Relative Biological Effectiveness of High LET Particles on the Reproductive System and Fetal Development
Abstract
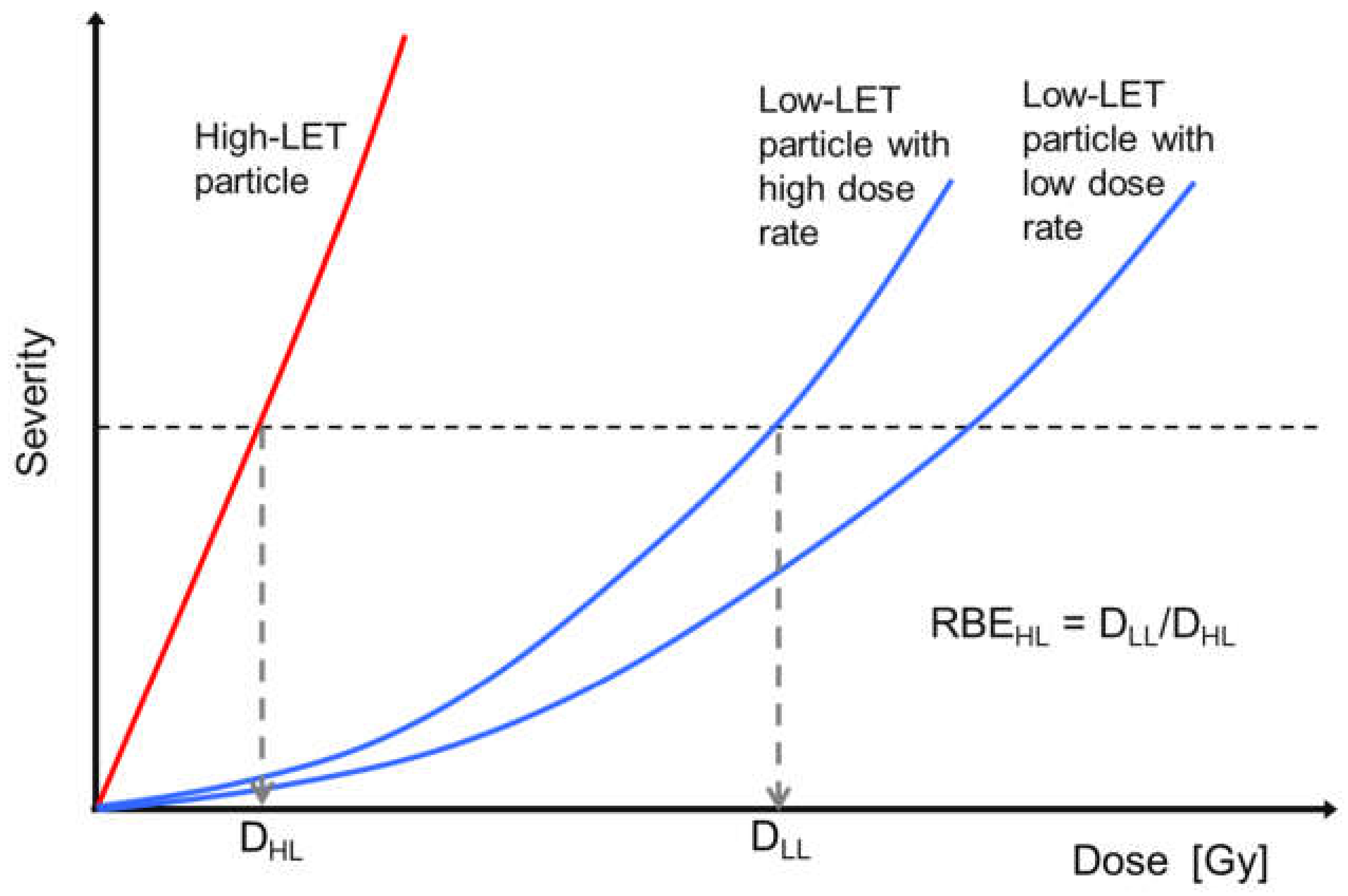
1. Introduction
2. On the Reproductive System
2.1. Effects on Female Reproductive System
RBE for Ovary
2.2. Effects on Male Reproductive System
RBE for Testis
3. On Embryonic and Fetal Development
3.1. Effects on the Developing Embryo and Fetus
RBE for Embryonic and Fetal Development
3.2. Effects on Developing Brain
3.3. Effects on Adult-Onset Noncancer Diseases
4. On Other Effects Related to Reproductive Ability
4.1. Lowering Fecundability
4.2. Sexual Dysfunction
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Council on Radiation Protection and Measurements (NCRP). Guidance on Radiation Received in Space Activities; NCRP Report No. 98; NCRP: Bethesda, MD, USA, 1989. [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP). Recommendations of Dose Limits for Low Earth Orbit; NCRP Report No. 132; NCRP: Bethesda, MD, USA, 2000. [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP). Operational Radiation Safety Program for Astronauts in Low-Earth Orbit: A Basic Framework; NCRP Report No. 142; NCRP: Bethesda, MD, USA, 2002. [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP). Information Needed to Make Radiation Protection Recommendations for Space Missions beyond Low-Earth Orbit; NCRP Report No. 153; NCRP: Bethesda, MD, USA, 2006. [Google Scholar]
- Nelson, G.A. Space radiation and human exposures, a primer. Radiat. Res. 2016, 185, 349–358. [Google Scholar] [CrossRef]
- Japan Aerospace Exploration Agency (JAXA). Rules on Radiation Exposure Management for the Astronauts Involved in the International Space Station; Rule no. 25–42; JAXA: Chofu, Tokyo, Japan, 2013; Available online: http://iss.jaxa.jp/med/research/radiation/pdf/kitei_130626_a.pdf (accessed on 15 October 2020). (In Japanese)
- International Commission on Radiological Protection (ICRP). Assessment of Radiation Exposure of Astronauts in Space; ICRP Publication 123; SAGE: London, UK, 2013. [Google Scholar]
- Badhwar, G.D.; O’Neill, P.M. Galactic cosmic radiation model and its applications. Adv. Space Res. 1996, 17, 7–17. [Google Scholar] [CrossRef]
- Benton, E.R.; Benton, E.V. Space radiation dosimetry in low-Earth orbit and beyond. Nucl. Instrum. Methods Phys. Res. B 2001, 184, 255–294. [Google Scholar] [CrossRef]
- Bourdarie, S.; Xapsos, M. The near-Earth space radiation environment. IEEE Transact. Nucl. Sci. 2008, 55, 1810–1832. [Google Scholar] [CrossRef]
- Parsons, J.L.; Townsend, L.W. Interplanetary crew dose rates for the August 1972 solar particle event. Radiat. Res. 2000, 153, 729–733. [Google Scholar] [CrossRef]
- Townsend, L.W.; Stephens, D.L., Jr.; Hoff, J.L.; Zapp, E.N.; Moussa, H.M.; Miller, T.M.; Campbell, C.E.; Nichols, T.F. The Carrington event: Possible doses to crews in space from a comparable event. Adv. Space Res. 2006, 38, 226–231. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Kim, M.H.Y.; Ren, L. Evaluating shielding effectiveness for reducing space radiation cancer risks. Radiat. Meas. 2006, 41, 1173–1185. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Kim, M.Y.; Willingham, V.; George, K.A. Physical and biological organ dosimetry analysis for International Space Station Astronauts. Radiat. Res. 2008, 170, 127–138. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Kim, M.-H.Y.; Chappell, L.J.; Huff, J.L. How safe is safe enough? Radiation risk for a human mission to Mars. PLoS ONE 2013, 8, e74988. [Google Scholar] [CrossRef]
- Zeitlin, C.; Hassler, D.M.; Cucinotta, F.A.; Ehresmann, B.; Wimmer-Schweingruber, R.F.; Brinza, D.E.; Kang, S.; Weigle, G.; Böttcher, S.; Böhm, E.; et al. Measurements of energetic particle radiation in transit to Mars on the Mars science laboratory. Science 2013, 340, 1080–1084. [Google Scholar] [CrossRef]
- Slaba, T.C.; Blattnig, S.R.; Norbury, J.W.; Rusek, A.; La Tessa, C.; Walker, S.A. GCR Simulator Reference Field and a Spectral Approach for Laboratory Simulation; NASA Report, No. L-20550; NASA Langley Research Center: Hampton, VA, USA, 2015. [Google Scholar]
- Sato, T.; Nagamatsu, A.; Ueno, H.; Kataoka, R.; Miyake, S.; Takeda, K.; Niita, K. Comparison of cosmic-ray environments on earth, moon, Mars and in spacecraft using PHITS. Radiat. Prot. Dosim. 2018, 180, 146–149. [Google Scholar] [CrossRef]
- Furukawa, S.; Nagamatsu, A.; Nenoi, M.; Fujimori, A.; Kakinuma, S.; Katsube, T.; Wang, B.; Tsuruoka, C.; Shirai, T.; Nakamura, A.J.; et al. Space radiation biology for “Living in Space”. BioMed Res. Int. 2020, 4703286. [Google Scholar] [CrossRef] [PubMed]
- Tobias, C.A.; Lyman, J.T.; Chatterjee, A.; Howard, J.; Maccabee, H.D.; Raju, M.R.; Smith, A.R.; Sperinde, J.M.; Welch, G.P. Radiological physics characteristics of the extracted heavy ion beams of the bevatron. Science 1971, 174, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Radiological Protection (ICRP). RBE for Deterministic Effects; ICRP Publication 58; Pergamon Press: Oxford, UK, 1989; pp. 10–35. [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP). The Relative Biological Effectiveness of Radiations of Different Quality; NCRP Report No. 104; NCRP: Bethesda, MD, USA, 1990. [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP). Preconception and Prenatal Radiation Exposure: Health Effects and Protective Guidance; NCRP Report No. 174; NCRP: Bethesda, MD, USA, 2013; pp. 1–150. [Google Scholar]
- Brent, R.L. Protection of the gametes embryo/fetus from prenatal radiation exposure. Health Phys. 2015, 108, 242–274. [Google Scholar] [CrossRef] [PubMed]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). UNSCEAR 1982 Report “Ionizing Radiation: Sources and Biological Effects”; United Nations: New York, NY, USA, 1982. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). UNSCEAR 1988 Report “Source, Effects, and Risks of Ionizing Radiation”; United Nations: New York, NY, USA, 1988. [Google Scholar]
- International Commission on Radiological Protection (ICRP). ICRP Statement on Tissue Reactions and Early and Late Effects of Radiation in Normal Tissues and Organs—Threshold Doses for Tissue Reactions in Radiation Protection Context; ICRP Publication 118; Elsevier: Amsterdam, The Netherlands, 2012; pp. 80–86. [Google Scholar]
- International Commission on Radiological Protection (ICRP). The 2007 Recommendations of the International Commission of Radiological Protection; ICRP Publication 103; Elsevier: Amsterdam, The Netherlands, 2007; pp. 57–59. [Google Scholar]
- Straume, T.; Dobson, R.L.; Kwan, T.C. Neutron RBEs and the radiosensitive target for mouse immature oocyte killing. Radiat. Res. 1987, 111, 47–57. [Google Scholar] [CrossRef]
- Satow, Y.; Hori, H.; Lee, J.Y. Teratogenic effect of fission neutron and tritium water on rat embryo. J. UOEH 1989, 11, 416–431. [Google Scholar]
- International Commission on Radiological Protection (ICRP). Relative Biological Effectiveness (RBE), Quality Factor (Q), and Radiation Weighting Factor (wR); ICRP Publication 92; Pergamon Press: Oxford, UK, 2003. [Google Scholar]
- Satow, Y.; Hori, H.; Lee, J.Y.; Ohtaki, M.; Sawada, S.; Nakamura, N.; Okada, S. Effect of tritiated water on female germ cells: Mouse oocyte killing and RBE. Int. J. Radiat. Biol. 1989, 56, 293–299. [Google Scholar] [CrossRef]
- Dobson, R.L.; Kwan, T.C. The RBE of tritium radiation measured in mouse oocytes: Increase at low exposure levels. Radiat. Res. 1976, 66, 615–625. [Google Scholar] [CrossRef]
- Dobson, R.L.; Kwan, T.C. The tritium RBE at low-level exposure: Variation with dose, dose rate, and exposure duration. Curr. Top. Radiat. Res. Q. 1978, 12, 44–62. [Google Scholar]
- Searle, A.G.; Beechey, C.V.; Green, D.; Howells, G.R. Comparative effects of protracted exposures to 60Co gamma-radiation and 239Pu alpha-radiation on breeding performance in female mice. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1980, 37, 189–200. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, X.; Yuan, Z.; Li, W.; Zhou, G.; Zhou, Q.; Bing, L.; Min, F.; Li, X.; Xie, Y. Chromosomal aberrations induced by 12C6+ ions and 60Co gamma-rays in mouse immature oocytes. Mutat. Res. 2006, 595, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.V.; Narra, V.R.; Howell, R.W.; Lanka, V.K.; Sastry, K.S. Induction of sperm head abnormalities by incorporated radionuclides: Dependence on subcellular distribution type of radiation dose rate and presence of radioprotectors. Radiat. Res. 1991, 125, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.V.; Govelitz, G.F.; Sastry, K.S. Radiotoxicity of thallium-201 in mouse testes: Inadequacy of conventional dosimetry. J. Nucl. Med. 1983, 24, 145–153. [Google Scholar] [PubMed]
- Kamiguchi, Y.; Tateno, H.; Mikamo, K. Dose-response relationship for the induction of structural chromosome aberrations in human spermatozoa after in vitro exposure to tritium beta-rays. Mutat. Res. 1990, 228, 125–131. [Google Scholar] [CrossRef]
- Rao, D.V.; Narra, V.R.; Howell, R.W.; Govelitz, G.F.; Sastry, K.S.R. In-vivo radiotoxicity of DNA incorporated 125I compared with that of densely ionising alpha-particles. Lancet 1989, 334, 650–653. [Google Scholar] [CrossRef]
- Howell, R.W.; Azure, M.T.; Narra, V.R.; Rao, D.V. Relative biological effectiveness of alpha-particle emitters in vivo at low doses. Radiat. Res. 1994, 137, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Howell, R.W.; Goddu, S.M.; Narra, V.R.; Fisher, D.R.; Schenter, R.E.; Rao, D.V. Radiotoxicity of gadolinium-148 and radium-223 in mouse testes: Relative biological effectiveness of alpha-particle emitters in vivo. Radiat. Res. 1997, 147, 342–348. [Google Scholar] [CrossRef]
- Tateno, H.; Kamiguchi, Y.; Watanabe, S.; Mikamo, K.; Sawada, S. Relative biological effectiveness (RBE) of 252Cf fission neutrons for the induction of chromosome damage in human spermatozoa. Int. J. Radiat. Biol. 1996, 70, 229–235. [Google Scholar] [CrossRef]
- Grahn, D.; Lee, C.H.; Farrington, B.F. Interpretation of cytogenetic damage induced in the germ line of male mice exposed for over 1 year to 239Pu alpha particles, fission neutrons, or 60Co gamma rays. Radiat. Res. 1983, 95, 566–583. [Google Scholar] [CrossRef]
- Pacchierotti, P.; Russo, A.; Metalli, P. Meiotic non-disjunction induced by fission neutrons relative to X-rays observed in mouse secondary spermatocytes. II. Dose-effect relationships after treatment of pachytene cells. Mutat. Res. 1987, 176, 233–241. [Google Scholar] [CrossRef]
- Matsuda, Y.; Ohara, H.; Tobari, I. Studies on radiation-induced chromosome aberrations in mouse spermatocytes. II. Dose-response relationships of chromosome aberrations induced at zygotene stage in mouse primary spermatocytes following fast neutron- and 60Co gamma-irradiations. Mutat. Res. 1987, 176, 251–257. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, J.-S.; Moon, C.; Kim, J.-C.; Jo, S.-K.; Kim, S.-H. Relative biological effectiveness of fast neutrons in a multiorgan assay for apoptosis in mouse. Environ. Toxicol. 2008, 23, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Hwang, N.H.; Feola, J.M.; Beach, J.L.; Maruyama, Y. RBE of CF-252 neutrons by mouse testes weight loss. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 901–905. [Google Scholar] [CrossRef]
- Gasinska, A.; De Ruiter-Bootsma, A.; Davids, J.A.G.; Folkard, M.; Fowler, J.F. Survival of mouse type B spermatogonia for the study of the biological effectiveness of 1 MeV, 2.3 MeV and 5.6 MeV fast neutrons. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1987, 52, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Gasinska, A. Mouse testis weight loss and survival of differentiated spermatogonia following irradiation with 250 kV X-rays and 5.5 MeV fast neutrons. Neoplasma 1985, 32, 443–449. [Google Scholar] [PubMed]
- Alpen, E.L.; Powers-Risius, P. The relative biological effect of high-Z, high-LET charged particles for spermatogonial killing. Radiat. Res. 1981, 88, 132–143. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, R.L.; Wang, R.Y.; Wei, Z.Q.; Li, W.J.; Gao, Q.X.; Chen, W.Q.; Wang, Z.H.; Han, G.W.; Liang, J.P. Chromosomal aberrations induced by 12C6+ heavy ion irradiation in spermatogonia and spermatocytes of mice. Mutat. Res. 1998, 398, 27–31. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, R.L.; Wei, Z.Q.; Li, W.J.; Gao, Q.X.; Chen, W.Q.; Wang, Z.H.; He, J.; Liang, J.P.; Han, G.W.; et al. Effects of pre-exposure of mouse testis with low-dose 16O8+ ions or 60Co gamma-rays on sperm shape abnormalities, lipid peroxidation and superoxide dismutase (SOD) activity induced by subsequent high-dose irradiation. Int. J. Radiat. Biol. 1998, 73, 163–167. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, W.; Zhou, X.-Y. Experimental studies on biological effects of tritium exposure in late last century in China. Indian J. Radiat. Res. 2007, 3, 26–33. [Google Scholar]
- International Commission on Radiological Protection (ICRP). Developmental Effects of Irradiation on the Brain of the Embryo and Fetus; ICRP Publication 49; Pregamon Press: Oxford, UK, 1986. [Google Scholar]
- International Commission on Radiological Protection (ICRP). Biological Effects after Prenatal Irradiation (Embryo and Fetus); ICRP Publication 90; Pergamon Press: Oxford, UK, 2003. [Google Scholar]
- Kozlowski, R.; Bouffler, S.D.; Haines, J.W.; Harrison, J.D.; Cox, R. In utero haemopoietic sensitivity to alpha, beta or X-irradiation in CBA/H mice. Int. J. Radiat. Biol. 2001, 77, 805–815. [Google Scholar] [CrossRef]
- Kuhne, W.W.; Gersey, B.B.; Wilkins, R.; Wu, H.; Wender, S.A.; George, V.; Dynan, W.S. Biological effects of high-energy neutrons measured in vivo using a vertebrate model. Radiat. Res. 2009, 172, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Szabó, E.R.; Brand, M.; Hans, S.; Hideghéty, K.; Karsch, L.; Lessmann, E.; Pawelke, J.; Schürer, M.; Beyreuther, E. Radiobiological effects and proton RBE determined by wildtype zebrafish embryos. PLoS ONE 2018, 813, e0206879. [Google Scholar] [CrossRef] [PubMed]
- Pampfer, S.; Streffer, C. Prenatal death and malformations after irradiation of mouse zygotes with neutrons or X-rays. Teratology 1988, 37, 599–607. [Google Scholar] [CrossRef]
- Hillebrandt, S.; Streffer, C. Relative biological effectiveness of neutrons: Induction of malformations in a mouse strain with genetic predisposition. Radiat. Res. 2000, 153, 595–598. [Google Scholar] [CrossRef]
- Streffer, C.; Molls, M. Cultures of preimplantation mouse embryos: A model for radiobiological studies. Adv. Radiat. Biol. 1987, 13, 169–213. [Google Scholar]
- Streffer, C.; Muller, W.-U. Malformations after radiation exposure of preimplantation stages. Int. J. Dev. Biol. 1996, 40, 355–360. [Google Scholar]
- Yamada, T.; Yukawa, O.; Asami, K.; Nakazawa, T. Effect of chronic HTO beta or 60Co gamma radiation on preimplantation mouse development in vitro. Radiat. Res. 1982, 92, 359–369. [Google Scholar] [CrossRef]
- Matsuda, Y.; Yamada, T.; Tobari, I. Chromosome aberrations induced by tritiated water or 60Co gamma-rays at early pronuclear stage in mouse eggs. Mutat. Res. 1986, 160, 87–93. [Google Scholar] [CrossRef]
- Weissenborn, U.; Streffer, C. Analysis of structural and numerical chromosomal anomalies at the first, second, and third mitosis after irradiation of one-cell mouse embryos with X-rays or neutrons. Int. J. Radiat. Biol. 1988, 54, 381–394. [Google Scholar] [CrossRef]
- Wang, B.; Watanabe, K.; Yamada, T.; Shima, A. Effects of beta radiation from organically bound tritium on cultured mouse embryonic mid brain cells. Health Phys. 1996, 71, 915–921. [Google Scholar] [CrossRef]
- Pampfer, S.; Müller, W.-U.; Streffer, C. Preimplantation growth delay and micronucleus formation after in vivo exposure of mouse zygotes to fast neutrons. Radiat. Res. 1992, 129, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Solomon, H.M.; Beckman, D.A.; Buck, S.J.; Gorson, R.O.; Mills, R.E.; Brent, R.L. Comparative effects of neutron irradiation and X irradiation on the embryonic development of the rat. Radiat. Res. 1994, 137, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Konermann, G. Brain development in mice after prenatal irradiations: Modes of effect manifestation, dose-response-relationship and RBE of neutrons. In Radiation Risk to the Developing Nervous System; Kriegel, H., Schmahl, W., Stieve, F.F., Gerber, G.B., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1986; pp. 93–116. [Google Scholar]
- Yasuda, T.; Oda, S.; Yasuda, H.; Hibi, Y.; Anzai, K.; Mitani, H. Neurocytotoxic effects of iron-ions on the developing brain measured in vivo using medaka (Oryzias latipes), a vertebrate model. Int. J. Radiat. Biol. 2011, 87, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Ohmachi, Y.; Nakata, Y.; Hiraoka, T.; Hamano, T.; Fushiki, S.; Ogiu, T. Dose-response and large relative biological effectiveness of fast neutrons with regard to mouse fetal cerebral neuron apoptosis. J. Radiat. Res. 2006, 47, 41–47. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Dong, J.C.; Zhou, S.Y.; Chen, J.D.; Guo, F.R. Experimental study on relative biological effectiveness of tritium and risk estimates of genetic damage. Chin. Med. J. 1989, 102, 872–878. [Google Scholar]
- Satow, Y.; Lee, J.Y.; Hori, H.; Okuda, H.; Tsuchimoto, S.; Sawada, S.; Yokoro, K. Teratogenic effect of californium-252 irradiation in rats. J. Radiat. Res. 1989, 30, 155–163. [Google Scholar] [CrossRef]
- Ward, W.F.; Aceto, H., Jr.; Jolly, R.; Buckle, D. Rbe and oer of extended-Bragg-peak helium ions: Survival and development of rat embryos. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1976, 30, 317–326. [Google Scholar] [CrossRef]
- Wang, B.; Murakami, M.; Eguchi-Kasai, K.; Nojima, K.; Shang, Y.; Tanaka, K.; Watanabe, K.; Fujita, K.; Moreno, S.G.; Coffigny, H.; et al. Effects of prenatal irradiation with an accelerated heavy-ion beam on postnatal development in rats: I. Neurophysiological alterations. Radiat. Res. 2005, 164, 561–566. [Google Scholar] [CrossRef]
- Wang, B.; Murakami, M.; Eguchi-Kasai, K.; Nojima, K.; Shang, Y.; Tanaka, K.; Watanabe, K.; Fujita, K.; Moreno, S.G.; Coffigny, H.; et al. Effects of prenatal irradiation with an accelerated heavy-ion beam on postnatal development in rats: II. Further study on neurophysiologic alterations. Adv. Space Res. 2007, 39, 994–1003. [Google Scholar] [CrossRef]
- Wang, B.; Tanaka, K.; Murakami, M.; Eguchi-Kasai, K.; Shang, Y.; Fujita, K.; Moreno, S.G.; Coffigny, H.; Hayata, I. Prenatal irradiations with accelerated-heavy-ion beams induced LET-dependent detrimental effects on prenatal development and postnatal neurophysiological accomplishment. Indian J. Radiat. Res. 2008, 5, 15–23. [Google Scholar]
- Sreetharan, S.; Thome, C.; Tharmalingam, S.; Jones, D.E.; Kulesza, A.V.; Khaper, N.; Lees, S.J.; Wilson, J.Y.; Boreham, D.R.; Tai, T.C. Ionizing radiation exposure during pregnancy: Effects on postnatal development and life. Radiat. Res. 2017, 187, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Dekaban, A.S. Abnormalities in children exposed to x-radiation during various stages of gestation: Tentative timetable of radiation injury to the human fetus, part I. J. Nucl. Med. 1968, 9, 471–477. [Google Scholar] [PubMed]
- Gustavson, K.H.; Jagell, S.; Blomquist, H.K.; Nordenson, I. Microcephaly, mental retardation and chromosomal aberrations in a girl following radiation therapy during late fetal life. Acta Radiol. Oncol. 1981, 20, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Nembhard, W.N.; McElfish, P.A.; Ayers, B.; Collins, R.T.; Shan, X.; Rabie, N.Z.; Zarate, Y.A.; Maity, S.; Cen, R.; Robbins, J.A. Nuclear radiation and prevalence of structural birth defects among infants born to women from the Marshall Islands. Birth Defects Res. 2019, 111, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Rugh, R.; Duhamel, L.; Chandler, A.; Varma, A. Cataract development after embryonic and fetal X-irradiation. Radiat. Res. 1964, 22, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Rugh, R.; Wohlfromm, M.; Varma, A.; Spencer, N.; Stanford, W. A reexamination of the mouse embryonic radiation cataract studies. Radiat. Res. 1971, 47, 182–190. [Google Scholar] [CrossRef]
- Nakashima, E.; Akahoshi, M.; Neriishi, K.; Fujiwara, S. Systolic blood pressure and systolic hypertension in adolescence of atomic bomb survivors exposed in utero. Radiat. Res. 2007, 168, 593–599. [Google Scholar] [CrossRef]
- Tatsukawa, Y.; Nakashima, E.; Yamada, M.; Funamoto, S.; Hida, A.; Akahoshi, M.; Sakata, R.; Ross, N.P.; Kasagi, F.; Fujiwara, S.; et al. Cardiovascular disease risk among atomic bomb survivors exposed in utero, 1978–2003. Radiat. Res. 2008, 170, 269–274. [Google Scholar] [CrossRef]
- Bakshi, M.V.; Azimzadeh, O.; Merl-Pham, J.; Verreet, T.; Hauck, S.M.; Benotmane, M.A.; Atkinson, M.J.; Tapio, S. In-utero low-dose irradiation leads to persistent alterations in the mouse heart proteome. PLoS ONE 2016, 11, e0156952. [Google Scholar] [CrossRef]
- Jennings, R.T.; Baker, E.S. Gynecological and reproductive issues for women in space: A review. Obstet. Gynecol. Surv. 2000, 55, 109–116. [Google Scholar] [CrossRef]
- Jones, J.A.; Jennings, R.; Pietryzk, R.; Ciftcioglu, N.; Stepaniak, P. Genitourinary issues during spaceflight: A review. Int. J. Impot. Res. 2005, 17, S64–S67. [Google Scholar] [CrossRef] [PubMed]
- Steller, J.G.; Alberts, J.R.; Ronca, A.E. Oxidative stress as cause, consequence, or biomarker of altered female reproduction and development in the space environment. Int. J. Mol. Sci. 2018, 19, 3729. [Google Scholar] [CrossRef] [PubMed]
- Nargund, V.H. Effects of psychological stress on male fertility. Nat. Rev. Urol. 2015, 12, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Sominsky, L.; Hodgson, D.M.; McLaughlin, E.A.; Smith, R.; Wall, H.M.; Spencer, S.J. Linking stress and infertility: A novel role for ghrelin. Endocr. Rev. 2017, 38, 432–467. [Google Scholar] [CrossRef]
- Mishra, B.; Luderer, U. Reproductive hazards of space travel in women and men. Nat. Rev. Endocrinol. 2019, 15, 713–730. [Google Scholar] [CrossRef]
- Choi, E.; Michaels, B.; Harris, K.; Gupta, N.; Mclean, K.; Wittman, D.; Sun, Y.; Jolly, S.; Maturen, K.E. High prevalence of sexual dysfunction among gynecologic cancer patients treated with radiation therapy: Role of treatment technique and time. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, E537. [Google Scholar] [CrossRef][Green Version]
- Stanton, A.M.; Handy, A.B.; Meston, C.M. Sexual function in adolescents and young adults diagnosed with cancer: A systematic review. J. Cancer Surviv. 2018, 12, 47–63. [Google Scholar] [CrossRef]
- Madan, R.; Dracham, C.B.; Khosla, D.; Goyal, S.; Yadav, A.K. Erectile dysfunction and cancer: Current perspective. Radiat. Oncol. J. 2020. [Google Scholar] [CrossRef]
- Ramirez-Fort, M.K.; Rogerse, M.J.; Santiago, R.; Mahase, S.S.; Mendez, M.; Zheng, Y.; Kong, X.; Kashanian, J.A.; Niaz, M.J.; McClelland, S., III; et al. Prostatic irradiation-induced sexual dysfunction: A review and multidisciplinary guide to management in the radical radiotherapy era (Part I defining the organ at risk for sexual toxicities). Rep. Pract. Oncol. Radiother. 2020, 25, 367–375. [Google Scholar] [CrossRef]
- The American Cancer Society medical and editorial content team. Sex and the Adult Male with Cancer; Available online: https://www.cancer.org/content/dam/CRC/PDF/Public/6709.00.pdf (accessed on 15 October 2020).
- Helgason, A.R.; Fredrikson, M.; Adolfsson, J.; Steineck, G. Decreased sexual capacity after external radiation therapy for prostate cancer impairs quality of life. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 33–39. [Google Scholar] [CrossRef]
- Beckendorf, V.; Hay, M.; Rozan, R.; Lagrange, J.L.; N’Guyen, T.; Giraud, B. Changes in sexual function after radiotherapy treatment of prostate cancer. Br. J. Urol. 1996, 77, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Joly, F.; Brune, D.; Coucette, J.E.; Lesaunier, F.; Héron, J.-F.; Pény, J.; Henry-Amar, M. Health-related quality of life and sequelae in patients treated with brachytherapy and external beam irradiation for localized prostate cancer. Ann. Oncol. 1998, 9, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Incrocci, L.; Slob, A.K.; Levendag, P.C. Sexual (dys) function after radiotherapy for prostate cancer: A review. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 681–693. [Google Scholar] [CrossRef]
- Mahmood, J.; Shamah, A.A.; Creed, T.M.; Pavlovic, R.; Matsui, H.; Kimura, M.; Molitoris, J.; Shukla, H.; Jackson, I.; Vujaskovic, Z. Radiation-induced erectile dysfunction: Recent advances and future directions. Adv. Radiat. Oncol. 2016, 1, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Flay, L.D.; Matthews, J.H.L. The effects of radiotherapy and surgery on the sexual function of women treated for cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 399–404. [Google Scholar] [CrossRef]
- Schover, L.R.; Fife, M.; Gershenson, D.M. Sexual dysfunction and treatment for early stage cervical cancer. Cancer 1989, 63, 204–212. [Google Scholar] [CrossRef]
- Nunns, D.; Williamson, K.; Swaney, L.; Davy, M. The morbidity of surgery and adjuvant radiotherapy in the management of endometrial carcinoma. Int. J. Gynecol. Cancer 2000, 10, 233–238. [Google Scholar] [CrossRef]
- Fisch, B.M.; Pickett, B.; Weinberg, V.; Roach, M. Dose of radiation received by the bulb of the penis correlates with risk of impotence after three-dimensional conformal radiotherapy for prostate cancer. Urology 2001, 57, 955–959. [Google Scholar] [CrossRef]
- Hoppe, B.S.; Nichols, R.C.; Henderson, R.H.; Morris, C.G.; Williams, C.R.; Costa, J.; Marcus, R.B., Jr.; Mendenhall, W.M.; Li, Z.; Mendenhall, N.P. Erectile function, incontinence, and other quality of life outcomes following proton therapy for prostate cancer in men 60 years old and younger. Cancer 2012, 118, 4619–4626. [Google Scholar] [CrossRef]
- Magli, A.; Giangreco, M.; Crespi, M.; Negri, A.; Ceschia, T.; De Giorgi, G.; Titone, F.; Parisi, G.; Fongione, S. Erectile dysfunction after prostate three-dimensional conformal radiation therapy—Correlation with the dose to the penile bulb. Strahlenther Onkol. 2012, 188, 997–1002. [Google Scholar] [CrossRef]
- Del Campo, E.R.; Thomas, K.; Weinberg, V.; Roach, M. Erectile dysfunction after radiotherapy for prostate cancer: A model assessing the conflicting literature on dose–volume effects. Int. J. Impot. Res. 2013, 25, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Tou, J.; Ronca, A.; Grindeland, R.; Wade, C. Models to study gravitational biology of mammalian reproduction. Biol. Reprod. 2001, 67, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Vernikos, J.; Schneider, V.S. Space, gravity and the physiology of aging: Parallel or convergent disciplines? A mini-review. Gerontology 2010, 56, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, H.; Liu, Z. Effects of real and simulated weightlessness on the cardiac and peripheral vascular functions of humans: A review. Int. J. Occup. Med. Environ. Health 2015, 28, 793–802. [Google Scholar] [CrossRef]
- Rydze, R.; Schutt, A.; Gibbons, W.; Nodler, J. Gravity and embryo development. Curr. Obstetr. Gynecol. Rep. 2017, 6, 51–54. [Google Scholar] [CrossRef]
- Yamanouchi, S.; Rhone, J.; Mao, J.-H.; Fujiwara, K.; Saganti, P.B.; Takahashi, A.; Hada, M. Simultaneous exposure of cultured human lymphoblastic cells to simulated microgravity and radiation increases chromosome aberrations. Life 2020, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Ogneva, I.V.; Usik, M.A.; Biryukov, N.S.; Zhdankina, Y.S. Sperm motility of mice under simulated microgravity and hypergravity. Int. J. Mol. Sci. 2020, 21, 5054. [Google Scholar] [CrossRef]
- Bertolet, A.; Carabe, A. Modelling dose effects from space irradiations: Combination of high-LET and low-LET radiations with a modified microdosimetric kinetic model. Life 2020, 10, 161. [Google Scholar] [CrossRef]
- Suzuki, M.; Uchihori, Y.; Kitamura, H.; Oikawa, M.; Konishi, T. Biologic Impact of Different Ultra-Low-Fluence Irradiations in Human Fibroblasts. Life 2020, 10, 154. [Google Scholar] [CrossRef]
- Schulte, P.; Alegret, L.; Arenillas, I.; Arz, J.A.; Barton, P.J.; Bown, P.R.; Bralower, T.J.; Christeson, G.L.; Claeys, P.; Cockell, C.S.; et al. The Chicxulub asteroid impact and mass extinction at the Cretaceous-Paleogene boundary. Science 2010, 327, 1214–1218. [Google Scholar] [CrossRef]
| Particle | Source or Energy (LET 1) | Biological Endpoint | RBE Value | ||
|---|---|---|---|---|---|
| of the Particle for the Specific Endpoint | of the Same Particle for All Endpoints | of All the Particles for All Endpoints | |||
| Electron | β-rays from HTO | Oocyte killing in mice | 1.1–3.5 [32] | 1.1–3.5 | 0.4–3.5 |
| β-rays from HTO | Oocyte killing in mice | 1.6–2.8 [33,34] | |||
| Neutron | 0.4 MeV | Oocyte killing in mice | 1.0 [31] | 1.0–3.5 | |
| 0.43 MeV | Oocyte killing in mice | 1.7 [29] | |||
| 14 MeV | Oocyte killing in mice | 1.3 [31] | |||
| from 252Cf | Oocyte killing in mice | 1.6–3.5 [30] | |||
| Helium | α-rays from 239Pu | Impairment of fertility in mice | 2.5 [35] | 2.5 | |
| Carbon | 80 MeV/amu (31 keV/µm) | Oocyte killing in mice | 1.32–1.49 [36] | 1.3–1.5 | |
| Neon | 450 MeV/amu (>30 keV/µm) | Oocyte killing in mice | 0.4–0.6 [21] | 0.4–0.6 | |
| Silicon | 670 MeV/amu (>50 keV/µm) | Oocyte killing in mice | 0.4–3.0 [21] | 0.4–3.0 | |
| Argon | 570 MeV/amu (>85 keV/µm) | Oocyte killing in mice | 0.4–2.2 [21] | 0.4–2.2 | |
| Particle | Source or Energy (LET 1) | Biological Endpoint | RBE Value | ||
|---|---|---|---|---|---|
| of the Particle for the Specific Endpoint | of the Same Particle for All ENDPOINTS | of All the Particles for All Endpoints | |||
| Electron | Auger electrons from 125I and 111In | Sperm head abnormalities in mice | 2.3–63 [37] | 1.0–63 | 0.9–270 |
| Auger electrons from 125I and 111In | Spermatogonium killing in mice | 1.0–7.9 [37] | |||
| β-rays from HTO | Spermatozoa chromosome damage in humans | 1.04–3.0 [39] | |||
| Neutron | from 252Cf | Spermatozoa chromosome damage in humans | 1.6–3.9 [43] | 1.6–24 | |
| from 252Cf | Testis weight loss in mice | 5.1 [48] | |||
| 0.4 MeV | Chromosome aberrations in secondary spermatocytes in mice | 5.65 [45] | |||
| 0.85 MeV | Chromosome aberrations in spermatogonia and primary spermatocytes in mice | 10–24 [44] | |||
| 1 MeV | Spermatogonium killing in mice | 5.7 [49] | |||
| 2.3 MeV | Spermatogonium killing in mice | 4.6 [49] | |||
| 5.5 MeV | Testis weight loss in mice | 4.25 [50] | |||
| 5.5 MeV | Spermatogonium killing in mice | 4.57 [50] | |||
| 5.6 MeV | Spermatogonium killing in mice | 3 [49] | |||
| ~50 MeV | Chromosome aberrations in spermatocytes in mice | 2.0–7.0 [21,46,47] | |||
| Helium | α-rays (3.2–8.8 MeV) | Sperm head killing in mice | ~7.4 [40,41,42] | ~270 | |
| α-rays from 210Po | Sperm head abnormalities in mice | 245 ± 23 [37] | |||
| α-rays from 210Po | Spermatogonium killing in mice | 6.7 [37] | |||
| 228 MeV/amu (~60 keV/µm) | Spermatogonium killing in mice | 1.15–1.3 [51] | |||
| Carbon | 50 MeV/amu (>45 keV/µm) | Chromosomal aberrations in spermatogonia in mice | 1.67 [52] | 0.9–3.0 | |
| 50 MeV/amu (>45 keV/µm) | Chromosomal aberrations in spermatocytes in mice | 1.66 [52] | |||
| 400 to 670 MeV/amu (>11 keV/µm) | Spermatogonium killing in mice | <3 [51] | |||
| 400 to 670 MeV/amu (>11 keV/µm) | Testis weight loss in mice | 2 [51] | |||
| 290 MeV/amu (13 keV/µm) | Gonocyte killing in mice | 1.0–1.4 [54] | |||
| 290 MeV/amu (13 keV/µm) | Impairment of fertility in mice | 0.9 [54] | |||
| Oxygen | 60 MeV/amu (>70 keV/μm) | Testis weight loss in mice | 1.84 [53] | 1.2–1.8 | |
| 60 MeV/amu (>70 keV/μm) | Sperm count decrease in mice | 1.22 [53] | |||
| 60 MeV/amu (>70 keV/μm) | Sperm abnormalities in mice | 1.29 [53] | |||
| Neon | 400 to 670 MeV/amu (> 30 keV/µm) | Spermatogonium killing in mice | <3 [51] | 1.0–3.0 | |
| 400 to 670 MeV/amu (>30 keV/µm) | Testis weight loss in mice | 2.2 [51] | |||
| 400 MeV/amu (40 keV/µm) | Gonocyte killing in mice | 1.0–1.3 [54] | |||
| 400 MeV/amu (40 keV/µm) | Impairment of fertility in mice | 1 [54] | |||
| Argon | 400 to 670 MeV/amu (>80 keV/µm) | Spermatogonium killing in mice | ~3 [51] | ~3.0 | |
| 400 to 670 MeV/amu (>80 keV/µm) | Testis weight loss in mice | 3 [51] | |||
| Particle | Source or Energy (LET 1) | Biological Endpoint | RBE Value | ||
|---|---|---|---|---|---|
| of the Particle for the Specific Endpoint | of the Same Particle for All Endpoints | of All the Particles for All Endpoints | |||
| Electron | β-rays from HTO | Chromosomal aberrations in bone marrow cells in mice | 1–2 [57] | 1.0–8.7 | 1.0–48 |
| β-rays from HTO | Embryo killing in mice | 1.0–1.7 [64] | |||
| β-rays from HTO | Chromosome aberrations in embryo cells in mice | 1.6–2.0 [65] | |||
| β-rays from HTO | Cell proliferation, differentiation, cellular DNA and protein contents in fetal midbrain in mice | 4.6–8.7 [67] | |||
| β-rays from HTO | Impairment of prenatal development and postnatal neurophysiological accomplishment in mice and rats | 2.3–3.0 [54,73] | |||
| Neutron | from 252Cf | Malformation in mice | 2.3–3.1 [30,74] | 2.3–48 | |
| 0.43 MeV | Formation of micronuclei, lethality, malformations, weight defects, brain structure changes in mice, | 1.8–7.4, 3.65 in average [69] | |||
| 1–800 MeV | Embryo killing in medaka fish | 48.1 [58] | |||
| 7 MeV | Prenatal mortality in mice | 2.3 [60] | |||
| 7 MeV | Malformation in mice | 2.0–2.8 [60] | |||
| 5.8–6.0 MeV | Malformation in mice | 2.0–3.67 [61] | |||
| Embryo killing in mice | 2.0–10 [31,62,63] | ||||
| 6.0 MeV | Chromosomal anomalies at the first mitosis in one-cell embryos in mice | 4.7 [66] | |||
| 6.0 MeV | Chromosomal anomalies at the second mitosis in one-cell embryos in mice | 4.8 [66] | |||
| 6.0 MeV | Chromosomal anomalies at the third mitosis in one-cell embryos in mice | 7.4 [66] | |||
| 6.0 MeV | Brain anatomical defects in mice | 3 in average [31,70] | |||
| 7.0 MeV | Micronucleus induction in pre-implantation embryos in mice | 2.5–3.5 [68] | |||
| Peak energy: 10 MeV | Induction of neuron apoptosis in fetal cerebral cortex in mice | 9.8 [72] | |||
| Proton | 150 MeV | Embryo killing in zebrafish | 1.13–1.2 [59] | 1.1–1.2 | |
| Helium | 530 MeV/amu (4–6% >40 keV/µm) | Fetal lethality in mice | 1.0–1.4 [75] | 1.0–1.4 | |
| Carbon | 290 MeV/amu (14 keV/µm) | Impairment of prenatal development and postnatal neurophysiological accomplishment in rats | 1.0–2.04 [76,77,78] | 1.0–2.0 | |
| Neon | 400 MeV/amu (40 keV/µm) | Impairment of prenatal development and postnatal neurophysiological accomplishment in rats | 1.0–2.14 [76,77,78] | 1.0–2.1 | |
| Iron | 500 MeV/amu (200 keV/µm) | Induction of apoptosis in the developing optic tectum in medaka fish | 3.7–4.2 [71] | 3.7–4.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Yasuda, H. Relative Biological Effectiveness of High LET Particles on the Reproductive System and Fetal Development. Life 2020, 10, 298. https://doi.org/10.3390/life10110298
Wang B, Yasuda H. Relative Biological Effectiveness of High LET Particles on the Reproductive System and Fetal Development. Life. 2020; 10(11):298. https://doi.org/10.3390/life10110298
Chicago/Turabian StyleWang, Bing, and Hiroshi Yasuda. 2020. "Relative Biological Effectiveness of High LET Particles on the Reproductive System and Fetal Development" Life 10, no. 11: 298. https://doi.org/10.3390/life10110298
APA StyleWang, B., & Yasuda, H. (2020). Relative Biological Effectiveness of High LET Particles on the Reproductive System and Fetal Development. Life, 10(11), 298. https://doi.org/10.3390/life10110298






