Methodologies of Primary HPV Testing Currently Applied for Cervical Cancer Screening
Abstract
1. Introduction
2. HPV Testing: Methodologies and Implementation in Screening Programs
2.1. Cervical Cytology and Reasons That Lead to HPV-Based Approaches
2.2. Reasoning of HPV Testing Implementation in Screening Programs
2.3. HPV Testing Assays and Validation
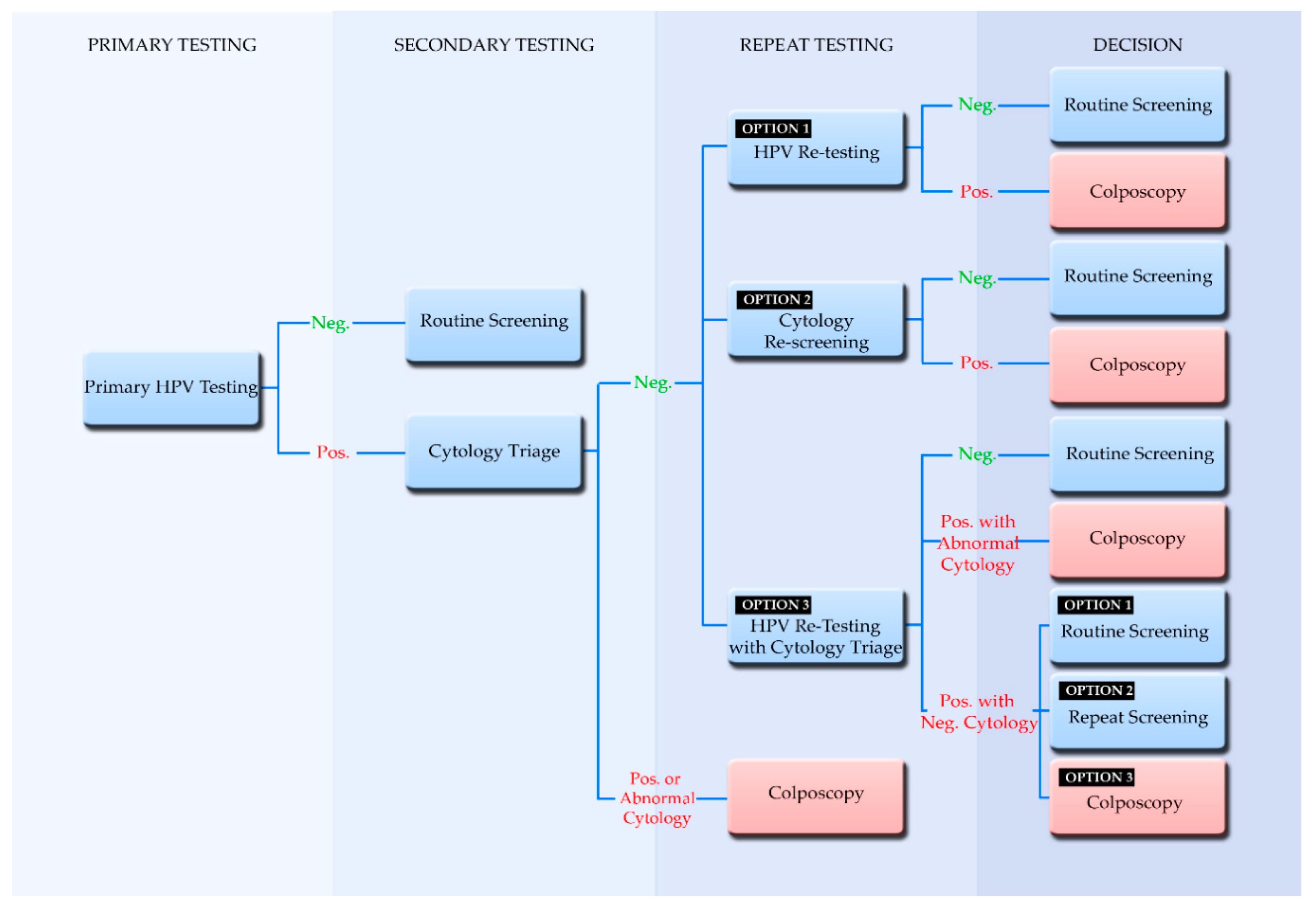
2.4. Screening Algorithms Employing Primary HPV Testing
2.5. Participation in Screening and the Implementation of Self-Sampling
2.6. Implementation of Primary HPV Testing in Europe
3. Emerging Diagnostic Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HPV | Human papillomavirus |
| Pap test | Papanicolaou test |
| Lr | Lower risk |
| Hr | High-risk |
| ASC-US | Atypical squamous cells of undetermined significance |
| NPV | Negative predictive value |
| LEEP | Loop electrosurgical excision procedure |
| RFLP | Restriction fragment length polymorphism |
| TMA | Transcription mediated amplification |
| NASBA | Nucleic-acid sequenced based amplification |
| VALGENT | Validation of HPV Genotyping Tests |
| HC2 | Hybrid Capture 2 |
| EIA | Enzyme Immunoassay |
| US FDA | United States Food and Drug Administration |
| LMNX | Luminex |
| IARC | International Agency for Research on Cancer |
| LBC | Liquid-Based Cytology |
| PCR | Polymerase Chain Reaction |
References
- Tommasino, M. The human papillomavirus family and its role in carcinogenesis. Semin. Cancer Biol. 2014, 26, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Bowden, S.J.; Kyrgiou, M. Human papillomavirus. Obstet. Gynaecol. Reprod. Med. 2020, 30, 109–118. [Google Scholar] [CrossRef]
- Vos, R.A.; Pasmans, H.; Tymchenko, L.; Janga-Jansen, A.V.A.; Baboe-Kalpoe, S.; Hulshof, K.; de Melker, H.E.; van der Klis, F.R.M. High seroprevalence of multiple high-risk human papillomavirus types among the general population of Bonaire, St. Eustatius and Saba, Caribbean Netherlands. Vaccine 2020, 38, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Moscicki, A.-B.; Schiffman, M.; Burchell, A.; Albero, G.; Giuliano, A.R.; Goodman, M.T.; Kjaer, S.K.; Palefsky, J. Updating the Natural History of Human Papillomavirus and Anogenital Cancers. Vaccine 2012, 30, F24–F33. [Google Scholar] [CrossRef]
- Ermel, A.; Shew, M.L.; Imburgia, T.M.; Brown, M.; Qadadri, B.; Tong, Y.; Brown, D.R. Redetection of human papillomavirus type 16 infections of the cervix in mid-adult life. Papillomavirus Res. 2018, 5, 75–79. [Google Scholar] [CrossRef]
- Egawa, N.; Doorbar, J. The low-risk papillomaviruses. Virus Res. 2017, 231, 119–127. [Google Scholar] [CrossRef]
- Flores-Miramontes, M.G.; Olszewski, D.; Artaza-Irigaray, C.; Willemsen, A.; Bravo, I.G.; Vallejo-Ruiz, V.; Leal-Herrera, Y.A.; Piña-Sánchez, P.; Molina-Pineda, A.; Cantón-Romero, J.C.; et al. Detection of Alpha, Beta, Gamma, and Unclassified Human Papillomaviruses in Cervical Cancer Samples From Mexican Women. Front. Cell. Infect. Microbiol. 2020, 10, 234. [Google Scholar] [CrossRef]
- Rantshabeng, P.; Kasvosve, I.; Ndlovu, A.; Gaseitsiwe, S.; Moyo, S. Prevalence of high-risk human papilloma virus in women with high-grade squamous cell intraepithelial lesions in Botswana using Abbott RealTime HPV assay. PLoS ONE 2019, 14, e0211260. [Google Scholar] [CrossRef]
- González, J.V.; Deluca, G.D.; Liotta, D.J.; Correa, R.M.; Basiletti, J.A.; Colucci, M.C.; Katz, N.; Vizzotti, C.; Picconi, M.A.; Giurgiovich, A.; et al. Baseline prevalence and type distribution of Human papillomavirus in sexually active non-vaccinated adolescent girls from Argentina. Rev. Argent. Microbiol. 2020. [Google Scholar] [CrossRef]
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Shingleton, H.M.; Patrick, R.L.; Johnston, W.W.; Smith, R.A. The current status of the Papanicolaou smear. CA Cancer J. Clin. 1995, 45, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Petry, K.U. HPV and cervical cancer. Scand. J. Clin. Lab. Invest. 2014, 74, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Durst, M.; Gissmann, L.; Ikenberg, H.; zur Hausen, H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc. Natl. Acad. Sci. USA 1983, 80, 3812–3815. [Google Scholar] [CrossRef]
- Chrysostomou, A.; Stylianou, D.; Constantinidou, A.; Kostrikis, L. Cervical Cancer Screening Programs in Europe: The Transition Towards HPV Vaccination and Population-Based HPV Testing. Viruses 2018, 10, 729. [Google Scholar] [CrossRef]
- Chantziantoniou, N.; Donnelly, A.D.; Mukherjee, M.; Boon, M.E.; Austin, R.M. Inception and Development of the Papanicolaou Stain Method. Acta Cytol. 2017, 61, 266–280. [Google Scholar] [CrossRef]
- Siebers, A.G.; Klinkhamer, P.J.J.M.; Grefte, J.M.M.; Massuger, L.F.A.G.; Vedder, J.E.M.; Beijers-Broos, A.; Bulten, J.; Arbyn, M. Comparison of Liquid-Based Cytology With Conventional Cytology for Detection of Cervical Cancer Precursors: A Randomized Controlled Trial. JAMA 2009, 302, 1757–1764. [Google Scholar] [CrossRef]
- Gibb, R.K.; Martens, M.G. The impact of liquid-based cytology in decreasing the incidence of cervical cancer. Rev. Obstet. Gynecol. 2011, 4, S2–S11. [Google Scholar]
- Thakur, M.; Guttikonda, V. Modified ultrafast Papanicolaou staining technique: A comparative study. J. Cytol. 2017, 34, 149. [Google Scholar] [CrossRef]
- Norimatsu, Y.; Yanoh, K.; Hirai, Y.; Kurokawa, T.; Kobayashi, T.K.; Fulciniti, F. A Diagnostic Approach to Endometrial Cytology by Means of Liquid-Based Preparations. Acta Cytol. 2020, 64, 195–207. [Google Scholar] [CrossRef]
- Raifu, A.O.; El-Zein, M.; Sangwa-Lugoma, G.; Ramanakumar, A.; Walter, S.D.; Franco, E.L. Determinants of Cervical Cancer Screening Accuracy for Visual Inspection with Acetic Acid (VIA) and Lugol’s Iodine (VILI) Performed by Nurse and Physician. PLoS ONE 2017, 12, e0170631. [Google Scholar] [CrossRef] [PubMed]
- Nayar, R.; Wilbur, D.C. The Bethesda System for Reporting Cervical Cytology: Definitions, Criteria, and Explanatory Notes; Nayar, R., Wilbur, D.C., Eds.; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Goodman, A. HPV testing as a screen for cervical cancer. BMJ 2015, 350, h2372. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Singhal, S. Primary HPV screening for cervical cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 65, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Tota, J.E.; Bentley, J.; Blake, J.; Coutlée, F.; Duggan, M.A.; Ferenczy, A.; Franco, E.L.; Fung-Kee-Fung, M.; Gotlieb, W.; Mayrand, M.-H.; et al. Introduction of molecular HPV testing as the primary technology in cervical cancer screening: Acting on evidence to change the current paradigm. Prev. Med. 2017, 98, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Von Karsa, L.; Arbyn, M.; De Vuyst, H.; Dillner, J.; Dillner, L.; Franceschi, S.; Patnick, J.; Ronco, G.; Segnan, N.; Suonio, E.; et al. European guidelines for quality assurance in cervical cancer screening. Summary of the supplements on HPV screening and vaccination. Papillomavirus Res. 2015, 1, 22–31. [Google Scholar] [CrossRef]
- Von Karsa, L.; Arbyn, M.; De Vuyst, H.; Dillner, J.; Dillner, L.; Franceschi, S.; Patnick, J.; Ronco, G.; Segnan, N.; Suonio, E.; et al. European Guidelines for Quality Assurance in Cervical Cancer Screening—Second Edition Supplements; EU: Luxembourg, 2015. [Google Scholar]
- Basu, P.; Mittal, S.; Bhadra Vale, D.; Chami Kharaji, Y. Secondary prevention of cervical cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 73–85. [Google Scholar] [CrossRef]
- Jin, X.W.; Lipold, L.; Foucher, J.; Sikon, A.; Brainard, J.; Belinson, J.; Schramm, S.; Nottingham, K.; Hu, B.; Rothberg, M.B. Cost-Effectiveness of Primary HPV Testing, Cytology and Co-testing as Cervical Cancer Screening for Women Above Age 30 Years. J. Gen. Intern. Med. 2016, 31, 1338–1344. [Google Scholar] [CrossRef]
- Basu, P.; Meheus, F.; Chami, Y.; Hariprasad, R.; Zhao, F.; Sankaranarayanan, R. Management algorithms for cervical cancer screening and precancer treatment for resource-limited settings. Int. J. Gynecol. Obstet. 2017, 138, 26–32. [Google Scholar] [CrossRef]
- Georgalis, L.; de Sanjosé, S.; Esnaola, M.; Bosch, F.X.; Diaz, M. Present and future of cervical cancer prevention in Spain: A Cost-Effectiveness Analysis. Eur. J. Cancer Prev. 2016, 25, 430–439. [Google Scholar] [CrossRef]
- Malagón, T.; Kulasingam, S.; Mayrand, M.-H.; Ogilvie, G.; Smith, L.; Bouchard, C.; Gotlieb, W.; Franco, E.L. Age at last screening and remaining lifetime risk of cervical cancer in older, unvaccinated women: A modelling study. Lancet Oncol. 2018, 19, 1569–1578. [Google Scholar] [CrossRef]
- Hermansson, R.S.; Olovsson, M.; Hoxell, E.; Lindström, A.K. HPV prevalence and HPV-related dysplasia in elderly women. PLoS ONE 2018, 13, e0189300. [Google Scholar] [CrossRef] [PubMed]
- Schlichte, M.; Guidry, J. Current Cervical Carcinoma Screening Guidelines. J. Clin. Med. 2015, 4, 918–932. [Google Scholar] [CrossRef] [PubMed]
- Poljak, M.; Oštrbenk Valenčak, A.; Gimpelj Domjanič, G.; Xu, L.; Arbyn, M. Commercially available molecular tests for human papillomaviruses: A global overview. Clin. Microbiol. Infect. 2020, 26, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Tsakogiannis, D.; Gartzonika, C.; Levidiotou-Stefanou, S.; Markoulatos, P. Molecular approaches for HPV genotyping and HPV-DNA physical status. Expert Rev. Mol. Med. 2017, 19, e1. [Google Scholar] [CrossRef]
- Meijer, C.J.L.M.; Berkhof, J.; Castle, P.E.; Hesselink, A.T.; Franco, E.L.; Ronco, G.; Arbyn, M.; Bosch, F.X.; Cuzick, J.; Dillner, J.; et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int. J. Cancer 2009, 124, 516–520. [Google Scholar] [CrossRef]
- Gautam, A.; Gedda, M.R.; Rai, M.; Sundar, S.; Chakravarty, J. Human Papillomavirus Genome based Detection and Typing: A Holistic Molecular Approach. Curr. Mol. Med. 2019, 19, 237–246. [Google Scholar] [CrossRef]
- Arbyn, M.; Snijders, P.J.F.; Meijer, C.J.L.M.; Berkhof, J.; Cuschieri, K.; Kocjan, B.J.; Poljak, M. Which high-risk HPV assays fulfil criteria for use in primary cervical cancer screening? Clin. Microbiol. Infect. 2015, 21, 817–826. [Google Scholar] [CrossRef]
- Arbyn, M.; Depuydt, C.; Benoy, I.; Bogers, J.; Cuschieri, K.; Schmitt, M.; Pawlita, M.; Geraets, D.; Heard, I.; Gheit, T.; et al. VALGENT: A protocol for clinical validation of human papillomavirus assays. J. Clin. Virol. 2016, 76, S14–S21. [Google Scholar] [CrossRef]
- Bonde, J.; Ejegod, D.M.; Cuschieri, K.; Dillner, J.; Heideman, D.A.M.; Quint, W.; Pavon Ribas, M.A.; Padalko, E.; Christiansen, I.K.; Xu, L.; et al. The Valgent4 protocol: Robust analytical and clinical validation of 11 HPV assays with genotyping on cervical samples collected in SurePath medium. J. Clin. Virol. 2018, 108, 64–71. [Google Scholar] [CrossRef]
- Maver, P.J.; Poljak, M. Primary HPV-based cervical cancer screening in Europe: Implementation status, challenges, and future plans. Clin. Microbiol. Infect. 2020, 26, 579–583. [Google Scholar] [CrossRef]
- PAHO Pan American Health Organization. Available online: https://www.paho.org/hq/index.php?option=com_content&view=article&id=11925:hpv-tests-for-cervical-cancer-screening&Itemid=41948&showall=1&lang=en (accessed on 2 November 2020).
- Isidean, S.D.; Coutlée, F.; Franco, E.L. cobas ® 4800 HPV Test, a real-time polymerase chain reaction assay for the detection of human papillomavirus in cervical specimens. Expert Rev. Mol. Diagn. 2014, 14, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Haedicke, J.; Iftner, T. A review of the clinical performance of the Aptima HPV assay. J. Clin. Virol. 2016, 76, S40–S48. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Vaughan, L.; Yanson, K.; Eckert, K.; Li, A.; Harris, J.; Ermel, A.; Williams, J.A.; Al-Ghoul, M.; Cammarata, C.L.; et al. Analytical and Clinical Sample Performance Characteristics of the Onclarity Assay for the Detection of Human Papillomavirus. J. Clin. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, A.T.; Heideman, D.A.M.; Berkhof, J.; Topal, F.; Pol, R.P.; Meijer, C.J.L.M.; Snijders, P.J.F. Comparison of the Clinical Performance of PapilloCheck Human Papillomavirus Detection with That of the GP5+/6+-PCR-Enzyme Immunoassay in Population-Based Cervical Screening. J. Clin. Microbiol. 2010, 48, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Ejegod, D.M.; Hansen, M.; Christiansen, I.K.; Pedersen, H.; Quint, W.; Xu, L.; Arbyn, M.; Bonde, J. Clinical validation of the Cobas 4800 HPV assay using cervical samples in SurePath medium under the VALGENT4 framework. J. Clin. Virol. 2020, 128, 104336. [Google Scholar] [CrossRef]
- Snijders, P.J.F.; van den Brule, A.J.C.; Jacobs, M.V.; Pol, R.P.; Meijer, C.J.L.M. HPV DNA Detection and Typing in Cervical Scrapes. In Human Papillomaviruses; Humana Press: Totowa, NJ, USA, 2005; pp. 101–114. [Google Scholar]
- Heideman, D.A.M.; Hesselink, A.T.; van Kemenade, F.J.; Iftner, T.; Berkhof, J.; Topal, F.; Agard, D.; Meijer, C.J.L.M.; Snijders, P.J.F. The Aptima HPV Assay Fulfills the Cross-Sectional Clinical and Reproducibility Criteria of International Guidelines for Human Papillomavirus Test Requirements for Cervical Screening. J. Clin. Microbiol. 2013, 51, 3653–3657. [Google Scholar] [CrossRef]
- Bonde, J.H.; Pedersen, H.; Quint, W.; Xu, L.; Arbyn, M.; Ejegod, D.M. Clinical and Analytical Performance of the BD Onclarity HPV Assay with Surepath Screening Samples from the Danish Cervical Screening Program Using the VALGENT Framework. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef]
- Lorincz, A.; Wheeler, C.M.; Cuschieri, K.; Geraets, D.; Meijer, C.J.L.M.; Quint, W. Chapter 7—Developing and Standardizing Human Papillomavirus Tests; Jenkins, D., Bosch, F.X.B.T.-H.P., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 111–130. [Google Scholar] [CrossRef]
- Salazar, K.L.; Duhon, D.J.; Olsen, R.; Thrall, M. A Review of the FDA-Approved Molecular Testing Platforms for Human Papillomavirus. J. Am. Soc. Cytopathol. 2019, 8, 284–292. [Google Scholar] [CrossRef]
- Wentzensen, N.; Schiffman, M.; Palmer, T.; Arbyn, M. Triage of HPV positive women in cervical cancer screening. J. Clin. Virol. 2016, 76, S49–S55. [Google Scholar] [CrossRef]
- D’Alessandro, P.; Arduino, B.; Borgo, M.; Saccone, G.; Venturella, R.; Di Cello, A.; Zullo, F. Loop electrosurgical excision procedure versus cryotherapy in the treatment of cervical intraepithelialneoplasia: A systematic review and meta-analysis of randomized controlled trials. Gynecol. Minim. Invasive Ther. 2018, 7, 145. [Google Scholar] [CrossRef]
- Onuki, M.; Matsumoto, K.; Sakurai, M.; Ochi, H.; Minaguchi, T.; Satoh, T.; Yoshikawa, H. Posttreatment human papillomavirus testing for residual or recurrent high-grade cervical intraepithelial neoplasia: A pooled analysis. J. Gynecol. Oncol. 2016, 27, e3. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Venturoli, S.; Origoni, M.; Preti, M.; Mariani, L.; Cristoforoni, P.; Sandri, M.T. Performance of HPV DNA testing in the follow-up after treatment of high-grade cervical lesions, adenocarcinoma in situ (AIS) and microinvasive carcinoma. Ecancermedicalscience 2015, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Satake, H.; Inaba, N.; Kanno, K.; Mihara, M.; Takagi, Y.; Kondo, N.; Sagae, S. Comparison Study of Self-Sampled and Physician-Sampled Specimens for High-Risk Human Papillomavirus Test and Cytology. Acta Cytol. 2020, 64, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Leinonen, M.K.; Schee, K.; Jonassen, C.M.; Lie, A.K.; Nystrand, C.F.; Rangberg, A.; Furre, I.E.; Johansson, M.J.; Tropé, A.; Sjøborg, K.D.; et al. Safety and acceptability of human papillomavirus testing of self-collected specimens: A methodologic study of the impact of collection devices and HPV assays on sensitivity for cervical cancer and high-grade lesions. J. Clin. Virol. 2018, 99, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Yeh, P.T.; Kennedy, C.E.; de Vuyst, H.; Narasimhan, M. Self-sampling for human papillomavirus (HPV) testing: A systematic review and meta-analysis. BMJ Glob. Health 2019, 4, e001351. [Google Scholar] [CrossRef]
- Arbyn, M.; Smith, S.B.; Temin, S.; Sultana, F.; Castle, P. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: Updated meta-analyses. BMJ 2018, 363, k4823. [Google Scholar] [CrossRef]
- Lefeuvre, C.; Pivert, A.; Le Guillou-Guillemette, H.; Lunel-Fabiani, F.; Veillon, P.; Le Duc-Banaszuk, A.-S.; Ducancelle, A. Urinary HPV DNA testing as a tool for cervical cancer screening in women who are reluctant to have a Pap smear in France. J. Infect. 2020, 81, 248–254. [Google Scholar] [CrossRef]
- Peeters, E.; Cornet, K.; Cammu, H.; Verhoeven, V.; Devroey, D.; Arbyn, M. Corrigendum to “Efficacy of strategies to increase participation in cervical cancer screening: GPs offering self-sampling kits for HPV testing versus recommendations to have a pap smear taken—A randomised controlled trial” [Papillomavirus Res. 9 (2020) 100194]. Papillomavirus Res. 2020, 9, 100201. [Google Scholar] [CrossRef]
- Tjalma, W.A.A.; Kim, E.; Vandeweyer, K. The impact on women’s health and the cervical cancer screening budget of primary HPV screening with dual-stain cytology triage in Belgium. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 212, 171–181. [Google Scholar] [CrossRef]
- Sroczynski, G.; Hillemanns, P.; Siebert, U. PCN43 new cervical cancer screening policy in germany—what is the impact on the benefit-harm balance? Value Health 2019, 22, S443. [Google Scholar] [CrossRef]
- Cervical Screening. Available online: https://deputyprimeminister.gov.mt/en/phc/nbs/Pages/Screening-Programmes/Cervical-Screening.aspx (accessed on 4 September 2020).
- Cox, B.; Sneyd, M.J. HPV screening, invasive cervical cancer and screening policy in Australia. J. Am. Soc. Cytopathol. 2018, 7, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.-W.; Kim, M.; Kim, Y.-H.; Kim, Y.B.; Kim, J.; Kim, J.-W.; Park, M.H.; Park, J.H.; Rhee, J.H.; Lim, M.C.; et al. High-risk human papillomavirus testing as a primary screening for cervical cancer: Position statement by the Korean Society of Obstetrics and Gynecology and the Korean Society of Gynecologic Oncology. J. Gynecol. Oncol. 2020, 31. [Google Scholar] [CrossRef] [PubMed]
- Gustinucci, D.; Rossi, P.G.; Cesarini, E.; Broccolini, M.; Bulletti, S.; Carlani, A.; D’angelo, V.; D’amico, M.R.; Di Dato, E.; Galeazzi, P.; et al. Use of Cytology, E6/E7 mRNA, and p16 INK4a –Ki-67 to Define the Management of Human Papillomavirus (HPV)–Positive Women in Cervical Cancer Screening. Am. J. Clin. Pathol. 2016, 145, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xu, M.; Wang, L.; Zhou, W.; Xiang, R.; Shi, Y.; Zhang, Y.; Piao, Y. Integrative analysis of DNA methylation and gene expression identified cervical cancer-specific diagnostic biomarkers. Signal Transduct. Target. Ther. 2019, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Shao, Y.; Chen, W.; Quan, C.; Zhu, Y.; Xu, X.; Zhou, Z.; Wang, S. Discovery of candidate gene expression signatures in peripheral blood for the screening of cervical cancer. Biomark. Med. 2020, 14, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Bi, H.; Zhang, X.; Zhao, Y.; Dong, Y.; Luo, X.; Zhou, D.; You, Z.; Wu, Y.; Liu, Z.; et al. Artificial intelligence-assisted cytology for detection of cervical intraepithelial neoplasia or invasive cancer: A multicenter, clinical-based, observational study. Gynecol. Oncol. 2020, 159, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Vink, F.J.; Meijer, C.J.L.M.; Clifford, G.M.; Poljak, M.; Oštrbenk, A.; Petry, K.U.; Rothe, B.; Bonde, J.; Pedersen, H.; Sanjosé, S.; et al. FAM19A4/miR124-2 methylation in invasive cervical cancer: A retrospective cross-sectional worldwide study. Int. J. Cancer 2020, 147, 1215–1221. [Google Scholar] [CrossRef]
- Kelly, H.; Benavente, Y.; Pavon, M.A.; De Sanjose, S.; Mayaud, P.; Lorincz, A.T. Performance of DNA methylation assays for detection of high-grade cervical intraepithelial neoplasia (CIN2+): A systematic review and meta-analysis. Br. J. Cancer 2019, 121, 954–965. [Google Scholar] [CrossRef]
- Song, F.; Du, H.; Xiao, A.; Wang, C.; Huang, X.; Yan, P.; Liu, Z.; Qu, X.; Belinson, J.L.; Wu, R. Evaluating the Performance of p16INK4a Immunocytochemistry in Cervical Cancer Screening. Cancer Manag. Res. 2020, 12, 9067–9075. [Google Scholar] [CrossRef]
- Bao, H.; Sun, X.; Zhang, Y.; Pang, B.; Li, H.; Zhou, L.; Wu, F.; Cao, D.; Wang, J.; Turic, B.; et al. The artificial intelligence-assisted cytology diagnostic system in large-scale cervical cancer screening: A population-based cohort study of 0.7 million women. Cancer Med. 2020, 9, 6896–6906. [Google Scholar] [CrossRef]

| Tests | Hybrid Capture 2 (Qiagen) | GP5+/6+ EIA a | Cobas 4800 HPV Test (Roche) | APTIMA HPV Assay (Hologic) | BD Onclarity HPV Assay |
|---|---|---|---|---|---|
| Type of assay | Signal amplification, hybrid capture | PCR, probe hybridization | Real-time PCR detection | Transcription mediated amplification, probe hybridization | Real-time PCR detection |
| Targets | DNA, Whole viral genome | L1 DNA, 150 bp | L1 DNA 200 bp | E6/E7 mRNA | E6 and E7 DNA |
| HPV Subtypes detected | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68 | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68. Individual genotyping for: 16, 18 | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68. Reflex Partial genotyping for: 16, 18–45 | 33–58; 56–59–66; 35–39–68 f. Individual genotyping for: 16, 18, 31, 45, 51, and 52 |
| Internal Controls Human genes | NO | NO | Internal human β-globin control | Internal RNA transcript (HPV16 E6/7) control | Internal human β-globin control |
| Capacity Batch size | 88 | 96 samples in 9.5 h e | 96 | Panther system 100 and 250 test /Tigris DTS system 250 | 46 |
| VALGENT Validation | Standard comparator tests for validation b | Standard comparator tests for validation b | YES | YES | YES |
| US FDA c Validation | YES | NO | YES | YES | YES |
| CE Mark d Validation | YES | YES | YES | YES | YES |
| Uses within a screening program | ASC-US Triage, test-of-cure | ASC-US Triage, test-of-cure | ASC-US Triage/co-testing/Primary testing | ASC-US Triage/co-testing | ASC-US Triage/co-testing/Primary testing |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrysostomou, A.C.; Kostrikis, L.G. Methodologies of Primary HPV Testing Currently Applied for Cervical Cancer Screening. Life 2020, 10, 290. https://doi.org/10.3390/life10110290
Chrysostomou AC, Kostrikis LG. Methodologies of Primary HPV Testing Currently Applied for Cervical Cancer Screening. Life. 2020; 10(11):290. https://doi.org/10.3390/life10110290
Chicago/Turabian StyleChrysostomou, Andreas C., and Leondios G. Kostrikis. 2020. "Methodologies of Primary HPV Testing Currently Applied for Cervical Cancer Screening" Life 10, no. 11: 290. https://doi.org/10.3390/life10110290
APA StyleChrysostomou, A. C., & Kostrikis, L. G. (2020). Methodologies of Primary HPV Testing Currently Applied for Cervical Cancer Screening. Life, 10(11), 290. https://doi.org/10.3390/life10110290






