Diacylglycerol Acetyltransferase Gene Isolated from Euonymus europaeus L. Altered Lipid Metabolism in Transgenic Plant towards the Production of Acetylated Triacylglycerols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Lipid Analysis
2.3. EeDAcT Gene Isolation, Sequencing, and Modification
2.4. EeDAcT Gene Synthesis and Expression in Tobacco
3. Results
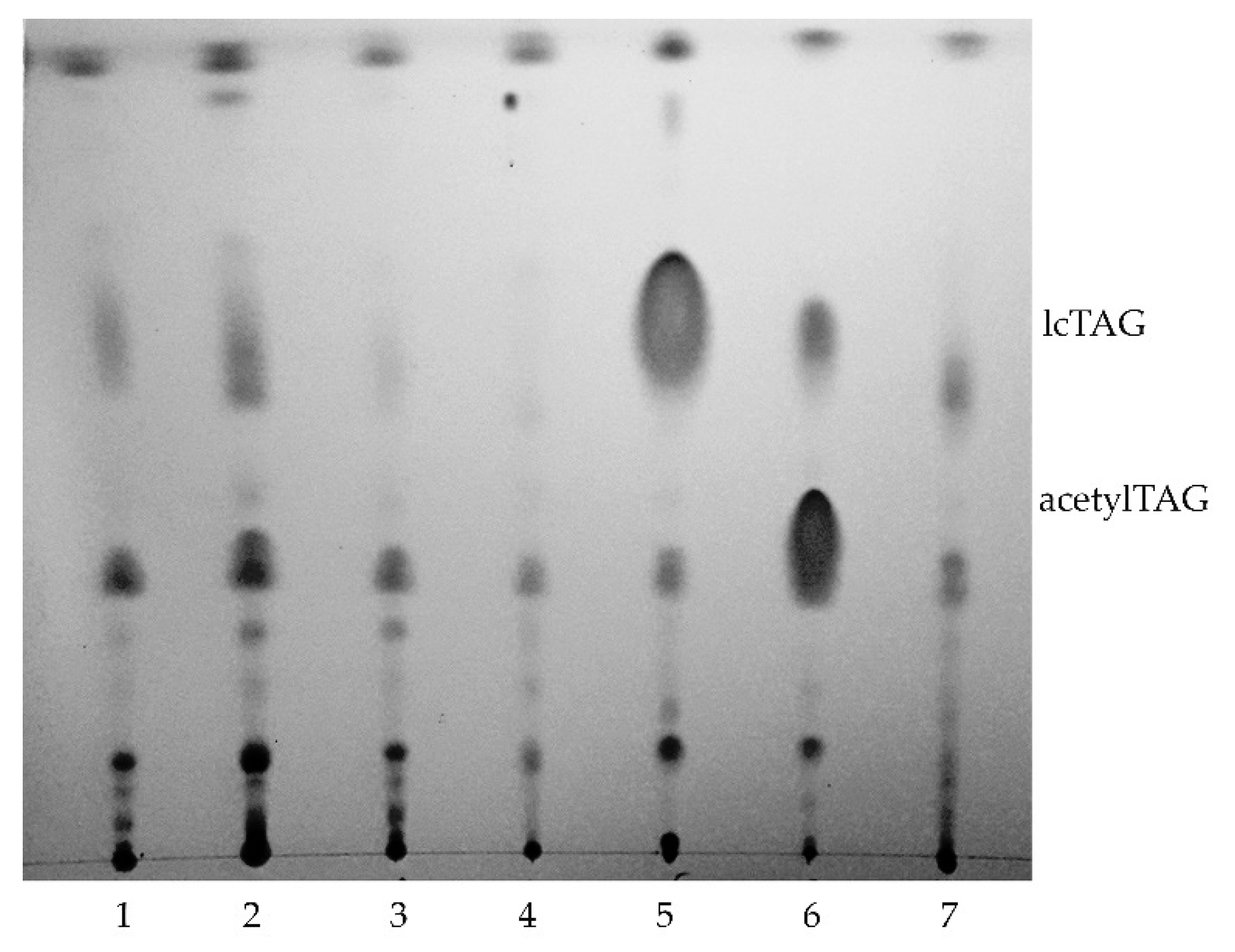
3.1. AcetylTAGs in E. europaeus
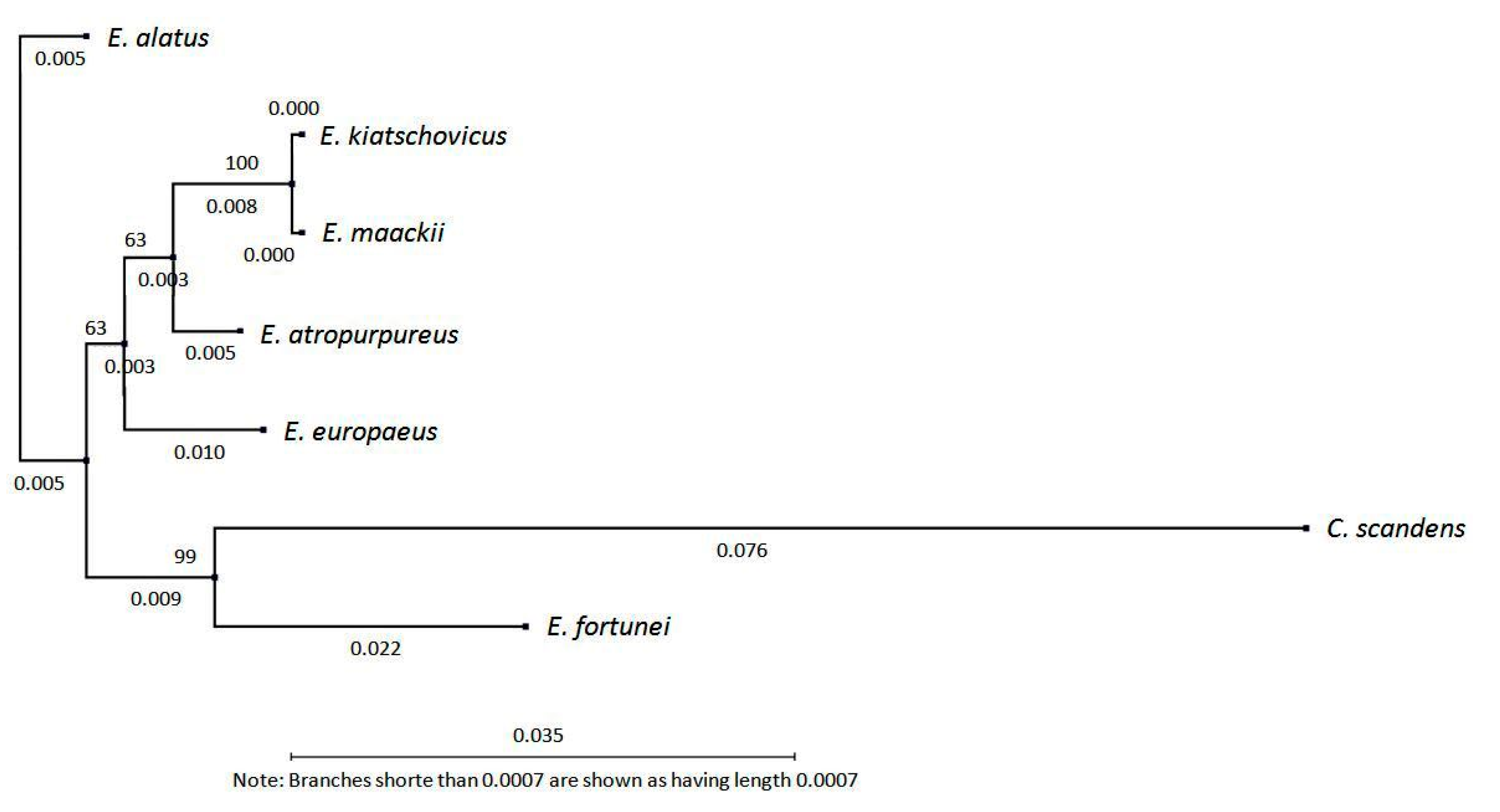
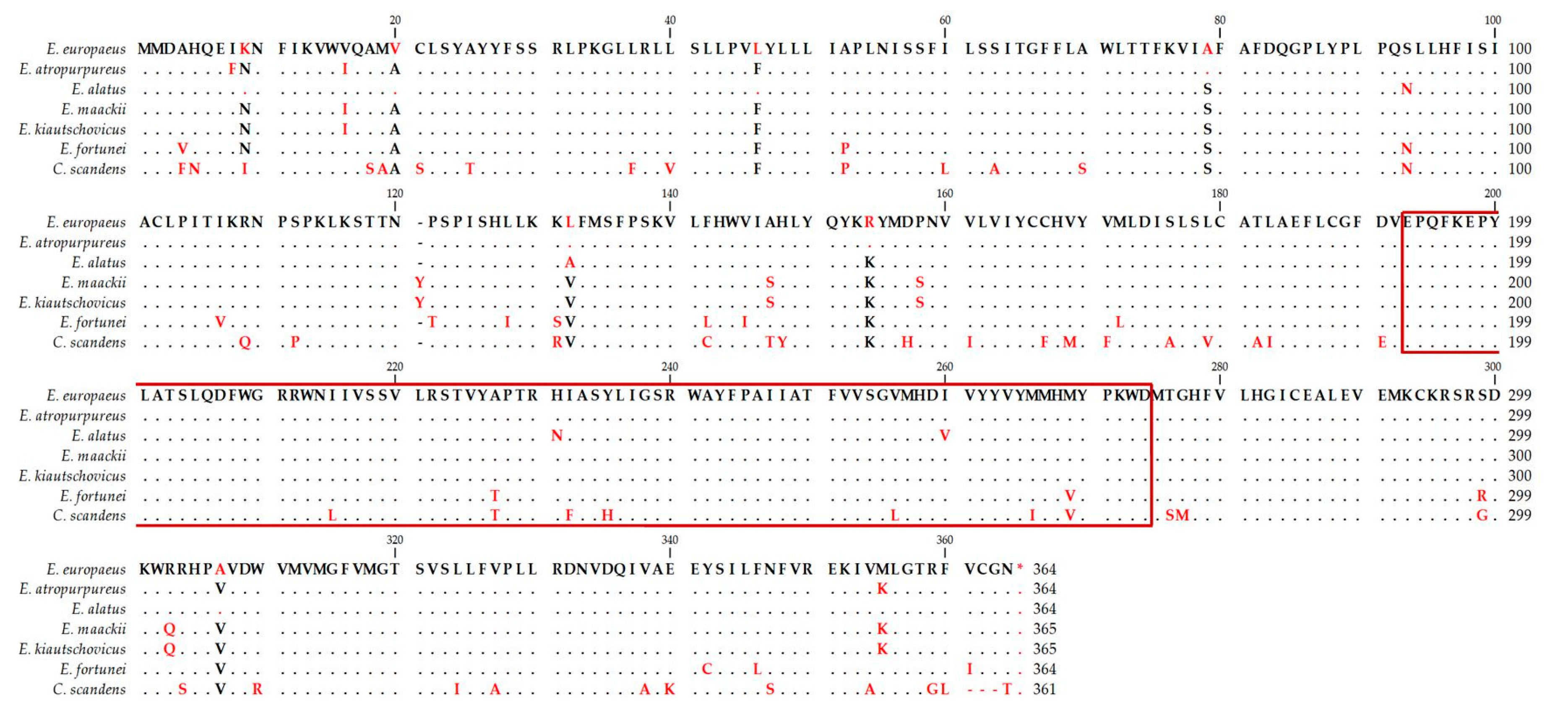
3.2. Diacylglycerol Acetyltransferase Gene from E. europaeus
3.3. Expression of EeDAcT Gene in Tobacco
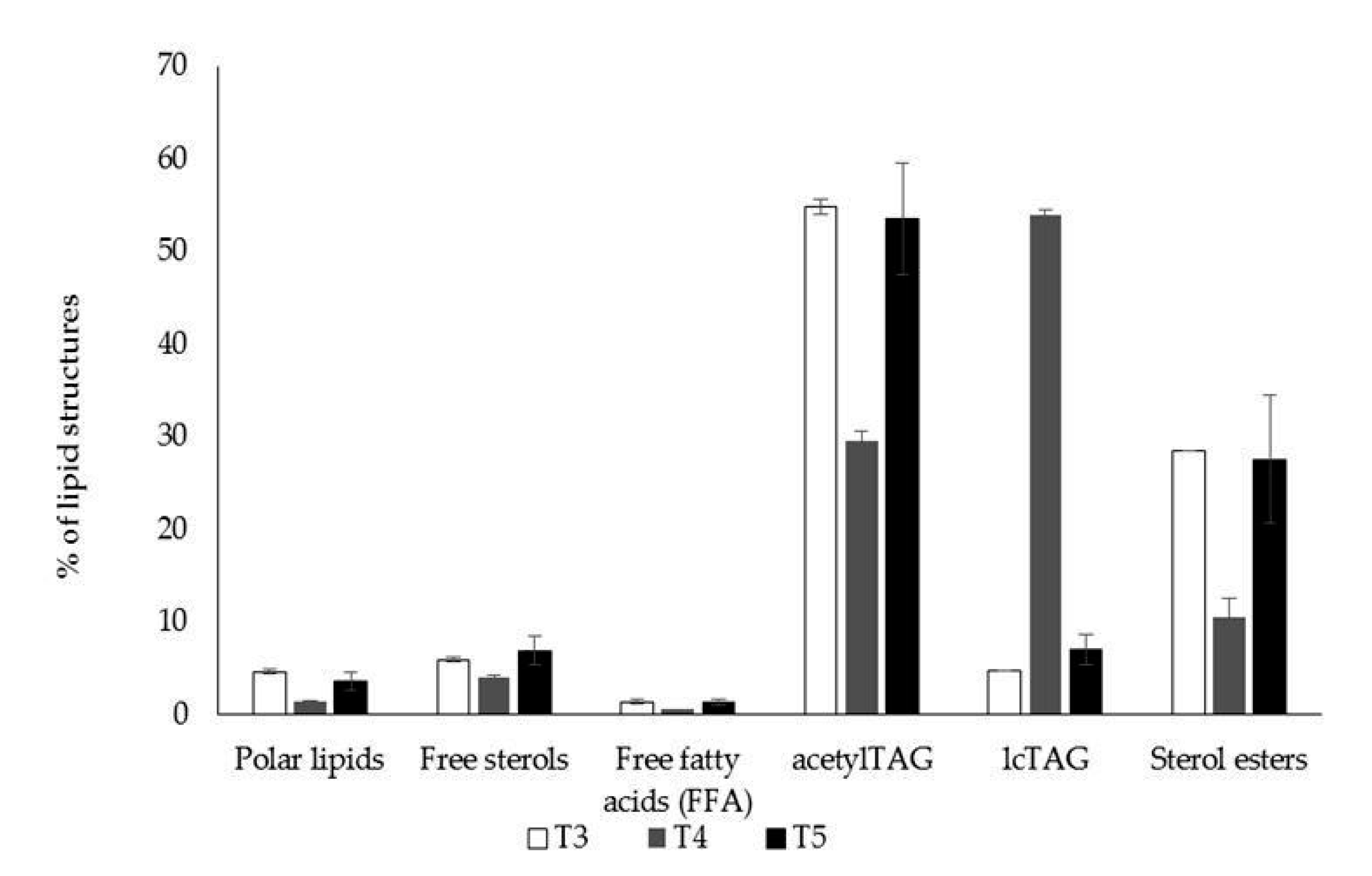
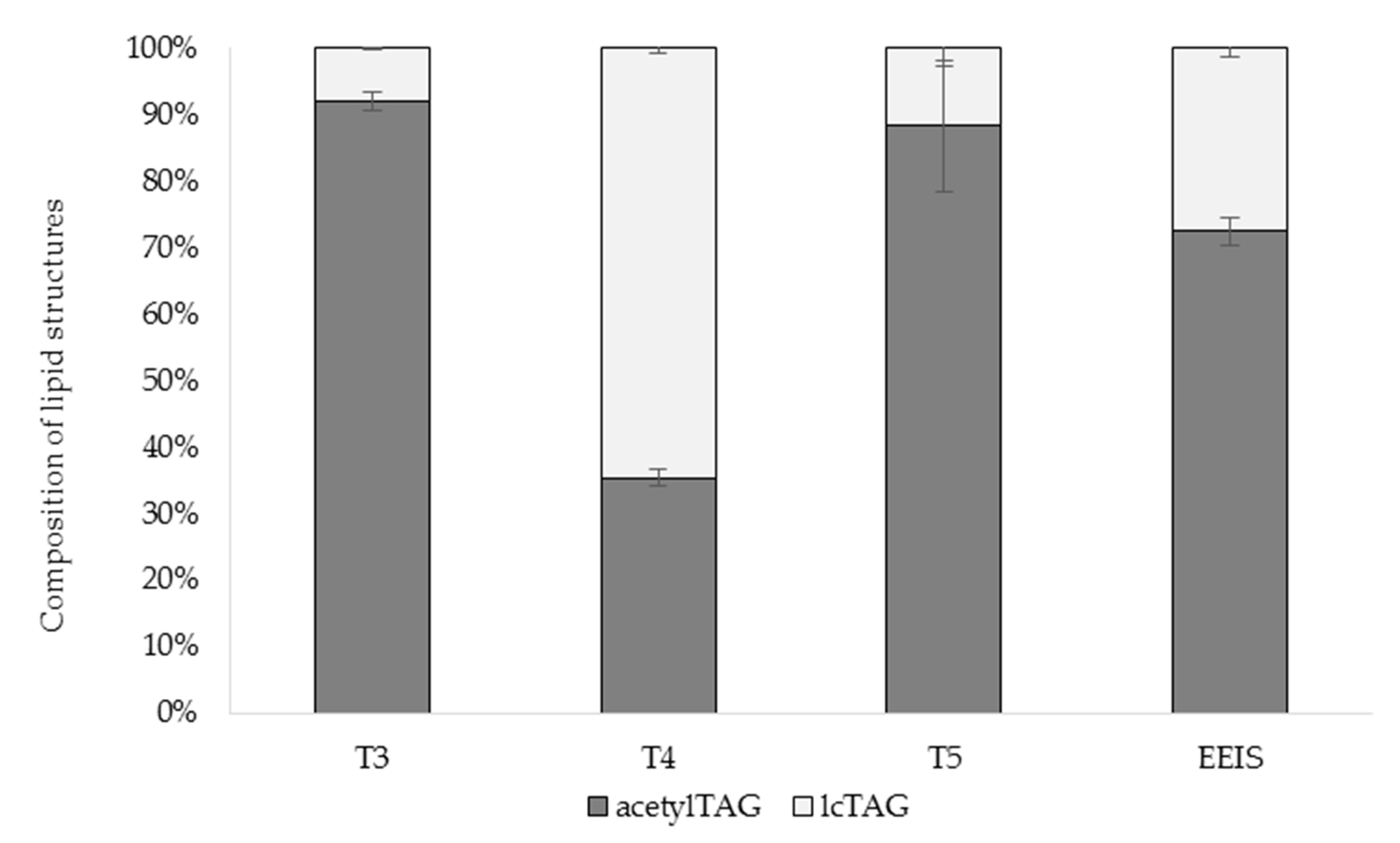
3.4. AcetylTAGs in Transgenic Tobacco
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, J.-X.; Qin, J.-J.; Chang, R.-J.; Zeng, Q.; Cheng, X.-R.; Zhang, F.; Jin, H.-Z.; Zhang, W.-D. Chemical constituents of plants from the genus Euonymus. Chem. Biodivers. 2012, 9, 1055–1076. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, C.; Ai, L.; Wang, L.; Li, L.; Fan, W.; Li, R.; He, L.; Wu, C.; Huang, Y. Traditional uses, botany, phytochemistry, pharmacology, separation and analysis Technologies of Euonymus alatus (Thunb.) Siebold: A comprehensive review. J. Ethnopharmacol. 2020, 259, 112942. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sati, S.C.; Sati, O.P.; Sati, M.D.; Kothiyal, S.K. Genus Euonymus: Chemical and pharmacological perception. Mini Rev. Org. Chem. 2012, 9, 341–351. [Google Scholar] [CrossRef]
- Popescu, I.; Caudullo, G.; de Rigo, D. Euonymus europaeus in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publ. Off. EU: Luxembourg, 2016; p. e01c0ac+. [Google Scholar]
- Kleiman, R.; Miller, R.W.; Earle, F.R.; Wolff, I.A. (S)-1,2-diacyl-3-acetins: Optically active triglycerides from Euonymus verrucosum seed oil. Lipids 1967, 2, 473–478. [Google Scholar] [CrossRef]
- Liu, J.; Rice, A.; McGlew, K.; Shaw, V.; Park, H.; Clemente, T.; Pollard, M.; Ohlrogge, J.; Durrett, T.P. Metabolic engineering of oilseed crops to produce high levels of novel acetyl glyceride oils with reduced viscosity, freezing point and calorific value. Plant Biotechnol. J. 2015, 13, 858–865. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Navarrete, J.M.; Serino, M.; Blasco-Baque, V.; Azalbert, V.; Barton, R.H.; Cardellini, M.; Latorre, J.; Ortega, F.; Sabater-Masdeu, M.; Burcelin, R.; et al. Gut microbiota interacts with markers of adipose tissue browning, insulin action and plasma acetate in morbid obesity. Mol. Nutr. Food Res. 2017, 62, 1700721. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, X.; Wan, X. Research progress on unusual acetyl triacylglycerol. Oil Crop Sci. 2017, 2, 211–216. [Google Scholar]
- Sidorov, R.A.; Zhukov, A.V.; Pchelkin, V.P.; Vereshchagin, A.G.; Tsydendambaev, V.D. Content of fatty acid composition of neutral acylglycerols in Euonymus fruits. J. Am. Oil Soc. 2014, 91, 805–814. [Google Scholar] [CrossRef]
- Sidorov, R.A.; Pchelkin, V.P.; Zhukov, A.V.; Tsydendambaev, V.D. Positional-species composition of diacylglycerol acetates from mature in Euonymus seeds. Chem. Biodivers. 2016, 13, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, Á. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Pinzi, S.; Garcia, I.L.; Lopez-Gimenez, F.J.; Luque de Castro, M.D.; Dorado, G.; Dorado, M.P. The ideal vegetable oil-based biodiesel composition: A review of social, economical and technical implications. Energy Fuels 2009, 23, 2325–2341. [Google Scholar] [CrossRef]
- Snyder, C.L.; Yurchenko, O.P.; Siloto, R.M.P.; Chen, X.; Liu, Q.; Mietkiewska, E.; Weselake, R.J. Acyltransferase action in the modification of seed oil biosynthesis. New Biotechnol. 2009, 26, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Napier, J.A.; Clemente, T.E.; Cahoon, E.B. New frontiers in oilseed biotechnology: Meeting the global demnad for vegetable oils for food, feed, biofuel, and industrial applications. Curr. Opin. Biotechnol. 2011, 22, 252–259. [Google Scholar] [CrossRef]
- Durrett, T.P.; McClosky, D.D.; Tumaney, A.W.; Elzinga, D.A.; Ohlrogge, J.; Pollard, M. A distinct DGAT with sn-3 acetyltransferase activity that synthesizes unusual, reduced-viscosity oils in Euonymus and trangenic seeds. Proc. Natl. Acad. Sci. USA 2010, 107, 9464–9469. [Google Scholar] [CrossRef] [Green Version]
- Milcamps, A.; Tumaney, A.W.; Paddock, T.; Pan, D.A.; Ohlrogge, J.; Pollard, M. Isolation of a gene encoding a 1,2-diacylglycerol-sn-acetyl-CoA acetyltransferase from developing seeds of Euonymus alatus. J. Biol. Chem. 2005, 280, 5370–5377. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.N.T.; Shelton, J.; Brown, S.; Durrett, T.P. Membrane topology and identification of key residues of EaDAcT, a plant MBOAT with unusual substrate specificity. Plant J. 2017, 92, 82–94. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Bansal, S.; Durrett, T.P. Rapid quantification of low-viscosity acetyl-triacylglycerols using electrospray ionization mass spectrometry. Lipids 2016, 51, 1093–1102. [Google Scholar] [CrossRef] [Green Version]
- May, R.A.; Stevenson, K.J. UN-SCAN-IT: Graph Digitizing Software. J. Am. Chem. Soc. 2008, 130, 7516. [Google Scholar] [CrossRef]
- Christoperson, S.W.; Glass, R.L. Preparation of milk fat methyl esters by alcoholysis in an essentially nonalcoholic solution. J. Dairy Sci. 1969, 52, 1289–1290. [Google Scholar] [CrossRef]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acid Res. 2013, 41, D36–D42. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, D.G.; Thompson, J.D.; Gibson, T.J. Using CLUSTAL for multiple sequence alignments. Methods Enzymol. 1996, 266, 383–402. [Google Scholar] [PubMed]
- Bertani, G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigel, D.; Glazebrook, J. Transformation of Agrobacterium using electroporation. Cold Spring Harb Protoc. 2006, 7, 1–13. [Google Scholar] [CrossRef]
- Horsch, R.B.; Fry, J.E.; Hoffmann, N.L.; Eichholz, D.; Rogers, S.G.; Fraley, R.T. A simple and general method for transferring genes into plants. Science 1985, 227, 1229–1231. [Google Scholar]
- Satoh, J.; Kato, K.; Shinmyo, A. The 5′-untranslated region of the tobacco alcohol dehydrogenase gene functions as an effective translational enhancer in plant. J. Biosci. Bioeng. 2004, 98, 1–8. [Google Scholar] [CrossRef]
- Yan, H.; Dai, X.; Feng, K.; Ma, Q.; Yin, T. IGDD: A database of intronless genes in dicots. BMC Bioinform. 2016, 17, 289. [Google Scholar] [CrossRef] [Green Version]
- Jain, M.; Khurana, P.; Tyagi, A.K.; Khurana, J.P. Genome-wide analysis of intronless genes in rice and Arabidopsis. Funct. Integr. Genom. 2008, 8, 69–78. [Google Scholar] [CrossRef]
- Mihálik, D.; Nogová, L.; Ondreičková, K.; Gubišová, M.; Gubiš, J.; Gregová, E.; Dokupilová, I.; Drška, R.; Kraic, J. A new high-molecularweight glutenin subunit from the slovak wheat (Triticum aestivum L.) cultivar ‘Trebišovská 76’. Food Sci. Biotechnol. 2013, 22, 33–37. [Google Scholar] [CrossRef]
- Andrianov, V.; Borisjuk, N.; Pogrebnyak, N.; Brinker, A.; Dixon, J.; Spitsin, S.; Flynn, J.; Matyszczuk, P.; Andryszak, K.; Laurelli, M.; et al. Tobacco as a production platform for biofuel: Overexpression of Arabidopsis DGAT and LEAC2 genes increases accumulation and shift the composition of lipid in green biomass. Plant Biotechnol. J. 2010, 8, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Matzke, A.J.; Matzke, M.A. Position effects and epigenetic silencing of plant transgenes. Curr. Opin. Plant Biol. 1998, 1, 142–148. [Google Scholar] [CrossRef]
- Kooter, J.M.; Matzke, M.A.; Meyer, P. Listening to the silent genes: Transgene silencing, gene regulation and pathogen control. Trends Plant Sci. 1999, 4, 340–347. [Google Scholar] [CrossRef]
- Rajeevkumar, S.; Anunanthini, P.; Sathishkumar, R. Epigenetic silencing in transgenic plants. Front. Plant Sci. 2015, 6, 693. [Google Scholar] [CrossRef] [Green Version]
- Jupe, F.; Rivkin, A.C.; Michael, T.P.; Zander, M.; Motley, S.T.; Sandoval, J.P.; Slotkin, R.K.; Chen, H.; Castanon, R.; Nery, J.R.; et al. The complex architecture and epigenomic impact of plant T-DNA insertions. PLoS Genet. 2019, 15, e1007819. [Google Scholar] [CrossRef] [Green Version]
- Zlatanov, M.; Angelova, M.; Antova, G. Lipid composition of tobacco seeds. Bulg. J. Agric. Sci. 2007, 13, 539–544. [Google Scholar]
- Zheng, P.; Allen, W.B.; Roesler, K.; Williams, M.E.; Zhang, S.; Li, J.; Glassman, K.; Ranch, J.; Nubel, D.; Solawetz, W.; et al. A phenylalanine in DGAT is a key determinant of oil content and composition in maize. Nat. Genet. 2008, 40, 367–372. [Google Scholar] [CrossRef]
- Iskandarov, U.; Silva, J.E.; Kim, H.J.; Andersson, M.; Cahoon, R.E.; Mockaitis, K.; Cahoon, E.B. A specialized diacylglycerol acyltransferase contributes to the extreme medium-chain fatty acid content of Cuphea seed oil. Plant Physiol. 2017, 174, 97–109. [Google Scholar] [CrossRef] [Green Version]



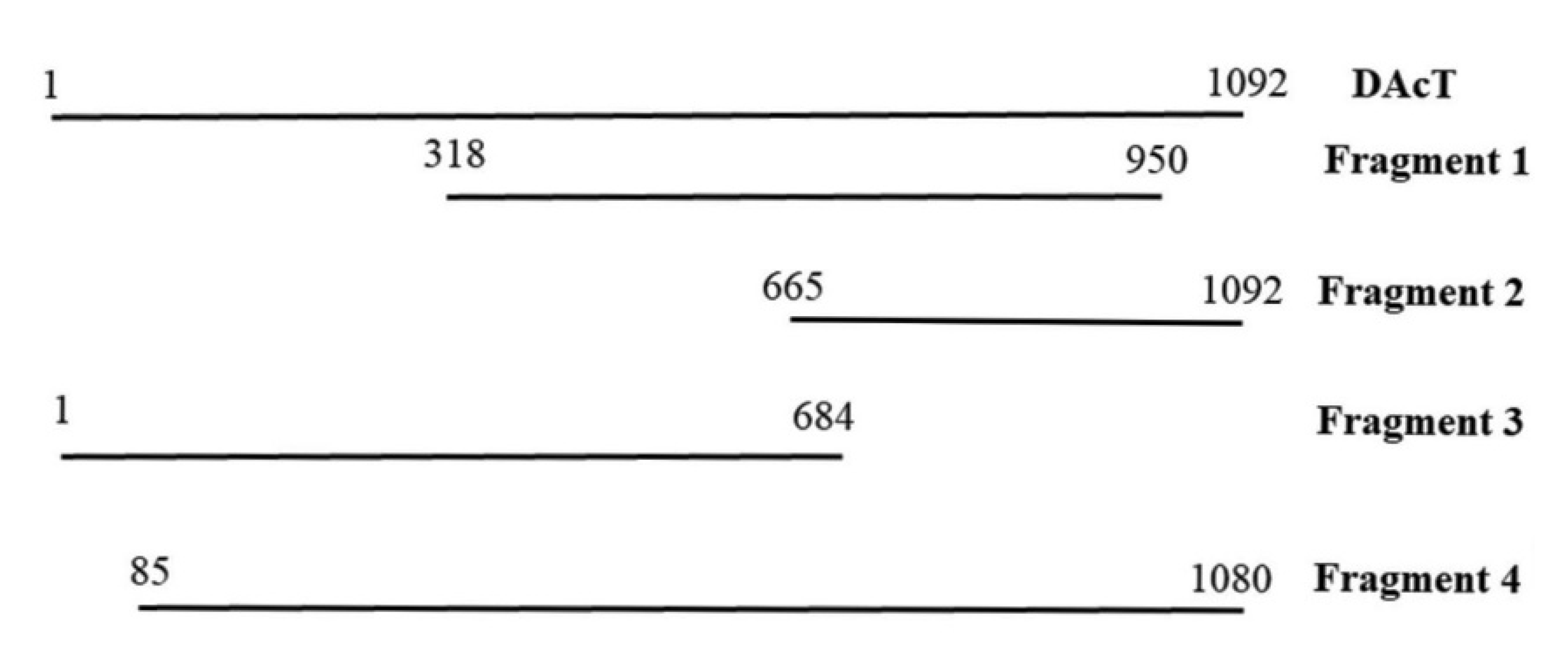


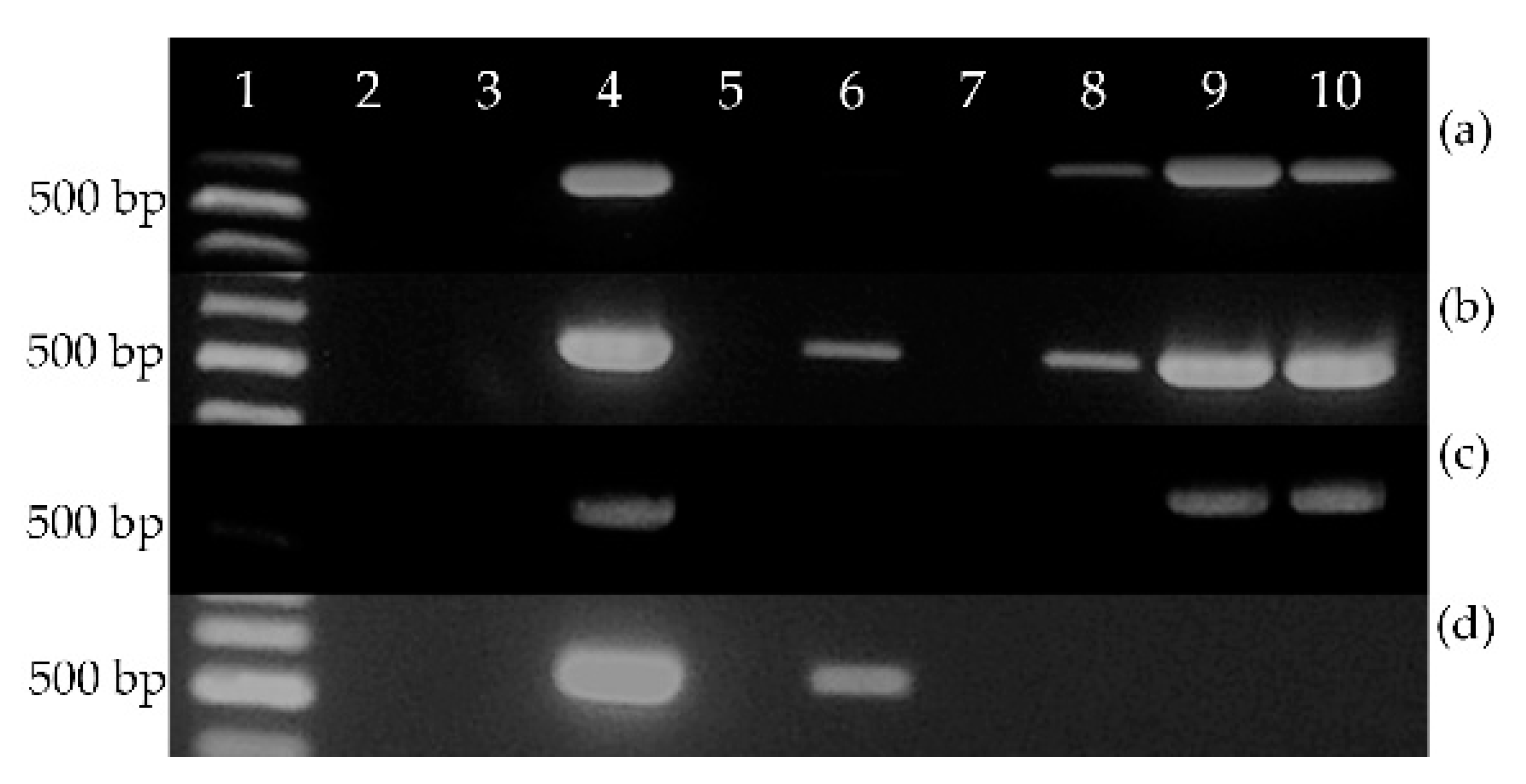

| Fragment | Primer Sequences (5′→3′) | PCR Product (bp) |
|---|---|---|
| 1 | F1 AATCAAGCGAAACCCATCAC R1 ATCACAAACCCCATCACCAT | 632 |
| 2 | F2 CGACTGTCTATGCCCCAACT R2 TCAATTTCCACACACAAA | 427 |
| 3 | F3 ATGATGGATGCTCATCAAGA R3 AGTTGGGGCATAGACAGTCG | 684 |
| 4 | F4 TCCTCAAGACTTCCAAAAGGA R4 CACAAACCTTGTTCCAAGCA | 1007 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihálik, D.; Lančaričová, A.; Mrkvová, M.; Kaňuková, Š.; Moravčíková, J.; Glasa, M.; Šubr, Z.; Predajňa, L.; Hančinský, R.; Grešíková, S.; et al. Diacylglycerol Acetyltransferase Gene Isolated from Euonymus europaeus L. Altered Lipid Metabolism in Transgenic Plant towards the Production of Acetylated Triacylglycerols. Life 2020, 10, 205. https://doi.org/10.3390/life10090205
Mihálik D, Lančaričová A, Mrkvová M, Kaňuková Š, Moravčíková J, Glasa M, Šubr Z, Predajňa L, Hančinský R, Grešíková S, et al. Diacylglycerol Acetyltransferase Gene Isolated from Euonymus europaeus L. Altered Lipid Metabolism in Transgenic Plant towards the Production of Acetylated Triacylglycerols. Life. 2020; 10(9):205. https://doi.org/10.3390/life10090205
Chicago/Turabian StyleMihálik, Daniel, Andrea Lančaričová, Michaela Mrkvová, Šarlota Kaňuková, Jana Moravčíková, Miroslav Glasa, Zdeno Šubr, Lukáš Predajňa, Richard Hančinský, Simona Grešíková, and et al. 2020. "Diacylglycerol Acetyltransferase Gene Isolated from Euonymus europaeus L. Altered Lipid Metabolism in Transgenic Plant towards the Production of Acetylated Triacylglycerols" Life 10, no. 9: 205. https://doi.org/10.3390/life10090205
APA StyleMihálik, D., Lančaričová, A., Mrkvová, M., Kaňuková, Š., Moravčíková, J., Glasa, M., Šubr, Z., Predajňa, L., Hančinský, R., Grešíková, S., Havrlentová, M., Hauptvogel, P., & Kraic, J. (2020). Diacylglycerol Acetyltransferase Gene Isolated from Euonymus europaeus L. Altered Lipid Metabolism in Transgenic Plant towards the Production of Acetylated Triacylglycerols. Life, 10(9), 205. https://doi.org/10.3390/life10090205





