Lactic Acid Bacteria Ameliorate Diesel Exhaust Particulate Matter-Exacerbated Allergic Inflammation in a Murine Model of Asthma
Abstract
:1. Introduction
2. Results
2.1. L. plantarum GCWB1001, P. acidilactici GCWB1085, and L. rhamnosus GCWB1156 are Safe Probiotic Strains
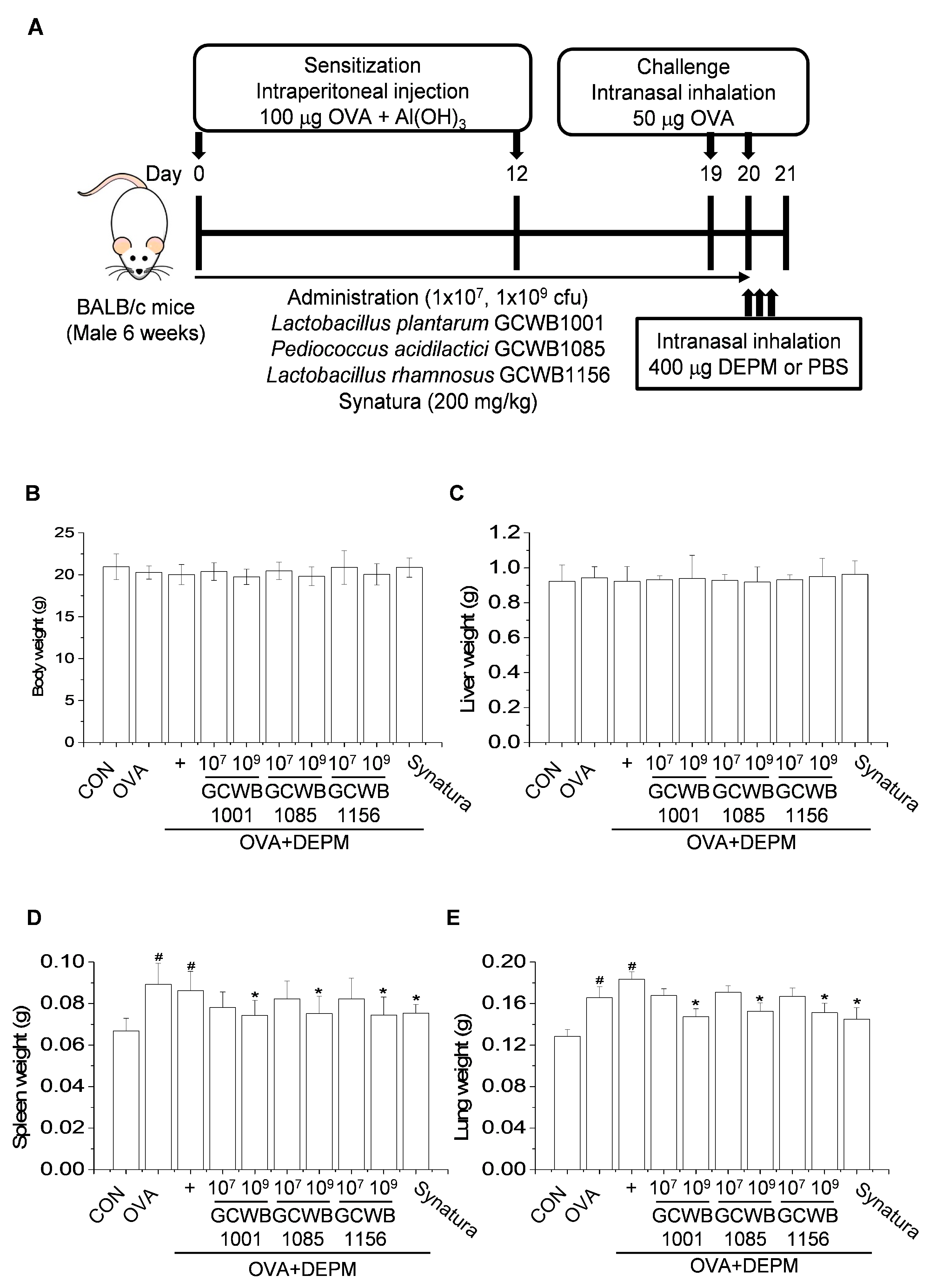
2.2. L. plantarum GCWB1001, P. acidilactici GCWB1085, and L. rhamnosus GCWB1156 Decrease the Weight of the Spleen and Lungs in a DEPM-Exacerbated Mouse Model of Asthma
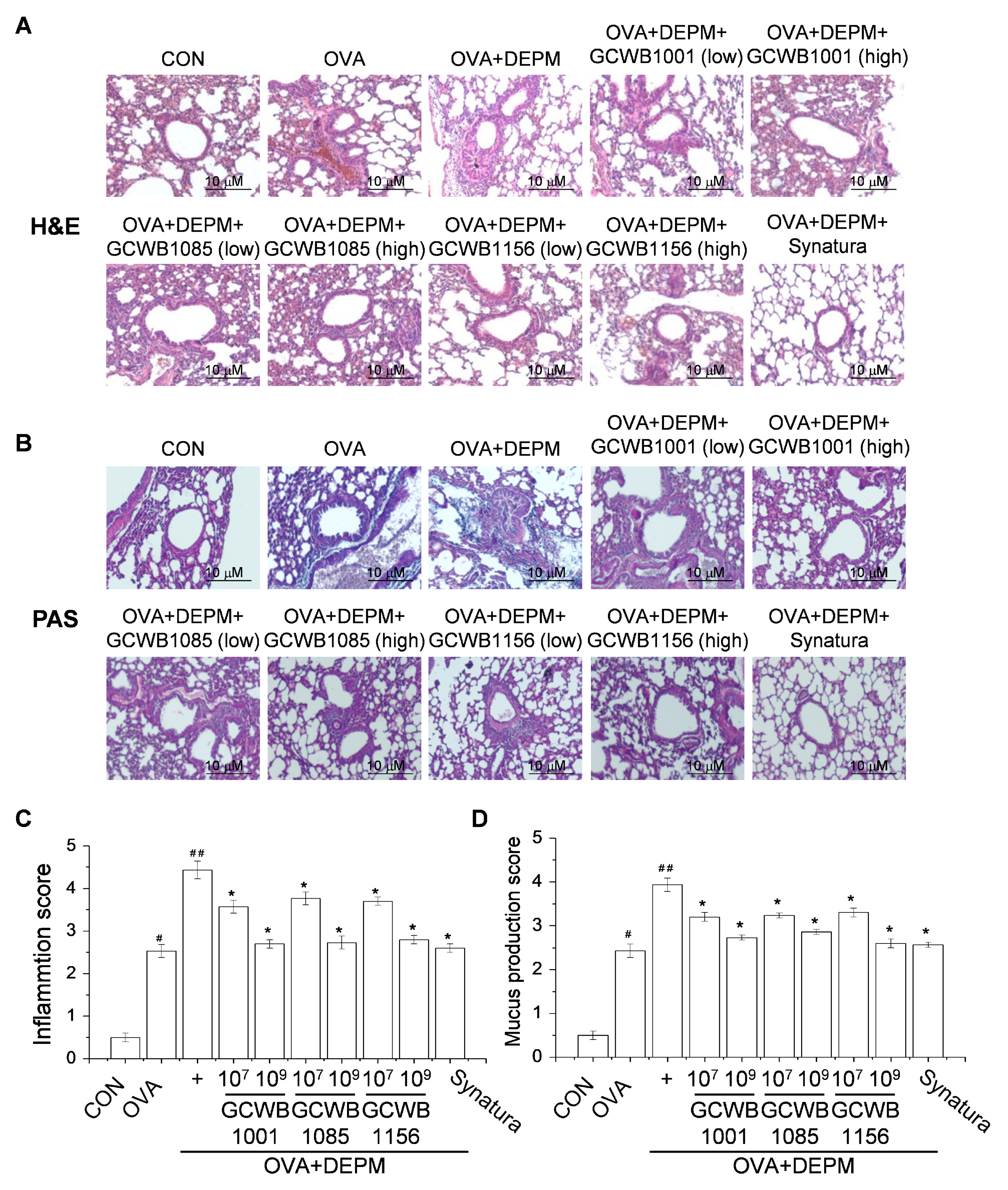
2.3. Administration of L. plantarum GCWB1001, P. acidilactici GCWB1085, and L. rhamnosus GCWB1156 Alleviated the Inflammatory Cell Infiltration Exacerbated by DEPM Exposure
2.4. Administration of L. plantarum GCWB1001, P. acidilactici GCWB1085, and L. rhamnosus GCWB1156 Inhibited DEPM-induced Exacerbation of Inflammatory Cytokines and Chemokines in BALF
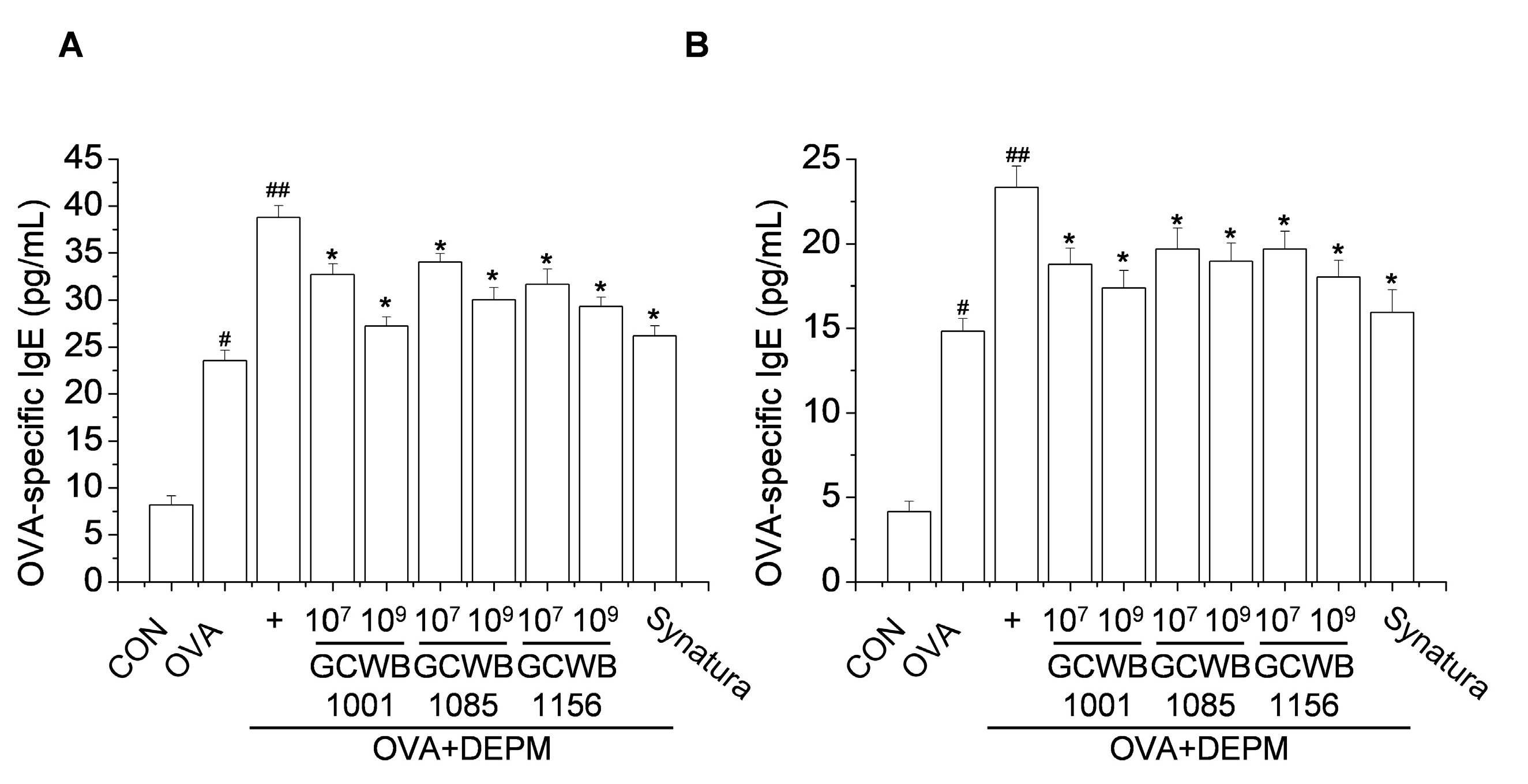
2.5. Administration of L. plantarum GCWB1001, P. acidilactici GCWB1085, and L. rhamnosus GCWB1156 Inhibited the IgE Level Increased by OVA Plus DEPM in BALF and Serum
2.6. The Effects of L. plantarum GCWB1001, P. acidilactici GCWB1085, and L. rhamnosus GCWB1156 on the Inflammatory Response and Mucus Production in Lungs Caused by DEPM
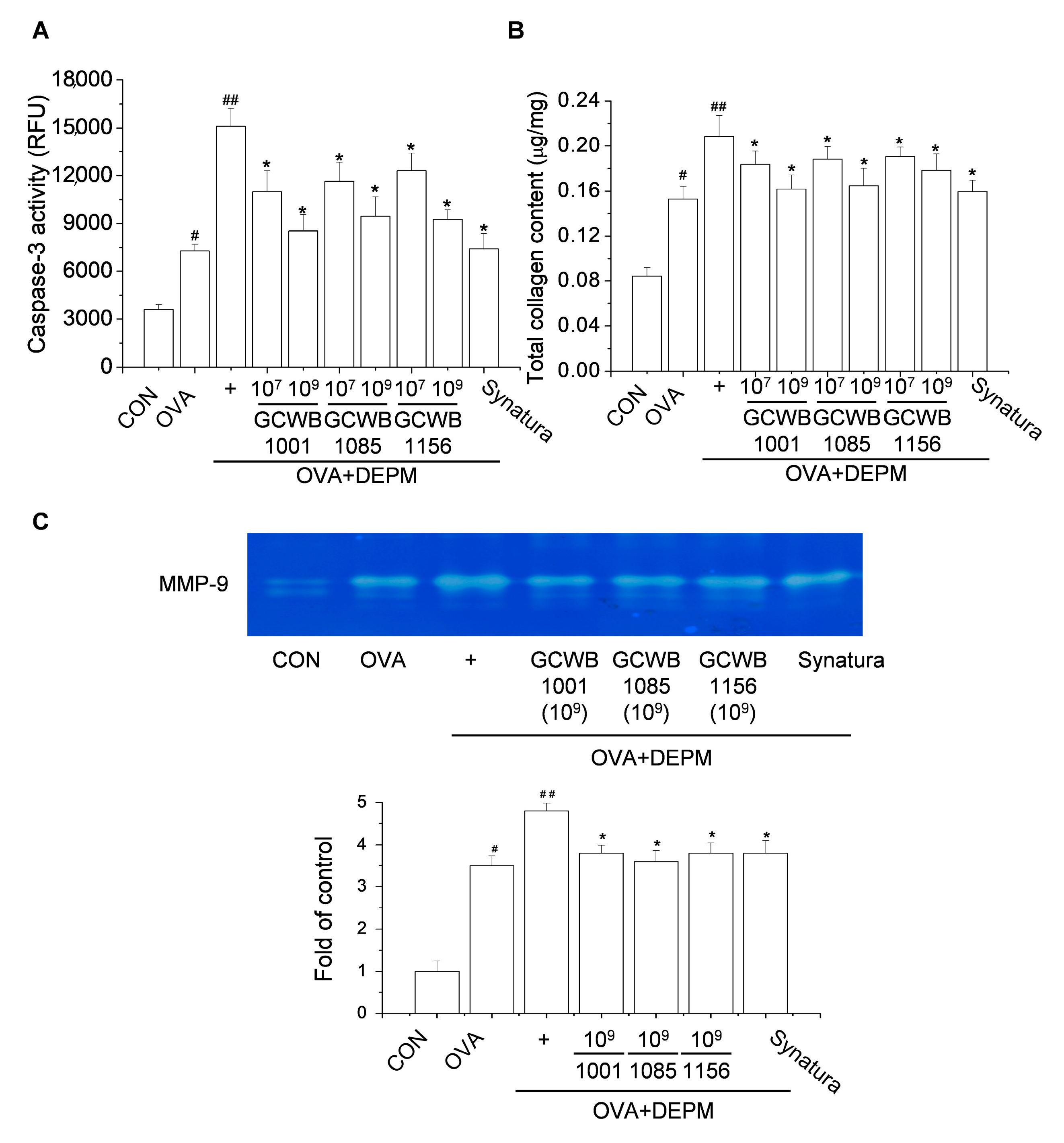
2.7. The Effects of L. plantarum GCWB1001, P. acidilactici GCWB1085, and L. rhamnosus GCWB1156 on Caspase-3 Activity, Total Collagen Level, and Matrix Metalloproteinase (MMP)-9 Activity in Lungs Treated with DEPM
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of DEPM Suspensions
4.3. Preparation of Lactic Acid Bacteria
4.4. Measurement of Cell Viability
4.5. Establishment of a DEPM-Exacerbated Mouse Model of Asthma and Probiotic Treatment
4.6. BALF Collection and Analysis of Cell Composition
4.7. Measurement of Hemolytic Activity
4.8. Measurement of the Total Lung Collagen Level and Caspase-3 Activity
4.9. Histological Examination of Lung Tissue
4.10. ELISA
4.11. Enzymatic Activity of MMP-9 by Gelatin Zymography
4.12. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AHR | Airway hyperresponsiveness |
| B(a)P | Benzo(a)pyrene |
| BALF | Bronchoalveolar lavage fluid |
| DEPM | Diesel exhaust particulate matter |
| ELISA | Enzyme-linked immunosorbent assay |
| LAB | Lactic acid bacteria |
| OVA | Ovalbumin |
| PAHs | Polycyclic aromatic hydrocarbons |
| PM | Particulate matter |
References
- Boulet, L.P. Airway remodeling in asthma: Update on mechanisms and therapeutic approaches. Curr. Opin. Pulm. Med. 2018, 24, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Olin, J.T.; Wechsler, M.E. Asthma: Pathogenesis and novel drugs for treatment. BMJ 2014, 349, g5517. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y.; DeKruyff, R.H.; Umetsu, D.T. The many paths to asthma: Phenotype shaped by innate and adaptive immunity. Nat. Immunol. 2010, 11, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Murphy, D.M.; O’Byrne, P.M. Recent advances in the pathophysiology of asthma. Chest 2010, 137, 1417–1426. [Google Scholar] [CrossRef]
- Steiner, S.; Bisig, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Diesel exhaust: Current knowledge of adverse effects and underlying cellular mechanisms. Arch. Toxicol. 2016, 90, 1541–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, X.; Barreiro, E.; Bustamante, V.; Lopez-Campos, J.L.; González-Barcala, F.J.; Cruz, M.J. Diesel exhausts particles: Their role in increasing the incidence of asthma. Reviewing the evidence of a causal link. Sci. Total Environ. 2019, 652, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Smith, C.B.; Madden, M.C. Diesel exhaust particles and airway inflammation. Curr. Opin. Pulm. Med. 2012, 18, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef] [Green Version]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalliomaki, M.; Salminen, S.; Poussa, T.; Arvilommi, H.; Isolauri, E. Probiotics and prevention of atopic disease: 4-year followup of a randomised placebo-controlled trial. Lancet 2013, 361, 1869–1871. [Google Scholar] [CrossRef]
- Wohlgemuth, S.; Loh, G.; Blaut, M. Recent developments and perspectives in the investigation of probiotic effects. Int. J. Med. Microbiol. 2010, 300, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Molimard, P.; Courau, S.; Crociani, J.; Dufour, C.; Le Vacon, F.; Carton, T. Beneficial effects of probiotics in upper respiratory tract infections and their mechanical actions to antagonize pathogens. J. Appl. Microbiol. 2012, 113, 1305–1318. [Google Scholar] [CrossRef] [PubMed]
- Żukiewicz-Sobczak, W.; Wróblewska, P.; Adamczuk, P.; Silny, W. Probiotic lactic acid bacteria and their potential in the prevention and treatment of allergic diseases. Cent. Eur. J. Immunol. 2014, 39, 104–108. [Google Scholar] [CrossRef]
- Jan, R.L.; Yeh, K.C.; Hsieh, M.H.; Lin, Y.L.; Kao, H.F.; Li, P.H.; Chang, Y.S.; Wang, J.Y. Lactobacillus gasseri suppresses Th17 proinflammatory response and attenuates allergen-induced airway inflammation in a mouse model of allergic asthma. Br. J. Nutr. 2012, 108, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julia, V.; Macia, L.; Dombrowicz, D. The impact of diet on asthma and allergic diseases. Nat. Rev. Immunol. 2015, 15, 308–322. [Google Scholar] [CrossRef]
- Nawaz, M.; Ma, C.; Basra, M.A.; Wang, J.; Xu, J. Amelioration of ovalbumin induced allergic symptoms in Balb/c mice by potentially probiotic strains of lactobacilli. Benef. Microbes 2015, 6, 669–678. [Google Scholar] [CrossRef]
- Wu, C.T.; Lin, F.H.; Lee, Y.T.; Ku, M.S.; Lue, K.H. Effect of Lactobacillus rhamnosus GG immunopathologic changes in chronic mouse asthma model. J. Microbiol. Immunol. Infect. 2019, 52, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Lin, H.C.; Hsueh, K.C.; Wu, S.F.; Fang, S.H. Oral administration of Lactobacillus salivarius inhibits the allergic airway response in mice. Can. J. Microbiol. 2010, 56, 373–379. [Google Scholar] [CrossRef]
- Wang, X.; Hui, Y.; Zhao, L.; Hao, Y.; Guo, H.; Ren, F.; Wang, X. Oral administration of Lactobacillus paracasei L9 attenuates PM2.5-induced enhancement of airway hyperresponsiveness and allergic airway response in murine model of asthma. PLoS ONE 2017, 12, e0171721. [Google Scholar]
- Pang, L.; Zou, S.; Shi, Y.; Mao, Q.; Chen, Y. Apigenin attenuates PM2.5-induced airway hyperresponsiveness and inflammation by down-regulating NF-κB in murine model of asthma. Int. J. Clin. Exp. Pathol. 2019, 12, 3700–3709. [Google Scholar]
- Wei, T.; Tang, M. Biological Effects of Airborne Fine Particulate Matter (PM2.5) Exposure on pulmonary immune system. Environ. Toxicol. Pharmacol. 2018, 60, 195–201. [Google Scholar] [PubMed]
- Mei, M.; Song, H.; Chen, L.; Hu, B.; Bai, R.; Xu, D.; Liu, Y.; Zhao, Y.; Chen, C. Early-life exposure to three size-fractionated ultrafine and fine atmospheric particulates in Beijing exacerbates asthma development in mature mice. Part. Fibre Toxicol. 2018, 15, 13. [Google Scholar] [PubMed] [Green Version]
- Chen, J.C.; Tsai, C.C.; Hsieh, C.C.; Lan, A.; Huang, C.C.; Leu, S.F. Multispecies probiotics combination prevents ovalbumin-induced airway hyperreactivity in mice. Allergol. Immunopathol. (Madr) 2018, 46, 354–360. [Google Scholar] [PubMed]
- Kim, W.G.; Kang, G.D.; Kim, H.I.; Han, M.J.; Kim, D.H. Bifidobacterium longum IM55 and Lactobacillus plantarum IM76 alleviate allergic rhinitis in mice by restoring Th2/Treg imbalance and gut microbiota disturbance. Benef. Microbes 2019, 10, 55–67. [Google Scholar]
- Li, N.; Xia, T.; Nel, A.E. The role of oxidative stress in ambient particulate matter induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic. Biol. Med. 2008, 44, 1689–1699. [Google Scholar]
- Chauhan, P.S.; Dash, D.; Singh, R. Intranasal Curcumin Inhibits Pulmonary Fibrosis by Modulating Matrix Metalloproteinase-9 (MMP-9) in Ovalbumin-Induced Chronic Asthma. Inflammation 2017, 40, 248–258. [Google Scholar]
- Morales-Bárcenas, R.; Chirino, Y.I.; Sánchez-Pérez, Y.; Osornio-Vargas, Á.R.; Melendez-Zajgla, J.; Rosas, I.; García-Cuellar, C.M. Particulate matter (PM10) induces metalloprotease activity and invasion in airway epithelial cells. Toxicol. Lett. 2015, 237, 167–173. [Google Scholar]
- Gauvreau, G.M.; Ellis, A.K.; Denburg, J.A. Haemopoietic processes in allergic disease, eosinophil/basophil development. Clin. Exp. Allergy 2009, 39, 1297–1306. [Google Scholar]
- Uhm, T.G.; Kim, B.S.; Chung, I.Y. Eosinophil development, regulation of eosinophil-specific genes, and role of eosinophils in the pathogenesis of asthma. Allergy Asthma Immunol. Res. 2012, 4, 68–79. [Google Scholar]
- Wang, P.; Nie, X.; Wang, Y.; Li, Y.; Ge, C.; Zhang, L.; Wang, L.; Bai, R.; Chen, A.; Zhao, Y.; et al. Multiwall carbon nanotubes mediate macrophage activation and promote pulmonary fibrosis through TGF-beta/Smad signaling pathway. Small 2013, 9, 3799–3811. [Google Scholar]
- Ozdemir, O. Various effects of different probiotic strains in allergic disorders: An update from laboratory and clinical data. Clin. Exp. Immunol. 2010, 160, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Liao, T.W.; Chen, Y.H.; Chiang, Y.C.; Tsai, Y.C. Oral administration of heat-inactivated Lactobacillus plantarum K37 modulated airway hyperresponsiveness in ovalbumin-sensitized BALB/c mice. PLoS ONE 2014, 9, e100105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Gangi, A.; Di Cicco, M.E.; Comberiati, P.; Peroni, D.G. Go With Your Gut: The Shaping of T-Cell Response by Gut Microbiota in Allergic Asthma. Front. Immunol. 2020, 11, 1485. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, Z.; Liu, X.; Hu, W.; Lu, W.; Lee, Y.K.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus reuteri attenuated allergic inflammation induced by HDM in the mouse and modulated gut microbes. PLoS ONE 2020, 15, e0231865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comhair, S.A.; Xu, W.; Ghosh, S.; Thunnissen, F.B.; Almasan, A.; Calhoun, W.J.; Janocha, A.J.; Zheng, L.; Hazen, S.L.; Erzurum, S.C. Superoxide dismutase inactivation in pathophysiology of asthmatic airway remodeling and reactivity. Am. J. Pathol. 2005, 166, 663–674. [Google Scholar] [CrossRef] [Green Version]
- Imaoka, H.; Hoshino, T.; Okamoto, M.; Sakazaki, Y.; Sawada, M.; Takei, S.; Kinoshita, T.; Kawayama, T.; Kato, S.; Aizawa, H. Endogenous and exogenous thioredoxin 1 prevents goblet cell hyperplasia in a chronic antigen exposure asthma model. Allergol. Int. 2009, 58, 403–410. [Google Scholar] [CrossRef] [Green Version]
- Voynow, J.A.; Fischer, B.M.; Malarkey, D.E.; Burch, L.H.; Wong, T.; Longphre, M.; Ho, S.B.; Foster, W.M. Neutrophil elastase induces mucus cell metaplasia in mouse lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L1293–L1302. [Google Scholar] [CrossRef] [Green Version]
- De Marco, V.G.; Habibi, J.; Whaley-Connell, A.T.; Schneider, R.I.; Sowers, J.R.; Andresen, B.T.; Gutweiler, A.A.; Ma, L.; Johnson, M.S.; Ferrario, C.M.; et al. Rosuvastatin ameliorates the development of pulmonary arterial hypertension in the transgenic (mRen2)27 rat. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1128–H1139. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Wang, M.; Bramble, L.A.; Schmitz, D.A.; Schauer, J.J.; Sioutas, C.; Harkema, R.H.; Nel, A.E. The adjuvant effect of ambient particulate matter is closely reflected by the particulate oxidant potential. Environ. Health Perspect. 2009, 117, 1116–1123. [Google Scholar] [CrossRef]
- Huang, K.L.; Liu, S.Y.; Chou, C.C.; Lee, Y.H.; Cheng, T.J. The effect of size segregated ambient particulate matter on Th1/Th2-like immune responses in mice. PLoS ONE 2017, 12, e0173158. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.G.; Rhee, C.K. The clinical efficacy of AG NPP709 (Synatura®) in patients with chronic bronchitis type stable chronic obstructive pulmonary disease. J. Thorac. Dis. 2020, 12, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Russell, W.M.; Douglas-Escobar, M.; Hauser, N.; Lopez, M.; Neu, J. Live and heat-killed Lactobacillus rhamnosus GG: Effects on proinflammatory and anti-inflammatory cytokines/chemokines in gastrostomy-fed infant rats. Pediatr. Res. 2009, 66, 203–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.S.; Kim, Y.; Han, K.S.; You, S.; Oh, S.; Kim, S.H. Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Lett. Appl. Microbiol. 2006, 42, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Kobatake, E.; Nakagawa, H.; Seki, T.; Miyazaki, T. Protective effects and functional mechanisms of Lactobacillus gasseri SBT2055 against oxidative stress. PLoS ONE 2017, 12, e0177106. [Google Scholar] [CrossRef]
- Gao, D.; Gao, Z.; Zhu, G. Antioxidant effects of Lactobacillus plantarum via activation of transcription factor Nrf2. Food Funct. 2013, 4, 982–989. [Google Scholar] [CrossRef]
- Saunders, V.; Breysse, P.; Clark, J.; Sproles, A.; Davila, M.; Wills-Karp, M. Particulate matter-induced airway hyperresponsiveness is lymphocyte dependent. Environ. Health Perspect. 2010, 118, 640–646. [Google Scholar] [CrossRef]
- Al-Ramli, W.; Prefontaine, D.; Chouiali, F.; Martin, J.G.; Olivenstein, R.; Lemiere, C.; Hamid, Q. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J. Allergy Clin. Immunol. 2009, 123, 1185–1187. [Google Scholar] [CrossRef]
- Lee, C.D.; Lee, J.K.; Jeong, J.H.; Park, E.S.; Sohn, U.D.; Lee, J.H.; Shin, J.W.; Lee, J.Y. Inhibitory effects of an extract mixture of ivy (Hedera helix) leaves and Coptidis rhizoma on ovalbumin-induced allergic lung inflammation by co-exposure to Asian sand dust in mice. Yakhak Hoeji 2018, 62, 21–29. [Google Scholar] [CrossRef]
- Yao, X.J.; Huang, K.W.; Li, Y.; Zhang, Q.; Wang, J.J.; Wang, W.; Liu, J.; Lv, Z.; An, Y.Q.; Ding, Y.Z.; et al. Direct comparison of the dynamics of IL-25- and ’allergen ’-induced airways inflammation, remodelling and hypersensitivity in a murine asthma model. Clin. Exp. Allergy 2014, 44, 765–777. [Google Scholar] [CrossRef]
- Ip, W.K.; Wong, C.K.; Lam, C.W. Interleukin (IL)-4 and IL-13 up-regulate monocyte chemoattractant protein-1 expression in human bronchial epithelial cells: Involvement of p38 mitogen-activated protein kinase, extracellular signal-regulated kinase 1/2 and Janus kinase-2 but not c-Jun NH2-terminal kinase 1/2 signalling pathways. Clin. Exp. Immunol. 2006, 145, 162–172. [Google Scholar]

| Strain | Positive | Negative | ||
|---|---|---|---|---|
| Alpha | Beta | Gamma | ||
| Control | Escherichia coli ATCC25922 | O | ||
| Staphylococcus aureus ATCC12600 | O | |||
| Enterococcus faecalis ATCC19433 | O | |||
| Test | Lactobacillus plantarum GCWB1001 | O | ||
| Pediococcus acidilactici GCWB1085 | O | |||
| Lactobacillus rhamnosus GCWB1156 | O | |||
| Group | TNF-α | IL-6 | IL-1β | IL-4 | IL-13 | MCP-1 | IFN-γ | |
|---|---|---|---|---|---|---|---|---|
| OVA+ DEPM+ | CON | 68 ± 3.1 | 19 ± 0.4 | 283 ± 24 | 12 ± 0.2 | 282 ± 73 | 120 ± 14 | 149 ± 17 |
| OVA | 562 ± 41 # | 169 ± 26 # | 610 ± 40 # | 14.7 ± 0.8 # | 2009 ± 307 # | 745 ± 58 # | 89 ± 8.1 # | |
| OVA+DEPM | 1381 ± 312 ## | 1028 ± 52 ## | 1028 ± 52 ## | 14.2 ± 0.6 # | 4498 ± 994 ## | 755 ± 105 ## | 81 ± 8.8 # | |
| GCWB1001-low | 884 ± 63 * | 429 ± 88 * | 889 ± 41 * | 13.5 ± 0.4 | 2690 ± 438 * | 507 ± 49 * | 100 ± 7.3 * | |
| GCWB1001-high | 743 ± 29 * | 119 ± 19 * | 795 ± 61 * | 12.6 ± 0.3 * | 1795 ± 152 * | 377 ± 29 * | 139 ± 13 * | |
| GCWB1085-low | 994 ± 148 * | 461 ± 74 * | 919 ± 51 * | 13.4 ± 0.3 ** | 2693 ± 360 * | 581 ± 48 * | 97 ± 7.9 * | |
| GCWB1085-high | 800 ± 46 * | 150 ± 23 * | 829 ± 43 * | 12.7 ± 0.2 * | 2193 ± 221 * | 436 ± 41 * | 115 ± 8.5 * | |
| GCWB1156-low | 915 ± 56 * | 475 ± 120 * | 892 ± 36 * | 13.0 ± 0.3 * | 2600 ± 431 * | 504 ± 36 * | 95 ± 5.2 * | |
| GCWB1156-high | 841 ± 51 * | 155 ± 23 * | 829 ± 37 * | 12.6 ± 0.3 * | 1922 ± 319 * | 379 ± 23 * | 120 ± 9.5 * | |
| Synatura (200 mg/kg) | 714 ± 33 * | 87 ± 11 * | 784 ± 53 * | 12.5 ± 0.2 * | 1722 ± 266 * | 336 ± 37 * | 130 ± 11 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.W.; Lee, G.H.; Jang, M.J.; Hong, G.E.; Kim, J.Y.; Park, G.D.; Jin, H.; Kim, H.S.; Choi, C.Y.; Choi, J.H.; et al. Lactic Acid Bacteria Ameliorate Diesel Exhaust Particulate Matter-Exacerbated Allergic Inflammation in a Murine Model of Asthma. Life 2020, 10, 260. https://doi.org/10.3390/life10110260
Jin SW, Lee GH, Jang MJ, Hong GE, Kim JY, Park GD, Jin H, Kim HS, Choi CY, Choi JH, et al. Lactic Acid Bacteria Ameliorate Diesel Exhaust Particulate Matter-Exacerbated Allergic Inflammation in a Murine Model of Asthma. Life. 2020; 10(11):260. https://doi.org/10.3390/life10110260
Chicago/Turabian StyleJin, Sun Woo, Gi Ho Lee, Min Jung Jang, Gyeong Eun Hong, Jae Young Kim, Gi Deok Park, Hui Jin, Hyun Su Kim, Chul Yung Choi, Jae Ho Choi, and et al. 2020. "Lactic Acid Bacteria Ameliorate Diesel Exhaust Particulate Matter-Exacerbated Allergic Inflammation in a Murine Model of Asthma" Life 10, no. 11: 260. https://doi.org/10.3390/life10110260







