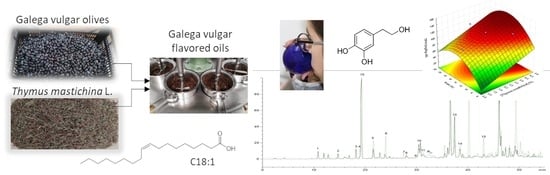
Co-Processed Olive Oils with Thymus mastichina L.—New Product Optimization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Milling of Thymus Mastichina L. and Particle Size Classification
2.3. Co-Processing
2.4. Shelf-Life Studies
2.5. Proximate Analysis of Olives and Thyme
2.6. Chemical and Sensory Characterisation of Flavored Oils
2.7. Statistical Analysis
3. Results and Discussion
3.1. Proximate Analysis of Olives and Thyme
3.2. Particle-Size Analysis of Milled Thyme
3.3. Optimization of Co-Processing Conditions
3.4. Characterization of Flavored Oils
3.5. Shelf-Life Studies: Quality, Phenol Composition, Sensory Analysis, and Oxidative Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchia, A.; Montedoro, G.F.; Morozzib, G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. J. Chromatogr. A 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Cornwell, D.G.; Ma, J. Nutritional Benefit of Olive Oil: The Biological Effects of Hydroxytyrosol and Its Arylating Quinone Adducts. J. Agric. Food Chem. 2008, 56, 8774–8786. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.; Keast, R. Biological Activities of Phenolic Compounds Present in Virgin Olive Oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Psaltopoulou, T.; Kosti, R.I.; Haidopoulos, D.; Dimopoulos, M.; Panagiotakos, D.B. Olive oil intake is inversely related to cancer prevalence: A systematic review and a meta-analysis of 13,800 patients and 23,340 controls in 19 observational studies. Lipids Health Dis. 2011, 10, 127. [Google Scholar] [CrossRef] [Green Version]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Caravaca, A.M.G.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic Molecules in Virgin Olive Oils: A Survey of Their Sensory Properties, Health Effects, Antioxidant Activity and Analytical Methods. An Overview of the Last Decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Peres, F.; Martins, L.L.; Mourato, M.; Vitorino, C.; Antunes, P.; Ferreira-Dias, S. Phenolic compounds of ‘Galega Vulgar’ and ‘Cobrançosa’ olive oils along early ripening stages. Food Chem. 2016, 211, 51–58. [Google Scholar] [CrossRef]
- Wani, T.A.; Masoodi, F.A.; Gani, A.; Baba, W.N.; Rahmanian, N.; Akhter, R.; Wani, I.A.; Ahmad, M. Olive oil and its principal bioactive compound: Hydroxytyrosol—A review of the recent literature. Trends Food Sci. Technol. 2018, 77, 77–90. [Google Scholar] [CrossRef]
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. 2006. Available online: http://data.europa.eu/eli/reg/2006/1924/oj (accessed on 4 January 2021).
- Commission Regulation (EU) No 432/2012 of 16 May 2012 Establishing a List of Permitted Health Claims Made on Foods, Other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. 2012. Available online: http://data.europa.eu/eli/reg/2012/432/oj (accessed on 4 January 2021).
- Baiano, A.; Terracone, C.; Gambacorta, G.; Notte, E.L. Changes in Quality Indices, Phenolic Content and Antioxidant Activity of Flavored Olive Oils during Storage. J. Am. Oil Chem. Soc. 2009, 86, 1083. [Google Scholar] [CrossRef]
- Mannina, L.; D'Imperio, M.; Gobbino, M.; D’Amico, I.; Casini, A.; Emanuele, M.C.; Sobolev, A.P. Nuclear magnetic resonance study of flavoured olive oils. Flavour Fragr. J. 2012, 27, 250–259. [Google Scholar] [CrossRef]
- Caporaso, N.; Paduano, A.; Nicoletti, G.; Sacchi, R. Capsaicinoids, antioxidant activity, and volatile compounds in olive oil flavored with dried chili pepper (Capsicum annuum). Eur. J. Lipid Sci. Technol. 2013, 115, 1434–1442. [Google Scholar] [CrossRef]
- Caponio, F.; Durante, V.; Varva, G.; Silletti, R.; Previtali, M.A.; Viggiani, I.; Squeo, G.; Summo, C.; Pasqualone, A.; Gomes, T.; et al. Effect of infusion of spices into the oil vs. combined malaxation of olive paste and spices on quality of naturally flavoured virgin olive oils. Food Chem. 2016, 202, 221–228. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; Figueiredo-González, M.; González-Barreiro, C.; Simal-Gándara, J.; Salvador, M.D.; Cancho-Grande, B.; Fregapane, G. State of the Art on Functional Virgin Olive Oils Enriched with Bioactive Compounds and Their Properties. Int. J. Mol. Sci. 2017, 18, 668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Gamallo, S.; Salvador, M.D.; Fregapane, G. Design and Characteristics of Novel Sensory and Nutritionally Oriented Olive, Seed, and Nut Virgin Oils’ Blendings. Eur. J. Lipid Sci. Technol. 2021, 123, 2100008. [Google Scholar] [CrossRef]
- Cherif, M.; Rodrigues, N.; Veloso, A.C.A.; Zaghdoudi, K.l.; Pereira, J.A.; Peres, A.M. Kinetic-thermodynamic study of the oxidative stability of Arbequina olive oils flavored with lemon verbena essential oil. LWT 2021, 140, 110711. [Google Scholar] [CrossRef]
- Sacchi, R.; Della Medaglia, D.; Paduano, A.; Caporaso, N.; Genovese, A. Characterisation of lemon-flavoured olive oils. LWT Food Sci. Technol. 2017, 79, 326–332. [Google Scholar] [CrossRef]
- Cerretani, L.; Bendini, A.; Poerio, A.; Toschi, T.G. Citric acid as co-adjuvant: Improvement of the antioxidant activity of edible olive oils. Agro Food Ind. Hi-Tech 2008, 19, 64–66. [Google Scholar]
- Clodoveo, M.L.; Dipalmo, T.; Crupi, P.; Durante, V.; Pesce, V.; Maiellaro, I.; Lovece, A.; Mercurio, A.; Laghezza, A.; Corbo, F.; et al. Comparison Between Different Flavored Olive Oil Production Techniques: Healthy Value and Process Efficiency. Plant Foods Hum. Nutr. 2016, 71, 81–87. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Taglieri, I.; Sgherri, C.; Flamini, G.; Macaluso, M.; Sanmartin, C.; Venturi, F.; Quartacci, M.F.; Pistelli, L.; Zinnai, A. Nutraceutical Oils Produced by Olives and Citrus Peel of Tuscany Varieties as Sources of Functional Ingredients. Molecules 2019, 24, 65. [Google Scholar] [CrossRef] [Green Version]
- Ergönül, P.G.; Sánchez, S. Evaluation of polycyclic aromatic hydrocarbons content in different types of olive and olive pomace oils produced in Turkey and Spain. Eur. J. Lipid Sci. Technol. 2013, 115, 1078–1084. [Google Scholar] [CrossRef]
- Bendini, A.; Di Lecce, G.; Valli, E.; Barbieri, S.; Tesini, F.; Gallina Toschi, T. Olive oil enriched in lycopene from tomato by-product through a co-milling process. Int. J. Food Sci. Nutr. 2015, 66, 371–377. [Google Scholar] [CrossRef]
- Méndez-Tovar, I.; Sponza, S.; Asensio-S-Manzanera, M.C.; Novak, J. Contribution of the main polyphenols of Thymus mastichina subsp. mastichina to its antioxidant properties. Ind. Crop. Prod. 2015, 66, 291–298. [Google Scholar] [CrossRef]
- Taghouti, M.; Martins-Gomes, C.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Chemical Characterization and Bioactivity of Extracts from Thymus mastichina: A Thymus with a Distinct Salvianolic Acid Composition. Antioxidants 2020, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Pardo-de-Santayana, M.; Tardío, J.; Blanco, E.; Carvalho, A.M.; Lastra, J.J.; San Miguel, E.; Morales, R. Traditional knowledge of wild edible plants used in the northwest of the Iberian Peninsula (Spain and Portugal): A comparative study. J. Ethnobiol. Ethnomedicine 2007, 3, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, A.P.; Roque, O.R. Especiarias e Plantas Condimentares. Origem, Composição e Utilizações; Fundação Calouste Gulbenkian: Lisboa, Portugal, 2015. [Google Scholar]
- Delgado, T.; Marinero, P.; Asensio, S.; Manzanera, M.C.; Asensio, C.; Herrero, B.; Pereira, J.A.; Ramalhosa, E. Antioxidant activity of twenty wild Spanish Thymus mastichina L. populations and its relation with their chemical composition. LWT-Food Sci. Technol. 2014, 57, 412–418. [Google Scholar] [CrossRef] [Green Version]
- Haaland, P.D. Experimental Design in Biotechnology; Marcel Dekker Inc.: New York, NY, USA, 1989. [Google Scholar]
- Montgomery, D.C. Design and analysis of Experiments, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2017. [Google Scholar]
- ISO 661. Animal and Vegetable Fats and Oils—Preparation of Test Sample; ISO: Geneve, Switzerland, 2003. [Google Scholar]
- Peres, F.; Martins, L.L.; Ferreira-Dias, S. Laboratory-scale optimization of olive oil extraction: Simultaneous addition of enzymes and microtalc improves the yield. Eur. J. Lipid Sci. Technol. 2014, 116, 1054–1062. [Google Scholar] [CrossRef]
- Pizarro, M.L.; Becerra, M.; Sayago, A.; Beltrán, M.; Beltrán, R. Comparison of Different Extraction Methods to Determine Phenolic Compounds in Virgin Olive Oil. Food Anal. Methods 2013, 6, 123–132. [Google Scholar] [CrossRef]
- Peres, F.; Talhinhas, P.; Afonso, H.; Alegre, H.; Oliveira, H.; Ferreira-Dias, S. Olive Oils from Fruits Infected with Different Anthracnose Pathogens Show Sensory Defects Earlier Than Chemical Degradation. Agronomy 2021, 11, 1041. [Google Scholar] [CrossRef]
- Pokorny, J.; Kalinová, L.; Dysseler, P. Determination of Chlorophyll pigments in Crude Vegetable Oils. Pure Appl. Chem. 1995, 67, 1781–1787. [Google Scholar]
- Official Journal of the European Union. Commission Delegated Regulation (EU) No 2019/1604 of 27 September 2019 amending Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis; Official Journal of the European Union: Brussels, Belgium, 2019; Volume L250, pp. 14–48. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R1604&from=EN (accessed on 4 January 2021).
- Cerretani, L.; Salvador, M.D.; Bendini, A.; Fregapane, G. Relationship Between Sensory Evaluation Performed by Italian and Spanish Official Panels and Volatile and Phenolic Profiles of Virgin Olive Oils. Chem. Percept. 2008, 1, 258–267. [Google Scholar] [CrossRef]
- IOC. Determination of Biophenols in Olive Oils by HPLC. COI/T20/DOC.29. 2009. Available online: https://www.oelea.de/downloads/COI-T20-DOC-29-2009-DETERMINATION-OF-BIOPHENOLS-IN-OLIVE-OILS-BY-HPLC.pdf (accessed on 4 January 2021).
- Pirisi, F.M.; Cabras, P.; Cao, C.F.; Migliorini, M.; Muggelli, M. Phenolic Compounds in Virgin Olive Oil. 2. Reappraisal of the Extraction, HPLC Separation, and Quantification Procedures. J. Agric. Food Chem. 2000, 48, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. From famine plants to tasty and fragrant spices: Three Lamiaceae of general dietary relevance in traditional cuisine of Trás-os-Montes (Portugal). LWT-Food Sci. Technol. 2011, 44, 543–548. [Google Scholar] [CrossRef] [Green Version]
- Kassegn, H.H.; Mekelle, E.P. Inorganic and phytochemical content analysis of the wild abyssinian thyme spice. Food Sci. Qua. Manage. 2016, 82–85. [Google Scholar]
- Kuçukbay, F.; Kuyumcu, E. Determination of trace element contents of Thymus species from Turkey. Turk. J. Chem. 2010, 34, 911–920. [Google Scholar]
- Squeo, G.; Difonzo, G.; Summo, C.; Crecchio, C.; Caponio, F. Study of the influence of technological coadjuvants on enzyme activities and phenolic and volatile compounds in virgin olive oil by a response surface methodology approach. LWT 2020, 133, 109887. [Google Scholar] [CrossRef]
- Sadkaoui, A.; Jiménez, A.; Pacheco, R.; Beltrán, G. Micronized natural talc affects the proteins and pectic cell wall polysaccharides during “Hojiblanca” virgin olive oil extraction. Eur. J. Lipid Sci. Technol. 2017, 119, 1600039. [Google Scholar] [CrossRef]
- Gambacorta, G.; Faccia, M.; Pati, S.; Lamacchia, C.; Baiano, A.; La Notte, E. Changes in the chemical and sensorial profile of extravirgin olive oils flavored with herbes and spices during storage. J. Food Lipids 2007, 14, 202–215. [Google Scholar] [CrossRef]
- Rodrigues, N.; Casal, S.; Pinho, T.; Peres, A.M.; Bento, A.; Baptista, P.; Pereira, J.A. Ancient olive trees as a source of olive oils rich in phenolic compounds. Food Chem. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayadi, M.A.; Grati-Kamoun, N.; Attia, H. Physico-chemical change and heat stability of extra virgin olive oils flavoured by selected Tunisian aromatic plants. Food Chem. Toxicol. 2009, 47, 2613–2619. [Google Scholar] [CrossRef] [PubMed]
- Issaoui, M.; Flamini, G.; Souid, S.; Bendini, A.; Barbieri, S.; Gharbi, I.; Toschi, T.G.; Cioni, P.L.; Hammami, M. How the Addition of Spices and Herbs to Virgin Olive Oil to Produce Flavored Oils Affects Consumer Acceptance. Nat. Prod. Commun. 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Diamantakos, P.; Giannara, T.; Skarkou, M.; Melliou, E.; Magiatis, P. Influence of Harvest Time and Malaxation Conditions on the Concentration of Individual Phenols in Extra Virgin Olive Oil Related to Its Healthy Properties. Molecules 2020, 25, 2449. [Google Scholar] [CrossRef]
- Guillaume, C.; Ravetti, L.; Gwyn, S. Characterisation of Phenolic Compounds in Oils Produced from Frosted Olives. J. Am. Oil Chem. Soc. 2010, 87, 247–254. [Google Scholar] [CrossRef]
- García-Vico, L.; García-Rodríguez, R.; Sanz, C.; Pérez, A.G. Biochemical aspects of olive freezing-damage: Impact on the phenolic and volatile profiles of virgin olive oil. LWT 2017, 86, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Baiano, A.; Previtali, M.A.; Viggiani, I.; Varva, G.; Squeo, G.; Paradiso, V.M.; Summo, C.; Gomes, T.; Caponio, F. As oil blending affects physical, chemical, and sensory characteristics of flavoured olive oils. Eur. Food Res. Technol. 2016, 242, 1693–1708. [Google Scholar] [CrossRef]
- Rubió, L.; Motilva, M.-J.; Macià, A.; Ramo, T.; Romero, M.-P. Development of a Phenol-Enriched Olive Oil with Both Its Own Phenolic Compounds and Complementary Phenols from Thyme. J. Agric. Food Chem. 2012, 60, 3105–3112. [Google Scholar] [CrossRef] [PubMed]
- Issaoui, M.; Flamini, G.; Hajaij, M.E.; Cioni, P.L.; Hammami, M. Oxidative Evolution of Virgin and Flavored Olive Oils under Thermo-oxidation Processes. J. Am. Oil Chem. Soc. 2011, 88, 1339–1350. [Google Scholar] [CrossRef]
- Bobiano, M.; Rodrigues, N.; Madureira, M.; Dias, L.G.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Unmasking Sensory Defects of Olive Oils Flavored with Basil and Oregano Using an Electronic Tongue-Chemometric Tool. J. Am. Oil Chem. Soc. 2019, 96, 751–760. [Google Scholar] [CrossRef] [Green Version]
- Khemakhem, I.; Yaiche, C.; Ayadi, M.A.; Bouaziz, M. Impact of Aromatization by Citrus limetta and Citrus sinensis Peels on Olive Oil Quality, Chemical Composition and Heat Stability. J. Am. Oil Chem. Soc. 2015, 92, 701–708. [Google Scholar] [CrossRef]
- Taoudiat, A.; Djenane, D.; Ferhat, Z.; Spigno, G. The effect of Laurus nobilis L. essential oil and different packaging systems on the photo-oxidative stability of Chemlal extra-virgin olive oil. J. Food Sci. Technol. 2018, 55, 4212–4222. [Google Scholar] [CrossRef]






| Experimental Points | Experiment Number | [Thyme] Coded Value | [Water] Coded Value | [Thyme] (%) Decoded Values | [Water] (%) Decoded Values |
|---|---|---|---|---|---|
| Factorial points | 1 | −1 | −1 | 1 | 10 |
| 2 | −1 | 1 | 1 | 18 | |
| 3 | 1 | −1 | 4 | 10 | |
| 4 | 1 | 1 | 4 | 18 | |
| Star points | 5 | 0 | 0.4 | 14 | |
| 6 | 0 | 4.6 | 14 | ||
| 7 | 0 | 2.5 | 8.3 | ||
| 8 | 0 | 2.5 | 19.7 | ||
| Central points | 9 | 0 | 0 | 2.5 | 14 |
| 10 | 0 | 0 | 2.5 | 14 | |
| 11 | 0 | 0 | 2.5 | 14 | |
| 12 | 0 | 0 | 2.5 | 14 | |
| 13 | 0 | 0 | 2.5 | 14 | |
| Control | 14 | _ | _ | - | 14 |
| Parameter | Unit | Olives | T. mastichina |
|---|---|---|---|
| Moisture | (%) | 42.31± 0.21 | 6.30 ± 0.04 |
| Fat | (%) | 39.62± 0.82 | 3.08 ± 0.01 |
| Ash | (%) | 3.67 ± 0.59 | 5.35 ± 0.06 |
| Protein | (%) | 5.00 ± 0.62 | 7.79 ± 0.15 |
| Cu | 9.2 ± 1.3 | 10.1 ± 1.1 | |
| Zn | 19.8 ± 3.0 | 83.2 ± 18.5 | |
| Fe | 91.0 ± 6.4 | 218.5 ± 4.5 | |
| Mn | 23.8 ± 1.3 | 292.7 ± 44.5 | |
| Na | 154.8 ± 7.6 | 205.5 ± 4.8 | |
| K | (mg/kg) | 16040 ± 199 | 13822 ± 1195 |
| Ca | 3288 ± 101 | 11118 ± 464 | |
| Mg | 472 ± 7 | 2178 ± 214 | |
| P | 1199 ± 17 | 1958 ± 260 | |
| S | 853 ± 33 | 1854 ± 107 |
| Experiment | Acidity | PV | K232 | K270 | C16:0 | C18:1 | C18:2 | C18:3 |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.17 | 4.5 | 1.54 | 0.13 | 11.59 | 77.03 | 5.06 | 0.48 |
| 2 | 0.18 | 4.3 | 1.57 | 0.11 | 11.54 | 76.99 | 5.00 | 0.58 |
| 3 | 0.19 | 4.5 | 1.60 | 0.13 | 12.04 | 77.31 | 4.73 | 0.76 |
| 4 | 0.21 | 4.3 | 1.59 | 0.12 | 12.06 | 77.21 | 4.66 | 0.75 |
| 5 | 0.18 | 4.2 | 1.58 | 0.11 | 11.59 | 77.09 | 4.86 | 0.59 |
| 6 | 0.18 | 4.7 | 1.61 | 0.15 | 12.22 | 77.42 | 4.56 | 0.82 |
| 7 | 0.21 | 4.7 | 1.62 | 0.14 | 11.85 | 77.07 | 4.85 | 0.67 |
| 8 | 0.17 | 4.3 | 1.60 | 0.12 | 11.97 | 77.10 | 4.63 | 0.67 |
| 9 | 0.18 | 4.5 | 1.61 | 0.14 | 11.74 | 77.28 | 4.57 | 0.70 |
| 10 | 0.17 | 4.9 | 1.61 | 0.14 | 11.87 | 77.16 | 4.73 | 0.72 |
| 11 | 0.19 | 4.2 | 1.61 | 0.14 | 11.93 | 77.04 | 4.73 | 0.70 |
| 12 | 0.18 | 4.7 | 1.63 | 0.14 | 11.97 | 77.08 | 4.77 | 0.71 |
| 13 | 0.18 | 4.6 | 1.61 | 0.14 | 11.88 | 77.14 | 4.70 | 0.73 |
| 14 (hammer mill) | 0.11 | 4.5 | 1.72 | 0.18 | 11.93 | 77.02 | 4.78 | 0.81 |
| 15 (VOO-control) | 0.20 | 4.4 | 1.59 | 0.12 | 11.47 | 76.94 | 4.86 | 0.62 |
| Experiment | Acidity | PV | K232 | K270 | C16:0 | C18:1 | C18:2 | C18:3 | TPH |
|---|---|---|---|---|---|---|---|---|---|
| 9 | 0.21 | 7.39 | 1.74 | 0.18 | 11.90 | 2.60 | 4.78 | 0.61 | 120.08 |
| 10 | 0.20 | 7.07 | 1.74 | 0.16 | 11.84 | 2.58 | 4.92 | 0.58 | 108.19 |
| 11 | 0.23 | 6.89 | 1.76 | 0.16 | 11.88 | 2.60 | 4.94 | 0.60 | 97.33 |
| 12 | 0.22 | 7.65 | 1.78 | 0.17 | 11.95 | 2.63 | 4.96 | 0.63 | 107.13 |
| 13 | 0.22 | 7.58 | 1.77 | 0.16 | 11.83 | 2.57 | 4.97 | 0.9 | 99.28 |
| 14 (hammer mill) | 0.25 | 6.7 | 1.78 | 0.19 | 11.83 | 2.62 | 4.62 | 0.70 | 158.60 |
| 15 (VOO-control) | 0.25 | 7.5 | 1.78 | 0.15 | 11.59 | 2.45 | 5.14 | 0.50 | 69.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peres, F.; Roldão, M.; Mourato, M.; Martins, L.L.; Ferreira-Dias, S. Co-Processed Olive Oils with Thymus mastichina L.—New Product Optimization. Life 2021, 11, 1048. https://doi.org/10.3390/life11101048
Peres F, Roldão M, Mourato M, Martins LL, Ferreira-Dias S. Co-Processed Olive Oils with Thymus mastichina L.—New Product Optimization. Life. 2021; 11(10):1048. https://doi.org/10.3390/life11101048
Chicago/Turabian StylePeres, Fátima, Marta Roldão, Miguel Mourato, Luisa L. Martins, and Suzana Ferreira-Dias. 2021. "Co-Processed Olive Oils with Thymus mastichina L.—New Product Optimization" Life 11, no. 10: 1048. https://doi.org/10.3390/life11101048