Quercitrin Ameliorates Hyperlipidemia and Hepatic Steatosis in Ovariectomized Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.2. Serum Analysis
2.3. Body Fat Composition and Liver Histological Analysis
2.4. Real-Time PCR
2.5. Statistical Analysis
3. Results
3.1. Quercitrin Inhibited Body Weight Gain, Epidermal Fat, and Liver Weight in OVX Mice
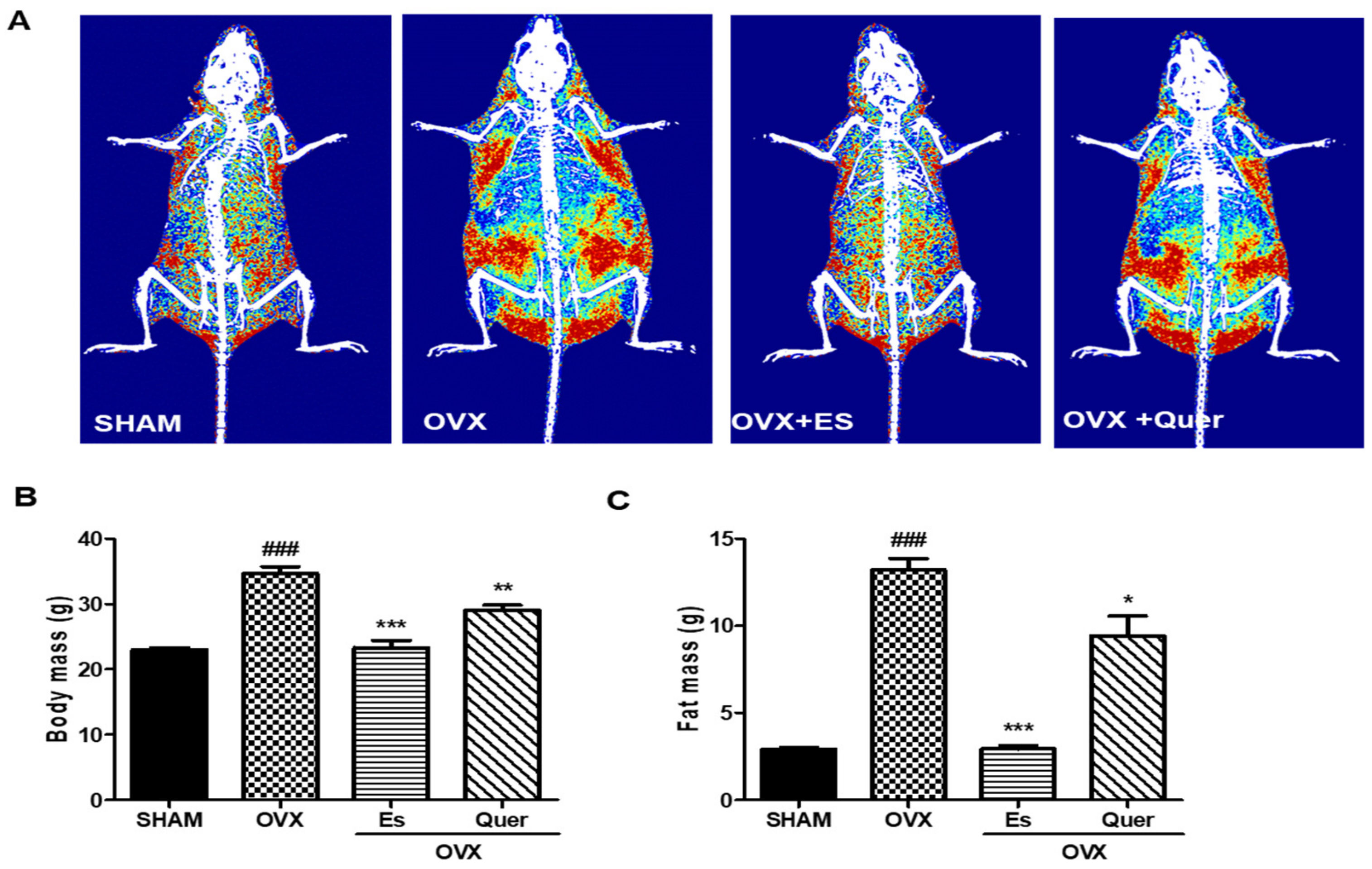
3.2. Quercitrin Disrupted the Distribution of Fat Mass and Body Mass in OVX Mice
3.3. Quercitrin Suppressed Serum Lipid Metabolite Levels in OVX Mice
3.4. Quercitrin Decreased Liver Injury in OVX Mice
3.5. Quercitrin Inhibited the Expression of Hepatic Inflammatory Molecules in OVX Mice
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Tanaka, N.; Takahashi, S.; Fang, Z.Z.; Matsubara, T.; Krausz, K.W.; Qu, A.; Gonzalez, F.J. Role of white adipose lipolysis in the development of NASH induced by methionine- and choline-deficient diet. Biochim. Biophys. Acta 2014, 1841, 1596–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazel, Y.; Koenig, A.B.; Sayiner, M.; Goodman, Z.D.; Younossi, Z.M. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism 2016, 65, 1017–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotronen, A.; Peltonen, M.; Hakkarainen, A.; Sevastianova, K.; Bergholm, R.; Johansson, L.M.; Lundbom, N.; Rissanen, A.; Ridderstråle, M.; Groop, L.; et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology 2009, 137, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Stubbins, R.E.; Najjar, K.; Holcomb, V.B.; Hong, J.; Nunez, N.P. Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes Obes. Metab. 2012, 14, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Venetsanaki, V.; Polyzos, S.A. Menopause and non-alcoholic fatty liver disease: A review focusing on therapeutic perspective. Curr. Vasc. Pharmacol. 2019, 17, 546–555. [Google Scholar] [CrossRef]
- Domínguez-López, I.; Yago-Aragón, M.; Salas-Huetos, A.; Tresserra-Rimbau, A.; Hurtado-Barroso, A. Effect of dietary phytoestrogens on hormones throughout a human lifespan: A review. Nutrients 2020, 12, 2456. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Yang, W.; Bosland, M.C. Soy isoflavones and prostate cancer: A review of molecular mechanisms. J. Steroid Biochem. Mol. Biol. 2014, 140, 116–132. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, I.L.; Savić-Gajić, I.M.; Tačić, A.D.; Savić, I.M. Classification and biological activity of phytoestrogens: A review. Adv. Technol. 2017, 6, 93–106. [Google Scholar] [CrossRef] [Green Version]
- Akhlaghi, M. Non-alcoholic fatty liver disease: Beneficial effects of flavonoids. Phytother. Res. 2016, 30, 1559–1571. [Google Scholar] [CrossRef]
- Salvamani, S.; Gunasekaran, B.; Shaharuddin, N.A.; Ahmad, S.A.; Shukor, M.Y. Antiartherosclerotic effects of plant flavonoids. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Musolino, V.; Gliozzi, M.; Scarano, F.; Bosco, F.; Scicchitano, M.; Nucera, S.; Carresi, C.; Ruga, S.; Zito, M.C.; Maiuolo, J.; et al. Bergamot polyphenols improve dyslipidemia and pathophysiological features in a mouse model of non-alcoholic fatty liver disease. Sci. Rep. 2020, 10, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chiow, K.H.; Phoon, M.C.; Putti, T.; Tan, B.K.; Chow, V.T. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac. J. Trop. Med. 2016, 9, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, C.C.; Oliveira, D.D.; Valdevite, M.; Saltoratto, A.L.; Pereira, S.I.; de França, S.C.; Pereira, A.M.; Pereira, P.S. The glycosylated flavonoids vitexin, isovitexin, and quercetrin isolated from Serjania erecta Radlk (Sapindaceae) leaves protect PC12 cells against amyloid-β25-35 peptide-induced toxicity. Food Chem. Toxicol. 2015, 86, 88–94. [Google Scholar] [CrossRef]
- Junior, W.A.R.; Piato, A.L.; Conterato, G.M.; Wildner, S.M.; Marcon, M.; Mocelin, R.; Emanuelli, M.P.; Emanuelli, T.; Nepel, A.; Barison, A.; et al. Hypolipidemic effects of Solidago chilensis hydroalcoholic extract and its major isolated constituent quercetrin in cholesterol-fed rats. Pharm. Biol. 2015, 53, 1488–1495. [Google Scholar] [CrossRef]
- Wier, B.V.D.; Koek, G.H.; Bast, A.; Haenen, G.R.M.M. The potential of flavonoids in the treatment of non-alcoholic fatty liver disease. Crit. Rev. Food Sci. Nutr. 2017, 57, 834–855. [Google Scholar] [CrossRef]
- Davaatseren, M.; Hur, H.J.; Yang, H.J.; Hwang, J.T.; Park, J.H.; Kim, H.J.; Kim, M.J.; Kwon, D.Y.; Sung, M.J. Taraxacum official (dandelion) leaf extract alleviates high-fat diet-induced nonalcoholic fatty liver. Food Chem. Toxicol. 2013, 58, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Morselli, E.; Santos, R.S.; Ávalos, Y.; Criollo, A.; Palmer, B.F.; Clegg, D.J. Impact of estrogens and estrogen receptor-α in brain lipid metabolism. Am. J. Phsiol. Endocrinol. Metab. 2018, 315, E7–E14. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-S.; Ko, S.-H. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients 2020, 12, 202. [Google Scholar]
- Chen, K.L.; Madak-Erdogan, Z. Estrogens and female liver health. Steroids 2018, 133, 38–43. [Google Scholar] [CrossRef]
- Chalvon-Demersay, T.; Blachier, F.; Tome, D.; Blais, A. Animal models for the study of the relationships between diet and obesity: A focus on dietary protein and estrogen deficiency. Front. Nutr. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Nanashima, N.; Horie, K.; Yamanouchi, K.; Tomisawa, T.; Kitajima, M.; Oey, I.; Maeda, H. Blackcurrant (Ribes nigrum) extract prevents dyslipidemia and hepatic steatosis in ovariectomized rats. Nutrients 2020, 12, 1541. [Google Scholar] [CrossRef] [PubMed]
- Koshy, S.M.; Bobby, Z.; Jacob, S.E.; Ananthanarayanan, P.H.; Sridhar, M.G.; Paulose, D.T. Amla prevents fructose-induced hepatic steatosis in ovariectomized rats: Role of liver FXR and LXRα. Climacteric 2015, 18, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Pappachan, J.M.; Babu, S.; Krishnan, B.; Ravindran, N.C. Non-alcoholic fatty liver disease: A clinical update. J. Clin. Transl. Hepatol. 2017, 5, 384–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, S.-H. Nonalcoholic fatty liver disease: Molecular mechanisms for the hepatic steatosis. Clin. Mol. Hepatol. 2013, 19, 210–215. [Google Scholar] [CrossRef]
- Jiang, W.; Guo, M.H.; Hai, X. Hepatoprotective and antioxidant effects of lycopene on non-alcoholic fatty liver disease in rat. World J. Gastroenterol. 2016, 22, 10180–10188. [Google Scholar] [CrossRef]
- Elshazly, S.M. Ameliorative effect of nicorandil on high fat diet induced non-alcoholic fatty liver disease in rats. Eur. J. Pharmacol. 2015, 748, 123–132. [Google Scholar] [CrossRef]
- Ambikairajah, A.; Walsh, E.; Cherbuin, N. Lipid profile differences during menopause: A review with meta-analysis. Menopause 2019, 26, 1327–1333. [Google Scholar] [CrossRef]
- Høegh-Andersen, P.; Tankó, L.B.; Andersen, T.L.; Lundberg, C.V.; Mo, J.A.; Heegaard, A.M.; Delaissé, J.M.; Christgau, S. Ovariectomized rats as a model of postmenopausal osteoarthritis: Validation and application. Arthritis Res. Ther. 2004, 6, R169. [Google Scholar] [CrossRef] [Green Version]
- Tilg, H. The role of cytokines in non-alcoholic fatty liver disease. Dig. Dis. 2010, 28, 179–185. [Google Scholar] [CrossRef]
- Braunersreuther, V.; Viviani, G.L.; Mach, F.; Montecucco, F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J. Gastroenterol. 2012, 18, 727–735. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Hur, H.J.; Jeon, E.J.; Park, S.J.; Hwang, J.T.; Lee, A.S.; Lee, K.W.; Sung, M.J. Honokiol improves liver steatosis in ovariectomized mice. Molecules 2018, 23, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertog, M.G.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr. Cancer 1993, 20, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Regerat, F.; Texier, O.; Agullo, G.; Demigne, C.; Remesy, C. Bioavailability, metabolism and physiological impact of 4-oxo-flavonoids. Nutr. Res. 1996, 16, 517–544. [Google Scholar] [CrossRef]
- Ma, J.-Q.; Luo, R.-Z.; Jiang, H.-Z.; Liu, C.-M. Quercitrin offers protection against brain injury in mice by inhibiting oxidative stress and inflammation. Food Func. 2016, 7, 549–556. [Google Scholar] [CrossRef]
- Li, W.; Zhang, M.; Wang, M.; Zhong, Y.; Wu, H.; Yang, Y.; Morel, L.; Wei, Q. Quercitrin ameliorates the development of systemic lupus erythematosus-like disease in a chronic graft-versus-host murine model. Am. J. Physio. Renal Physiol. 2016, 311, F217–F226. [Google Scholar] [CrossRef] [Green Version]
- Comalada, M.; Camuesco, D.; Sierra, A.; Ballester, I.; Xaus, J.; Galvez, J.; Zarzuelo, A. Quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur. J. Immunol. 2005, 35, 584–592. [Google Scholar] [CrossRef]
- Ricci, M. Dosing animals via diet. ALN World 2012, 5, 5. [Google Scholar]


| Ingredient | SH, OVX | OVX + ES | OVX + Qtr |
|---|---|---|---|
| Cornstarch | 397.486 | 397.486 | 397.486 |
| Casein | 200.000 | 200.000 | 200.000 |
| Dextrin | 132.000 | 132.000 | 132.000 |
| Sucrose | 100.000 | 100.000 | 100.000 |
| Soybean oil (no additives) | 70.000 | 70.000 | 70.000 |
| Fiber | 50.000 | 50.000 | 50.000 |
| Mineral mix (AIN-93G-MX) | 35.000 | 35.000 | 35.000 |
| Vitamin mix (AIN-93-vx) | 10.000 | 10.000 | 10.000 |
| l-Cystine | 3.000 | 3.000 | 3.000 |
| Choline bitartrate | 2.500 | 2.500 | 2.500 |
| tert-Butylhydroquinone | 0.014 | 0.014 | 0.014 |
| Quer | 0 | 0 | 0.5 |
| Es | 0 | 0.0004 | 0 |
| Total | 1000 | 1000.0004 | 1000.5 |
| Parameters | SHAM | OVX | OVX + ES | OVX + Quer |
|---|---|---|---|---|
| Initial body weight (g) | 19.53 ± 0.76 | 19.81 ± 1.15 | 19.63 ± 0.85 | 19.57 ± 1.06 |
| Weight gain (g) | 3.13 ± 0.5 | 14.10 ± 1.03 ### | 4.49 ± 1.01 * | 8.59 ± 1.23 ** |
| Epidermal fat (g) | 0.258 ± 0.027 | 1.17 ± 0.162 ### | 0.172 ± 0.025 *** | 0.942 ± 0.163 * |
| Liver (g) | 0.13 ± 0.13 | 1.89 ± 0.33 ### | 1.25 ± 0.13 ** | 1.33 ± 0.12 * |
| Parameters | SHAM | OVX | OVX + ES | OVX + Quer |
|---|---|---|---|---|
| TC (mg/dL) | 128.0 ± 9.8 | 215.3 ± 13.9 ### | 175.3 ± 10.7 ** | 171.0 ± 9.9 ** |
| HDL-C (mg/dL) | 106.7 ± 11.3 | 86.7 ± 5.2 | 87.6 ± 7.2 | 104.3 ± 12 |
| LDL-C (mg/dL) | 28.9 ± 2.3 | 108.6 ± 12.5 ### | 93.3 ± 9.4 | 79.7 ± 15.4 ** |
| TG (mg/dL) | 62.4 ± 6.3 | 80.9 ± 6.5 # | 100.7 ± 10.6 | 66.1 ± 6.4 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hur, H.J.; Jeong, Y.-H.; Lee, S.H.; Sung, M.J. Quercitrin Ameliorates Hyperlipidemia and Hepatic Steatosis in Ovariectomized Mice. Life 2020, 10, 243. https://doi.org/10.3390/life10100243
Hur HJ, Jeong Y-H, Lee SH, Sung MJ. Quercitrin Ameliorates Hyperlipidemia and Hepatic Steatosis in Ovariectomized Mice. Life. 2020; 10(10):243. https://doi.org/10.3390/life10100243
Chicago/Turabian StyleHur, Haeng Jeon, Yeon-Hui Jeong, Sang Hee Lee, and Mi Jeong Sung. 2020. "Quercitrin Ameliorates Hyperlipidemia and Hepatic Steatosis in Ovariectomized Mice" Life 10, no. 10: 243. https://doi.org/10.3390/life10100243
APA StyleHur, H. J., Jeong, Y.-H., Lee, S. H., & Sung, M. J. (2020). Quercitrin Ameliorates Hyperlipidemia and Hepatic Steatosis in Ovariectomized Mice. Life, 10(10), 243. https://doi.org/10.3390/life10100243





