Microgravity Modulates Effects of Chemotherapeutic Drugs on Cancer Cell Migration
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
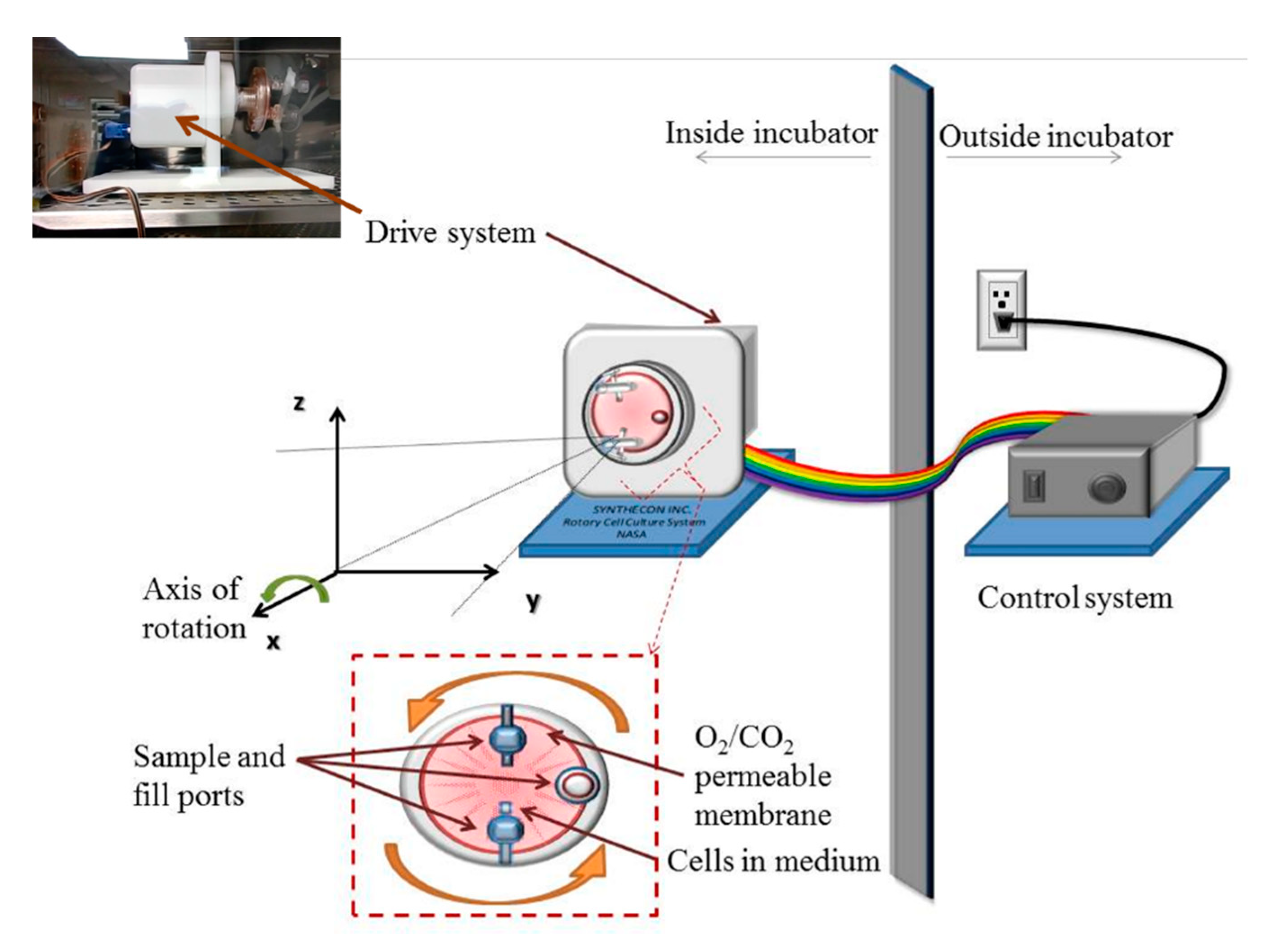
2.2. Simulation of Microgravity
2.3. Chemotherapy and Other Drug Treatments
2.4. Assessment of Reactive Oxygen Species
2.5. Viability Tests, Morphometry and Migration Assay
2.6. Measurement of Cell Deformability
2.7. Statistical Analysis
3. Results
3.1. Cell Viability and Morphometry Post-Microgravity and Post-Microgravity Chemotherapy
3.2. Post-Microgravity ROS Generation is Cell-Type Dependent
3.3. Both Doxorubicin and Daunorubicin Enhance Post-Microgravity Migration of Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yi, Z.-C.; Xia, B.; Xue, M.; Zhang, G.Y.; Wang, H.; Zhou, H.M.; Sun, Y.; Zhuang, F.-Y. Simulated microgravity inhibits the proliferation of K562 erythroleukemia cells but does not result in apoptosis. Adv. Sp. Res. 2009, 44, 233–244. [Google Scholar] [CrossRef]
- Becker, J.L.; Souza, G.R. Using space-based investigations to inform cancer research on Earth. Nat. Rev. Cancer 2013, 13, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Jhala, D.V.; Kale, R.K.; Singh, R.P. Microgravity alters cancer growth and progression. Curr. Cancer Drug Targets 2014, 14, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Ahn, C.B.; Son, K.H.; Yi, E.; Son, H.S.; Kim, H.; Lee, S.H. Simulated microgravity effects on nonsmall cell lung cancer cell proliferation and migration. Aerosp. Med. Hum. Perform. 2017, 882, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Xu, H.; Guo, Y.; Jiang, X.; Liu, Y.; Li, K.; Pan, C.; Yuan, M.; Wang, J.; Li, T.; et al. Simulated microgravity alters the metastatic potential of a human lung adenocarcinoma cell line. Vitr. Cell. Dev. Biol. Anim. 2013, 493, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Xu, A.; Zhao, T.; Zhao, Q.; Zhang, J.; Fan, C.; Deng, Y.; Freywald, A.; Genth, H.; Xiang, J. Simulated microgravity inhibits cell focal adhesions leading to reduced melanoma cell proliferation and metastasis via FAK/RhoA-regulated mTORC1 and AMPK pathways. Sci. Rep. 2018, 81, 3769. [Google Scholar] [CrossRef]
- Zhao, T.; Li, R.; Tan, X.; Zhang, J.; Fan, C.; Zhao, Q.; Deng, Y.; Xu, A.; Lukong, K.E.; Genth, H.; et al. Simulated microgravity reduces focal adhesions and alters cytoskeleton and nuclear positioning leading to enhanced apoptosis via suppressing FAK/RhoA-mediated mTORC1/NF-κB and ERK1/2 pathways. Int. J. Mol. Sci. 2018, 19, 1994. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Kang, B.-B.; Chen, J.H. Transcriptional regulation of human osteopontin promoter by C/EBPalpha and AML-1 in metastatic cancer cells. Oncogene 2004, 23, 278–288. [Google Scholar] [CrossRef]
- Wirtz, D.; Konstantopoulos, K.; Searson, P.C. The physics of cancer: The role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer 2011, 11, 512–522. [Google Scholar] [CrossRef]
- Fritsch, A.; Höckel, M.; Kiessling, T.; Nnetu, K.D.; Wetzel, F.; Zink, M.; Käs, J.A. Are biomechanical changes necessary for tumour progression? Nat. Phys. 2010, 6, 730–732. [Google Scholar] [CrossRef]
- Geldof, A.A.; Rao, B.R. Doxorubicin treatment increases metastasis of prostate tumor (R3327-MatLyLu). Anticancer Res. 1988, 8, 1335–1339. [Google Scholar] [PubMed]
- Volk-Draper, L.; Hall, K.; Griggs, C.; Rajput, S.; Kohio, P.; DeNardo, D.; Ran, S. Paclitaxel therapy promotes breast cancer metastasis in a TLR4-dependent manner. Cancer Res. 2014, 74, 5421–5434. [Google Scholar] [CrossRef] [PubMed]
- Ran, S. The role of TLR4 in chemotherapy-driven metastasis. Cancer Res. 2015, 75, 2405–2410. [Google Scholar] [CrossRef]
- Prathivadhi-Bhayankarama, S.V.; Ning, J.; Mimlitz, M.; Taylor, C.; Gross, E.; Nichols, M.; Guck, J.; Ekpenyong, A.E. Chemotherapy impedes in Vitro microcirculation and promotes migration of leukemic cells with impact on metastasis. Biochem. Biophys. Res. Commun. 2016, 479, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.F. Why does cancer therapy lack effective anti-metastasis drugs? Cancer Lett. 2013, 328, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Koumoutsakos, P.; Pivkin, I.; Milde, F. The fluid mechanics of cancer and its therapy. Annu. Rev. Fluid. Mech. 2013, 45, 325–355. [Google Scholar] [CrossRef]
- Chaffer, C.; Weinberg, R. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1565. [Google Scholar] [CrossRef]
- De Angelis, A.; Urbanek, K.; Cappetta, D.; Piegari, E.; Ciuffreda, L.P.; Rivellino, A.; Russo, R.; Esposito, G.; Rossi, F.; Berrino, L. Doxorubicin cardiotoxicity and target cells: A broader perspective. Cardio Oncol. 2016, 2, 2. [Google Scholar] [CrossRef]
- Lam, W.A.; Rosenbluth, M.J.; Fletcher, D.A. Chemotherapy exposure increases leukemia cell stiffness. Blood 2007, 109, 3505–3508. [Google Scholar] [CrossRef]
- Zucker, R.M.; Whittington, K.; Price, B.J. Differentiation of HL-60 cells: Cell volume and cell cycle changes. Cytometry 1983, 3, 414–418. [Google Scholar] [CrossRef]
- Collins, S.J. The HL-60 promyelocytic leukemia cell line: Proliferation, differentiation, and cellular oncogene expression. Blood 1987, 70, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Ekpenyong, A.E.; Whyte, G.; Chalut, K.; Pagliara, S.; Lautenschläger, F.; Fiddler, C.; Paschke, S.; Keyser, U.F.; Chilvers, E.R.; Guck, J. Viscoelastic properties of differentiating blood cells are fate- and function-dependent. PLoS ONE 2012, 7, e45237. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.J.; Ekpenyong, A.E.; Golfier, S.; Li, W.; Chalut, K.J.; Otto, O.; Elgeti, J.; Guck, J.; Lautenschläger, F. Myosin II activity softens cells in suspension. Biophys. J. 2015, 108, 1856–1869. [Google Scholar] [CrossRef] [PubMed]
- Ekpenyong, A.E. Viscoelastic and Optical Properties of Blood Stem Cells: From Differentiation to Activation and Infection. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2012. [Google Scholar]
- Lee, B.H.; Suresh, S.; Ekpenyong, A. Fluorescence intensity modulation of CdSe/ZnS quantum dots assesses ROS during chemotherapy and radiotherapy for cancer cells. J. Biophotonics 2019, 12, e201800172. [Google Scholar]
- Klein, E.; Vánky, F.; Ben-Bassat, H.; Neumann, H.; Ralph, P.; Zeuthen, J.; Polliack, A. Properties of the K562 cell line, derived from a patient with chronic myeloid leukemia. Int. J. Cancer 1976, 18, 421–431. [Google Scholar] [CrossRef]
- Saland, E.; Boutzen, H.; Castellano, R.; Pouyet, L.; Griessinger, E.; Larrue, C.; De Toni, F.; Scotland, S.; David, M.; Danet-Desnoyers, G.; et al. A robust and rapid xenograft model to assess efficacy of chemotherapeutic agents for human acute myeloid leukemia. Blood Cancer J. 2015, 5, e297. [Google Scholar] [CrossRef]
- Wang, C.; Li, N.; Zhang, C.; Sun, S.; Gao, Y.; Long, M. Effects of simulated microgravity on functions of neutrophil-like HL-60 cells. Microgravity Sci. Technol. 2015, 27, 515–527. [Google Scholar] [CrossRef]
- Schwarz, R.P.; Goodwin, T.J.; Wolf, D.A. Cell culture for three-dimensional modeling in rotating-wall vessels: An application of simulated microgravity. J. Tissue Cult. Methods 1992, 14, 51–57. [Google Scholar] [CrossRef]
- Margolis, L.; Hatfill, S.; Chuaqui, R.; Vocke, C.; Emmert-Buck, M.; Linehan, W.M.; Duray, P.H. Long term organ culture of human prostate tissue in a NASA-designed rotating wall bioreactor. J. Urol. 1999, 161, 290–297. [Google Scholar] [CrossRef]
- Zhang, Y.; Sang, C.; Paulsen, K.; Arenz, A.; Zhao, Z.; Jia, X.; Ullrich, O.; Zhuang, F. ICAM-1 expression and organization in human endothelial cells is sensitive to gravity. Acta Astronaut. 2010, 67, 1073–1080. [Google Scholar] [CrossRef]
- Stojak, M.; Mazur, L.; Opydo-Chanek, M.; Lukawska, M.; Oszczapowicz, I. Effects of structural modifications of daunorubicin on in Vitro antileukemic activity. Anticancer Res. 2012, 32, 5271–5277. [Google Scholar] [PubMed]
- Von Maltzahn, G.; Park, J.H.; Lin, K.Y.; Singh, N.; Schwöppe, C.; Mesters, R.; Berdel, W.E.; Ruoslahti, E.; Sailor, M.J.; Bhatia, S.N. Nanoparticles that communicate in vivo to amplify tumour targeting. Nat. Mater. 2011, 10, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa-Nowak, D.; Paine, M.J.I.; Wolf, C.R.; Tarasiuk, J. The role of bioreductive activation of doxorubicin in cytotoxic activity against leukaemia HL60-sensitive cell line and its multidrug-resistant sublines. Br. J. Cancer 2005, 93, 89–97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Man, S.M.; Ekpenyong, A.; Tourlomousis, P.; Achouri, S.; Cammarota, E.; Hughes, K.; Rizzo, A.; Ng, G.; Wright, J.A.; Cicuta, P.; et al. Actin polymerization as a key innate immune effector mechanism to control Salmonella infection. Proc. Natl. Acad. Sci. USA 2014, 111, 17588–17593. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Ran, F.; An, L.; Fan, Y.; Hang, H.; Wang, S. Simulated microgravity potentiates generation of reactive oxygen species in cells. Biophys. Rep. 2016, 2, 100–105. [Google Scholar] [CrossRef]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In vitro cell migration and invasion assays. J. Vis. Exp. 2014, 88, 51046. [Google Scholar] [CrossRef]
- Wu, P.H.; Aroush, D.R.B.; Asnacios, A.; Chen, W.C.; Dokukin, M.E.; Doss, B.L.; Durand-Smet, P.; Ekpenyong, A.; Guck, J.; Guz, N.V.; et al. A comparison of methods to assess cell mechanical properties. Nat. Methods 2018, 15, 491–498. [Google Scholar] [CrossRef]
- Ekpenyong, A.E.; Toepfner, N.; Fiddler, C.; Herbig, M.; Li, W.; Cojoc, G.; Summers, C.; Guck, J.; Chilvers, E.R. Mechanical deformation induces depolarization of neutrophils. Sci. Adv. 2017, 3, e1602536. [Google Scholar] [CrossRef]
- Perbal, G.; Driss-Ecole, D. Mechanotransduction in gravisensing cells. Trends Plant Sci. 2003, 8, 498–504. [Google Scholar] [CrossRef]
- Thiel, C.S.; de Zélicourt, D.; Tauber, S.; Adrian, A.; Franz, M.; Simmet, D.M.; Schoppmann, K.; Hauschild, S.; Krammer, S.; Christen, M.; et al. Rapid adaptation to microgravity in mammalian macrophage cells. Sci. Rep. 2017, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Tauber, S.; Lauber, B.A.; Paulsen, K.; Layer, L.E.; Lehmann, M.; Hauschild, S.; Shepherd, N.R.; Polzer, J.; Segerer, J.; Thiel, C.S.; et al. Cytoskeletal stability and metabolic alterations in primary human macrophages in long-term microgravity. PLoS ONE 2017, 12, e0175599. [Google Scholar] [CrossRef] [PubMed]
- Janmaleki, M.; Pachenari, M.; Seyedpour, S.M.; Shahghadami, R.; Sanati-Nezhad, A. Impact of simulated microgravity on cytoskeleton and viscoelastic properties of endothelial cell. Sci. Rep. 2016, 6, 32418. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhara, T.; Takeda, M.; Yamaguchi, S.; Manabe, T.; Matsumoto, M.; Kawahara, Y.; Yuge, L.; Kurisu, K. Simulated microgravity facilitates cell migration and neuroprotection after bone marrow stromal cell transplantation in spinal cord injury. Stem Cell Res. Ther. 2013, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.E.; Langer, R.; Martin, I.; Pellis, N.R.; Vunjak-Novakovic, G. Tissue engineering of cartilage in space. Proc. Natl. Acad. Sci. USA 1997, 94, 13885–13890. [Google Scholar] [CrossRef]
- Unsworth, B.; Lelkes, P. Growing tissues in microgravity. Nat. Med. 1998, 4, 901–907. [Google Scholar] [CrossRef]
- Tivnan, A.; Heilinger, T.; Lavelle, E.C.; Prehn, J.H.M. Advances in immunotherapy for the treatment of glioblastoma. J. Neurooncol. 2017, 131, 1–9. [Google Scholar] [CrossRef]
- Wojcik, T.; Buczek, E.; Majzner, K.; Kolodziejczyk, A.; Miszczyk, J.; Kaczara, P.; Kwiatek, W.; Baranska, M.; Szymonski, M.; Chlopicki, S. Comparative endothelial profiling of doxorubicin and daunorubicin in cultured endothelial cells. Toxicol. Vitr. 2015, 29, 512–521. [Google Scholar] [CrossRef]
- DiFrancesco, J.; Olson, J. The economics of microgravity research. NPJ Microgravity 2015, 1, 15001. [Google Scholar] [CrossRef]
- Blue, R.S.; Bayuse, T.M.; Daniels, V.R.; Wotring, V.E.; Suresh, R.; Mulcahy, R.A.; Antonsen, E.L. Supplying a pharmacy for NASA exploration spaceflight: Challenges and current understanding. NPJ Microgravity 2019. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, K.; Wei, D.; Tian, Y.; Gao, Y.; Chen, Z.; Qian, A. The impact of spaceflight and simulated microgravity on cell adhesion. Int. J. Mol. Sci. 2020, 21, 3031. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasanth, D.; Suresh, S.; Prathivadhi-Bhayankaram, S.; Mimlitz, M.; Zetocha, N.; Lee, B.; Ekpenyong, A. Microgravity Modulates Effects of Chemotherapeutic Drugs on Cancer Cell Migration. Life 2020, 10, 162. https://doi.org/10.3390/life10090162
Prasanth D, Suresh S, Prathivadhi-Bhayankaram S, Mimlitz M, Zetocha N, Lee B, Ekpenyong A. Microgravity Modulates Effects of Chemotherapeutic Drugs on Cancer Cell Migration. Life. 2020; 10(9):162. https://doi.org/10.3390/life10090162
Chicago/Turabian StylePrasanth, Devika, Sindhuja Suresh, Sruti Prathivadhi-Bhayankaram, Michael Mimlitz, Noah Zetocha, Bong Lee, and Andrew Ekpenyong. 2020. "Microgravity Modulates Effects of Chemotherapeutic Drugs on Cancer Cell Migration" Life 10, no. 9: 162. https://doi.org/10.3390/life10090162
APA StylePrasanth, D., Suresh, S., Prathivadhi-Bhayankaram, S., Mimlitz, M., Zetocha, N., Lee, B., & Ekpenyong, A. (2020). Microgravity Modulates Effects of Chemotherapeutic Drugs on Cancer Cell Migration. Life, 10(9), 162. https://doi.org/10.3390/life10090162





