Quality-of-Life Impairment among Patients with Hidradenitis Suppurativa: A Cross-Sectional Study of 1795 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Quality of Life
2.3. Statistical Analysis
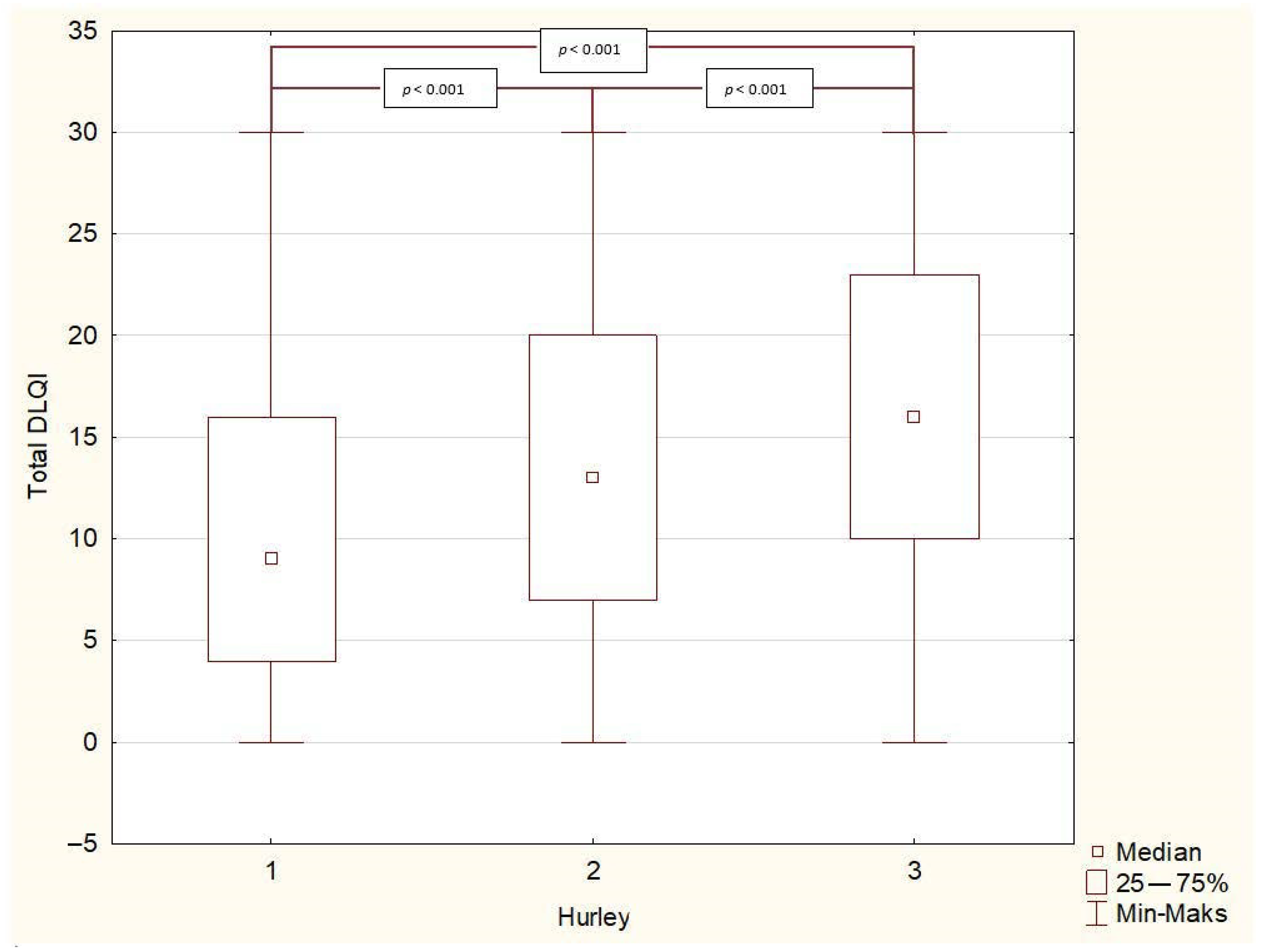
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Vere Hunt, I.J.; Howard, E.; McPherson, T. The impact of chronic skin disease in adolescence and the need for specialist adolescent services. Clin. Exp. Dermatol. 2020, 45, 5–9. [Google Scholar] [CrossRef]
- Camfferman, D.; Kennedy, J.D.; Gold, M.; Martin, A.J.; Winwood, P.; Lushington, K. Eczema, sleep, and behavior in children. J. Clin. Sleep Med. 2010, 6, 581–588. [Google Scholar] [CrossRef]
- Chernyshov, P.V. Stigmatization and self-perception in children with atopic dermatitis. Clin. Cosmet. Investig. Dermatol. 2016, 9, 159–166. [Google Scholar] [CrossRef]
- Cuenca-Barrales, C.; Molina-Leyva, A. Risk Factors of Sexual Dysfunction in Patients with Hidradenitis Suppurativa: A Cross-Sectional Study. Dermatology 2020, 236, 37–45. [Google Scholar] [CrossRef]
- Matusiak, L.; Bieniek, A.; Szepietowski, J.C. Psychophysical aspects of hidradenitis suppurativa. Acta Derm. Venereol. 2010, 90, 264–268. [Google Scholar] [CrossRef]
- Wolkenstein, P.; Loundou, A.; Barrau, K.; Auquier, P.; Revuz, J. Quality of Life Group of the French Society of Dermatology. Quality of life impairment in hidradenitis suppurativa: A study of 61 cases. J. Am. Acad. Dermatol. 2007, 56, 621–623. [Google Scholar] [CrossRef]
- Matusiak, L.; Bieniek, A.; Szepietowski, J.C. Hidradenitis suppurativa markedly decreases quality of life and professional activity. J. Am. Acad. Dermatol. 2010, 62, 706–708. [Google Scholar] [CrossRef] [PubMed]
- Kaaz, K.; Szepietowski, J.C.; Matusiak, L. Influence of Itch and Pain on Sleep Quality in Patients with Hidradenitis Suppurativa. Acta Derm. Venereol. 2018, 98, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.M.; Lindso Andersen, P.; Sigsgaard, V.; Theut Riis, P.; Jemec, G.B. Pain perception in patients with hidradenitis suppurativa. Br. J. Dermatol. 2020, 182, 166–174. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Del Marmol, V.; Mrowietz, U.; Prens, E.P.; Tzellos, T.; Jemec, G.B. Hidradenitis Suppurativa/Acne Inversa: Criteria for Diagnosis, Severity Assessment, Classification and Disease Evaluation. Dermatology 2015, 231, 184–190. [Google Scholar] [CrossRef]
- Horvath, B.; Janse, I.C.; Sibbald, G.R. Pain management in patients with hidradenitis suppurativa. J. Am. Acad. Dermatol. 2015, 73, S47–S51. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Tzellos, T.; Kyrgidis, A.; Jemec, G.B.E.; Bechara, F.G.; Giamarellos-Bourboulis, E.J.; Ingram, J.R.; Kanni, T.; Karagiannidis, I.; Martorell, A.; et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br. J. Dermatol. 2017, 177, 1401–1409. [Google Scholar] [CrossRef]
- Chien, C.W.; Bagraith, K.S.; Khan, A.; Deen, M.; Syu, J.J.; Strong, J. Establishment of cutpoints to categorize the severity of chronic pain using composite ratings with Rasch analysis. Eur. J. Pain 2017, 21, 82–91. [Google Scholar] [CrossRef]
- Chernyshov, P.V.; Zouboulis, C.C.; Tomas-Aragones, L.; Jemec, G.B.; Svensson, A.; Manolache, L.; Tzellos, T.; Sampogna, F.; Pustisek, N.; Van der Zee, H.H.; et al. Quality of life measurement in hidradenitis suppurativa: Position statement of the European Academy of Dermatology and Venereology task forces on Quality of Life and Patient-Oriented Outcomes and Acne, Rosacea and Hidradenitis Suppurativa. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1633–1643. [Google Scholar] [CrossRef]
- Mattei, P.L.; Corey, K.C.; Kimball, A.B. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): The correlation between disease severity and psychological burden in patients treated with biological therapies. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 333–337. [Google Scholar] [CrossRef]
- Janmohamed, S.R.; Gwillim, E.C.; Yousaf, M.; Patel, K.R.; Silverberg, J.I. The impact of prurigo nodularis on quality of life: A systematic review and meta-analysis. Arch. Dermatol. Res. 2020. [Google Scholar] [CrossRef]
- Misery, L.; Seneschal, J.; Reguiai, Z.; Merhand, S.; Heas, S.; Huet, F.; Taieb, C.; Ezzedine, K. The impact of atopic dermatitis on sexual health. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 428–432. [Google Scholar] [CrossRef]
- Langley, R.G.; Tsai, T.F.; Flavin, S.; Song, M.; Randazzo, B.; Wasfi, Y.; Jiang, J.; Li, S.; Puig, L. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: Results of the randomized, double-blind, phase III NAVIGATE trial. Br. J. Dermatol. 2018, 178, 114–123. [Google Scholar] [CrossRef]
- Cork, M.J.; Eckert, L.; Simpson, E.L.; Armstrong, A.; Barbarot, S.; Puig, L.; Girolomoni, G.; De Bruin-Weller, M.; Wollenberg, A.; Kataoka, Y.; et al. Dupilumab improves patient-reported symptoms of atopic dermatitis, symptoms of anxiety and depression, and health-related quality of life in moderate-to-severe atopic dermatitis: Analysis of pooled data from the randomized trials SOLO 1 and SOLO 2. J. Dermatolog. Treat 2020, 31, 606–614. [Google Scholar] [CrossRef]
- Sisic, M.; Kirby, J.S.; Boyal, S.; Plant, L.; McLellan, C.; Tan, J. Development of a Quality-of-Life Measure for Hidradenitis Suppurativa. J. Cutan. Med. Surg. 2017, 21, 152–155. [Google Scholar] [CrossRef]
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI)—A simple practical measure for routine clinical use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Desai, N.; Emtestam, L.; Hunger, R.E.; Ioannides, D.; Juhasz, I.; Lapins, J.; Matusiak, L.; Prens, E.P.; Revuz, J.; et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 619–644. [Google Scholar] [CrossRef]
- Vazquez, B.G.; Alikhan, A.; Weaver, A.L.; Wetter, D.A.; Davis, M.D. Incidence of hidradenitis suppurativa and associated factors: A population-based study of Olmsted County, Minnesota. J. Invest. Dermatol. 2013, 133, 97–103. [Google Scholar] [CrossRef]
- Schneider-Burrus, S.; Lux, G.; Van der Linde, K.; Barbus, S.; Huss-Marp, J.; Tsaousi, A.; Wasem, J.; Wolff, B.; Sabat, R. Hidradenitis suppurativa-prevalence analyses of German statutory health insurance data. J. Eur. Acad. Dermatol. Venereol. 2020. [Google Scholar] [CrossRef]
- Jemec, G.B. The symptomatology of hidradenitis suppurativa in women. Br. J. Dermatol. 1988, 119, 345–350. [Google Scholar] [CrossRef]
- Frings, V.G.; Bauer, B.; Gloditzsch, M.; Goebeler, M.; Presser, D. Assessing the psychological burden of patients with hidradenitis suppurativa. Eur. J. Dermatol. 2019, 29, 294–301. [Google Scholar] [CrossRef]
- Jorgensen, A.R.; Holm, J.G.; Ghazanfar, M.N.; Yao, Y.; Ring, H.C.; Thomsen, S.F. Factors affecting quality of life in patients with hidradenitis suppurativa. Arch. Dermatol. Res. 2020, 312, 427–436. [Google Scholar] [CrossRef]
- Kouris, A.; Platsidaki, E.; Christodoulou, C.; Efstathiou, V.; Dessinioti, C.; Tzanetakou, V.; Korkoliakou, P.; Zisimou, C.; Antoniou, C.; Kontochristopoulos, G. Quality of Life and Psychosocial Implications in Patients with Hidradenitis Suppurativa. Dermatology 2016, 232, 687–691. [Google Scholar] [CrossRef]
- Von der Werth, J.M.; Jemec, G.B. Morbidity in patients with hidradenitis suppurativa. Br. J. Dermatol. 2001, 144, 809–813. [Google Scholar] [CrossRef]
- Onderdijk, A.J.; Van der Zee, H.H.; Esmann, S.; Lophaven, S.; Dufour, D.N.; Jemec, G.B.; Boer, J. Depression in patients with hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 473–478. [Google Scholar] [CrossRef]
- Hazarika, N.; Archana, M. The Psychosocial Impact of Acne Vulgaris. Indian J. Dermatol. 2016, 61, 515–520. [Google Scholar] [CrossRef]
- Rencz, F.; Gulacsi, L.; Pentek, M.; Wikonkal, N.; Baji, P.; Brodszky, V. Alopecia areata and health-related quality of life: A systematic review and meta-analysis. Br. J. Dermatol. 2016, 175, 561–571. [Google Scholar] [CrossRef]
- Itakura, A.; Tani, Y.; Kaneko, N.; Hide, M. Impact of chronic urticaria on quality of life and work in Japan: Results of a real-world study. J. Dermatol. 2018, 45, 963–970. [Google Scholar] [CrossRef]
- Revicki, D.; Willian, M.K.; Saurat, J.H.; Papp, K.A.; Ortonne, J.P.; Sexton, C.; Camez, A. Impact of adalimumab treatment on health-related quality of life and other patient-reported outcomes: Results from a 16-week randomized controlled trial in patients with moderate to severe plaque psoriasis. Br. J. Dermatol. 2008, 158, 549–557. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Gelfand, J.M.; Margolis, D.J.; Boguniewicz, M.; Fonacier, L.; Grayson, M.H.; Simpson, E.L.; Ong, P.Y.; Chiesa Fuxench, Z.C. Patient burden and quality of life in atopic dermatitis in US adults: A population-based cross-sectional study. Ann. Allergy Asthma Immunol. 2018, 121, 340–347. [Google Scholar] [CrossRef]
- Kluger, N.; Ranta, M.; Serlachius, M. The Burden of Hidradenitis Suppurativa in a Cohort of Patients in Southern Finland: A Pilot Study. Skin Appendage Disord. 2017, 3, 20–27. [Google Scholar] [CrossRef]
- Esmann, S.; Jemec, G.B. Psychosocial impact of hidradenitis suppurativa: A qualitative study. Acta Derm. Venereol. 2011, 91, 328–332. [Google Scholar] [CrossRef]
- Rosen, S.; Ham, B.; Mogil, J.S. Sex differences in neuroimmunity and pain. J. Neurosci. Res. 2017, 95, 500–508. [Google Scholar] [CrossRef]
- Bidaki, R.; Majidi, N.; Moghadam Ahmadi, A.; Bakhshi, H.; Sadr Mohammadi, R.; Mostafavi, S.A.; Kazemi Arababadi, M.; Hadavi, M.; Mirzaei, A. Vitiligo and social acceptance. Clin. Cosmet. Investig. Dermatol. 2018, 11, 383–386. [Google Scholar] [CrossRef]
- Lakuta, P.; Marcinkiewicz, K.; Bergler-Czop, B.; Brzezinska-Wcislo, L. How does stigma affect people with psoriasis? Postepy Dermatol. Alergol. 2017, 34, 36–41. [Google Scholar] [CrossRef]
- Nicholas, M.N.; Gooderham, M.J. Atopic Dermatitis, Depression, and Suicidality. J. Cutan. Med. Surg. 2017, 21, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Wang, A.P.; Shi, T.Y.; Zhang, J.; Xu, H.; Wang, D.Q.; Feng, L. The psychosocial adaptation of patients with skin disease: A scoping review. BMC Public Health 2019, 19, 1404. [Google Scholar] [CrossRef] [PubMed]
- Racine, M.; Castarlenas, E.; De la Vega, R.; Tome-Pires, C.; Sole, E.; Miro, J.; Jensen, M.P.; Moulin, D.E.; Nielson, W.R. Sex differences in psychological response to pain in patients with fibromyalgia syndrome. Clin. J. Pain. 2015, 31, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Sabat, R.; Jemec, G.B.E.; Matusiak, L.; Kimball, A.B.; Prens, E.; Wolk, K. Hidradenitis suppurativa. Nat. Rev. Dis. Primers 2020, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Hana, A.; Booken, D.; Henrich, C.; Gratchev, A.; Maas-Szabowski, N.; Goerdt, S.; Kurzen, H. Functional significance of non-neuronal acetylcholine in skin epithelia. Life Sci. 2007, 80, 2214–2220. [Google Scholar] [CrossRef] [PubMed]
- Gerner, R.R.; Wieser, V.; Moschen, A.R.; Tilg, H. Metabolic inflammation: Role of cytokines in the crosstalk between adipose tissue and liver. Can. J. Physiol. Pharmacol. 2013, 91, 867–872. [Google Scholar] [CrossRef]
- Da Silva, N.; Augustin, M.; Langenbruch, A.; Mrowietz, U.; Reich, K.; Thaci, D.; Boehncke, W.H.; Kirsten, N.; Danckworth, A.; Sommer, R. Sex-related impairment and patient needs/benefits in anogenital psoriasis: Difficult-to-communicate topics and their impact on patient-centred care. PLoS ONE 2020, 15, e0235091. [Google Scholar] [CrossRef]


| DLQI | Total (n = 1795) | Female (n = 1152) | Male (n = 643) | Female vs. Male |
|---|---|---|---|---|
| DLQI Total | 13.2 ± 8.1 | 14.2 ± 8.0 | 11.5 ± 8.0 | 0.000000 |
| DLQI Item 1 | 1.7 ± 1.0 | 1.7 ± 1.0 | 1.5 ± 1.0 | 0.000055 |
| DLQI Item 2 | 1.4 ± 1.2 | 1.5 ± 1.2 | 1.1 ± 1.1 | 0.000000 |
| DLQI Item 3 | 1.1 ± 1.1 | 1.2 ± 1.1 | 1.0 ± 1.1 | 0.001810 |
| DLQI Item 4 | 1.7 ± 1.2 | 1.9 ± 1.1 | 1.4 ± 1.2 | 0.000000 |
| DLQI Item 5 | 1.4 ± 1.2 | 1.5 ± 1.2 | 1.3 ± 1.2 | 0.001835 |
| DLQI Item 6 | 1.5 ± 1.3 | 1.6 ± 1.3 | 1.5 ± 1.3 | 0.147319 |
| DLQI Item 7 | 1.1 ± 1.2 | 1.1 ± 1.1 | 1.1 ± 1.2 | 0.997689 |
| DLQI Item 8 | 0.9 ± 1.1 | 1.1 ± 1.2 | 0.7 ± 1.0 | 0.000000 |
| DLQI Item 9 | 1.2 ± 1.3 | 1.4 ± 1.3 | 0.9 ± 1.2 | 0.000000 |
| DLQI Item 10 | 1.1 ± 1.1 | 1.2 ± 1.2 | 1.0 ± 1.1 | 0.006825 |
| DOMAINS | ||||
| Symptoms and feelings | 3 ± 1.8 | 3.2 ± 1.8 | 2.6 ± 1.7 | 0.000000 |
| Daily activities | 2.8 ± 2.0 | 3.1 ± 1.9 | 2.8 ± 2.2 | 0.000000 |
| Leisure | 2.9 ± 2.2 | 3 ± 2.2 | 2.4 ± 2.0 | 0.010427 |
| Work and school | 1.1 ± 1.2 | 1.1 ± 1.1 | 1.1 ± 1.2 | 0.997689 |
| Personal relationships | 2.2 ± 2.2 | 2.5 ± 2.3 | 1.6 ± 2.0 | 0.000000 |
| Treatment | 1.1 ± 1.1 | 1.2 ± 1.2 | 1 ± 1.1 | 0.006825 |
| Affected Body Area | Singular | Multiple |
|---|---|---|
| Head (mean ± SD points) | 14.9 ± 10.1 (n = 10) | 13.3 ± 8.3 (n = 789) |
| Neck (mean ± SD points) | 10 (n = 1) | 14.7 ± 8.3 (n = 129) |
| Upper extremities (mean ± SD points) | 11.3 ± 8.3 (n = 13) | 13.3 ± 8.2 (n = 706) |
| Armpits (mean ± SD points) | 12.28 ± 7.1 (n = 28) | 13.3 ± 8.2 (n = 876) |
| Breast area (mean ± SD points) | 15.1 ± 9 (n = 24) | 13.3 ± 8.1 (n = 1213) |
| Anogenital area (mean ± SD points) | 15.2 ± 7.1 (n = 16) | 13.5 ± 8 (n = 1151) |
| Lower extremities (mean ± SD points) | 9 ± 7.1 (n = 2) | 11.9 ± 7.9 (n = 347) |
| Back (mean ± SD points) | 7.3 ± 4.5 (n = 8) | 12.5 ± 7.7 (n = 35) |
| Buttocks (mean ± SD points) | - | 15.8 ± 10.9 (n = 4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajewski, P.K.; Matusiak, Ł.; von Stebut, E.; Schultheis, M.; Kirschner, U.; Nikolakis, G.; Szepietowski, J.C. Quality-of-Life Impairment among Patients with Hidradenitis Suppurativa: A Cross-Sectional Study of 1795 Patients. Life 2021, 11, 34. https://doi.org/10.3390/life11010034
Krajewski PK, Matusiak Ł, von Stebut E, Schultheis M, Kirschner U, Nikolakis G, Szepietowski JC. Quality-of-Life Impairment among Patients with Hidradenitis Suppurativa: A Cross-Sectional Study of 1795 Patients. Life. 2021; 11(1):34. https://doi.org/10.3390/life11010034
Chicago/Turabian StyleKrajewski, Piotr K., Łukasz Matusiak, Esther von Stebut, Michael Schultheis, Uwe Kirschner, Georgios Nikolakis, and Jacek C. Szepietowski. 2021. "Quality-of-Life Impairment among Patients with Hidradenitis Suppurativa: A Cross-Sectional Study of 1795 Patients" Life 11, no. 1: 34. https://doi.org/10.3390/life11010034
APA StyleKrajewski, P. K., Matusiak, Ł., von Stebut, E., Schultheis, M., Kirschner, U., Nikolakis, G., & Szepietowski, J. C. (2021). Quality-of-Life Impairment among Patients with Hidradenitis Suppurativa: A Cross-Sectional Study of 1795 Patients. Life, 11(1), 34. https://doi.org/10.3390/life11010034






