High-Throughput Sequencing of Circulating MicroRNAs in Plasma and Serum during Pregnancy Progression
Abstract
:1. Introduction
2. Results
2.1. Characteristics of Subjects
2.2. Small RNA Sequencing Results
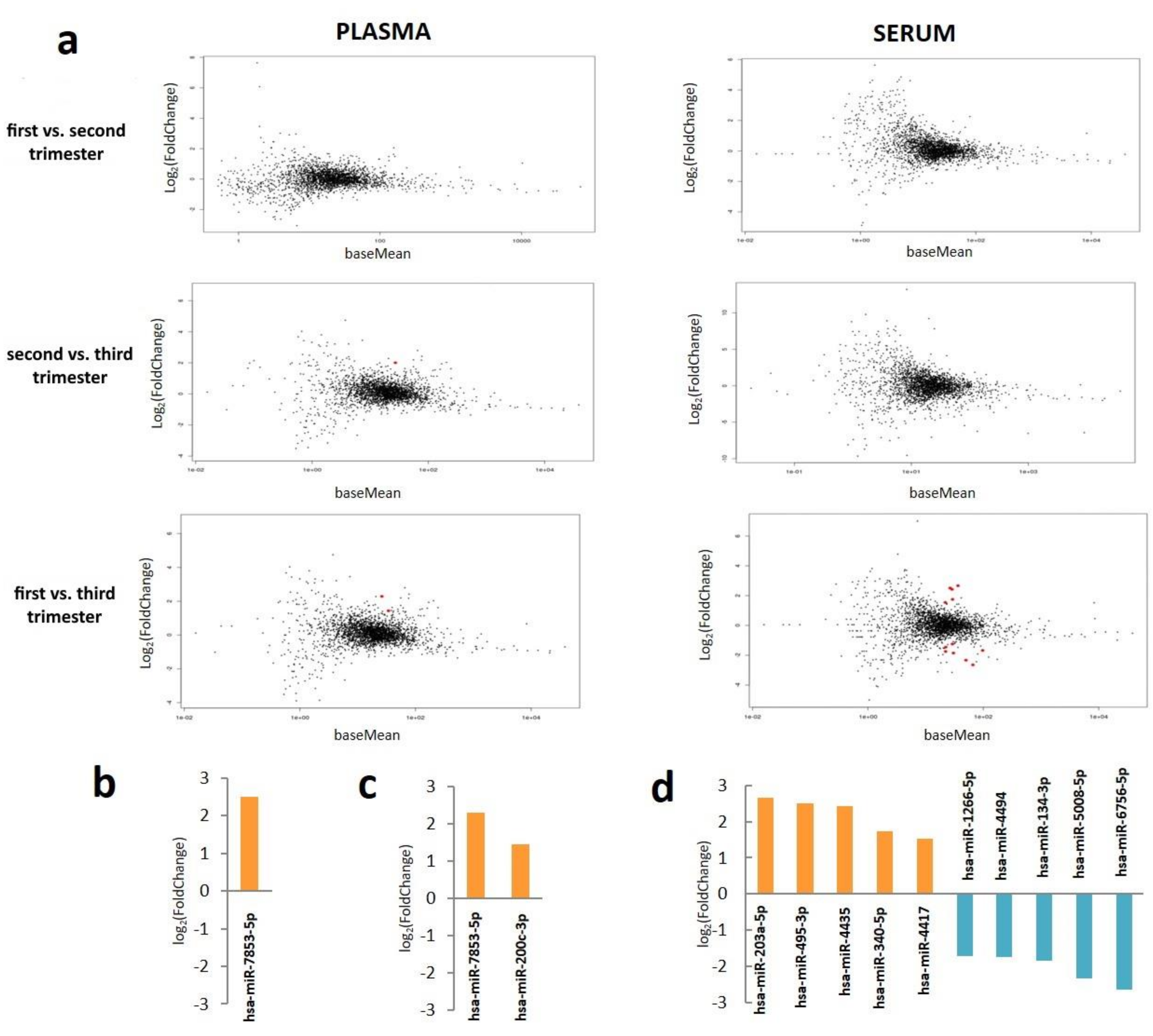
2.3. Plasma and Serum miRNA Profiles in Different Stages of Pregnancy
2.4. Comparison of miRNA Profiles between Plasma and Serum
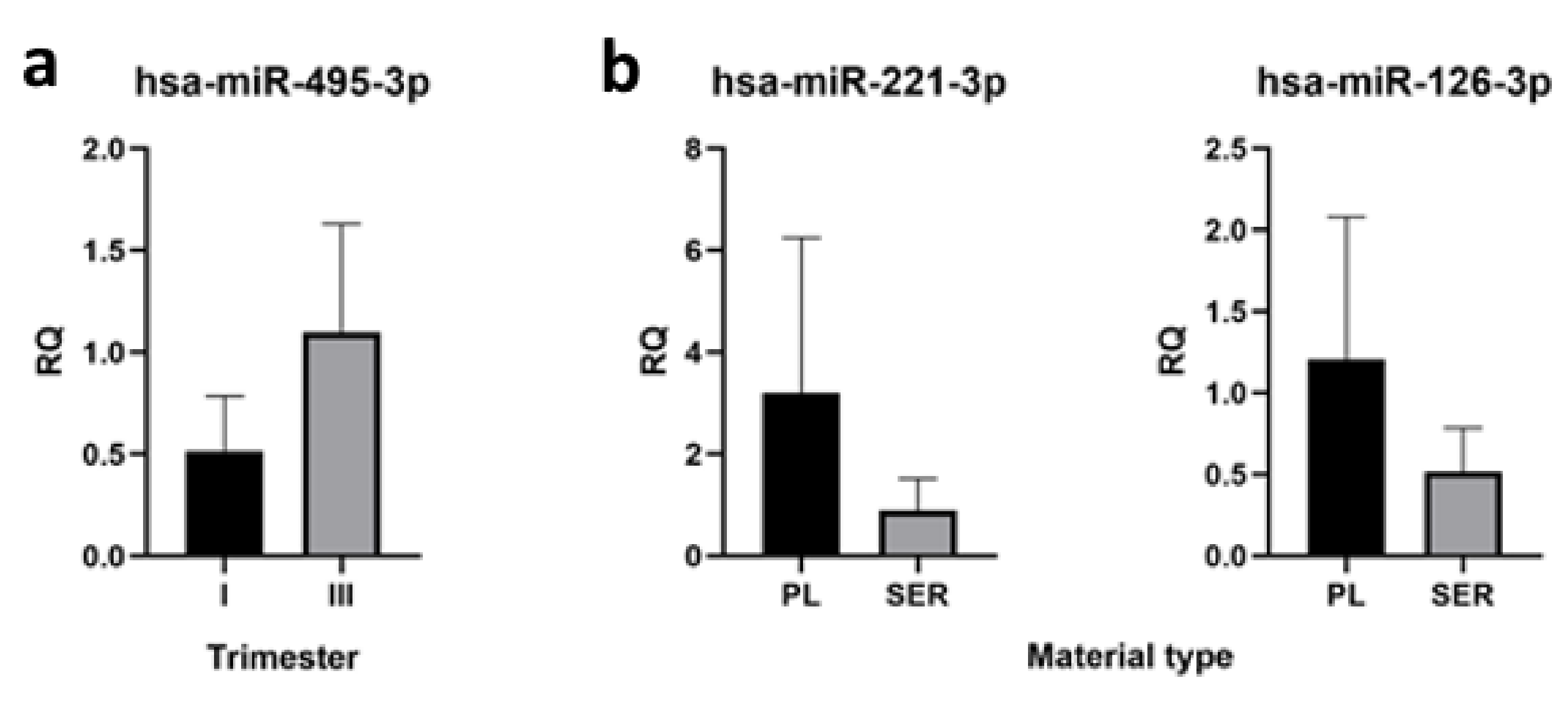
2.5. qRT-PCR Verification of High-Throughput Sequencing Data
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Sample Preparation
4.3. Small RNA Isolation and Library Preparation for Sequencing
4.4. Illumina Sequencing
4.5. Clinical Data Analysis
4.6. Sequencing Data Analysis
4.7. qRT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.; Moley, K.H.; Gronowski, A.M. Diagnostic potential for miRNAs as biomarkers for pregnancy-specific diseases. Clin. Biochem. 2013, 46, 953–960. [Google Scholar] [CrossRef]
- Chen, D.B.; Wang, W. Human placental microRNAs and preeclampsia. Biol. Reprod. 2013, 88, 130. [Google Scholar] [CrossRef] [PubMed]
- Gunel, T.; Zeybek, Y.; Akcakaya, P.; Kalelioglu, I.; Benian, A.; Ermis, H.; Aydinli, K. Serum microRNA expression in pregnancies with preeclampsia. Genet Mol. Res. 2011, 10, 4034–4040. [Google Scholar] [CrossRef] [PubMed]
- Morales-Prieto, D.M.; Ospina-Prieto, S.; Chaiwangyen, W.; Schoenleben, M.; Markert, U.R. Pregnancy-associated miRNA-clusters. J. Reprod. Immunol. 2013, 97, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Barchitta, M.; Maugeri, A.; Quattrocchi, A.; Agrifoglio, O.; Agodi, A. The Role of miRNAs as Biomarkers for Pregnancy Outcomes: A Comprehensive Review. Int. J. Genom. 2017, 2017, 8067972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.D.; Pillman, K.; Jankovic-Karasoulos, T.; McAninch, D.; Wan, Q.; Bogias, K.J.; McCullough, D.; Bianco-Miotto, T.; Breen, J.; Roberts, C.T. Large-scale transcriptome-wide profiling of microRNAs in human placenta and maternal plasma at early to mid gestation. medRxiv 2020. [Google Scholar] [CrossRef]
- Vashukova, E.; Glotov, A.; Fedotov, P.V.; Efimova, O.; Pakin, V.; Mozgovaya, E.V.; Pendina, A.; Tikhonov, A.; Koltsova, A.; Baranov, V. Placental microRNA expression in pregnancies complicated by superimposed pre-eclampsia on chronic hypertension. Mol. Med. Rep. 2016, 14, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A.; Illarionov, R.; Vashukova, E.; Glotov, A. Publication-based analysis of miR-210 dependent biomarkers of pre-eclampsia. Biol. Commun. 2020, 65, 163–177. [Google Scholar] [CrossRef]
- Alexovič, M.; Sabo, J.; Longuespée, R. Microproteomic sample preparation. Proteomics 2021, 21, e2000318. [Google Scholar] [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, P.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [Green Version]
- Heneghan, H.M.; Miller, N.; Lowery, A.J.; Sweeney, K.J.; Newell, J.; Kerin, M.J. Circulating microRNAs as novel minimally invasive biomarkers for breast cancer. Ann. Surg. 2010, 251, 499–505. [Google Scholar] [CrossRef]
- Brase, J.C.; Johannes, M.; Schlomm, T.; Fälth, M.; Haese, A.; Steuber, T.; Beißbarth, T.; Kuner, R.; Sültmann, H. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int. J. Cancer 2011, 128, 608–616. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandra, Y.; Devanna, P.; Limana, F.; Straino, S.; Di Carlo, A.; Brambilla, P.G.; Rubino, M.; Carena, M.C.; Spazzafumo, L.; De Simone, M.; et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur. Heart J. 2010, 31, 2765–2773. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Yuan, Y.; Cho, J.H.; McClarty, S.; Baxter, D.; Galas, D.J. Comparing the MicroRNA spectrum between serum and plasma. PLoS ONE 2012, 7, e41561. [Google Scholar] [CrossRef]
- Willeit, P.; Zampetaki, A.; Dudek, K.; Kaudewitz, D.; King, A.; Kirkby, N.S.; Crosby-Nwaobi, R.; Prokopi, M.; Drozdov, I.; Langley, S.R.; et al. Circulating microRNAs as novel biomarkers for platelet activation. Circ. Res. 2013, 112, 595–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Q.; Shen, Y.; Tian, F.; Lu, J.; Bai, Y.; Lu, Z. Profiling circulating microRNAs in maternal serum and plasma. Mol. Med. Rep. 2015, 12, 3323–3330. [Google Scholar] [CrossRef] [Green Version]
- Parker, V.L.; Gavriil, E.; Marshall, B.; Pacey, A.; Heath, P.R. Profiling microRNAs in uncomplicated pregnancies: Serum vs. plasma. Biomed. Rep. 2021, 14, 24. [Google Scholar] [CrossRef]
- Gilad, S.; Meiri, E.; Yogev, Y.; Benjamin, S.; Lebanony, D.; Yerushalmi, N.; Benjamin, H.; Kushnir, M.; Cholakh, H.; Melamed, N.; et al. Serum microRNAs are promising novel biomarkers. PLoS ONE 2008, 3, e3148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, K.; Miura, S.; Yamasaki, K.; Higashijima, A.; Kinoshita, A.; Yoshiura, K.-I.; Masuzaki, H. Identification of pregnancy-associated microRNAs in maternal plasma. Clin. Chem. 2010, 56, 1767–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Guo, L.; Wu, Q.; Lu, J.; Ge, Q.; Lu, Z. A comprehensive survey of maternal plasma miRNAs expression profiles using high-throughput sequencing. Clin. Chim. Acta. 2012, 413, 568–576. [Google Scholar] [CrossRef]
- Menon, R.; Debnath, C.; Lai, A.; Guanzon, D.; Bhatnagar, S.; Kshetrapal, P.K.; Sheller-Miller, S.; Salomon, C.; The Garbhini Study Team. Circulating Exosomal miRNA Profile during Term and Preterm Birth Pregnancies: A Longitudinal Study. Endocrinology 2019, 160, 249–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coenen-Stass, A.M.; Magen, I.; Brooks, T.; Ben-Dov, I.; Greensmith, L.; Hornstein, E.; Fratta, P. Evaluation of methodologies for microRNA biomarker detection by next generation sequencing. RNA Biol. 2018, 15, 1133–1145. [Google Scholar] [CrossRef]
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Song, X.; Zhang, Q.; Li, Y.; Chen, Y.; Du, W.; Zhang, Z.; Jin, L. miR-203a-5p suppresses cervical cancer tumorigenesis and EMT via targeting MAPK1. Acta Med. Mediterr. 2020, 36, 3599. [Google Scholar]
- DaI, S.G.; Guo, L.L.; XIa, X.; Pan, Y. Long non-coding RNA WT1-AS inhibits cell aggressiveness via miR-203a-5p/FOXN2 axis and is associated with prognosis in cervical cancer. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 486–495. [Google Scholar] [PubMed]
- Ostadrahimi, S.; Abedi-Valugerdi, M.; Hassan, M.; Haddad, G.; Fayaz, S.; ParvizHamidi, M.; Mahdian, R.; Fard-Esfahani, P. miR-1266-5p and miR-185-5p Promote Cell Apoptosis in Human Prostate Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2018, 19, 2305–2311. [Google Scholar] [CrossRef]
- Mosapour, A.; Karami Tehrani, F.S.; Atri, M. Differential expression of miR-1297, miR-3191-5p, miR-4435, and miR-4465 in malignant and benign breast tumors. Iran J. Basic Med. Sci. 2020, 23, 1045–1052. [Google Scholar] [CrossRef]
- Chang, C.; Liu, T.; Huang, Y.; Qin, W.; Yang, H.; Chen, J. MicroRNA-134-3p is a novel potential inhibitor of human ovarian cancer stem cells by targeting RAB27A. Gene 2017, 605, 99–107. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Bai, J.; He, A. Expression, regulation and function of miR-495 in healthy and tumor tissues. Oncol. Lett. 2017, 13, 2021–2026. [Google Scholar] [CrossRef] [Green Version]
- Paquette, A.G.; Shynlova, O.; Wu, X.; Kibschull, M.; Wang, K.; Price, N.D.; Lye, S.J. MicroRNA-transcriptome networks in whole blood and monocytes of women undergoing preterm labour. J. Cell. Mol. Med. 2019, 23, 6835–6845. [Google Scholar] [CrossRef]
- Hu, T.-X.; Wang, G.; Guo, X.-J.; Sun, Q.-Q.; He, P.; Gu, H.; Huang, Y.; Gao, L.; Ni, X. MiR 20a,-20b and -200c are involved in hydrogen sulfide stimulation of VEGF production in human placental trophoblasts. Placenta 2016, 39, 101–110. [Google Scholar] [CrossRef]
- Enquobahrie, D.A.; Abetew, D.F.; Sorensen, T.K.; Willoughby, D.; Chidambaram, K.; Williams, M.A. Placental microRNA expression in pregnancies complicated by preeclampsia. Am. J. Obstet. Gynecol. 2011, 204, 178.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayor-Lynn, K.; Toloubeydokhti, T.; Cruz, A.C.; Chegini, N. Expression profile of microRNAs and mRNAs in human placentas from pregnancies complicated by preeclampsia and preterm labor. Reprod. Sci. 2011, 18, 46–56. [Google Scholar] [CrossRef]
- Ge, Q.; Zhu, Y.; Li, H.; Tian, F.; Xie, X.; Bai, Y. Differential expression of circulating miRNAs in maternal plasma in pregnancies with fetal macrosomia. Int. J. Mol. Med. 2015, 35, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Q.; Zhang, D.; Yang, Y.U.; Cui, X.; Sun, J.; Liang, C.; Qin, H.; Yang, X.; Liu, S.; Yan, Q. MicroRNA-200c impairs uterine receptivity formation by targeting FUT4 and α1,3-fucosylation. Cell Death Differ. 2017, 24, 2161–2172. [Google Scholar] [CrossRef] [Green Version]
- Fallen, S.; Baxter, D.; Wu, X.; Kim, T.; Shynlova, O.; Lee, M.Y.; Scherler, K.; Lye, S.; Hood, L.; Wang, K. Extracellular vesicle RNAs reflect placenta dysfunction and are a biomarker source for preterm labour. J. Cell Mol. Med. 2018, 22, 2760–2773. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.Y.; Lim, S.M.; Lee, H.J.; Hong, J.S.; Kim, G.J. Identification of microRNAs and their target genes in the placenta as biomarkers of inflammation. Clin. Exp. Reprod. Med. 2020, 47, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Stirm, L.; Huypens, P.; Sass, S.; Batra, R.; Fritsche, L.; Brucker, S.; Abele, H.; Hennige, A.M.; Theis, F.; Beckers, J.; et al. Maternal whole blood cell miRNA-340 is elevated in gestational diabetes and inversely regulated by glucose and insulin. Sci. Rep. 2018, 8, 1366. [Google Scholar] [CrossRef] [PubMed]
- Winger, E.E.; Reed, J.L.; Ji, X. Early first trimester peripheral blood cell microRNA predicts risk of preterm delivery in pregnant women: Proof of concept. PLoS ONE 2017, 12, e0180124. [Google Scholar] [CrossRef] [Green Version]
- Dufourd, T.; Robil, N.; Mallet, D.; Carcenac, C.; Boulet, S.; Brishoual, S.; Rabois, E.; Houeto, J.-L.; De La Grange, P.; Carnicella, S. Plasma or serum? A qualitative study on rodents and humans using high-throughput microRNA sequencing for circulating biomarkers. Biol. Methods Protoc. 2019, 4, bpz006. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, J.-Q.; Zhang, J.-T.; Li, Q.; Li, Y.; He, J.; Qin, Y.-W.; Jing, Q. Circulating microRNA: A novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 2010, 31, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. Clusterprofiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]

| Characteristic | Research Group (n = 7) |
|---|---|
| Maternal Characteristics | |
| Age, years | 32.71 ± 1.98 |
| Ethnicity | Russian, 7/7 |
| Weight, kg | 65.43 ± 2.70 |
| Height, m | 1.67 ± 0.03 |
| BMI, kg/m2 | 23.53 ± 0.79 |
| Gestational Age of Sampling | |
| First trimester, weeks | 11.07 ± 0.41 |
| Second trimester, weeks | 21.77 ± 1.11 |
| Third trimester, weeks | 32.54 ± 0.89 |
| Pregnancy Outcome | |
| Mode of delivery | Vaginal delivery/Cesarean section, 6/1 |
| Gestational age at delivery, weeks | 39.90 ± 0.35 |
| Fetal weight, g | 3481.43 ± 125.12 |
| Fetal height, cm | 51.57 ± 0.57 |
| Upregulated in Plasma Compared with Serum | Downregulated in Plasma Compared with Serum | ||||
|---|---|---|---|---|---|
| miRNA | log2 (fold change) | Adjusted p-value | miRNA | log2 (fold change) | Adjusted p-value |
| hsa-miR-195-5p | 2.1037 | 4.173E-05 | hsa-miR-130b-5p | −1.9032 | 0.0023 |
| hsa-miR-103a-3p | 1.9215 | 0.0138 | hsa-miR-4513 | −2.1794 | 0.0062 |
| hsa-miR-103b | 1.8966 | 0.0159 | hsa-miR-134-3p | −1.7922 | 0.0090 |
| hsa-miR-20a-5p | 1.8640 | 0.0024 | hsa-miR-6811-3p | −2.2019 | 0.0090 |
| hsa-miR-101-3p | 1.8614 | 0.0004 | hsa-miR-6780b-5p | −1.6910 | 0.0104 |
| hsa-miR-26a-5p | 1.8337 | 0.0022 | hsa-miR-5698 | −3.0858 | 0.0125 |
| hsa-miR-126-3p | 1.8255 | 0.0090 | hsa-miR-4742-5p | −2.0452 | 0.0149 |
| hsa-miR-16-5p | 1.8214 | 0.0164 | hsa-miR-138-5p | −2.1160 | 0.0149 |
| hsa-miR-451a | 1.8079 | 0.0279 | hsa-miR-6756-5p | −2.3664 | 0.0159 |
| hsa-miR-320a | 1.7348 | 0.0390 | hsa-miR-3151-5p | −2.1801 | 0.0180 |
| hsa-miR-221-3p | 1.6173 | 0.0390 | hsa-miR-7152-5p | −2.2668 | 0.0215 |
| hsa-let-7d-5p | 1.6133 | 0.0245 | hsa-miR-571 | −1.6354 | 0.0249 |
| hsa-miR-144-3p | 1.5750 | 0.0125 | hsa-miR-4650-5p | −1.5487 | 0.0304 |
| hsa-miR-32-5p | 1.5547 | 0.0225 | hsa-miR-5008-5p | −2.2514 | 0.0305 |
| hsa-miR-320b | 1.5117 | 0.0390 | hsa-miR-8054 | −1.6772 | 0.0367 |
| hsa-miR-1266-5p | −1.5223 | 0.0390 | |||
| hsa-miR-181a-3p | −1.8287 | 0.0390 | |||
| hsa-miR-3689d | −1.6338 | 0.0420 | |||
| hsa-miR-6754-3p | −1.6791 | 0.0429 | |||
| hsa-miR-6748-5p | −2.1050 | 0.0500 | |||
| hsa-miR-518c-3p | −1.7148 | 0.0496 | |||
| GO ID | GO Term | Gene Number for Term | Adjusted p-Value |
|---|---|---|---|
| GO:0003712 | Transcription coregulator activity | 208 | 2.192646 × 10−14 |
| GO:0000987 | Proximal promoter sequence-specific DNA binding | 193 | 2.831005 × 10−14 |
| GO:0004674 | Protein serine/threonine kinase activity | 155 | 1.493812 × 10−10 |
| GO:0001228 | DNA-binding transcription activator activity, RNA polymerase II-specific | 160 | 3.029877 × 10−10 |
| GO:0019199 | Transmembrane receptor protein kinase activity | 39 | 3.064696 × 10−7 |
| GO:0051020 | GTPase binding | 160 | 5.003948 × 10−7 |
| GO:0046332 | SMAD binding | 40 | 2.917633 × 10−6 |
| GO:0019003 | GDP binding | 33 | 2.894027 × 10−5 |
| GO:0003682 | Chromatin binding | 155 | 3.486248 × 10−5 |
| GO:0019787 | Ubiquitin-like protein transferase activity | 121 | 6.411654 × 10−5 |
| GO:0003924 | GTPase activity | 88 | 8.003687 × 10−5 |
| GO:0008013 | Beta-catenin binding | 39 | 8.173702 × 10−5 |
| GO:0050839 | Cell adhesion molecule binding | 153 | 1.619180 × 10−4 |
| GO:0035091 | Phosphatidylinositol binding | 84 | 1.799412 × 10−4 |
| GO:0032550 | Purine ribonucleoside binding | 115 | 1.799412 × 10−4 |
| KEGG ID | KEGG Term | Gene Number for Term | Adjusted p-Value |
|---|---|---|---|
| hsa04151 | PI3K-Akt signaling pathway | 143 | 8.615448 × 10−12 |
| hsa04010 | MAPK signaling pathway | 129 | 8.204628 × 10−14 |
| hsa05165 | Human papillomavirus infection | 120 | 6.230594 × 10−7 |
| hsa04144 | Endocytosis | 98 | 1.338059 × 10−7 |
| hsa05205 | Proteoglycans in cancer | 96 | 1.371966 × 10−12 |
| hsa04360 | Axon guidance | 93 | 3.946275 × 10−15 |
| hsa04810 | Regulation of actin cytoskeleton | 92 | 3.863385 × 10−9 |
| hsa04014 | Ras signaling pathway | 92 | 1.404485 × 10−7 |
| hsa04510 | Focal adhesion | 88 | 9.398793 × 10−10 |
| hsa05163 | Human cytomegalovirus infection | 86 | 2.219365 × 10−6 |
| hsa04015 | Rap1 signaling pathway | 82 | 1.411583 × 10−6 |
| hsa04020 | Calcium signaling pathway | 82 | 3.687535 × 10−4 |
| hsa04024 | cAMP signaling pathway | 81 | 1.014159 × 10−5 |
| hsa05166 | Human T-cell leukemia virus 1 infection | 78 | 1.141134 × 10−4 |
| hsa05131 | Shigellosisr | 78 | 5.208530 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vashukova, E.S.; Kozyulina, P.Y.; Illarionov, R.A.; Yurkina, N.O.; Pachuliia, O.V.; Butenko, M.G.; Postnikova, T.B.; Ivanova, L.A.; Eremeeva, D.R.; Zainulina, M.S.; et al. High-Throughput Sequencing of Circulating MicroRNAs in Plasma and Serum during Pregnancy Progression. Life 2021, 11, 1055. https://doi.org/10.3390/life11101055
Vashukova ES, Kozyulina PY, Illarionov RA, Yurkina NO, Pachuliia OV, Butenko MG, Postnikova TB, Ivanova LA, Eremeeva DR, Zainulina MS, et al. High-Throughput Sequencing of Circulating MicroRNAs in Plasma and Serum during Pregnancy Progression. Life. 2021; 11(10):1055. https://doi.org/10.3390/life11101055
Chicago/Turabian StyleVashukova, Elena S., Polina Y. Kozyulina, Roman A. Illarionov, Natalya O. Yurkina, Olga V. Pachuliia, Mariya G. Butenko, Tatyana B. Postnikova, Lada A. Ivanova, Dina R. Eremeeva, Marina S. Zainulina, and et al. 2021. "High-Throughput Sequencing of Circulating MicroRNAs in Plasma and Serum during Pregnancy Progression" Life 11, no. 10: 1055. https://doi.org/10.3390/life11101055







