Biochemical Characterization of a Bifunctional Enzyme Constructed by the Fusion of a Glucuronan Lyase and a Chitinase from Trichoderma sp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmids Constructions and Pichia Pastoris Transformation
2.2. Production and Purification of Recombinant Parental and Chimeric Enzymes
2.3. Enzyme Assays
2.4. pH and Temperature Stability
2.5. Kinetic Parameters Analysis of Glucuronan Lyase
2.6. 3D Structure Simulation of Chimeric Enzyme
3. Results
3.1. Expression of Fused TrGL-Thchit42 Constructions in Pichia Pastoris
3.2. Enzymatic Activities of TrGL-ThCHIT42 Chimeras
3.3. Kinetic Parameters of Glucuronan Lyase
3.4. Temperature and pH Stability of TrGL-ThCHIT42 Chimeras vs. Parental Enzymes
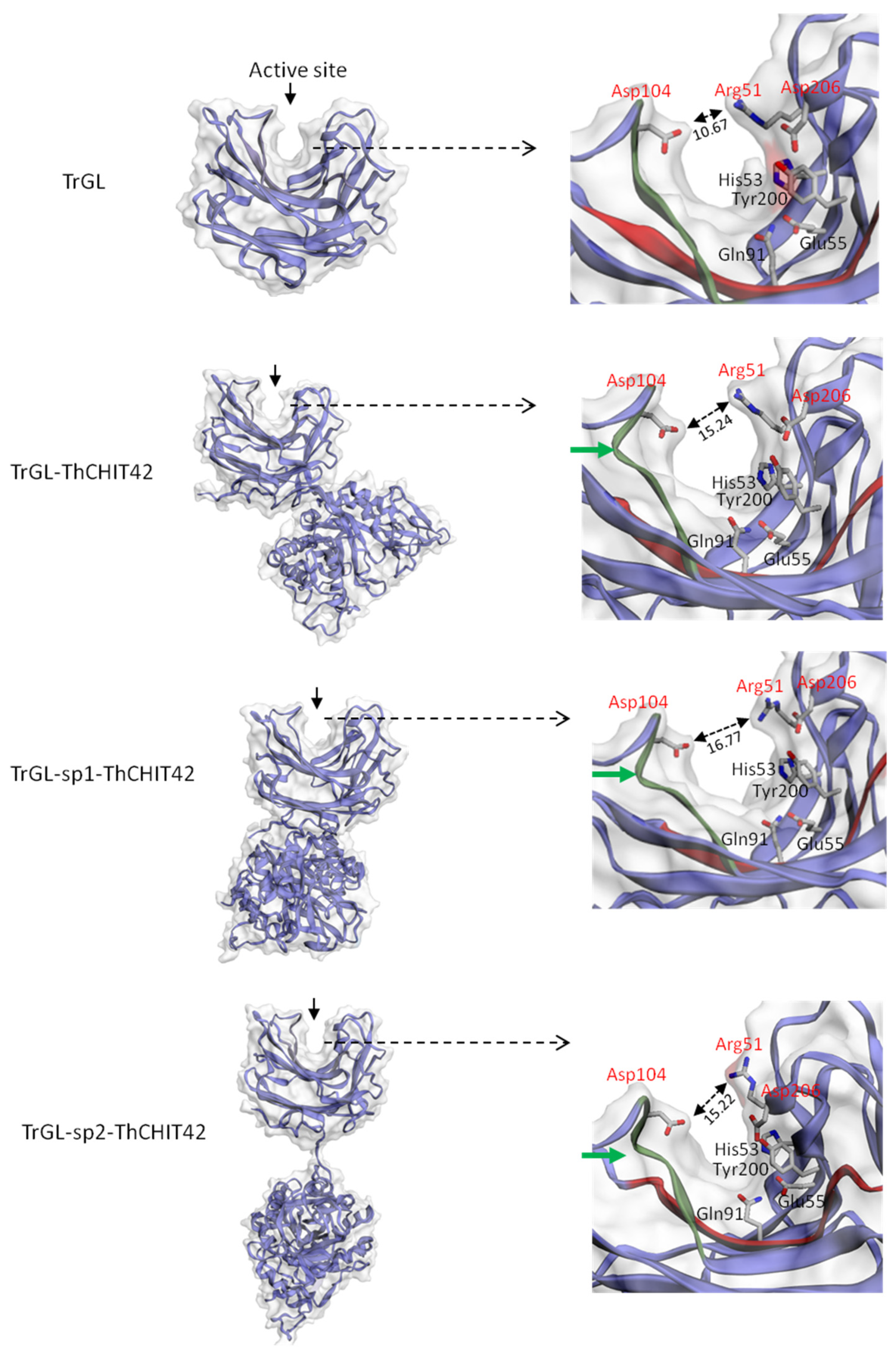
3.5. 3D structure of Chimeric Enzymes and Glucuronan Lyase
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Samuels, G.J. Trichoderma: A review of biology and systematic of the genus. Mycol. Res. 1996, 100, 923–935. [Google Scholar] [CrossRef]
- Benitez, T.; Rincon, A.M.; Limon, M.C.; Codon, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar] [PubMed]
- Gajera, H.; Domadiya, R.; Patel, S.; Kapopara, M.; Golakiya, B. Molecular mechanism of Trichoderma as bio-control agent against phytopathogen system—A review. Curr. Res. Microbiol. 2013, 1, 133–142. [Google Scholar]
- Guthrie, J.L.; Castle, A.J. Chitinase production during interaction of Trichoderma aggressivum and Agaricus bisporus. Can. J. Microbiol. 2006, 52, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.V.; Kim, Y.J.; Oh, K.T.; Jung, W.J.; Park, R.D. Antifungal activity of chitinases from Trichoderma aureoviride DY-59 and Rhizopus microsporus VS-9. Curr. Microbiol. 2008, 56, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Marco, J.L.D.; Valadares-Inglis, M.C.; Felix, C.R. Production of hydrolytic enzymes by Trichoderma isolates with antagonistic activity against Crinipillis perniciosa the causal agent of witches broom of cocoa. Braz. J. Microbiol. 2003, 34, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Sanz, L.; Montero, M.; Roedondo, J.; Llobell, A.; Monte, E. Expression of an alpha-1,3-glucanase during mycoparasitisc interaction of Trichoderma asperellum. FEBS J. 2005, 272, 493–499. [Google Scholar] [CrossRef]
- Mercado, J.A.; Barcelo, M.; Pliego, C.; Rey, M.; Caballero, J.L.; Munoz-Blanco, J.; Ruano-Rosa, D.; Lopez-Herrera, C.; de Los Santos, B.; Romero-Munoz, F.; et al. Expression of the β-1,3-glucanase from Trichoderma harzanium in strawberry increases tolerance to crown rot diseases but interferes with plant growth. Trangenic Res. 2015, 24, 979–989. [Google Scholar] [CrossRef] [Green Version]
- Howell, C.R. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Kredics, L.; Antal, Z.; Szekeres, A.; Hatvani, L.; Manczinger, L.; Vagvolgyi, C.; Nagy, E. Extracellular proteases of Trichoderma species. A review. Acta Microbiol. Immunol. Hung. 2005, 52, 169–184. [Google Scholar] [CrossRef]
- Suarez, M.B.; Vizcaino, J.A.; Llobell, A.; Monte, E. Characterization of genes encoding novel peptidases in the biocontrol fungus Trichoderma harzanium CECT 2413 using the TrichoEST functional genomics approach. Curr. Genet. 2007, 51, 331–342. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species-opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Viterbo, A.; Ramot, O.; Chemin, L.; Chet, I. Significance of lytic enzymes from Trichoderma spp. in the biocontrol of fungal plant pathogens. Ant. Van Leeuw. 2002, 81, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Langner, T.; Göhre, V. Fungal chitinases: Function, regulation and potential roles in plant/pathogens interactions. Curr. Genet. 2016, 62, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Brasselet, C.; Pierre, G.; Dubessay, P.; Dols-Lafargue, M.; Coulon, J.; Maupeu, J.; Vallet-Courbin, A.; De Baynast, H.; Docco, T.; Michaud, P.; et al. Modification of chitosan for the generation of functional derivatives. Appl. Sci. 2019, 9, 1321. [Google Scholar] [CrossRef] [Green Version]
- Oyeleye, A.; Normi, Y.M. Chitinase: Diversity, limitations and trends in engineering for suitable applications. Biosci. Rep. 2018, 38, 1–21. [Google Scholar] [CrossRef]
- Kim, D.J.; Baek, J.M.; Uribe, P.; Kenerley, C.M.; Cook, D.R. Cloning and characterization of multiple glycosyl hydrolase genes in Trichoderma virens. Current. Genet. 2002, 40, 374–380. [Google Scholar] [CrossRef]
- Chen, F.; Chen, X.Z.; Qin, L.N.; Tao, Y.; Dong, Z.Y. Characterization and homologeous overexpression of an N-acetylglucosaminidase Nag1 from Trichoderma reesei. Biochem. Biophys. Res. Commun. 2015, 459, 184–188. [Google Scholar] [CrossRef]
- Haran, S.; Schickler, H.; Oppenheim, A.; Chet, I. Differential expression of Trichoderma harzanium chitinases during mycoparasitism. Phytopathology 1996, 86, 980–985. [Google Scholar] [CrossRef]
- De la Cruz, J.; Hidalgo-Gallego, A.; Lora, J.M.; Benítez, T.; Pintor-Toro, J.A.; Llobell, A. Isolation and characterization of three chitinases from Trichoderma harzianum. Eur. J. Biochem. 1992, 206, 859–867. [Google Scholar] [CrossRef]
- Limon, M.C.; Chacon, M.R.; Meijas, R.; Delgado-Jarana, J.; Rincon, A.M.; Codon, A.C.; Benitez, T. Increased antifungal and chitinase specific activities of Trichoderma harzanium CECT 2413 by addition of a cellulose binding domain. Appl. Microbiol. Biotechnol. 2004, 64, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Carsolio, C.; Benhamou, N.; Haran, S.; Cortes, C.; Gutierrez, A.; Chet, I.; Herrera-Estrella, A. Role of Trichoderma harzanium endochitinase gene, ech42, in mycoparasitism. Appl. Environ. Microbiol. 1999, 65, 929–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delattre, C.; Michaud, P.; Keller, C.; Elboutachfaiti, R.; Beven, L.; Courtois, B.; Courtois, J. Purification and characterization of a novel glucuronan lyase from Trichoderma sp. GL2. Appl. Microbiol. Biotechnol. 2006, 70, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Konno, N.; Igarashi, K.; Habu, N.; Samejima, M.; Isogai, A. Cloning of Trichoderma reseei cDNA encoding a glucuronan lyase belonging to a novel polysaccharide lyase family. Appl. Environ. Microbiol. 2009, 75, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Guo, X.; Zhu, M.; Chen, C.; Li, D. Polysaccharide monooxygenase-catalysed oxidation of cellulose to glucuronic acid-containing cello-oligosaccharides. Biotechnol. Biofuels 2019, 12, 42–59. [Google Scholar] [CrossRef]
- Heyraud, A.; Courtois, J.; Dantas, L.; Colin-Morel, P.; Courtois, B. Structural characterization and rheological properties of an extracellular glucuronan produced by a Rhizobium meliloti M5N1 mutant strain. Carbohydr. Res. 1993, 240, 71–78. [Google Scholar] [CrossRef]
- Aono, R. The poly-α and β-1,4-glucuronic acid moiety of teichuronopeptide from the cell wall of the alkalophilic Bacillus strain C-125. Biochem. J. 1990, 270, 363–367. [Google Scholar] [CrossRef]
- De Ruiter, G.A.; Josso, S.L.; Colquhoun, I.J.; Voragen, A.G.J.; Rombouts, F.M. Isolation and characterization of β(1-4)-d-glucuronans from extracellular polysaccharides of moulds belonging to Mucorales. Carbohydr. Polym. 1992, 18, 1–7. [Google Scholar] [CrossRef]
- Lecointe, K.; Cornu, M.; Leroy, J.; Coulon, P.; Sendid, B. Polysaccharides cell wall architecture of Mucorales. Front. Microbiol. 2019, 10, 469. [Google Scholar] [CrossRef]
- Elleuche, S. Bringing functions together with fusion enzymes—From nature’s inventions to biotechnological applications. Appl. Microbiol. Biotechnol. 2015, 99, 1545–1556. [Google Scholar] [CrossRef]
- Kidibule, P.A.; Santos-Moriano, P.; Jimenez-Ortega, E.; Ramirez-Escudero, M.; Limon, M.C.; Remacha, M.; Plou, F.J.; Sanz-Aparicio, J.; Fernadez-Lobato, M. Use of chitin and chitosan to produce new chitooligosaccharides by chitinase Chit42: Enzymatic activity and structural basis of protein specificity. Microb. Cell Fact 2018, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Waffenshchmidt, S.; Jaenicke, L. Assay of reducing sugars in the nanomole range with 2, 2′-bicinchoninate. Anal. Biochem. 1987, 165, 337–340. [Google Scholar] [CrossRef]
- Konno, N.; Ishida, T.; Igarashi, K.; Fushinobu, S.; Habu, N.; Samejima, M.; Isogai, A. Crystal structure of polysaccharide lyase family 20 endo-β-1,4-glucuronan lyase from the filamentous fungus Trichoderma reesei. FEBS Lett. 2009, 583, 1323–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadhikolaei, K.K.; Noghabi, K.A.; Zahiri, H.S. Development of a bi-fucntional xylanase-cellulase chimera with enhanced activity on rice and barley straws using a modular xylanase and an endoglucanase procured from camel rumen metagenome. Appl. Microbiol. Biotechnol. 2017, 101, 6929–6939. [Google Scholar]
- Furtado, G.P.; Ribeiro, L.F.; Lourenzoni, M.R.; Ward, R.J. A designed bifunctional laccase/β-1,3-1,4-glucanase enzyme shows synergistic sugar release from milled sugarcane bagasse. Prot. Eng. Des. Sel. 2013, 26, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Nath, P.; Dhillon, A.; Kumar, K.; Sharma, K.; Jamaldhenn, S.B.; Moholkar, V.S.; Goyal, A. Developpement of bi-functional chimeric enzyme (CtGH1-L1-CtGH5F194A) from endoglucanase (CtGH5) mutant F194A and β-1,4-glucosidase (CtGH1) from Clostridium thermocellum with enhanced activity and structural activity. Bioresour. Technol. 2019, 282, 494–501. [Google Scholar] [CrossRef]
- Eiben, S.; Bartelmas, H.; Urlacher, V.B. Construction of a thermostable cytochrome P450 chimera derived from self-sufficient mesophilic parents. Appl. Microbiol. Biotechnol. 2007, 75, 1055–1061. [Google Scholar] [CrossRef]
- Lee, H.L.; Chang, C.K.; Teng, K.H.; Liang, P.H. Construction and characterization of different fusion proteins between cellulases and β-glucosidase to improve glucose production and thermostability. Bioresour. Technol. 2011, 102, 3973–3976. [Google Scholar] [CrossRef]
- Herod, M.R.; Prince, C.A.; Skilton, R.J.; Ward, V.K.; Cooper, J.B.; Clarke, I.N. Structure-based design and functional studies of novel noroviral 3C protease chimaeras offer insights into substrate specificity. Biochem. J. 2014, 464, 461–472. [Google Scholar]
- Subbayya, N.S.; Sukumaran, S.; Shivashankar, K.; Balaram, H. Unusual substrate specificity of a chimeric hypoxanthine-guanine phosphoribosyltransferase containing segments from Plasmodium falciparum and human enzymes. Biochem. Biophys. Res. Commun. 2000, 272, 596–602. [Google Scholar] [CrossRef]
- Adlakha, N.; Sawant, S.; Anil, A.; Lali, A.; Yazdani, S.S. Specific fusion of β-1,4-endoglucanase and β-1,4-glucosidase enhances cellulolytic activity and helps in channeling of intermediates. Appl. Environ. Microbiol. 2012, 78, 7447–7454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Z.; Wagschal, K.; Lee, C.C.; Kong, Q.; Shen, K.A.; Maiti, I.B.; Yuang, L. The construction and characterization of two xylan-degrading chimeric enzymes. Biotechnol. Bioeng. 2009, 102, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Limon, M.C.; Margolles-Clark, E.; Benitez, T.; Pentilla, M. Addition of substrate-binding domains increases substrate binding capacity and specific activity of a chitinase from Trichoderma harzanium. FEMS Microbiol. Lett. 2001, 198, 57–63. [Google Scholar] [CrossRef]
- Kowsari, M.; Motallebi, M.; Zamani, M. Protein engineering of chit42 towards improvement of chitinase antifungal activities. Curr. Microbiol. 2014, 68, 495–502. [Google Scholar] [CrossRef]
- Lu, P.; Feng, M.G. Bifunctional enhancement of β-glucanase-xylanase fusion enzyme by optimization of peptides linkers. Appl. Microbiol. Biotechnol. 2008, 79, 579–587. [Google Scholar] [CrossRef]
- Ribeiro, L.F.; Futado, G.P.; Lourenzoni, M.R.; Costa-Filho, A.J.; Santos, C.R.; Nogeira, S.C.; Betini, J.A.; Polizeli, L.; Murakami, M.T.; Ward, R.J. Engineering bifunctional laccase-xylanase chimeras for improved catalytic performence. J. Biol. Chem. 2011, 16, 43026–43038. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.Y.; Lee, J.S.; Cho, K.M.; Math, R.K.; Kim, Y.H.; Hong, S.J.; Cho, Y.U.; Kim, H.; Yun, H.D. Assembling a novel bifunctonal cellulase-xylanase from Thermotoga maritima by end-to-end fusion. Biotechnol. Lett. 2006, 28, 1857–1862. [Google Scholar] [CrossRef]





| Gene | Primer | Nucleotide Sequence (5’-3’) | Cloning Sites | Final Plasmid |
|---|---|---|---|---|
| TrGL | XTrGL-Fwd | TTTCTCGAGAAAAGAACCCGCAGCTTCTACAACGACGG | XhoI | pPICZαA/TrGL |
| XbTrGL-Rev | AAATCTAGATTAAGCCTGGTCAGGGTCGACATCG | XbaI | ||
| Thchit42 | EThchit42-Fwd | GGGGAATTCGCTAGTGGTTACGCTAACGC | EcorI | pPICZαA/Thchit42 |
| XbThchit42-Rev | AAATCTAGATTAAGACCACTACGAATGTTATC | XbaI | ||
| Thchit42 | XbThchit42-Fwd | AAATCTAGAGGCTAGTGGTTACGCTAACGCT | XbaI | pPICZαA/TrGL-Thchit42 |
| XbThchit42-Rev | AAATCTAGATTAAGACCACTACGAATGTTATC | XbaI | ||
| Thchit42 | Xbsp1Thchit42-Fwd | AAATCTAGAGGGTGGCGGTGGCTCGGCTAGTGGTTACGCTAACGCT | XbaI | pPICZαA/TrGL-sp1-Thchit42 |
| XbThchit42-Rev | AAATCTAGATTAAGACCACTACGAATGTTATC | XbaI | ||
| Thchit42 | Xbsp2Thchit42-Fwd | AAATCTAGAGGGCGGTGGTGGGTCGGGTGGCGGTGGCTCGGCTAGTGGTTACGCTAACGCT | XbaI | pPICZαA/TrGL-sp2-Thchit42 |
| XbThchit42-Rev | AAATCTAGATTAAGACCACTACGAATGTTATC | XbaI |
| Enzymes | kcat [Min−1] | Km (g/L) | kcat/Km [Min−1. (g/L)−1] |
|---|---|---|---|
| TrGL | 0.299 ± 0.033 | 0.096 ± 0.029 | 3.105 |
| TrGL-ThCHIT42 | 0.503 ± 0.019 | 0.092 ± 0.002 | 5.423 |
| TrGL-sp1-ThCHIT42 | 0.444 ± 0.014 | 0.076 ± 0.004 | 5.847 |
| TrGL-sp2-ThCHIT42 | 0.493 ± 0.107 | 0.088 ± 0.024 | 5.570 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baklouti, Z.; Delattre, C.; Pierre, G.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Dubessay, P. Biochemical Characterization of a Bifunctional Enzyme Constructed by the Fusion of a Glucuronan Lyase and a Chitinase from Trichoderma sp. Life 2020, 10, 234. https://doi.org/10.3390/life10100234
Baklouti Z, Delattre C, Pierre G, Gardarin C, Abdelkafi S, Michaud P, Dubessay P. Biochemical Characterization of a Bifunctional Enzyme Constructed by the Fusion of a Glucuronan Lyase and a Chitinase from Trichoderma sp. Life. 2020; 10(10):234. https://doi.org/10.3390/life10100234
Chicago/Turabian StyleBaklouti, Zeineb, Cédric Delattre, Guillaume Pierre, Christine Gardarin, Slim Abdelkafi, Philippe Michaud, and Pascal Dubessay. 2020. "Biochemical Characterization of a Bifunctional Enzyme Constructed by the Fusion of a Glucuronan Lyase and a Chitinase from Trichoderma sp." Life 10, no. 10: 234. https://doi.org/10.3390/life10100234
APA StyleBaklouti, Z., Delattre, C., Pierre, G., Gardarin, C., Abdelkafi, S., Michaud, P., & Dubessay, P. (2020). Biochemical Characterization of a Bifunctional Enzyme Constructed by the Fusion of a Glucuronan Lyase and a Chitinase from Trichoderma sp. Life, 10(10), 234. https://doi.org/10.3390/life10100234









