A New Record for Microbial Perchlorate Tolerance: Fungal Growth in NaClO4 Brines and its Implications for Putative Life on Mars
Abstract
1. Introduction
2. Materials and Methods
2.1. Organisms and Culture Conditions
2.2. Determination of Perchlorate Tolerances
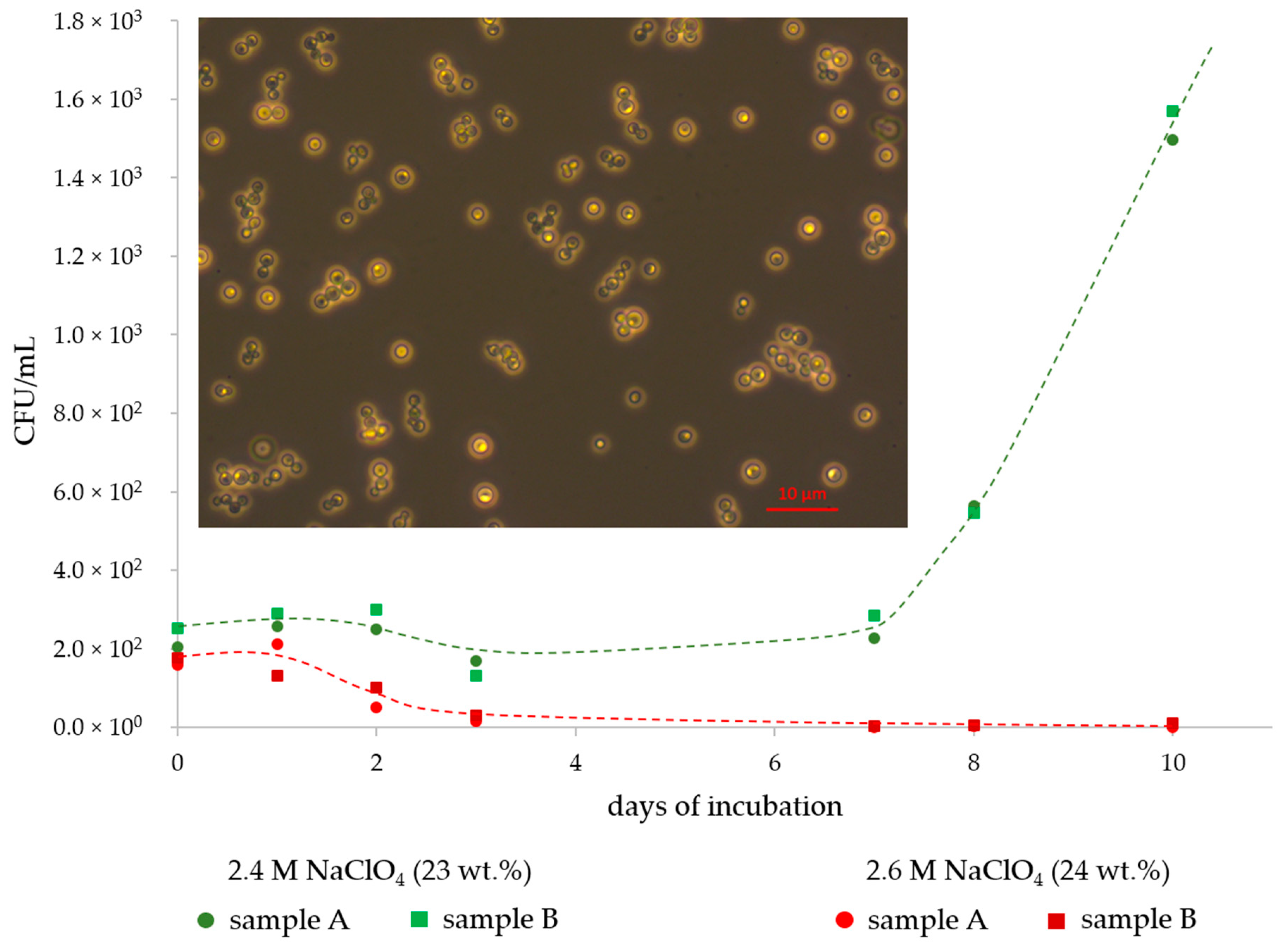
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Masson, P.; Carr, M.H.; Costard, F.; Greeley, R.; Hauber, E.; Jaumann, R. Geomorphologic Evidence for Liquid Water. Space Sci. Rev. 2001, 96, 333–364. [Google Scholar] [CrossRef]
- Christensen, P.R.; Bandfield, J.L.; Clark, R.N.; Edgett, K.S.; Hamilton, V.E.; Hoefen, T.; Kieffer, H.H.; Kuzmin, R.O.; Lane, M.D.; Malin, M.C.; et al. Detection of crystalline hematite mineralization on Mars by the Thermal Emission Spectrometer: Evidence for near-surface water. J. Geophys. Res. 2000, 105, 9623–9642. [Google Scholar] [CrossRef]
- Fairén, A.G. A cold and wet Mars. Icarus 2010, 208, 165–175. [Google Scholar] [CrossRef]
- Wordsworth, R.D. The Climate of Early Mars. Annu. Rev. Earth Planet. Sci. 2016, 44, 381–408. [Google Scholar] [CrossRef]
- Vaisberg, O. Mars atmospheric losses induced by the solar wind: Comparison of observations with models. Planet. Space Sci. 2015, 119, 69–91. [Google Scholar] [CrossRef]
- Jakosky, B.M.; Slipski, M.; Benna, M.; Mahaffy, P.; Elrod, M.; Yelle, R.; Stone, S.; Alsaeed, N. Mars’ atmospheric history derived from upper-atmosphere measurements of 38Ar/36Ar. Science 2017, 355, 1408–1410. [Google Scholar] [CrossRef]
- Davila, A.F.; Schulze-Makuch, D. The Last Possible Outposts for Life on Mars. Astrobiology 2016, 16, 159–168. [Google Scholar] [CrossRef]
- Martínez, G.M.; Renno, N.O. Water and Brines on Mars: Current Evidence and Implications for MSL. Space Sci. Rev. 2013, 175, 29–51. [Google Scholar] [CrossRef]
- Martín-Torres, F.J.; Zorzano, M.-P.; Valentín-Serrano, P.; Harri, A.-M.; Genzer, M.; Kemppinen, O.; Rivera-Valentin, E.G.; Jun, I.; Wray, J.; Bo Madsen, M.; et al. Transient liquid water and water activity at Gale crater on Mars. Nat. Geosci. 2015, 8, 357–361. [Google Scholar] [CrossRef]
- Ojha, L.; Wilhelm, M.B.; Murchie, S.L.; McEwen, A.S.; Wray, J.J.; Hanley, J.; Massé, M.; Chojnacki, M. Spectral evidence for hydrated salts in recurring slope lineae on Mars. Nat. Geosci. 2015, 8, 829–832. [Google Scholar] [CrossRef]
- Kounaves, S.P.; Hecht, M.H.; Kapit, J.; Gospodinova, K.; DeFlores, L.; Quinn, R.C.; Boynton, W.V.; Clark, B.C.; Catling, D.C.; Hredzak, P.; et al. Wet Chemistry experiments on the 2007 Phoenix Mars Scout Lander mission: Data analysis and results. J. Geophys. Res. 2010, 115, E00E10. [Google Scholar] [CrossRef]
- Hecht, M.H.; Kounaves, S.P.; Quinn, R.C.; West, S.J.; Young, S.M.M.; Ming, D.W.; Catling, D.C.; Clark, B.C.; Boynton, W.V.; Hoffman, J.; et al. Detection of perchlorate and the soluble chemistry of martian soil at the Phoenix lander site. Science 2009, 325, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Pestova, O.N.; Myund, L.A.; Khripun, M.K.; Prigaro, A.V. Polythermal Study of the Systems M(ClO4)2-H2O (M2+ = Mg2+, Ca2+, Sr2+, Ba2+). Russ. J. Appl. Chem. 2005, 78, 409–413. [Google Scholar] [CrossRef]
- Davila, A.F.; Gómez-Silva, B.; de los Rios, A.; Ascaso, C.; Olivares, H.; McKay, C.P.; Wierzchos, J. Facilitation of endolithic microbial survival in the hyperarid core of the Atacama Desert by mineral deliquescence. J. Geophys. Res. 2008, 113. [Google Scholar] [CrossRef]
- Wierzchos, J.; Ascaso, C.; McKay, C.P. Endolithic cyanobacteria in halite rocks from the hyperarid core of the Atacama Desert. Astrobiology 2006, 6, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Maus, D.; Heinz, J.; Schirmack, J.; Airo, A.; Kounaves, S.P.; Wagner, D.; Schulze-Makuch, D. Methanogenic Archaea Can Produce Methane in Deliquescence-Driven Mars Analog Environments. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. The dying Dead Sea: The microbiology of an increasingly extreme environment. Lakes Reserv. Res. Manag. 2010, 15, 215–222. [Google Scholar] [CrossRef]
- Pontefract, A.; Zhu, T.F.; Walker, V.K.; Hepburn, H.; Lui, C.; Zuber, M.T.; Ruvkun, G.; Carr, C.E. Microbial Diversity in a Hypersaline Sulfate Lake: A Terrestrial Analog of Ancient Mars. Front. Microbiol. 2017, 8, 1819. [Google Scholar] [CrossRef]
- Marion, G.M. A theoretical evaluation of mineral stability in Don Juan Pond, Wright Valley, Victoria Land. Antarct. Sci. 1997, 9, 92–99. [Google Scholar] [CrossRef]
- Dickson, J.L.; Head, J.W.; Levy, J.S.; Marchant, D.R. Don Juan Pond, Antarctica: Near-surface CaCl(2)-brine feeding Earth’s most saline lake and implications for Mars. Sci. Rep. 2013, 3, 1166. [Google Scholar] [CrossRef]
- Hallsworth, J.E.; Yakimov, M.M.; Golyshin, P.N.; Gillion, J.L.M.; D’Auria, G.; de Lima Alves, F.; La Cono, V.; Genovese, M.; McKew, B.A.; Hayes, S.L.; et al. Limits of life in MgCl2-containing environments: Chaotropicity defines the window. Environ. Microbiol. 2007, 9, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Life in Magnesium- and Calcium-Rich Hypersaline Environments: Salt Stress by Chaotropic Ions. In Polyextremophiles: Life under Multiple Forms of Stress; Seckbach, J., Oren, A., Stan-Lotter, H., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 215–232. ISBN 978-94-007-6487-3. [Google Scholar]
- Samarkin, V.A.; Madigan, M.T.; Bowles, M.W.; Casciotti, K.L.; Priscu, J.C.; McKay, C.P.; Joye, S.B. Abiotic nitrous oxide emission from the hypersaline Don Juan Pond in Antarctica. Nat. Geosci. 2010, 3, 341–344. [Google Scholar] [CrossRef]
- Fox-Powell, M.G.; Hallsworth, J.E.; Cousins, C.R.; Cockell, C.S. Ionic Strength Is a Barrier to the Habitability of Mars. Astrobiology 2016, 16, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Ericksen, G.E. Geology and Origin of the Chilean Nitrate Deposits; U.S. Department of the Interior, U.S. Government Publishing Office: Washington, DC, USA, 1981.
- Catling, D.C.; Claire, M.W.; Zahnle, K.J.; Quinn, R.C.; Clark, B.C.; Hecht, M.H.; Kounaves, S. Atmospheric origins of perchlorate on Mars and in the Atacama. J. Geophys. Res. 2010, 115, E00E11. [Google Scholar] [CrossRef]
- Schulze-Makuch, D.; Wagner, D.; Kounaves, S.P.; Mangelsdorf, K.; Devine, K.G.; de Vera, J.-P.; Schmitt-Kopplin, P.; Grossart, H.-P.; Parro, V.; Kaupenjohann, M.; et al. Transitory microbial habitat in the hyperarid Atacama Desert. Proc. Natl. Acad. Sci. USA 2018, 115, 2670–2675. [Google Scholar] [CrossRef]
- Kounaves, S.P.; Stroble, S.T.; Anderson, R.M.; Moore, Q.; Catling, D.C.; Douglas, S.; McKay, C.P.; Ming, D.W.; Smith, P.H.; Tamppari, L.K.; et al. Discovery of natural perchlorate in the Antarctic Dry Valleys and its global implications. Environ. Sci. Technol. 2010, 44, 2360–2364. [Google Scholar] [CrossRef]
- Heinz, J.; Schirmack, J.; Airo, A.; Kounaves, S.P.; Schulze-Makuch, D. Enhanced Microbial Survivability in Subzero Brines. Astrobiology 2018, 18, 1171–1180. [Google Scholar] [CrossRef]
- Heinz, J.; Waajen, A.C.; Airo, A.; Alibrandi, A.; Schirmack, J.; Schulze-Makuch, D. Bacterial Growth in Chloride and Perchlorate Brines: Halotolerances and Salt Stress Responses of Planococcus halocryophilus. Astrobiology 2019, 19, 1377–1387. [Google Scholar] [CrossRef]
- Oren, A.; Elevi Bardavid, R.; Mana, L. Perchlorate and halophilic prokaryotes: Implications for possible halophilic life on Mars. Extremophiles 2014, 18, 75–80. [Google Scholar] [CrossRef]
- Laye, V.J.; DasSarma, S. An Antarctic Extreme Halophile and Its Polyextremophilic Enzyme: Effects of Perchlorate Salts. Astrobiology 2018, 18, 412–418. [Google Scholar] [CrossRef]
- Al Soudi, A.F.; Farhat, O.; Chen, F.; Clark, B.C.; Schneegurt, M.A. Bacterial growth tolerance to concentrations of chlorate and perchlorate salts relevant to Mars. Int. J. Astrobiol. 2017, 16, 229–235. [Google Scholar] [CrossRef]
- Matsubara, T.; Fujishima, K.; Saltikov, C.W.; Nakamura, S.; Rothschild, L.J. Earth analogues for past and future life on Mars: Isolation of perchlorate resistant halophiles from Big Soda Lake. Int. J. Astrobiol. 2017, 16, 218–228. [Google Scholar] [CrossRef]
- Beblo-Vranesevic, K.; Huber, H.; Rettberg, P. High Tolerance of Hydrogenothermus marinus to Sodium Perchlorate. Front. Microbiol. 2017, 8, 1369. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakova, V.; Oshurkova, V.; Yoshimura, Y. The Effects of Perchlorates on the Permafrost Methanogens: Implication for Autotrophic Life on Mars. Microorganisms 2015, 3, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Flores, N.; Hoyos, S.; Venegas, M.; Galetović, A.; Zúñiga, L.M.; Fábrega, F.; Paredes, B.; Salazar-Ardiles, C.; Vilo, C.; Ascaso, C.; et al. Haloterrigena sp. Strain SGH1, a Bacterioruberin-Rich, Perchlorate-Tolerant Halophilic Archaeon Isolated From Halite Microbial Communities, Atacama Desert, Chile. Front. Microbiol. 2020, 11, 324. [Google Scholar] [CrossRef]
- Kral, T.A.; Goodhart, T.H.; Harpool, J.D.; Hearnsberger, C.E.; McCracken, G.L.; McSpadden, S.W. Sensitivity and adaptability of methanogens to perchlorates: Implications for life on Mars. Planet. Space Sci. 2016, 120, 87–95. [Google Scholar] [CrossRef]
- Coates, J.D.; Achenbach, L.A. Microbial perchlorate reduction: Rocket-fueled metabolism. Nat. Rev. Microbiol. 2004, 2, 569–580. [Google Scholar] [CrossRef]
- Bardiya, N.; Bae, J.-H. Dissimilatory perchlorate reduction: A review. Microbiol. Res. 2011, 166, 237–254. [Google Scholar] [CrossRef]
- Liebensteiner, M.G.; Pinkse, M.W.H.; Schaap, P.J.; Stams, A.J.M.; Lomans, B.P. Archaeal (per)chlorate reduction at high temperature: An interplay of biotic and abiotic reactions. Science 2013, 340, 85–87. [Google Scholar] [CrossRef]
- Zajc, J.; Džeroski, S.; Kocev, D.; Oren, A.; Sonjak, S.; Tkavc, R.; Gunde-Cimerman, N. Chaophilic or chaotolerant fungi: A new category of extremophiles? Front. Microbiol. 2014, 5, 708. [Google Scholar] [CrossRef]
- Gunde-Cimerman, N.; Zalar, P. Extremely halotolerant and halophilic fungi inhabit brine in solar salterns around the globe. Food Technol. Biotech. 2014, 52, 170–179. [Google Scholar]
- Breuer, U.; Harms, H. Debaryomyces hansenii—an extremophilic yeast with biotechnological potential. Yeast 2006, 23, 415–437. [Google Scholar] [CrossRef] [PubMed]
- Nobre, M.F.; Costa, M.S.d. The accumulation of polyols by the yeast Debaryomyces hansenii in response to water stress. Can. J. Microbiol. 1985, 31, 1061–1064. [Google Scholar] [CrossRef]
- Ramos, J. Introducing Debaryomyces Hansenii, a Salt Loving Yeast. In Adaptation to Life at High Salt Concentrations in Archaea, Bacteria, and Eukarya; Gunde-Cimerman, N., Oren, A., Plemenitaš, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 441–451. ISBN 1-4020-3632-9. [Google Scholar]
- Prista, C.; Michán, C.; Miranda, I.M.; Ramos, J. The halotolerant Debaryomyces hansenii, the Cinderella of non-conventional yeasts. Yeast 2016, 33, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Lotlikar, N.P.; Damare, S.R. Variability in Protein Expression in Marine-Derived Purpureocillium lilacinum Subjected to Salt and Chromium Stresses. Indian J. Microbiol. 2018, 58, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Arpini, C.M.; Nóbrega, Y.C.; Castheloge, V.D.; Neves, D.S.; Tadokoro, C.E.; Costa, G.L.d.; Oliveira, M.M.E.; Santos, M.R.d.D. Purpuriocillium lilacinum infection in captive loggerhead sea turtle hatchlings. Med. Mycol. Case Rep. 2019, 23, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Kounaves, S.P.; Chaniotakis, N.A.; Chevrier, V.F.; Carrier, B.L.; Folds, K.E.; Hansen, V.M.; McElhoney, K.M.; O’Neil, G.D.; Weber, A.W. Identification of the perchlorate parent salts at the Phoenix Mars landing site and possible implications. Icarus 2014, 232, 226–231. [Google Scholar] [CrossRef]
- Toner, J.D.; Catling, D.C.; Light, B. Modeling salt precipitation from brines on Mars: Evaporation versus freezing origin for soil salts. Icarus 2015, 250, 451–461. [Google Scholar] [CrossRef]
| Domain | Organism | NaClO4 Tolerance | Literature | ||
|---|---|---|---|---|---|
| (mol/L) | (wt.%) | (wt./vol.%) | |||
| Archaea | Haloferax mediterranei | 0.6 | 6.8 | 7.3 | [31] |
| Halorubrum lacusprofundi | 0.8 | 8.9 | 9.8 | [32] | |
| Bacteria | Halomonas venusta | 1.0 | 10.9 | 12.2 | [33] |
| Planococcus halocryophilus | 1.1 | 12.0 | 13.6 | [30] | |
| Eukarya (Fungi) | Purpureocillium lilacinum | 1.9 | 19.0 | 23.5 | This study |
| Debaryomyces hansenii | 2.4 | 23.0 | 29.9 | This study | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinz, J.; Krahn, T.; Schulze-Makuch, D. A New Record for Microbial Perchlorate Tolerance: Fungal Growth in NaClO4 Brines and its Implications for Putative Life on Mars. Life 2020, 10, 53. https://doi.org/10.3390/life10050053
Heinz J, Krahn T, Schulze-Makuch D. A New Record for Microbial Perchlorate Tolerance: Fungal Growth in NaClO4 Brines and its Implications for Putative Life on Mars. Life. 2020; 10(5):53. https://doi.org/10.3390/life10050053
Chicago/Turabian StyleHeinz, Jacob, Tim Krahn, and Dirk Schulze-Makuch. 2020. "A New Record for Microbial Perchlorate Tolerance: Fungal Growth in NaClO4 Brines and its Implications for Putative Life on Mars" Life 10, no. 5: 53. https://doi.org/10.3390/life10050053
APA StyleHeinz, J., Krahn, T., & Schulze-Makuch, D. (2020). A New Record for Microbial Perchlorate Tolerance: Fungal Growth in NaClO4 Brines and its Implications for Putative Life on Mars. Life, 10(5), 53. https://doi.org/10.3390/life10050053






