Defining Lyfe in the Universe: From Three Privileged Functions to Four Pillars
Abstract
:1. Introduction: The Need for a New Definition of Life
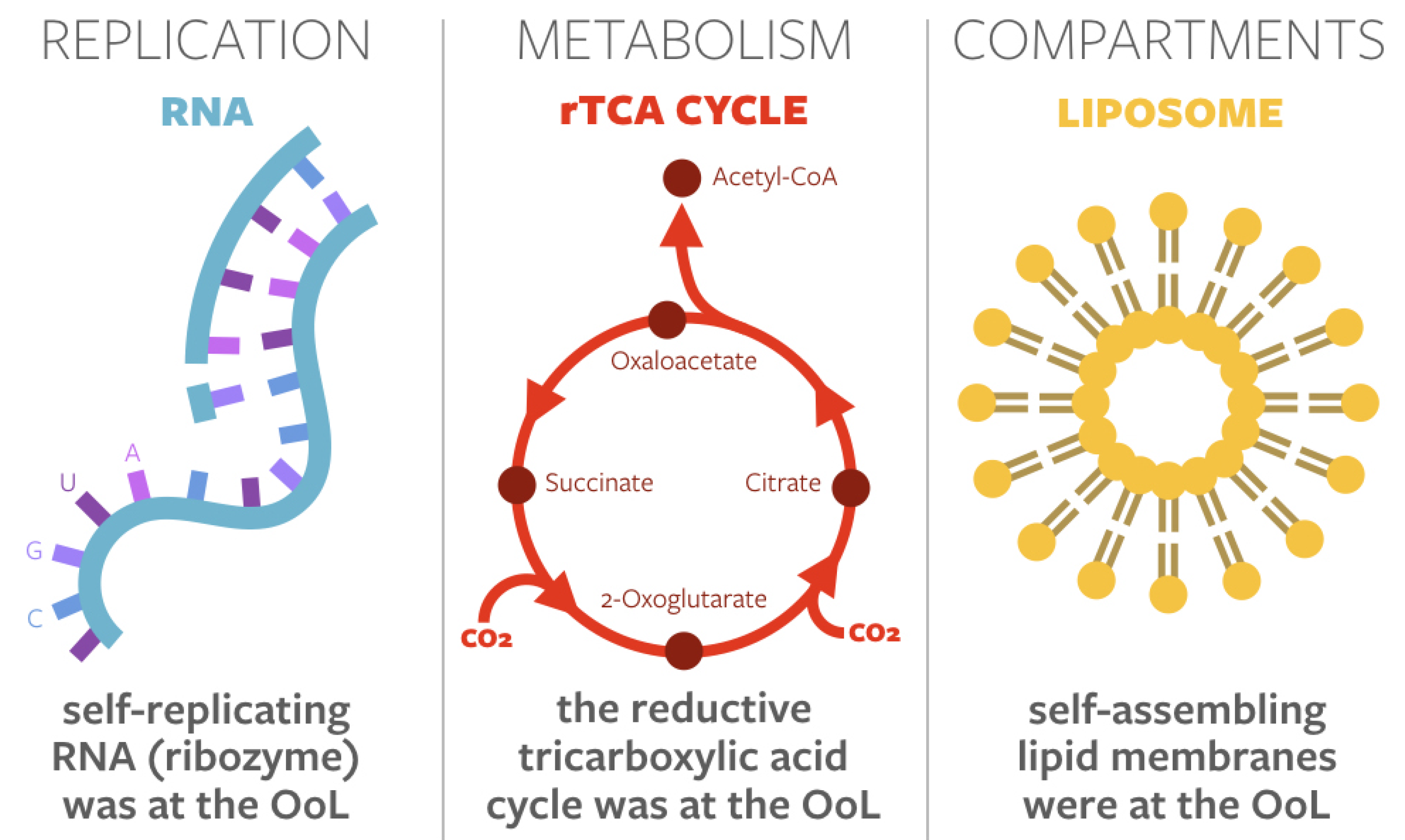
1.1. Privileged Functions at the Origin of Life
1.2. The Event Horizon in Origins-of-Life Research
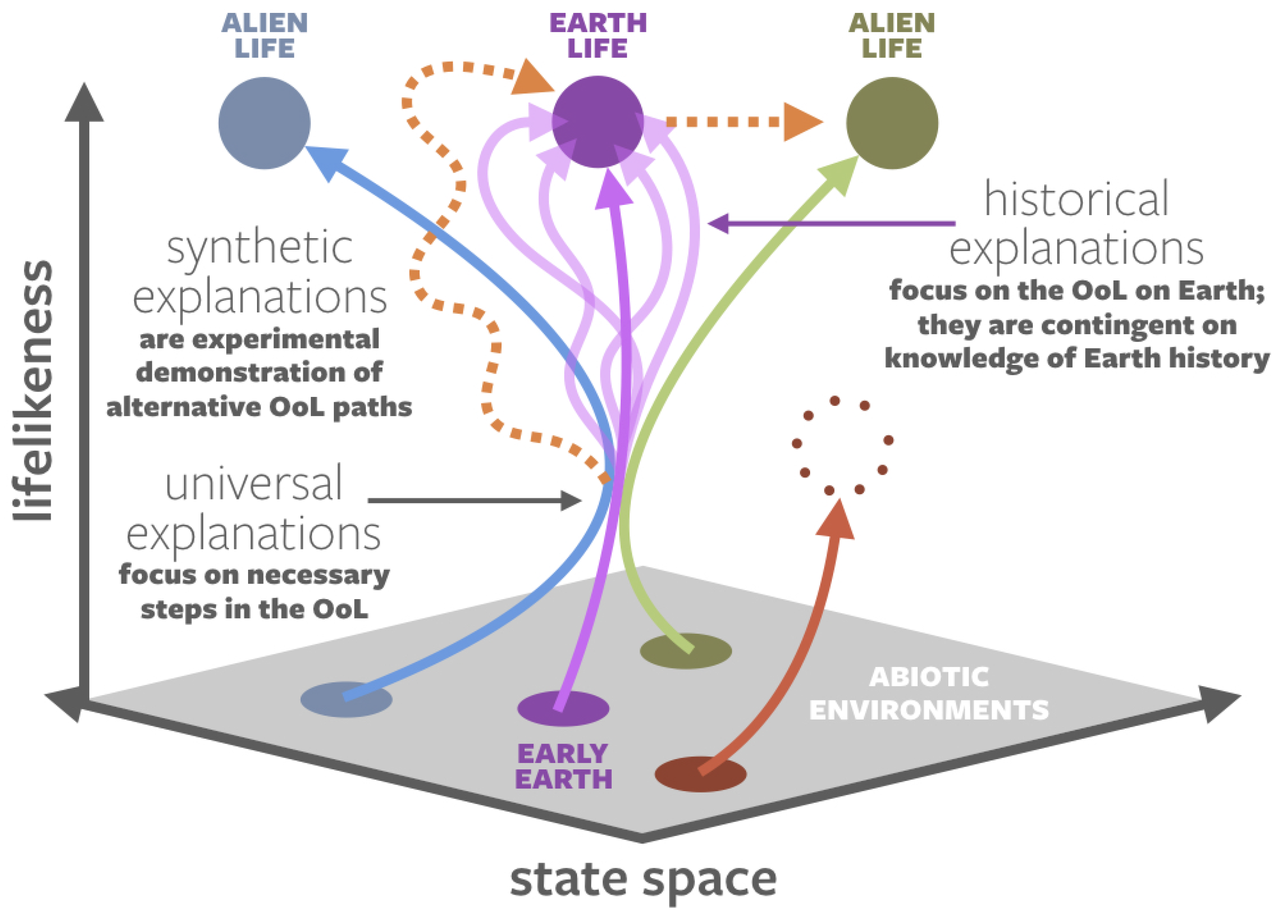
1.3. Historical vs. Synthetic vs. Universal Origin Narratives
2. The Definition of Lyfe
- Life represents life as we know it; it uses the specific disequilibria and classes of components of earthly life. Life is an autocatalytic network of organometallic chemicals in aqueous solution that records and processes information about its environment in molecular form and achieves dynamical order by dissipating any subset of the following disequilibria: redox gradients, chemiosmotic gradients, visible/thermal photons, etc.
- Lyfe represents any hypothetical phenomenon in the universe that fulfills the fundamental processes of the living state, regardless of the disequilibria or components that it harnesses or uses. Lyfe is any hypothetical phenomenon that maintains a low-entropy state via dissipation and disequilibria conversions, utilizes autocatalytic networks to achieve nonlinear growth and proliferation, employs homeostatic regulatory mechanisms to maintain stability and mitigate external perturbations, and acquires and processes functional information about its environment.
- Dissipation—Lyfe cannot exist at equilibrium. The second law of thermodynamics, in the presence of free energy transduction mechanisms, permits the coupling of exergonic processes to the endergonic, organized configurations of lyfe.Using an array of nanoscale molecular machines, life dissipates external chemical disequilibria and/or converts low-entropy photons into high-entropy waste heat, transducing these disequilibria into other disequilibria (e.g., endergonically building up proton gradients and high ). To perform useful work, life converts , which dissipates the disequilibrium [28,29].
- Autocatalysis—The ability of a system to exhibit exponential growth of representative measures of size or population in ideal conditions. The property of autocatalysis can appear in different forms—including self-catalysis, cross-catalysis, and network autocatalysis—as long as the effect leads to exponential growth of a suitable metric under ideal conditions.A cultured system of microorganisms exhibits autocatalytic population growth due to cellular replication in resource-abundant conditions.
- Homeostasis—The ability of a system to maintain key internal variables within ranges of ideal set points. In a dynamic world of perturbations, coupled with the exponential growth described above, a lyving system must have means to limit the variation of its internal systems when external conditions change.Life performs homeostasis with networks of sensors, receptors, and effectors. The substance under homeostatic regulation (e.g., calcium ions) typically binds with receptors and promotes the release of further substances (e.g., hormones). These indicator compounds then stimulate an appropriate response mechanism to return the substance level to within the desired window.
- Learning—The ability of a system to record information about its external and internal environment, process that information, and carry out actions that feed back positively on its probability of surviving/proliferating.Darwinian evolution is one commonly cited biological learning process (e.g., [30,31,32]) among a much larger set of learning processes that living systems perform. For example, there are widely studied examples of biological learning within the realm of neuroscience, permitted by a range of neuronal and synaptic interactions (e.g., [33,34,35]). In addition, there is a growing list of non-neural learning systems, including gene regulatory networks [36,37,38], protein interaction networks [39,40], and other epigenetic mechanisms (e.g., [41,42]). Many examples fall under the general framework of associative learning, which has been exhibited by non-neural organisms such as slime moulds [43,44]. Darwinism mingles with these other learning processes (and perhaps other hitherto undiscovered forms) to create the incredible diversity and complexity of the biosphere. Hence, “learning” is an umbrella term for this large and incompletely understood set of processes.
2.1. Sublyfe
- Dissipation only: Thermal diffusion, or any thermodynamically irreversible process.
- Homeostasis only: An ideal gas at equilibrium. An isolated system such as this always relaxes back to equilibrium after an internal or external fluctuation.
- Dissipation and autocatalysis: Fire is a frequently discussed example of dissipation and autocatalysis. It exhibits homeostasis of certain variables (e.g., burn temperature naturally stays within certain bounds), but its inability to fully regulate its behavior or learn from experience keeps it relegated to the nonliving world. Another relevant example would by the exponential growth of products in nonlinear chemical reactions (e.g., the formose reaction).
- Dissipation and homeostasis: A damped harmonic oscillator converts kinetic energy to thermal energy and always returns to its equilibrium position.
- Dissipation and learning: An artificial neural network is an example system that learns and is dissipative but does not necessarily exhibit autocatalytic growth or homeostasis (e.g., it does not by itself maintain the temperature of its own hardware). One could argue that their usefulness compels us to produce them at an exponential rate, but that is another discussion.
- Dissipation, autocatalysis, and learning: A living system that wipes itself out by tragedy of the commons. Examples might include invasive species introduced to an island that destroy their food sources so fast that the food sources are damaged beyond recovery. One might also suggest anthropic climate change as another example. Note that these cases depend critically on where one draws the boundary of the system (e.g., to include humans or not). Indeed, this form of sublyfe or sublife is less likely to occur because if the system is capable of learning, then in principle it could learn how to regulate itself homeostatically (unless it cannot learn fast enough).
- Dissipation, homeostasis, and learning: A “smart” house thermostat that monitors occupant behavior over time. This system cannot replicate but consumes free energy, is capable of primitive learning, and can regulate its local temperature.
- All four: Lyfe (which includes life).
2.2. Lyfe and Origins-of-Life Studies
3. Imagining Lyfe
3.1. Examples of Alternative Components in Origins-of-Life Hypotheses
3.2. Lyfe on Titan
3.3. Mechanotrophs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LUCA | Last Universal Common Ancestor |
References
- Walker, S.I.; Davies, P.C.W. The algorithmic origins of life. J. R. Soc. Interface 2013, 10, 20120869. [Google Scholar] [CrossRef]
- Lanier, K.A.; Williams, L.D. The Origin of Life: Models and Data. J. Mol. Evol. 2017, 84, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulkidjanian, A.Y.; Bychkov, A.Y.; Dibrova, D.V.; Galperin, M.Y.; Koonin, E.V. Origin of first cells at terrestrial, anoxic geothermal fields. Proc. Natl. Acad. Sci. USA 2012, 109, E821–E830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulkidjanian, A.Y.; Galperin, M.Y. Physico-chemical and evolutionary constraints for the formation and selection of first biopolymers: towards the consensus paradigm of the abiogenic origin of life. Chem. Biodivers. 2007, 4, 2003–2015. [Google Scholar] [CrossRef] [PubMed]
- Benner, S.A.; Bell, E.A.; Biondi, E.; Brasser, R.; Carell, T.; Kim, H.J.; Mojzsis, S.J.; Omran, A.; Pasek, M.A.; Trail, D. When Did Life Likely Emerge on Earth in an RNA-First Process? ChemSystemsChem 2019. [Google Scholar] [CrossRef]
- Russell, M.J.; Barge, L.M.; Bhartia, R.; Bocanegra, D.; Bracher, P.J.; Branscomb, E.; Kidd, R.; McGlynn, S.; Meier, D.H.; Nitschke, W.; et al. The Drive to Life on Wet and Icy Worlds. Astrobiology 2014, 14, 308–343. [Google Scholar] [CrossRef]
- Branscomb, E.; Russell, M.J. Turnstiles and bifurcators: The disequilibrium converting engines that put metabolism on the road. Biochim. Biophys. Acta Bioenergy 2013, 1827, 62–78. [Google Scholar] [CrossRef] [Green Version]
- Branscomb, E.; Biancalani, T.; Goldenfeld, N.; Russell, M. Escapement mechanisms and the conversion of disequilibria; the engines of creation. Phys. Rep. 2017, 677, 1–60. [Google Scholar] [CrossRef]
- Dzieciol, A.J.; Mann, S. Designs for life: protocell models in the laboratory. Chem. Soc. Rev. 2012, 41, 79–85. [Google Scholar] [CrossRef]
- Saha, R.; Pohorille, A.; Chen, I.A. Molecular Crowding and Early Evolution. Orig. Life Evol. Biosph. 2015, 44, 319–324. [Google Scholar] [CrossRef]
- Saha, R.; Verbanic, S.; Chen, I.A. Lipid vesicles chaperone an encapsulated RNA aptamer. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.D. Opinion: Studies on the origin of life—The end of the beginning. Nat. Rev. Chem. 2017, 1, 0012. [Google Scholar] [CrossRef]
- Damer, B.; Deamer, D. Coupled Phases and Combinatorial Selection in Fluctuating Hydrothermal Pools: A Scenario to Guide Experimental Approaches to the Origin of Cellular Life. Life 2015, 5, 872–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornish-Bowden, A.; Cárdenas, M.L. Life before LUCA. J. Theor. Biol. 2017, 434, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.C.; Benner, S.A.; Cleland, C.E.; Lineweaver, C.H.; McKay, C.P.; Wolfe-Simon, F. Signatures of a Shadow Biosphere. Astrobiology 2009, 9, 241–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baross, J.A.; Martin, W.F. The Ribofilm as a Concept for Life’s Origins. Cell 2015, 162, 13–15. [Google Scholar] [CrossRef] [Green Version]
- Fournier, G.P.; Andam, C.P.; Gogarten, J.P. Ancient horizontal gene transfer and the last common ancestors. BMC Evol. Biol. 2015, 15, 70. [Google Scholar] [CrossRef] [Green Version]
- Mariscal, C.; Barahona, A.; Aubert-Kato, N.; Aydinoglu, A.U.; Bartlett, S.; Cárdenas, M.L.; Chandru, K.; Cleland, C.; Cocanougher, B.T.; Comfort, N.; et al. Hidden Concepts in the History and Philosophy of Origins-of-Life Studies: A Workshop Report. Orig. Life Evol. Biosph. 2019, 49, 111–145. [Google Scholar] [CrossRef]
- Scharf, C.; Virgo, N.; Cleaves, H.J.; Aono, M.; Aubert-Kato, N.; Aydinoglu, A.; Barahona, A.; Barge, L.M.; Benner, S.A.; Biehl, M.; et al. A Strategy for Origins of Life Research. Astrobiology 2015, 15, 1031–1042. [Google Scholar] [CrossRef] [Green Version]
- Arnold, F.H. Directed Evolution: Bringing New Chemistry to Life. Angew. Chem. Int. Ed. 2018, 57, 4143–4148. [Google Scholar] [CrossRef] [Green Version]
- Hoshika, S.; Leal, N.A.; Kim, M.J.; Kim, M.S.; Karalkar, N.B.; Kim, H.J.; Bates, A.M.; Watkins, N.E.; SantaLucia, H.A.; Meyer, A.J.; et al. Hachimoji DNA and RNA: A genetic system with eight building blocks. Science 2019, 363, 884–887. [Google Scholar] [CrossRef] [PubMed]
- England, J.L. Statistical physics of self-replication. J. Chem. Phys. 2013, 139, 121923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- England, J.L. Dissipative adaptation in driven self-assembly. Nat. Nanotechnol. 2015, 10, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.I. Origins of life: A problem for physics, a key issues review. Rep. Prog. Phys. 2017, 80, 092601. [Google Scholar] [CrossRef]
- Kim, H.; Smith, H.B.; Mathis, C.; Raymond, J.; Walker, S.I. Universal scaling across biochemical networks on Earth. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Russell, M.J. Life is a verb, not a noun. Geology 2017, 45, 1143–1144. [Google Scholar] [CrossRef]
- Schrödinger, E. What Is Life?: The Physical Aspect of the Living Cell; Cambridge University: Cambridge, UK, 1944. [Google Scholar]
- Branscomb, E.; Russell, M.J. Frankenstein or a Submarine Alkaline Vent: Who Is Responsible for Abiogenesis? BioEssays 2018, 40, 1700179. [Google Scholar] [CrossRef]
- Branscomb, E.; Russell, M.J. Frankenstein or a Submarine Alkaline Vent: Who is Responsible for Abiogenesis? BioEssays 2018, 40, 1700182. [Google Scholar] [CrossRef]
- Valiant, L. Probably Approximately Correct: Nature’s Algorithms for Learning and Prospering in a Complex World; Basic Books: New York, NY, USA, 2013. [Google Scholar]
- Watson, R.A.; Mills, R.; Buckley, C.; Kouvaris, K.; Jackson, A.; Powers, S.T.; Cox, C.; Tudge, S.; Davies, A.; Kounios, L.; et al. Evolutionary connectionism: algorithmic principles underlying the evolution of biological organisation in evo-devo, evo-eco and evolutionary transitions. Evol. Biol. 2016, 43, 553–581. [Google Scholar] [CrossRef] [Green Version]
- Watson, R.A.; Szathmáry, E. How can evolution learn? Trends Ecol. Evol. 2016, 31, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Hopfield, J.J. Neurons, Dyanmics, and Computation. Phys. Today 1994, 40–46. [Google Scholar] [CrossRef]
- Purves, D.; Cabeza, R.; Huettel, S.; LaBar, K.; Platt, M.; Woldorff, M. Principles of Cognitive Neuroscience; Sinauer: Sunderland, MA, USA, 2013. [Google Scholar]
- Tononi, G.; Boly, M.; Massimini, M.; Koch, C. Integrated information theory: from consciousness to its physical substrate. Nat. Rev. Neurosci. 2016, 17, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Fernando, C.T.; Liekens, A.M.; Bingle, L.E.; Beck, C.; Lenser, T.; Stekel, D.J.; Rowe, J.E. Molecular circuits for associative learning in single-celled organisms. J. R. Soc. Interface 2009, 6, 463–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhi, N.; Ashkenasy, G.; Tannenbaum, E. Associative learning in biochemical networks. J. Theor. Biol. 2007, 249, 58–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagkopoulos, I.; Liu, Y.C.; Tavazoie, S. Predictive behavior within microbial genetic networks. Science 2008, 320, 1313–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, D. Protein molecules as computational elements in living cells. Nature 1995, 376, 307–312. [Google Scholar] [CrossRef]
- Bray, D. Wetware: A Computer in Every Living Cell; Yale University Press: New Haven, CT, USA, 2009. [Google Scholar]
- Arkin, A.; Ross, J. Computational functions in biochemical reaction networks. Biophys. J. 1994, 67, 560–578. [Google Scholar] [CrossRef] [Green Version]
- Turner, C.; Robling, A.; Duncan, R.; Burr, D. Do bone cells behave like a neuronal network? Calcif. Tissue Int. 2002, 70, 435. [Google Scholar] [CrossRef]
- Adamatzky, A. Advances in Physarum Machines: Sensing and Computing with Slime Mould; Springer: Berlin/Heidelberg, Germany, 2016; Volume 21. [Google Scholar] [CrossRef]
- Boisseau, R.P.; Vogel, D.; Dussutour, A. Habituation in non-neural organisms: evidence from slime moulds. Proc. R. Soc. Biol. Sci. 2016, 283, 20160446. [Google Scholar] [CrossRef]
- Breitbart, M.; Bonnain, C.; Malki, K.; Sawaya, N.A. Phage puppet masters of the marine microbial realm. Nat. Microbiol. 2018, 3. [Google Scholar] [CrossRef]
- Smith, E.; Morowitz, H.J. The Origin and Nature of Life on Earth: The Emergence of the Fourth Geosphere; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Owen, T. Life as a planetary phenomenon. Orig. Life Evol. Biosph. 1985, 15, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Kalita, N.K.; Kalamdhad, A.; Katiyar, V. Recent Trends and Advances in the Biodegradation of Conventional Plastics. In Advances in Sustainable Polymers; Springer: Berlin/Heidelberg, Germany, 2020; pp. 389–404. [Google Scholar] [CrossRef]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, D.; Li, Q.; Zhao, Y.; Li, L.; Lin, H.; Bi, Q.; Zhao, Y. Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci. Total Environ. 2020, 704, 135931. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses REVIEW. Nat. Med. 2004, 10, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, S. Why Is life? An Assessment of the Thermodynamic Properties of Dissipative, Pattern-Forming Systems. Ph.D. Thesis, University of Southampton, Southampton, UK, 2014. [Google Scholar]
- Bartlett, S.; Bullock, S. Emergence of competition between different dissipative structures for the same free energy source. In Proceedings of the Artificial Life Conference, York, UK, 20–24 July 2015; pp. 415–422. [Google Scholar] [CrossRef]
- Bartlett, S.; Bullock, S. A precarious existence: Thermal homeostasis of simple dissipative structures. In Proceedings of the Artificial Life Conference 2016, Cancúun, Mexico, 4 July 4–8 August 2016; pp. 608–615. [Google Scholar] [CrossRef]
- Bartlett, S. Delving deeper into homeostatic dynamics of reaction diffusion systems with a general fluid dynamics and artificial chemistry model. In Proceedings of the Artificial Life Conference, Lyon, France, 4–8 September 2017; pp. 52–59. [Google Scholar] [CrossRef] [Green Version]
- Landauer, R. Irreversibility and heat generation in the computing process. IBM J. Res. Dev. 1961, 5, 183–191. [Google Scholar] [CrossRef]
- Greenwald, J.; Riek, R. On the possible amyloid origin of protein folds. J. Mol. Biol. 2012, 421, 417–426. [Google Scholar] [CrossRef]
- Maury, C.P.J. Self-propagating β-sheet polypeptide structures as prebiotic informational molecular entities: The amyloid world. Orig. Life Evol. Biosph. 2009, 39, 141–150. [Google Scholar] [CrossRef]
- Rout, S.K.; Friedmann, M.P.; Riek, R.; Greenwald, J. A prebiotic template-directed peptide synthesis based on amyloids. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Feschotte, C.; Pritham, E.J. DNA transposons and the evolution of eukaryotic genomes. Annu. Rev. Genet. 2007, 41, 331–368. [Google Scholar] [CrossRef] [Green Version]
- Fischer, M.G.; Suttle, C.A. A virophage at the origin of large DNA transposons. Science 2011, 332, 231–234. [Google Scholar] [CrossRef]
- Iranzo, J.; Puigbò, P.; Lobkovsky, A.E.; Wolf, Y.I.; Koonin, E.V. Inevitability of genetic parasites. Genome Biol. Evol. 2016, 8, 2856–2869. [Google Scholar] [CrossRef] [Green Version]
- Kapitonov, V.V.; Jurka, J. Self-synthesizing DNA transposons in eukaryotes. Proc. Natl. Acad. Sci. USA 2006, 103, 4540–4545. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, P.A.; Luisi, P.L.; Lang, J. Autocatalytic self-replicating micelles as models for prebiotic structures. Nature 1992, 357, 57–59. [Google Scholar] [CrossRef]
- Hanczyc, M.M.; Szostak, J.W. Replicating vesicles as models of primitive cell growth and division. Curr. Opin. Chem. Biol. 2004, 8, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, S.J.; Beckett, P. Probing complexity: Thermodynamics and computational mechanics approaches to origins studies. Interface Focus 2019, 9, 20190058. [Google Scholar] [CrossRef] [PubMed]
- Maturana, H.R.; Varela, F.J. Autopoiesis and Cognition: The Realization of the Living; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1991; Volume 42. [Google Scholar]
- Gánti, T. Organization of chemical reactions into dividing and metabolizing units: the chemotons. BioSystems 1975, 7, 15–21. [Google Scholar] [CrossRef]
- Hartman, H. Speculations on the origin and evolution of metabolism. J. Mol. Evol. 1975, 4, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Hartman, H.; Smith, T.F. Origin of the Genetic Code Is Found at the Transition between a Thioester World of Peptides and the Phosphoester World of Polynucleotides. Life 2019, 9, 69. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, C.; Mohan, S.; Kalahar, B.K.; Williams, L.D. Peeling the onion: ribosomes are ancient molecular fossils. Mol. Biol. Evol. 2009, 26, 2415–2425. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.; Szathmary, E. The Major Transitions in Evolution; OUP: Oxford, UK, 1997. [Google Scholar]
- Cairns-Smith, A.G. Seven Clues to the Origin of Life: A Scientific Detective Story; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Talori, Y.S.; Zhao, J.S.; Liu, Y.F.; Lu, W.X.; Li, Z.H.; O’Connor, J.K. Identification of avian flapping motion from non-volant winged dinosaurs based on modal effective mass analysis. PLoS Comput. Biol. 2019, 15. [Google Scholar] [CrossRef] [Green Version]
- Lingappa, U.F.; Monteverde, D.R.; Magyar, J.S.; Valentine, J.S.; Fischer, W.W. How manganese empowered life with dioxygen (and vice versa). Free. Radic. Biol. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Vrba, E.S. Exaptation—A missing term in the science of form. Paleobiology 1982, 8, 4–15. [Google Scholar] [CrossRef]
- Duval, S.; Baymann, F.; Schoepp-Cothenet, B.; Trolard, F.; Bourrié, G.; Grauby, O.; Branscomb, E.; Russell, M.J.; Nitschke, W. Fougerite: The not so simple progenitor of the first cells. Interface Focus 2019, 9, 20190063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandru, K.; Gilbert, A.; Butch, C.; Aono, M.; Cleaves, H.J. The abiotic chemistry of thiolated acetate derivatives and the origin of life. Sci. Rep. 2016, 6, 29883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keefe, A.D.; Miller, S.L. Are polyphosphates or phosphate esters prebiotic reagents? J. Mol. Evol. 1995, 41, 693–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, A.W. Phosphorus in prebiotic chemistry. Philos. Trans. R. Soc. Biol. Sci. 2006, 361, 1743–1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldford, J.E.; Hartman, H.; Smith, T.F.; Segrè, D. Remnants of an ancient metabolism without phosphate. Cell 2017, 168, 1126–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazen, R.M.; Sverjensky, D.A. Mineral Surfaces, Geochemical Complexities, and the Origins of Life. Cold Spring Harb. Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef] [Green Version]
- Martin, W.; Russell, M.J. On the origin of biochemistry at an alkaline hydrothermal vent. Philos. Trans. R. Soc. Biol. Sci. 2007, 362, 1887–1926. [Google Scholar] [CrossRef]
- Wächtershäuser, G. Evolution of the first metabolic cycles. Proc. Natl. Acad. Sci. USA 1990, 87, 200–204. [Google Scholar] [CrossRef] [Green Version]
- Goldford, J.E.; Hartman, H.; Marsland, R.; Segrè, D. Environmental boundary conditions for the origin of life converge to an organo-sulfur metabolism. Nat. Ecol. Evol. 2019, 3, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, H.C.; Coveney, P.V. Layered double hydroxide minerals as possible prebiotic information storage and transfer compounds. Orig. Life Evol. Biosph. 2006, 36, 13–37. [Google Scholar] [CrossRef] [PubMed]
- Hartman, H.; Cairns-Smith, A.G. Clay Minerals and the Origin of Life; CUP Archive: Cambridge, UK, 1986. [Google Scholar] [CrossRef]
- Swadling, J.B.; Coveney, P.V.; Greenwell, H.C. Clay Minerals Mediate Folding and Regioselective Interactions of RNA: A Large-Scale Atomistic Simulation Study. J. Am. Chem. Soc. 2010, 132, 13750–13764. [Google Scholar] [CrossRef]
- Biondi, E.; Branciamore, S.; Fusi, L.; Gago, S.; Gallori, E. Catalytic activity of hammerhead ribozymes in a clay mineral environment: Implications for the RNA world. Gene 2007, 389, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Russell, M. Green Rust: The Simple Organizing Seed of All Life? Life 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.C.B.; Guldberg, S.; Erbs, M.; Koch, C.B. Kinetics of nitrate reduction by green rusts–effects of interlayer anion and Fe(II):Fe(III) ratio. Appl. Clay Sci. 2001, 18, 81–91. [Google Scholar] [CrossRef]
- Trolard, F.; Bourrié, G. Fougerite a natural layered double hydroxide in gley soil: Habitus, structure, and some properties. In Clay Minerals in Nature–Their Characterization, Modification and Application; IntechOpen: Rijeka, Croatia, 2012; pp. 171–188. [Google Scholar] [CrossRef] [Green Version]
- Russell, M.J. Figuring out how life first took off is (much like) rocket science! Planet. Space Sci. 2019, 175, 13–20. [Google Scholar] [CrossRef]
- Arrhenius, G.O. Crystals and Life. Helv. Chim. Acta 2003, 86, 1569–1586. [Google Scholar] [CrossRef]
- Martin, W.; Russell, M.J. On the origins of cells: A hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos. Trans. R. Soc. Lond. Ser. Biol. Sci. 2003, 358, 59–85. [Google Scholar] [CrossRef] [Green Version]
- Kurland, C.G. The RNA dreamtime: Modern cells feature proteins that might have supported a prebiotic polypeptide world but nothing indicates that RNA world ever was. Bioessays 2010, 32, 866–871. [Google Scholar] [CrossRef]
- Shapiro, R. A simpler origin for life. Sci. Am. 2007, 296, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Feldmann, J.; Wiedemann, S.; Okamura, H.; Schneider, C.; Iwan, K.; Crisp, A.; Rossa, M.; Amatov, T.; Carell, T. Unified prebiotically plausible synthesis of pyrimidine and purine RNA ribonucleotides. Science 2019, 366, 76–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiti, F.; Dobson, C.M. Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Maynard-Casely, H.E.; Hodyss, R.; Cable, M.L.; Vu, T.H.; Rahm, M. A co-crystal between benzene and ethane: A potential evaporite material for Saturn’s moon Titan. IUCrJ 2016, 3, 192–199. [Google Scholar] [CrossRef]
- Maynard-Casely, H.E.; Cable, M.L.; Malaska, M.J.; Vu, T.H.; Choukroun, M.; Hodyss, R. Prospects for mineralogy on Titan. Am. Mineral. J. Earth Planet. Mater. 2018, 103, 343–349. [Google Scholar] [CrossRef]
- Lv, K.P.; Norman, L.; Li, Y.L. Oxygen-Free Biochemistry: The Putative CHN Foundation for Exotic Life in a Hydrocarbon World? Astrobiology 2017, 17, 1173–1181. [Google Scholar] [CrossRef]
- Stevenson, J.; Lunine, J.; Clancy, P. Membrane alternatives in worlds without oxygen: Creation of an azotosome. Sci. Adv. 2015, 1, e1400067. [Google Scholar] [CrossRef] [Green Version]
- Palmer, M.Y.; Cordiner, M.A.; Nixon, C.A.; Charnley, S.B.; Teanby, N.A.; Kisiel, Z.; Irwin, P.G.J.; Mumma, M.J. ALMA detection and astrobiological potential of vinyl cyanide on Titan. Sci. Adv. 2017, 3. [Google Scholar] [CrossRef] [Green Version]
- Sandström, H.; Rahm, M. Can polarity-inverted membranes self-assemble on Titan? Sci. Adv. 2020, 6. [Google Scholar] [CrossRef] [Green Version]
- Nealson, K.H.; Rowe, A.R. Electromicrobiology: Realities, grand challenges, goals and predictions. Microb. Biotechnol. 2016, 9, 595–600. [Google Scholar] [CrossRef]
- Liao, J.C.; Beal, D.N.; Lauder, G.V.; Triantafyllou, M.S. Fish exploiting vortices decrease muscle activity. Science 2003, 302, 1566–1569. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Chwang, A.T. Extraction of flow energy by fish and birds in a wavy stream. In Swimming and Flying in Nature; Springer: Berlin/Heidelberg, Germany, 1975; pp. 687–702. [Google Scholar] [CrossRef]
- Yoshida, M.; Muneyuki, E.; Hisabori, T. ATP synthase—A marvellous rotary engine of the cell. Nat. Rev. Mol. Cell Biol. 2001, 2, 669–677. [Google Scholar] [CrossRef] [PubMed]
- DeRosier, D.J. The turn of the screw: the bacterial flagellar motor. Cell 1998, 93, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Sowa, Y.; Berry, R.M. Bacterial flagellar motor. Q. Rev. Biophys. 2008, 41, 103–132. [Google Scholar] [CrossRef] [Green Version]
- Lauga, E. Bacterial hydrodynamics. Annu. Rev. Fluid Mech. 2016, 48, 105–130. [Google Scholar] [CrossRef] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartlett, S.; Wong, M.L. Defining Lyfe in the Universe: From Three Privileged Functions to Four Pillars. Life 2020, 10, 42. https://doi.org/10.3390/life10040042
Bartlett S, Wong ML. Defining Lyfe in the Universe: From Three Privileged Functions to Four Pillars. Life. 2020; 10(4):42. https://doi.org/10.3390/life10040042
Chicago/Turabian StyleBartlett, Stuart, and Michael L. Wong. 2020. "Defining Lyfe in the Universe: From Three Privileged Functions to Four Pillars" Life 10, no. 4: 42. https://doi.org/10.3390/life10040042
APA StyleBartlett, S., & Wong, M. L. (2020). Defining Lyfe in the Universe: From Three Privileged Functions to Four Pillars. Life, 10(4), 42. https://doi.org/10.3390/life10040042




