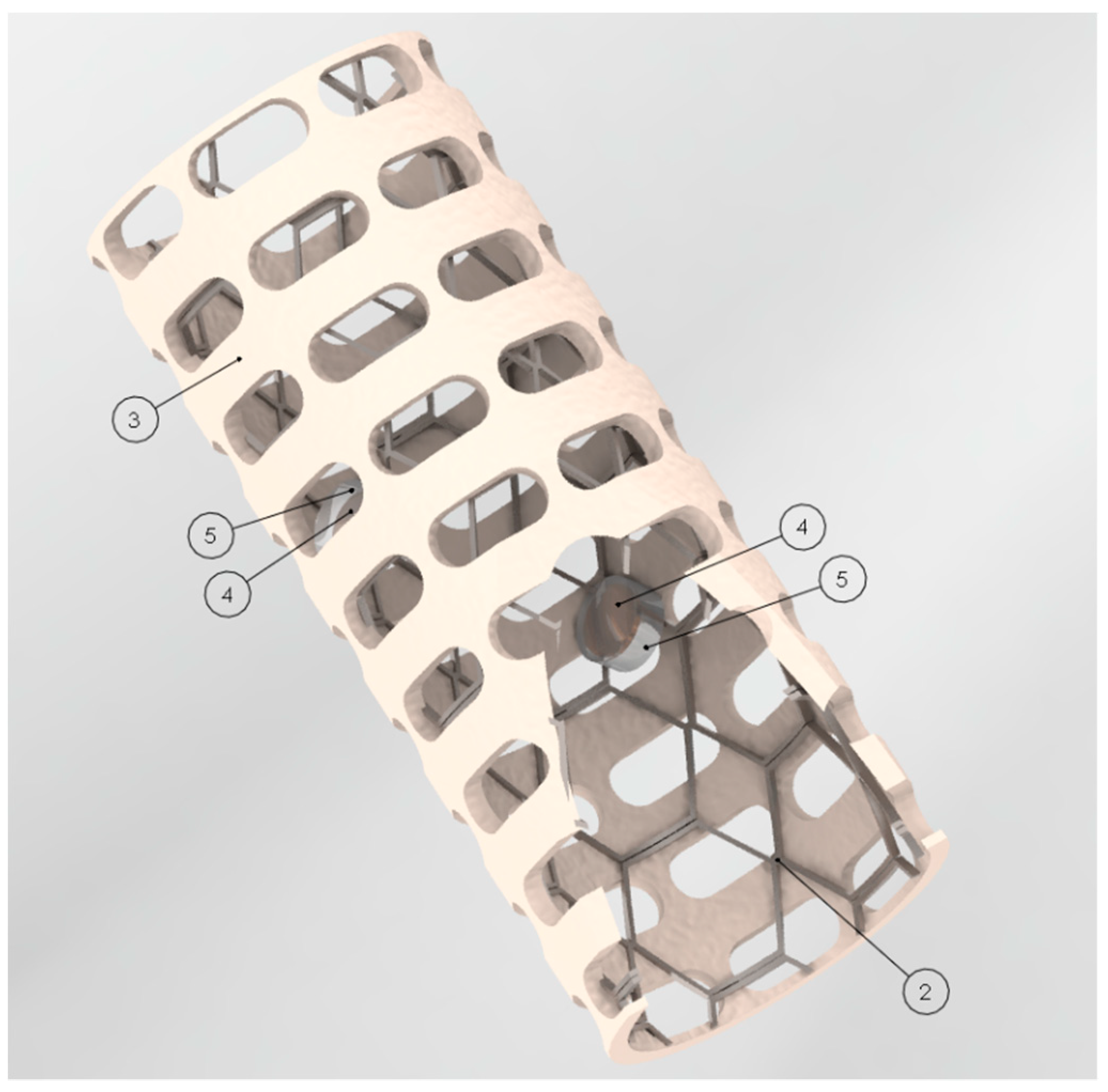
Figure 1 shows the designed smart stent. In
Figure 1, the stent parts are numbered in order to describe it. The device is composed of a metal mesh structure with cylindrical shape (2), a biocompatible and adaptable envelope (3) and pair-operated ultrasonic sensors (4) [
16,
17]. Two or more pair-operated ultrasonic sensors (4) can be used to measure the velocity of the blood flow at different places on the stent. The material of the metal structure can be titanium and the material of the envelope can be silicon elastomer. However, other biocompatible material could be used to build the device. The device can be implanted into lumen organs or blood vessels. The diameter of the stent can be expanded and adapted according to the treated lumen. The biocompatible and adaptable envelope (3) wraps the metal structural mesh (2). Two or more pair-operated ultrasonic sensors (4) are incorporated into the envelope (3) and covered by a flexible film made of biocompatible material (5). The ultrasonic sensors use a beam of ultrasonic energy that allow one to calculate the velocity of the blood flow of the blood.
Figure 2 shows the device implanted in the descending thoracic aorta. The transducers are connected to a small controller unit via catheterized millimeter wires. The wires are connected to the controller unit for power supply and signal acquisition. The controller unit is external to the patient and it can be permanently connected for continuous monitoring if required or it can be intermittently connected/disconnected for periodical monitoring. To obtain velocity of the blood flow, the small controller unit acquires the signal from the transducers, performs the required calculation and sends the data via Wi-Fi to a computer. A user interface shows real-time graphs of the velocity trend of the blood that can be saved for further processing. A small 3.7 V Li-Po battery is used by the tiny controller unit to ensure portability.
Figure 3 shows a scheme of two ultrasonic sensors that operate in pairs for calculating velocity of the blood flow based on transit time. The velocity of the blood flow can be calculated using the transit time of the signal
t [
16]:
where
t is the transit time,
D is the distance between transducers,
c is the sound velocity and
u is the velocity of the blood flow. When the blood flows in the same direction as the transmitted signal, the observed transit time is shortened, otherwise it lengthened. The
t value is obtained through the catheterized small wires of millimeter diameter that are connected to the transducers. Subsequently, with the obtained
t value and the other known variables, the velocity of the blood flow can be calculated.
2.2. Simulation Procedure
Continuous monitoring of restenosis post-implantation is important to prevent health complications. However, it is also important to evaluate if the stent design itself will not cause complications such as restenosis or thrombosis. Since velocity of the flow blood is an important parameter to evaluate the restenosis and thrombus formation, the velocity of the blood flow was evaluated along an aorta artery and along the smart stent that is inserted in the descending thoracic section of the aorta. In addition, pressures were computed in order to test pressure changes after stent implantation. Computational fluid dynamic (CFD) analysis was carried out using the SolidWorks Flow Simulation tool in SolidWorks software (version 2018, Dassault Systems, S.A., Suresnes, France). In SolidWorks Flow Simulation, it is possible to use blood fluid for simulation purposes. The blood model available in SolidWorks Flow Simulation includes blood parameters that are shown in
Table 1. In addition, gravity and well-known parameters such as blood velocity, environmental pressure, and environmental temperature in the human body circulatory system were taken into account as initial conditions in the simulation according to the literature [
18,
19,
20].
The SolidWorks Flow Simulation technology is based upon the use of Cartesian-based meshes, and meshing is one of the key elements of the CAD/CFD bridge for CAD-embedded CFD.
As a result of using Cartesian-based meshes, cells which are located fully in solid bodies (solid cells), in the fluid (fluid cells) and cells intersected the immersed boundary. In the simplest case, the partial cell consists of 2 control volumes (CV): a fluid CV and a solid CV. Each CV is then fully solid or fully fluid. For each CV, all necessary geometrical parameters such as volume and the coordinates of the cell center are computed.
Multiple layers of CVs are essential not only for fluid flow modelling but also for heat transfer phenomena, including the contact resistances and Joule heating calculations within a solid body [
21].
For all the physical phenomena, the native CAD geometry is the source of initial geometric information. In fluid regions, SolidWorks Flow Simulation solves the Navier–Stokes equations, which are formulations of mass, momentum and energy conservation laws:
Thus, the fluid region represents the main computational challenge from the point of view of algorithmic complexity and of calculation overhead. Using the given CAD as a source of geometric information, it is essential to pay specific attention to the robustness and efficiency of the numerical methods used. SolidWorks Flow Simulation uses the solver and related numerical mentioned algorithms for modelling fluid flows.
The input parameters to define initial conditions of the blood during the simulation are shown in
Table 2. The proposed smart stent was put in the descending thoracic section of a virtually reconstructed aorta. The aorta came from a computed tomography (CT) scan, the scan was converted to a stereolithography (STL) file and the boundaries were closed to allow an internal flow analysis. The parameters of the analysis mesh were set for an optimal simulation (
Table 3).
Effects of loads on the solid body (i.e., the stent) can be investigated by exporting the results of the computational fluid dynamic (CFD) analysis from “SolidWorks Flow Simulation” to “SolidWorks Simulation”. Therefore, loads such as thermal effect, environmental pressure, fluid pressure and velocity along the path coming from flow scenarios in SolidWorks Flow Simulation can be exported to SolidWorks Simulation in order to perform a stress analysis of the stent. SolidWorks Simulation is a finite element analysis (FEA) package that can analyze outputs on the solid body such as stress and factor of safety (FOS) when a linear static study is defined. The benefit of this method versus just defining the pressure and/or temperature load in a static study is that these imported loads are non-uniform. SolidWorks Flow Simulation exports all the gradients across the surfaces. This produces more realistic loads on the model. However, in the static analysis, fixtures do have to be added since it was not previously necessary to define these, as all physical geometry was stationary in the CFD tool. The static study requires fixtures to avoid an infinite movement of the geometry as a response to the loads. All the imported loads are applied to the model at the same time. The order does not matter since the laws of superposition compound all stresses. In terms of the effects of the loads imported, the reference temperature and/or pressure of the imported loads were specified. These values are used to determine how charges impact the state of the zero pressure. Zero strain arises when no stress on the model is applied. The Von Mises stress function was used as a criterion for the stress analysis. The Von Mises stress function,
σvm, can be expressed in the form [
22]:
where
σ1,
σ2 and
σ3 are the three principal stresses acting on the x-, y-, and z-axes of the solid body. Von Mises stress is a non-negative, scalar stress measure that evaluates elasto-plastic properties. This number function represents a stress magnitude, which can be compared against the yield strength of the material in order to determine whether or not failure by yielding is predicted. On the other hand, using the maximum Von Mises stress criteria [
22], the condition of safe design can be expressed as:
If the factor of safety (FOS) < 1, the material fails; if FOS = 0, the material is in a critical condition; and if FOS > 1, the material supports the applied loads without material failure.
Stress and FOS analysis were worked out through FEA modelling.
Table 4 shows the mesh information used for the stress and FOS simulation. To establish a more complete image of the simulated environment, it was important to connect both simulations. Doing so, it was possible not only to determine the flow of the fluids, but also to study the stresses on geometry. In the simulation settings, titanium was used for the metal structure of the smart stent and silicon elastomer for the envelope.
Table 5 and
Table 6 show the mechanical properties of the titanium and the silicon elastomer used for the stent stress simulation, respectively.