A Focus on the Nowadays Potential Antiviral Strategies in Early Phase of Coronavirus Disease 2019 (Covid-19): A Narrative Review
Abstract
:1. Introduction
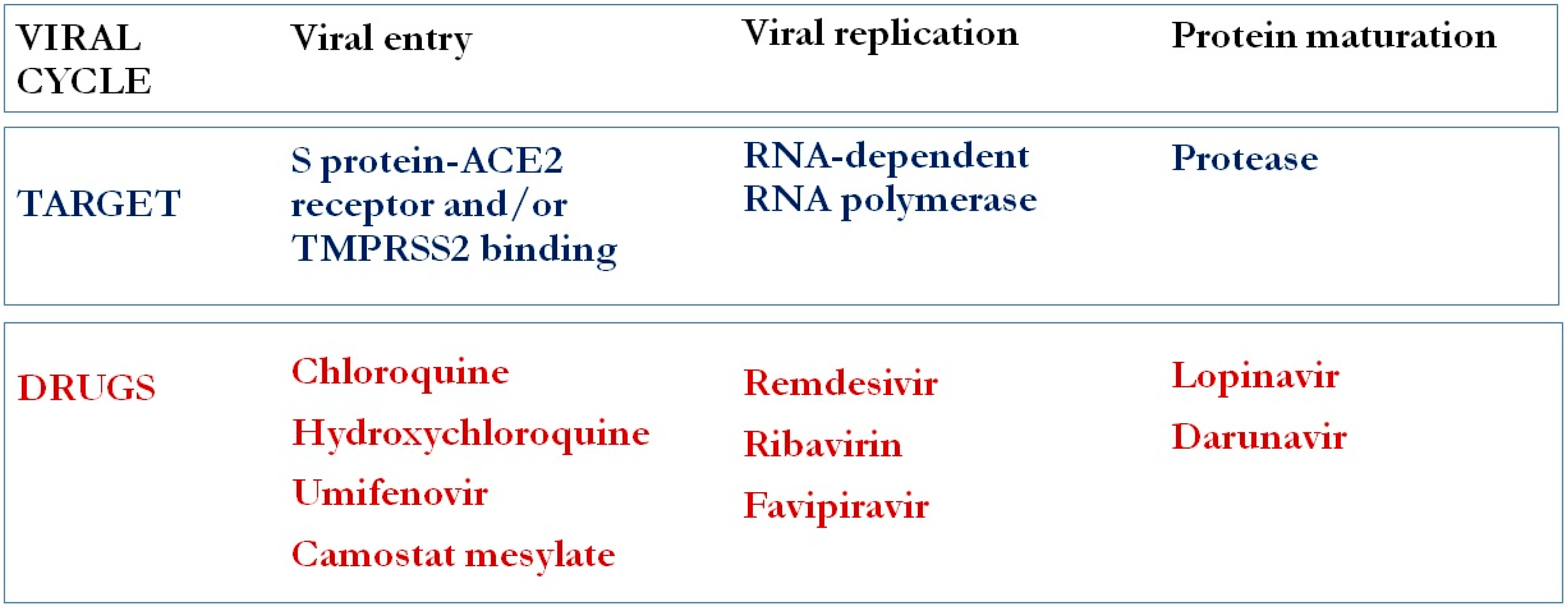
2. Viral Cycle Pathogenesis
3. Natural History and Proposed Clinical Staging
4. Aim of the Narrative Review
5. Materials and Methods
6. Antiviral Agents
6.1. Chloroquine and Hydroxychloroquine
6.2. Lopinavir/Ritonavir (LPV/r) and Other Protease Inhibitors (PIs)
6.3. Remdesivir
6.4. Other Antivirals
7. Ongoing Clinical Trials
8. Current Recommendations
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- WHO. WHO Announces COVID-19 Outbreak A Pandemic; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. Coronavirus Disease (COVID-19) Situation Report—193; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020. [Google Scholar] [CrossRef]
- Sanders, J.M.; Monogue, M.L.; Jodlowski, T.Z.; Cutrell, J.B. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 323, 1824–1836. [Google Scholar] [CrossRef]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J. Heart Lung Transpl. 2020, 39, 405. [Google Scholar] [CrossRef] [Green Version]
- Linton, N.M.; Kobayashi, T.; Yang, Y.; Hayashi, K.; Akhmetzhanov, A.R.; Jung, S.M.; Yuan, B.; Kinoshita, R.; Nishiura, H. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: A statistical analysis of publicly available case data. J. Clin. Med. 2020, 9, 538. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Chen, X.; Cai, Y.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; Zhang, Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020. [Google Scholar] [CrossRef] [Green Version]
- Gilead Sciences. Remdesivir (GS-5734) Investigator’s Brochure, 5th ed.; Personal Communication, 21 February 2020; Gilead Sciences: Foster City, CA, USA, 2020. [Google Scholar]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Dai, S.M.; Tong, Q. COVID-19: A recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J. Antimicrob. Chemother. 2020. [Google Scholar] [CrossRef]
- Devaux, C.A.; Rolain, J.M.; Colson, P.; Raoult, D. New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? Int. J. Antimicrob. Agents 2020, 55, 105938. [Google Scholar] [CrossRef]
- Mo, Y.; Fisher, D. A review of treatment modalities for Middle East respiratory syndrome. J. Antimicrob. Chemother. 2016, 71, 3340–3350. [Google Scholar] [CrossRef]
- Savarino, A.; Boelaert, J.R.; Cassone, A.; Majori, G.; Cauda, R. Effects of chloroquine on viral infections: An old drug against today’s diseases. Lancet Infect. Dis. 2003, 3, 722–727. [Google Scholar] [CrossRef]
- Maisonnasse, P.; Guedj, J.; Contreras, V.; Behillil, S.; Solas, C.; Marlin, R.; Naninck, T.; Pizzorno, A.; Lemaitre, J.; Gonçalves, A. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature 2020, 1–8. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Gautret, P.; Lagier, J.C.; Parola, P.; Meddeb, L.; Sevestre, J.; Mailhe, M.; Doudier, B.; Aubry, C.; Amrane, S.; Seng, P. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med. Infect. Dis. 2020, 101663. [Google Scholar] [CrossRef]
- Gautret, P.; Lagier, J.C.; Parola, P.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; Dupont, H.T. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 105949. [Google Scholar] [CrossRef]
- Gao, J.; Tian, Z.; Yang, X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends 2020. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Hu, J.; Zhang, Z.; Jiang, S.; Han, S.; Yan, D.; Zhuang, R.; Hu, B.; Zhang, Z. Efficacy of hydroxychloroquine in patients with COVID-19: Results of a randomized clinical trial. MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Geleris, J.; Sun, Y.; Platt, J.; Zucker, J.; Baldwin, M.; Hripcsak, G.; Labella, A.; Manson, D.; Kubin, C.; Barr, R.G.; et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Arshad, S.; Kilgore, P.; Chaudhry, Z.S.; Jacobsen, G.; Wang, D.D.; Huitsing, K.; Brar, I.; Alangaden, G.J.; Ramesh, M.S.; McKinnon, J.E. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Skipper, C.P.; Pastick, K.A.; Engen, N.W.; Bangdiwala, A.S.; Abassi, M.; Lofgren, S.M.; Williams, D.A.; Okafor, E.C.; Pullen, M.F.; Nicol, M.R. Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19: A Randomized Trial. Ann. Intern. Med. 2020. [Google Scholar] [CrossRef]
- Mehra, M.R.; Desai, S.S.; Ruschitzka, F.; Patel, A.N. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis. Lancet 2020. [Google Scholar] [CrossRef]
- Mehra, M.R.; Ruschitzka, F.; Patel, A.N. Retraction-Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis. Lancet 2020, 395, 1820. [Google Scholar] [CrossRef]
- WHO. “Solidarity” Clinical Trial for COVID-19 Treatments; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Oxford University. RECOVERY Trial—Randomized Evaluation of COVID-10 Therapy; Oxford University: Oxford, UK, 2020. [Google Scholar]
- Ader, F.; Espérou, H. Trial of Treatments for COVID-19 in Hospitalized Adults (DisCoVeRy). Clin. Trial Gov. 2020. [Google Scholar]
- Sinha, N.; Balayla, G. Hydroxychloroquine and covid-19. Postgrad. Med. J. 2020, 0, 1–6. [Google Scholar] [CrossRef] [Green Version]
- De Wilde, A.H.; Jochmans, D.; Posthuma, C.C.; Zevenhoven-Dobbe, J.C.; van Nieuwkoop, S.; Bestebroer, T.M.; van den Hoogen, B.G.; Neyts, J.; Snijder, E.J. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014, 58, 4875–4884. [Google Scholar] [CrossRef] [Green Version]
- Nukoolkarn, V.; Lee, V.S.; Malaisree, M.; Aruksakulwong, O.; Hannongbua, S. Molecular dynamic simulations analysis of ritronavir and lopinavir as SARS-CoV 3CLpro inhibitors. J. Theor. Biol. 2008, 254, 861–867. [Google Scholar] [CrossRef]
- Chen, F.; Chan, K.; Jiang, Y.; Kao, R.; Lu, H.; Fan, K.; Cheng, V.; Tsui, W.; Hung, I.; Lee, T. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 2004, 31, 69–75. [Google Scholar] [CrossRef]
- Chu, C.; Cheng, V.; Hung, I.; Wong, M.; Chan, K.; Chan, K.; Kao, R.; Poon, L.; Wong, C.; Guan, Y. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax 2004, 59, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Choy, K.T.; Wong, A.Y.L.; Kaewpreedee, P.; Sia, S.F.; Chen, D.; Hui, K.P.Y.; Chu, D.K.W.; Chan, M.C.W.; Cheung, P.P.-H.; Huang, X. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020, 104786. [Google Scholar] [CrossRef]
- Que, T.; Wong, V.; Yuen, K. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: A multicentre retrospective matched cohort study. Hong Kong Med. J. 2003, 9, 399–406. [Google Scholar]
- Young, B.; Ong, S.; Kalimuddin, S.; Low, J.; Tan, S.; Loh, J.; Ng, O.; Marimuthu, K.; Ang, L.; Mak, T. Singapore Novel Coronavirus Outbreak Research T. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA 2020, 323, 1488–1494. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Xu, A.; Zhang, Y.; Xuan, W.; Yan, T.; Pan, K.; Yu, W.; Zhang, J. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Lee, Y.-L.; Chen, C.-P.; Lin, Y.-C.; Liu, C.-E.; Liao, C.-h.; Cheng, S.-H. Lopinavir/ritonavir did not shorten the duration of SARS CoV-2 shedding in patients with mild pneumonia in Taiwan. J. Microbiol. Immunol. Infect. 2020. [Google Scholar] [CrossRef]
- Yao, T.T.; Qian, J.D.; Zhu, W.Y.; Wang, Y.; Wang, G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus—A possible reference for coronavirus disease-19 treatment option. J. Med. Virol. 2020. [Google Scholar] [CrossRef] [Green Version]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Oxford University. No Clinical Benefit from Use of Lopinavir-Ritonavir in Hospitalised COVID-19 Patients Studied in RECOVERY; Oxford University: Oxford, UK, 2020. [Google Scholar]
- Dong, L.; Hu, S.; Gao, J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov. Ther. 2020, 14, 58–60. [Google Scholar] [CrossRef] [Green Version]
- Riva, A.; Conti, F.; Bernacchia, D.; Pezzati, L.; Sollima, S.; Merli, S.; Siano, M.; Lupo, A.; Rusconi, S.; Cattaneo, D. Darunavir does not prevent SARS-CoV-2 infection in HIV patients. Pharmacol. Res. 2020, 104826. [Google Scholar] [CrossRef]
- FDA. KALETRA (Lopinavir and Ritonavir) Tablet and Oral Solution, for Oral Use; FDA: Silver Spring, MD, USA, 2020.
- Agostini, M.L.; Andres, E.L.; Sims, A.C.; Graham, R.L.; Sheahan, T.P.; Lu, X.; Smith, E.C.; Case, J.B.; Feng, J.Y.; Jordan, R. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio 2018, 9, e00221-18. [Google Scholar] [CrossRef] [Green Version]
- Sheahan, T.P.; Sims, A.C.; Graham, R.L.; Menachery, V.D.; Gralinski, L.E.; Case, J.B.; Leist, S.R.; Pyrc, K.; Feng, J.Y.; Trantcheva, I. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Sheahan, T.P.; Sims, A.C.; Leist, S.R.; Schäfer, A.; Won, J.; Brown, A.J.; Montgomery, S.A.; Hogg, A.; Babusis, D.; Clarke, M.O. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Williamson, B.; Feldmann, F.; Schwarz, B.; Meade-White, K.; Porter, D.; Schulz, J.; Van Doremalen, N.; Leighton, I.; Yinda, C.K.; Perez-Perez, L. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. BioRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, A.; Padey, B.; Julien, T.; Trouillet-Assant, S.; Traversier, A.; Errazuriz-Cerda, E.; Fouret, J.; Dubois, J.; Gaymard, A.; Lescure, X. Characterization and treatment of SARS-CoV-2 in nasal and bronchial human airway epithelia. BioRxiv 2020. [Google Scholar] [CrossRef]
- Holshue, M.L.; DeBolt, C.; Lindquist, S.; Lofy, K.H.; Wiesman, J.; Bruce, H.; Spitters, C.; Ericson, K.; Wilkerson, S.; Tural, A. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kujawski, S.A.; Wong, K.K.; Collins, J.P.; Epstein, L.; Killerby, M.E.; Midgley, C.M.; Abedi, G.R.; Ahmed, N.S.; Almendares, O.; Alvarez, F.N. First 12 patients with coronavirus disease 2019 (COVID-19) in the United States. MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.-X. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S. Remdesivir for the Treatment of Covid-19—Preliminary Report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- FDA. Remdesivir EUA Letter of Authorization—FDA; FDA: Silver Spring, MD, USA, 2020.
- Goldman, J.D.; Lye, D.C.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.D.; Galli, M.; Ahn, M.-Y.; Nahass, R.G. Remdesivir for 5 or 10 days in patients with severe Covid-19. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- McKee, D.L.; Sternberg, A.; Stange, U.; Laufer, S.; Naujokat, C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol. Res. 2020, 104859. [Google Scholar] [CrossRef]
- Pécheur, E.-I.; Borisevich, V.; Halfmann, P.; Morrey, J.D.; Smee, D.F.; Prichard, M.; Mire, C.E.; Kawaoka, Y.; Geisbert, T.W.; Polyak, S.J. The synthetic antiviral drug arbidol inhibits globally prevalent pathogenic viruses. J. Virol. 2016, 90, 3086–3092. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Li, C.; Zeng, Q.; Liu, X.; Li, X.; Zhang, H.; Hong, Z.; Xia, J. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J. Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Lu, Z.; Xu, T.; Chen, C.; Yang, G.; Zha, T.; Xue, Y. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, B.; Li, Q.; Wen, L.; Zhang, R. Clinical Features of 69 Cases With Coronavirus Disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, N.; Xie, H.; Lin, S.; Huang, J.; Zhao, J.; Lin, Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: A retrospective study. Clin. Microbiol. Infect. 2020. [Google Scholar] [CrossRef]
- Furuta, Y.; Komeno, T.; Nakamura, T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B 2017, 93, 449–463. [Google Scholar] [CrossRef] [Green Version]
- Eloy, P.; Solas, C.; Touret, F.; Mentré, F.; Malvy, D.; de Lamballerie, X.; Guedj, J. Dose rationale for favipiravir use in patients infected with SARS-CoV-2. Clin. Pharmacol. Ther. 2020. [Google Scholar] [CrossRef]
- Cai, Q.; Yang, M.; Liu, D.; Chen, J.; Shu, D.; Xia, J.; Liao, X.; Gu, Y.; Cai, Q.; Yang, Y. Experimental treatment with favipiravir for COVID-19: An open-label control study. Engineering 2020. [Google Scholar] [CrossRef]
- Huang, J.; Song, W.; Huang, H.; Sun, Q. Pharmacological Therapeutics Targeting RNA-Dependent RNA Polymerase, Proteinase and Spike Protein: From Mechanistic Studies to Clinical Trials for COVID-19. J. Clin. Med. 2020, 9, 1131. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Schroeder, S.; Kleine-Weber, H.; Müller, M.A.; Drosten, C.; Pöhlmann, S. Nafamostat mesylate blocks activation of SARS-CoV-2: New treatment option for COVID-19. Antimicrob. Agents Chemother. 2020. [Google Scholar] [CrossRef] [Green Version]
- Falzarano, D.; De Wit, E.; Rasmussen, A.L.; Feldmann, F.; Okumura, A.; Scott, D.P.; Brining, D.; Bushmaker, T.; Martellaro, C.; Baseler, L. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV–infected rhesus macaques. Nat. Med. 2013, 19, 1313–1317. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Mandourah, Y.; Al-Hameed, F.; Sindi, A.A.; Almekhlafi, G.A.; Hussein, M.A.; Jose, J.; Pinto, R.; Al-Omari, A.; Kharaba, A. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am. J. Respir. Crit. Care Med. 2018, 197, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Stockman, L.J.; Bellamy, R.; Garner, P. SARS: Systematic review of treatment effects. PLoS Med. 2006, 3, e343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, U.J.; Won, E.J.; Kee, S.J.; Jung, S.I.; Jang, H.C. Case report Combination therapy with lopinavir/ritonavir, ribavirin and interferon-α for Middle East respiratory syndrome. Antivir. Ther. 2016, 21, 455–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.F.; Chan, K.H.; Kao, R.Y.; To, K.K.; Zheng, B.J.; Li, C.P.; Li, P.T.; Dai, J.; Mok, F.K.; Chen, H. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J. Infect. 2013, 67, 606–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.F.W.; Yao, Y.; Yeung, M.L.; Deng, W.; Bao, L.; Jia, L.; Li, F.; Xiao, C.; Gao, H.; Yu, P. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 2015, 212, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 104787. [Google Scholar] [CrossRef]
- Jans, D.A.; Martin, A.J.; Wagstaff, K.M. Inhibitors of nuclear transport. Curr. Opin. Cell Biol. 2019, 58, 50–60. [Google Scholar] [CrossRef]
- Schmith, V.D.; Zhou, J.; Lohmer, L.R. The Approved Dose of Ivermectin Alone is not the Ideal Dose for the Treatment of COVID-19. Clin. Pharmacol. Ther. 2020. [Google Scholar] [CrossRef]
- Stauffer, W.M.; Alpern, J.D.; Walker, P.F. COVID-19 and Dexamethasone: A Potential Strategy to Avoid Steroid-Related Strongyloides Hyperinfection. JAMA 2020. [Google Scholar] [CrossRef]
- Kelleni, M. Nitazoxanide/Azithromycin combination for COVID-19: A suggested new protocol for COVID-19 early management. Preprints 2020. [Google Scholar] [CrossRef] [Green Version]
- Rossignol, J.F. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J. Infect. Public Health 2016, 9, 227–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IDSA. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19; IDSA: Arlington, VA, USA, 2020. [Google Scholar]
- NIH. COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/therapeutic-options-under-investigation/antiviral-therapy/ (accessed on 23 April 2020).
- WHO. Off-label use of medicines for COVID-19. In Scientific Brief, 31 March 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. Clinical Management of COVID-19—Interim Guidance; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Sardu, C.; D’Onofrio, N.; Balestrieri, M.L.; Barbieri, M.; Rizzo, M.R.; Messina, V.; Maggi, P.; Coppola, N.; Paolisso, G.; Marfella, R. Outcomes in Patients With Hyperglycemia Affected by COVID-19: Can We Do More on Glycemic Control? Diabetes Care 2020, 43, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Macera, M.; De Angelis, G.; Sagnelli, C.; Coppola, N.; COVID, V. Clinical Presentation of COVID-19: Case Series and Review of the Literature. Int. J. Environ. Res. Public Health 2020, 17, 5062. [Google Scholar] [CrossRef] [PubMed]
- Barillari, M.R.; Bastiani, L.; Lechien, J.R.; Mannelli, G.; Molteni, G.; Cantarella, G.; Coppola, N.; Costa, G.; Trecca, E.M.; Grillo, C.; et al. A structural equation model to examine the clinical features of mild-to-moderate COVID-19: A multicenter Italian study. J. Med. Virol. 2020, 25. [Google Scholar] [CrossRef]
| Drug Name | Mechanism of Action | Dosing * | Adverse Events | |
|---|---|---|---|---|
| Chloroquine (CQ) and Hydroxychloroquine (HCQ) |
|
| HCQ has a lower incidence of toxicity than CQ. Cardiological: QTc prolongation, torsade de pointes, ventricular arrythmia and cardiac deaths. Baseline and follow-up electrocardiogram (ECG) are recommended especially when coadministered with other QT-interval prolonging drugs [antibiotics (ex. azithromycin), antifungals, antiarrhythmics, antipsychotics] Gastrointestinal: nausea, diarrhea, vomiting Others: hemolysis (if G6PD-deficiency), hypoglycemia, pruritus and dermatological alterations (rash), retinopathy, bone marrow suppression, proximal muscles neuromyopathy, likely with long-term use. | |
| Azithromycin |
| tab 500 mg: 1 tab QD | When used with HCQ: Cardiological: QTc prolongation (in particular when coadministered with other QT-interval prolonging drugs) Gastrointestinal: nausea, diarrhea, vomiting Hepatotoxicity | |
| HCQ + Azithromycin | See above | |||
| HIV Protease Inhibitors (LPV/r and DRV/c) | Possible inhibition of SARS-CoV-2 3-chymotrisyn-like (3CL)-protease and papain-like protease Lopinavir is excreted in the gastrointestinal (GI) tract, and thus coronavirus-infected enterocytes might be exposed to higher concentrations of the drug | LPV/r tab 200/50 mg: 2 tab BID LPV/r oral sol 80/20 mg: 5 mL BID DRV/cobi tab 800/150 mg: 1 tab QD | Gastrointestinal: diarrhea, nausea, vomiting, increased amylase, lipase, total cholesterol and triglycerides (risk factor for pancreatitis) Hepatotoxicity: increasing in GGT, AST, ALT, total bilirubin, hepatitis Cardiological: QT- and PR-interval prolongation, hypertension, bradyarrhytmias; torsade de pointes have been reported in patients treated with LPV/r Metabolical: hyperglycemia and diabetes mellitus, increased uric acid | |
| Remdesivir (GS-5734) | Adenosine nucleotide analog prodrug, which inhibits viral RNA-dependent RNA polymerase (RdRp). Potent in vitro activity demonstrated in SARS-CoV-2-infected Vero E6 cells In vitro and in vivo activity vs. SARS-CoV and MERS-CoV | fl 150 mg: LD 200 mg on day 1, then 100 mg QD on days 2-10 | Gastrointestinal: nausea, vomiting Hepatotoxicity: transient elevation of ALT and AST (grade 1 or 2), typically after multiple days of treatment [9] Hematological: mild, reversible prolonged prothrombin time (PT) without INR change (Gilead 2020) Renal: potential toxicity due to accumulation of sulfobutyl ether β-cyclodextrin sodium (SBECD) in moderate to severe renal impairment | |
| CQ or HCQ | HCQ + Azithromicyn | LPV/r or Others PIs | Remdesivir | |
|---|---|---|---|---|
| WHO | None of the drugs proposed as potential therapies against COVID-19 have been shown to be safe and effective. Many agents are now being or will soon be studied in clinical trials (including the SOLIDARITY trial). “We recommend that the following drugs not be administered as treatment or prophylaxis for COVID-19, outside of the context of clinical trials. … If it is not possible to give the treatment as part of a clinical trial, appropriate records of the use of the medicine must be kept, in compliance with national law, and outcomes for patients should be monitored and recorded. If early results from an unproven or experimental treatment are promising, the treatment should be studied in the context of a formal clinical trial to establish its safety, efficacy, risks, and benefits.” | |||
| IDSA | The panel recommends HCQ or CQ in the context of a clinical trial among hospitalized patients with COVID 19 | The panel recommends HCQ or CQ + azithromycin only in the context of a clinical trial among hospitalized patients with COVID-19 | The panel recommends HCQ or CQ in the context of a clinical trial among hospitalized patients with COVID | / |
| NIH | There are insufficient clinical data to recommend either FOR or AGAINST CQ or HCQ for the treatment of COVID-19 (AIII). When they are used, monitor adverse effects, especially QTc interval prolongation (AIII). | The panel recommends AGAINST the use of HCQ plus azithromycin, except in the context of a clinical trial (AIII). | The panel recommends AGAINST the use of lopinavir/ritonavir (AI) or other HIV protease inhibitors (AIII), except in the context of a clinical trial. | There are insufficient clinical data to recommend either FOR or AGAINST the use of remdesivir for the treatment of COVID-19 (AIII). |
| Early (Viral) Phase | Intermediate (Pulmonary) Phase | Late (Cytokine Storm) Phase | |
|---|---|---|---|
| Pathogenesis | Viral entry in host cells (S protein-ACE2 or—TMPRSS2 binding) | Viral replication, mainly in lower respiratory airways | Systemic hyper-inflammation syndrome and cytokine-storm, mainly in the lung |
| Clinical presentation | Mild upper airways symptoms: dry cough, sore throat, conjunctivitis, malaise ±hypo- or a-nosmia ±hypo- or a-geusia Mild constitutional symptoms: fever, headache, muscle ache and less frequently nausea or diarrhea | Fever (recurrent or persistent) Lower respiratory tract involvement (viral pneumonia) In late phase, possible dyspnoea, shortness of breath and hypoxia ±extra-pulmonary symptoms | ARDS Shock ±Multi-organ failure may appear in most severe cases |
| Biochemical presentation | Leucopenia with lymphopenia, Mild increase in: prothrombin time, D-dimer, C reactive protein (CRP) and LDH | Lymphopenia Increase in: ALT, AST Increased systemic inflammatory markers: CRP, ferritin, LDH, D-dimer, IL-6 In late phase, possible hypoxemia | Lymphopenia Significant increased systemic inflammatory markers: CRP, ferritin, LDH, D-dimer, cytokines (IL2, IL6, IL7, TNFα, granulocyte-colony stimulating, etc.), procalcitonin Increase in: troponin, NT-proBNP, ALT, AST Severe hypoxemia |
| Imaging (RX, Tc) signs | None | Pneumonia with bilateral ground glass opacities (GGO) and/or consolidations | Increasing in number, dimension and density of bilateral consolidations until ARDS scenario |
| Treatment strategy |
|
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monari, C.; Gentile, V.; Camaioni, C.; Marino, G.; Coppola, N.; Vanvitelli COVID-19 group. A Focus on the Nowadays Potential Antiviral Strategies in Early Phase of Coronavirus Disease 2019 (Covid-19): A Narrative Review. Life 2020, 10, 146. https://doi.org/10.3390/life10080146
Monari C, Gentile V, Camaioni C, Marino G, Coppola N, Vanvitelli COVID-19 group. A Focus on the Nowadays Potential Antiviral Strategies in Early Phase of Coronavirus Disease 2019 (Covid-19): A Narrative Review. Life. 2020; 10(8):146. https://doi.org/10.3390/life10080146
Chicago/Turabian StyleMonari, Caterina, Valeria Gentile, Clarissa Camaioni, Giulia Marino, Nicola Coppola, and Vanvitelli COVID-19 group. 2020. "A Focus on the Nowadays Potential Antiviral Strategies in Early Phase of Coronavirus Disease 2019 (Covid-19): A Narrative Review" Life 10, no. 8: 146. https://doi.org/10.3390/life10080146
APA StyleMonari, C., Gentile, V., Camaioni, C., Marino, G., Coppola, N., & Vanvitelli COVID-19 group. (2020). A Focus on the Nowadays Potential Antiviral Strategies in Early Phase of Coronavirus Disease 2019 (Covid-19): A Narrative Review. Life, 10(8), 146. https://doi.org/10.3390/life10080146






