Ubiquitous Electron Transport in Non-Electron Transfer Proteins
Abstract
:1. Introduction
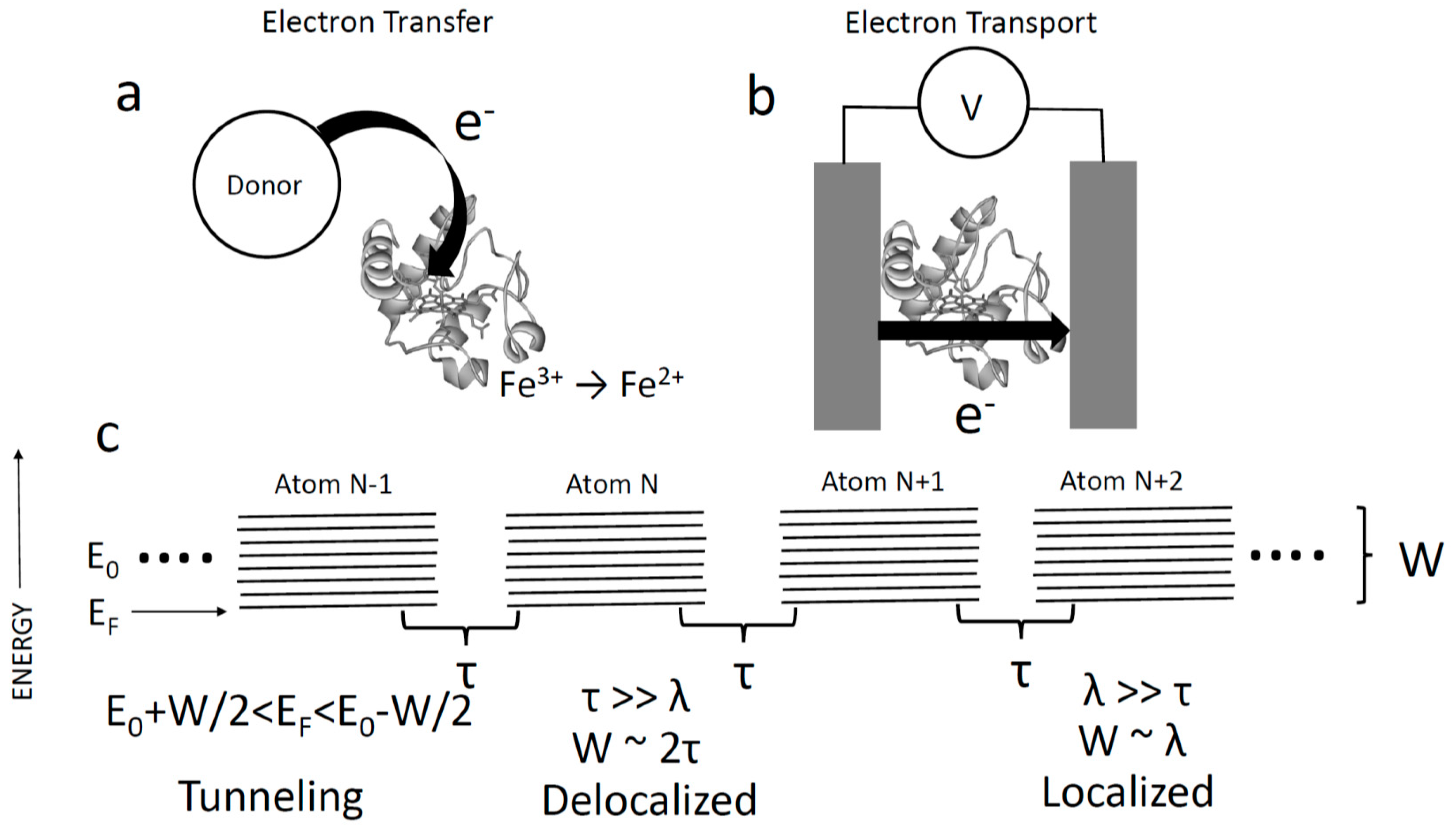
2. Evidence That Proteins Conduct
3. Single Molecule Measurements
- (a)
- Water is often present (and, if not, should be to maintain protein folding), giving rise to the possibility that current is carried by ions. To address this, we carried out measurements with electrodes submerged in electrolyte, and under electrochemical potential control, such that electrode potentials are maintained outside the region where Faradaic currents are generated.
- (b)
- Almost all of the proteins in the prior studies referred to were redox active, forming part of the biological electron transfer chain. We were concerned that some (unknown) nanoscale mechanism might involve transport via rapid reduction and oxidation of the redox active sites. For this reason, we chose to study proteins that were electrochemically inert, eliminating the complications of redox co-factor mediated transport.
- (c)
- Non-specific adsorption and denaturation of proteins are common problems on electrode surfaces, often overcome by treating the surface with specific ligands for the target protein [24] an approach we have adopted in our work, testing the specificity with non-binding control proteins, in order to show that binding is selective on the electrode surface.
- (d)
- The surface chemistry of proteins is complex, so, as the use of specific binding ligands is absent, the chemical nature of the contact between the metal and the protein is unknown.
4. Contacts and Protein Conductance
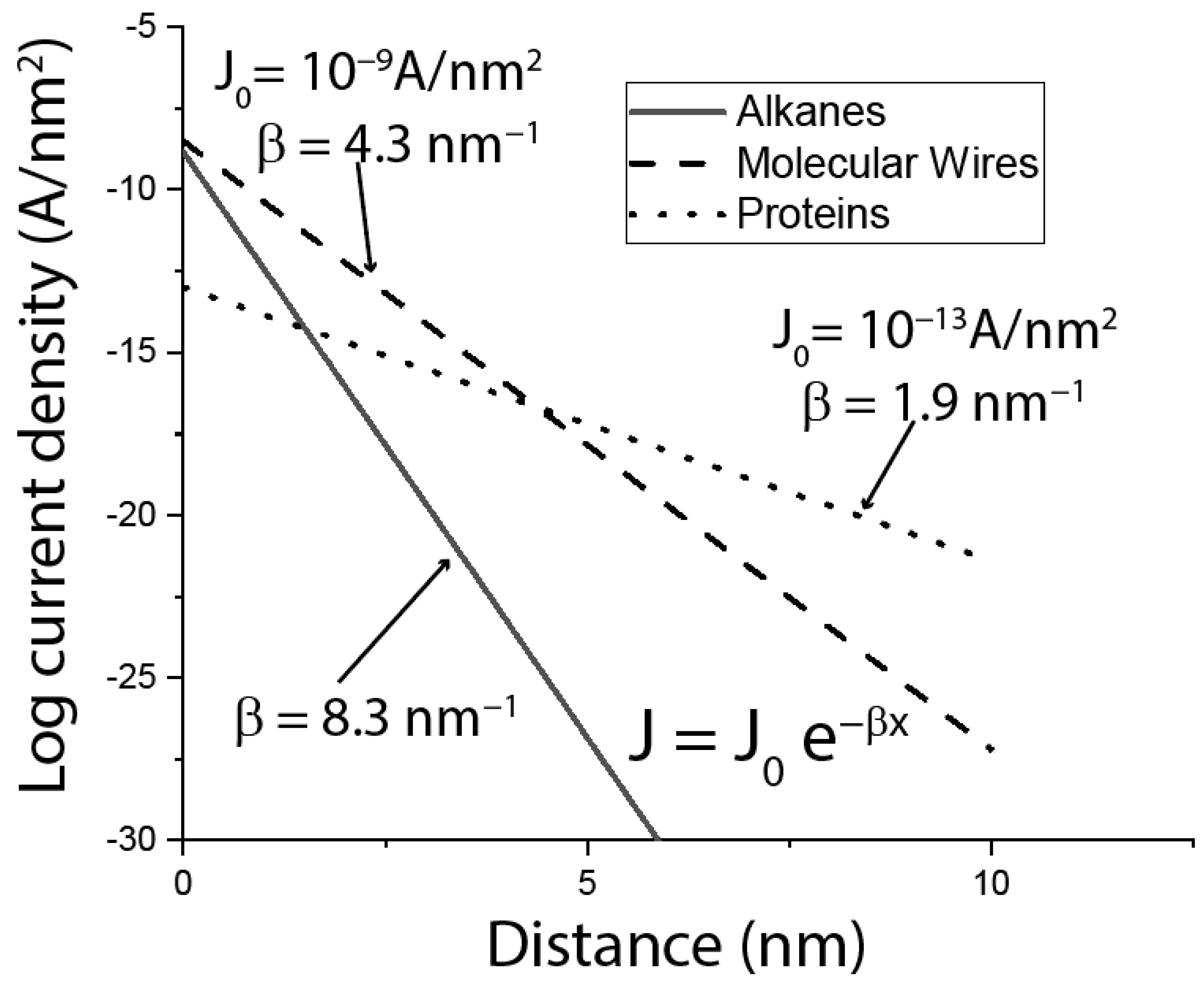
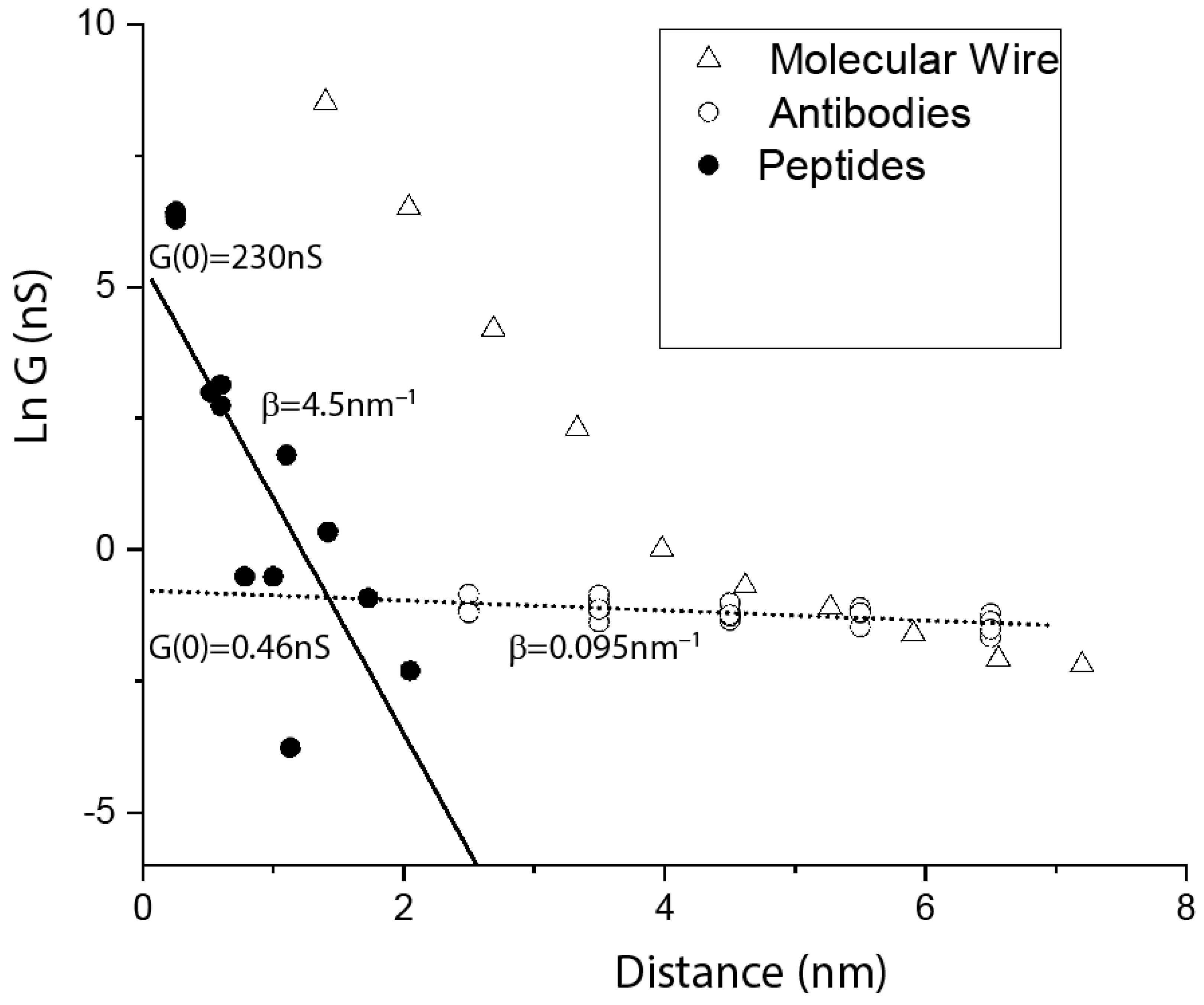
5. Distance Dependence of Protein Conductance
6. Energy of the States Responsible for Transport
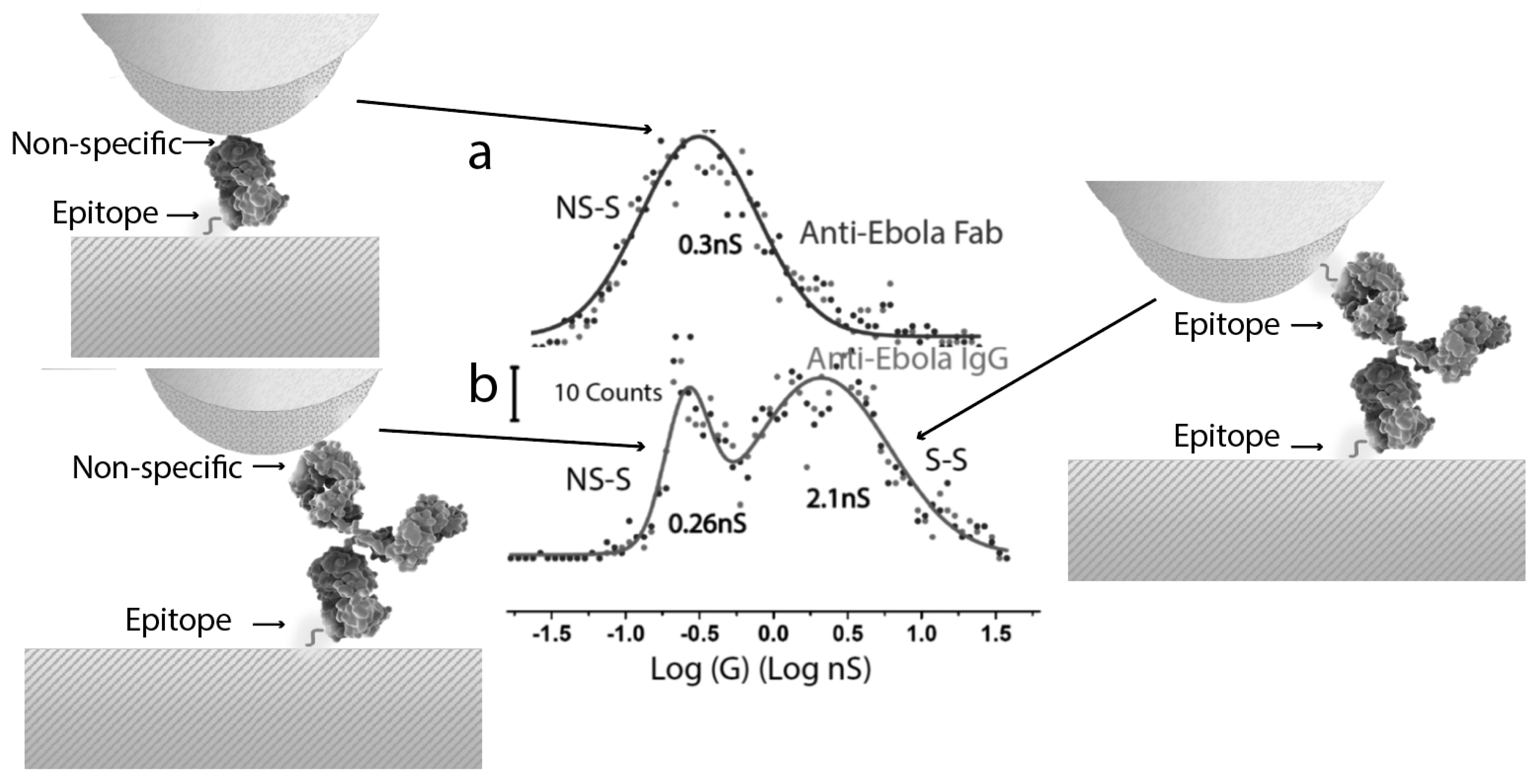
7. Conformation and Conductance
8. Mechanisms
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Ing, N.L.; El-Naggar, M.Y.; Hochbaum, A.I. Going the Distance: Long-Range Conductivity in Protein and Peptide Bioelectronic Materials. J. Phys. Chem. B 2018, 122, 10403–10423. [Google Scholar] [CrossRef] [Green Version]
- Malvankar, N.S.; Vargas, M.; Nevin, K.P.; Franks, A.E.; Leang, C.; Kim, B.-C.; Inoue, K.; Mester, T.; Covalla, S.F.; Johnson, J.P.; et al. Tunable metallic-like conductivity in microbial nanowire networks. Nat. Nanotechnol. 2011, 6, 573–579. [Google Scholar] [CrossRef]
- Wang, F.; Gu, Y.; O’Brien, J.P.; Yi, S.M.; Yalcin, S.E.; Srikanth, V.; Shen, C.; Vu, D.; Ing, N.L.; Hochbaum, A.I.; et al. Structure of Microbial Nanowires Reveals Stacked Hemes that Transport Electrons over Micrometers. Cell 2019, 177, 361–369.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meysman, F.J.R.; Cornelissen, R.; Trashin, S.; Bonné, R.; Martinez, S.H.; Van Der Veen, J.; Blom, C.J.; Karman, C.; Hou, J.-L.; Eachambadi, R.T.; et al. A highly conductive fibre network enables centimetre-scale electron transport in multicellular cable bacteria. Nat. Commun. 2019, 10, 4120–4128. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, S. Introduction to Nanoscience; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Szent-Györgyi, The Study of Energy Levels in Biochemistry. Nature 1941, 148, 157–159. [CrossRef]
- Duan, H.-G.; Prokhorenko, V.I.; Cogdell, R.J.; Ashraf, K.; Stevens, A.L.; Thorwart, M.; Miller, R.J.D. Nature does not rely on long-lived electronic quantum coherence for photosynthetic energy transfer. Proc. Natl. Acad. Sci. USA 2017, 114, 8493–8498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lever, G.; Cole, D.J.; Hine, N.D.M.; Haynes, P.; Payne, M.C. Electrostatic Considerations Affecting the Calculated HOMO–LUMO Gap in Protein Molecules. J. Phys. Condens. Matter 2013, 25, 152101. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.A. On the Theory of Oxidation-Reduction Reactions Involving Electron Transfer. I. J. Chem. Phys. 1956, 24, 966. [Google Scholar] [CrossRef] [Green Version]
- Bostick, C.D.; Mukhopadhyay, S.; Pecht, I.; Sheves, M.; Cahen, D.; Lederman, D. Protein bioelectronics: A review of what we do and do not know. Rep. Prog. Phys. 2018, 81, 026601. [Google Scholar] [CrossRef] [Green Version]
- Amdursky, N.; Marchak, D.; Sepunaru, L.; Pecht, I.; Sheves, M.; Cahen, D. Electronic Transport via Proteins. Adv. Mater. 2014, 26, 7142–7161. [Google Scholar] [CrossRef]
- Sepunaru, L.; Pecht, I.; Sheves, M.; Cahen, D. Solid-State Electron Transport across Azurin: From a Temperature-Independent to a Temperature-Activated Mechanism. J. Am. Chem. Soc. 2011, 133, 2421–2423. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, A.; Salerno, M.; Frabboni, S.; Facci, P. Single-metalloprotein Wet Biotransistor. Appl. Phys. Lett. 2005, 86, 133902. [Google Scholar] [CrossRef]
- Alessandrini, A.; Corni, S.; Facci, P. Unravelling Single Metalloprotein Electron Transfer by Scanning Probe Techniques. Phys. Chem. Chem. Phys. 2006, 8, 4383. [Google Scholar] [CrossRef] [PubMed]
- Chi, Q.; Farver, O.; Ulstrup, J. Long-Range Protein Electron Transfer Observed at the Single-Molecule Level: In Situ Mapping of Redox-Gated Tunneling Resonance. Proc. Natl. Acad. Sci. USA 2005, 102, 16203–16208. [Google Scholar] [CrossRef] [Green Version]
- Artés, J.M.; Díez-Pérez, I.; Sanz, F.; Gorostiza, P. Direct Measurement of Electron Transfer Distance Decay Constants of Single Redox Proteins by Electrochemical Tunneling Spectroscopy. ACS Nano 2011, 5, 2060–2066. [Google Scholar] [CrossRef]
- Madden, C.; Vaughn, M.D.; Díez-Pérez, I.; Brown, K.A.; King, P.W.; Gust, D.; Moore, A.L.; Moore, T.A. Catalytic Turnover of [FeFe]-Hydrogenase Based on Single-Molecule Imaging. J. Am. Chem. Soc. 2011, 134, 1577–1582. [Google Scholar] [CrossRef]
- Chi, Q.; Zhang, J.; Arslan, T.; Borg, L.; Pedersen, G.W.; Christensen, H.E.M.; Nazmudtinov, R.R.; Ulstrup, J. Approach to Interfacial and Intramolecular Electron Transfer of the Diheme Protein Cytochromec4Assembled on Au(111) Surfaces. J. Phys. Chem. B 2010, 114, 5617–5624. [Google Scholar] [CrossRef]
- Karlsson, J.-J.; Nielsen, M.F.; Thuesen, M.H.; Ulstrup, J. Electrochemistry of Cytochrome c4 from Pseudomonas stutzeri. J. Phys. Chem. B 1997, 101, 2430–2436. [Google Scholar] [CrossRef]
- Wang, M.; Bugarski, S.; Stimming, U. Topological and Electron-Transfer Properties of Glucose Oxidase Adsorbed on Highly Oriented Pyrolytic Graphite Electrodes. J. Phys. Chem. C 2008, 112, 5165–5173. [Google Scholar] [CrossRef]
- Vaz-Domínguez, C.; Pita, M.; de Lacey, A.L.; Shleev, S.; Cuesta, A. Combined ATR-SEIRAS and EC-STM Study of the Immobilization of Laccase on Chemically Modified Au Electrodes. J. Phys. Chem. C 2012, 116, 16532–16540. [Google Scholar] [CrossRef]
- Ruiz, M.P.; Aragonès, A.C.; Camarero, N.; Vilhena, J.G.; Ortega, M.; Zotti, L.A.; Perez, R.; Cuevas, J.C.; Gorostiza, P.; Díez-Pérez, I. Bioengineering a Single-Protein Junction. J. Am. Chem. Soc. 2017, 139, 15337–15346. [Google Scholar] [CrossRef] [PubMed]
- Della Pia, E.A.; Chi, Q.; Macdonald, J.; Ulstrup, J.; Jones, D.D.; Elliott, M. Fast Electron Transfer through a Single Molecule Natively Structured Redox Protein. Nanoscale 2012, 4, 7106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras-Naranjo, J.E.; Aguilar, O. Suppressing Non-Specific Binding of Proteins onto Electrode Surfaces in the Development of Electrochemical Immunosensors. Biosensors 2019, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Song, W.; Pang, P.; Zhao, Y.; Zhang, P.; Csabai, I.; Vattay, G.; Lindsay, S. Observation of Giant Conductance Fluctuations in a Protein. Nano Future 2017, 1, 035002. [Google Scholar] [CrossRef]
- Zhang, B.; Song, W.; Pang, P.; Lai, H.; Chen, Q.; Zhang, P.; Lindsay, S. The Role of Contacts in Long-Range Protein Conductance. Proc. Natl. Acad. Sci. USA 2019, 116, 5886–5891. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Lindsay, S. Electronic Decay Length in a Protein Molecule. Nano Lett. 2019, 19, 4017–4022. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, B.; Frisbie, C.D.; Miles, L.; Kapos, V. Electrical Resistance of Long Conjugated Molecular Wires. Science 2008, 320, 1482–1486. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, B.; Tao, N. Conductance Titration of Single-Peptide Molecules. J. Am. Chem. Soc. 2004, 126, 5370–5371. [Google Scholar] [CrossRef]
- Sepunaru, L.; Refaely-Abramson, S.; Lovrincic, R.; Gavrilov, Y.; Agrawal, P.; Levy, Y.; Kronik, L.; Pecht, I.; Sheves, M.; Cahen, D. Electronic Transport via Homopeptides: The Role of Side Chains and Secondary Structure. J. Am. Chem. Soc. 2015, 137, 9617–9626. [Google Scholar] [CrossRef]
- Brisendine, J.M.; Refaely-Abramson, S.; Liu, Z.-F.; Cui, J.; Ng, F.; Neaton, J.B.; Koder, R.L.; Venkataraman, L. Probing Charge Transport through Peptide Bonds. J. Phys. Chem. Lett. 2018, 9, 763–767. [Google Scholar] [CrossRef]
- Sek, S.; Misicka, A.; Swiatek, K.; Maicka, E. Conductance of α-Helical Peptides Trapped within Molecular Junctions. J. Phys. Chem. B 2006, 110, 19671–19677. [Google Scholar] [CrossRef] [PubMed]
- Juhaniewicz-Debinska, J.; Sek, S. Peptide Molecular Junctions: Distance Dependent Electron Transmission through Oligoprolines. Bioelectrochemistry 2012, 87, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Song, W.; Brown, J.; Nemanich, R.J.; Lindsay, S. Electronic Conductance Resonance in Non-Redox-Active Proteins. J. Am. Chem. Soc. 2020, 142, 6432–6438. [Google Scholar] [CrossRef] [PubMed]
- Salomon, A.; Cahen, D.; Lindsay, S.; Tomfohr, J.; Engelkes, V.; Frisbie, C. Comparison of Electronic Transport Measurements on Organic Molecules. Adv. Mater. 2003, 15, 1881–1890. [Google Scholar] [CrossRef]
- Harriman, A. Further Comments on the Redox Potentials of Tryptophan and Tyrosine. J. Phys. Chem. 1987, 91, 6102–6104. [Google Scholar] [CrossRef]
- Odella, E.; Mora, S.J.; Wadsworth, B.L.; Huynh, M.T.; Goings, J.J.; Liddell, P.A.; Groy, T.L.; Gervaldo, M.A.; Sereno, L.E.; Gust, D.; et al. Controlling Proton-Coupled Electron Transfer in Bioinspired Artificial Photosynthetic Relays. J. Am. Chem. Soc. 2018, 140, 15450–15460. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, H.; Mukherjee, S.; Song, W.; Wang, X.; Lindsay, S. Engineering an Enzyme for Direct Electrical Monitoring of Activity. ACS Nano 2019, 14, 1360–1368. [Google Scholar] [CrossRef]
- Aubert, C.; Vos, M.H.; Mathis, P.; Eker, A.P.M.; Brettel, K. Intraprotein radical transfer during photoactivation of DNA photolyase. Nature 2000, 405, 586–590. [Google Scholar] [CrossRef]
- Matyushov, D.V. Protein Electron Transfer: Dynamics and Statistics. J. Chem. Phys. 2013, 139, 25102. [Google Scholar] [CrossRef]
- Kuznetsov, A.M.; Ulstrup, J. Dissipative Relaxation of a Low-Energy Intermediate Electronic State in Three-Level Electron Transfer. Chem. Phys. 1991, 157, 25–33. [Google Scholar] [CrossRef]
- Vattay, G.; Salahub, D.; Csabai, I.; Nassimi, A.; Kaufmann, S.A. Quantum Criticality at the Origin of Life. J. Phys. Conf. Ser. 2015, 626, 012023. [Google Scholar] [CrossRef]



© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindsay, S. Ubiquitous Electron Transport in Non-Electron Transfer Proteins. Life 2020, 10, 72. https://doi.org/10.3390/life10050072
Lindsay S. Ubiquitous Electron Transport in Non-Electron Transfer Proteins. Life. 2020; 10(5):72. https://doi.org/10.3390/life10050072
Chicago/Turabian StyleLindsay, Stuart. 2020. "Ubiquitous Electron Transport in Non-Electron Transfer Proteins" Life 10, no. 5: 72. https://doi.org/10.3390/life10050072
APA StyleLindsay, S. (2020). Ubiquitous Electron Transport in Non-Electron Transfer Proteins. Life, 10(5), 72. https://doi.org/10.3390/life10050072




