Insights into the Resistome and Phylogenomics of a ST195 Multidrug-Resistant Acinetobacter baumannii Clinical Isolate from the Czech Republic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Antimicrobial Susceptibility Testing
2.2. Detection of β-Lactamase Genes by PCR
2.3. Whole-Genome Sequencing, Annotation and Molecular Analysis
2.4. Genome Analysis and Phylogenetic Analysis
2.5. Data Availability
3. Results
3.1. Antimicrobial Susceptibility Testing
3.2. PCR Detection of β-Lactamase Genes
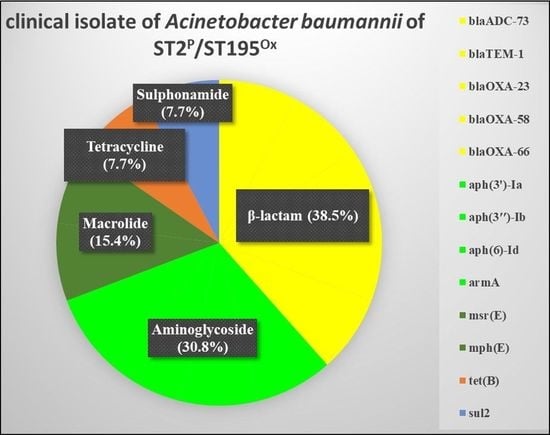
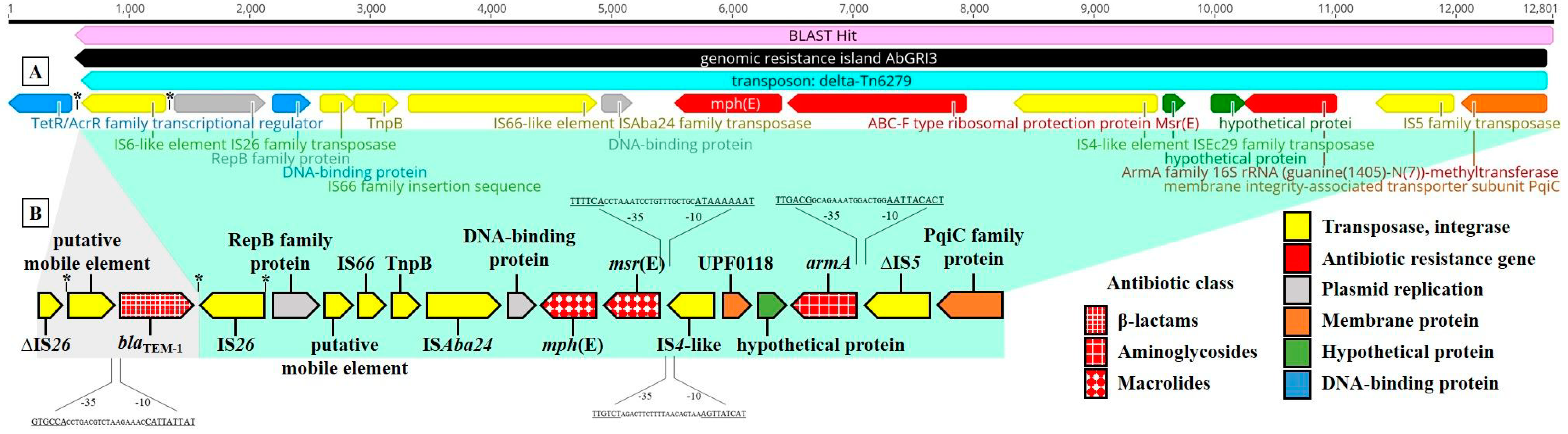
3.3. Whole-Genome Analysis of MDR A. baumannii 11069/A including Antibiotic Resistance Genes and Associated Genes
3.4. Phylogenetic Analysis of A. baumannii 11069/A
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansen, G.T. Continuous Evolution: Perspective on the Epidemiology of Carbapenemase Resistance Among Enterobacterales and Other Gram-Negative Bacteria. Infect. Dis. Ther. 2021, 10, 75–92. [Google Scholar] [CrossRef]
- Tenover, F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Med. 2006, 119, S3–S10. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, M.D.; Goglin, K.; Molyneaux, N.; Hujer, K.M.; Lavender, H.; Jamison, J.J.; MacDonald, I.J.; Martin, K.M.; Russo, T.; Campagnari, A.A.; et al. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J. Bacteriol. 2008, 190, 8053–8064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ECDC. European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe 2018; ECDC: Stockholm, Sweden, 2019; Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2018 (accessed on 20 April 2020).
- Dortet, L.; Agathine, A.; Naas, T.; Cuzon, G.; Poirel, L.; Nordmann, P. Evaluation of the RAPIDEC (R) CARBA NP, the Rapid CARB Screen (R) and the Carba NP test for biochemical detection of carbapenemase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2015, 70, 3014–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mlynarcik, P.; Bardon, J.; Htoutou Sedlakova, M.; Prochazkova, P.; Kolar, M. Identification of novel OXA-134-like beta-lactamases in Acinetobacter lwoffii and Acinetobacter schindleri isolated from chicken litter. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech. Repub. 2019, 163, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Mlynarcik, P.; Dolejska, M.; Vagnerova, I.; Kutilova, I.; Kolar, M. Detection of clinically important beta-lactamases by using PCR. FEMS Microbiol. Lett. 2021, 368, fnab068. [Google Scholar] [CrossRef] [PubMed]
- Kolar, M.; Htoutou Sedlakova, M.; Urbanek, K.; Mlynarcik, P.; Roderova, M.; Hricova, K.; Mezerova, K.; Kucova, P.; Zapletalova, J.; Fiserova, K.; et al. Implementation of Antibiotic Stewardship in a University Hospital Setting. Antibiotics 2021, 10, 93. [Google Scholar] [CrossRef]
- Lee, C.R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell. Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mlynarcik, P.; Chalachanova, A.; Vagnerova, I.; Holy, O.; Zatloukalova, S.; Kolar, M. PCR Detection of Oxacillinases in Bacteria. Microb. Drug Resist. 2020, 26, 1023–1037. [Google Scholar] [CrossRef] [Green Version]
- Mlynarcik, P.; Roderova, M.; Kolar, M. Primer Evaluation for PCR and its Application for Detection of Carbapenemases in Enterobacteriaceae. Jundishapur J. Microbiol. 2016, 9, e29314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagani, L.; Dell’Amico, E.; Migliavacca, R.; D’Andrea, M.M.; Giacobone, E.; Amicosante, G.; Romero, E.; Rossolini, G.M. Multiple CTX-M-Type extended-spectrum b-lactamases in nosocomial isolates of Enterobacteriaceae from a hospital in northern Italy. J. Clin. Microbiol. 2003, 41, 4264–4269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirel, L.; Nordmann, P. Genetic structures at the origin of acquisition and expression of the carbapenem-hydrolyzing oxacillinase gene bla(OXA-58) in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2006, 50, 1442–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Naas, T.; Oueslati, S.; Bonnin, R.A.; Dabos, M.L.; Zavala, A.; Dortet, L.; Retailleau, P.; Iorga, B.I. Beta-lactamase database (BLDB)—Structure and function. J. Enzyme Inhib. Med. Chem. 2017, 32, 917–919. [Google Scholar] [CrossRef]
- Solovyev, V.; Salamov, A. Automatic annotation of microbial genomes and metagenomic sequences. In Metagenomics and its Applications in Agriculture, Biomedicine and Environmental Studies; Li, R.W., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 61–78. [Google Scholar]
- Falagas, M.E.; Bliziotis, I.A. Pandrug-resistant Gram-negative bacteria: The dawn of the post-antibiotic era? Int. J. Antimicrob. Agents 2007, 29, 630–636. [Google Scholar] [CrossRef]
- Poirel, L.; Bercot, B.; Millemann, Y.; Bonnin, R.A.; Pannaux, G.; Nordmann, P. Carbapenemase-producing Acinetobacter spp. in cattle, France. Emerg. Infect. Dis. J. 2012, 18, 523–525. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.; Zhang, Q.; Qi, J.; Liu, H.; Wang, Y.; He, T.; Ma, L.; Lai, J.; Shen, Z.; et al. Identification of New Delhi metallo-beta-lactamase 1 in Acinetobacter lwoffii of food animal origin. PLoS ONE 2012, 7, e37152. [Google Scholar] [CrossRef] [Green Version]
- Bardon, J.; Mlynarcik, P.; Procházkova, P.; Roderova, M.; Mezerova, K.; Kolar, M. Occurrence of bacteria with a dangerous extent of antibiotic resistance in poultry in the Central Region of Moravia. Acta Vet. Brno 2018, 87, 165–172. [Google Scholar] [CrossRef]
- Qu, J.; Du, Y.; Yu, R.; Lu, X. The First Outbreak Caused by Acinetobacter baumannii ST208 and ST195 in China. Biomed. Res. Int 2016, 2016, 9254907. [Google Scholar] [CrossRef] [Green Version]
- Hammerum, A.M.; Hansen, F.; Skov, M.N.; Stegger, M.; Andersen, P.S.; Holm, A.; Jakobsen, L.; Justesen, U.S. Investigation of a possible outbreak of carbapenem-resistant Acinetobacter baumannii in Odense, Denmark using PFGE, MLST and whole-genome-based SNPs. J. Antimicrob. Chemother. 2015, 70, 1965–1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karah, N.; Haldorsen, B.; Hermansen, N.O.; Tveten, Y.; Ragnhildstveit, E.; Skutlaberg, D.H.; Tofteland, S.; Sundsfjord, A.; Samuelsen, O. Emergence of OXA-carbapenemase- and 16S rRNA methylase-producing international clones of Acinetobacter baumannii in Norway. J. Med. Microbiol. 2011, 60, 515–521. [Google Scholar] [CrossRef]
- Quale, J.; Bratu, S.; Gupta, J.; Landman, D. Interplay of efflux system, ampC, and oprD expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 2006, 50, 1633–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirel, L.; Nordmann, P. Carbapenem resistance in Acinetobacter baumannii: Mechanisms and epidemiology. Clin. Microbiol. Infect. 2006, 12, 826–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirel, L.; Pitout, J.D.; Nordmann, P. Carbapenemases: Molecular diversity and clinical consequences. Future Microbiol. 2007, 2, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Ford, P.J.; Avison, M.B. Evolutionary mapping of the SHV beta-lactamase and evidence for two separate IS26-dependent blaSHV mobilization events from the Klebsiella pneumoniae chromosome. J. Antimicrob. Chemother. 2004, 54, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Villodres, A.; Gil-Marques, M.L.; Alvarez-Marin, R.; Bonnin, R.A.; Pachon-Ibanez, M.E.; Aguilar-Guisado, M.; Naas, T.; Aznar, J.; Pachon, J.; Lepe, J.A.; et al. Extended-spectrum resistance to beta-lactams/beta-lactamase inhibitors (ESRI) evolved from low-level resistant Escherichia coli. J. Antimicrob. Chemother. 2020, 75, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.J.; Chen, X.Y.; Hou, P.F. Mutation of CarO participates in drug resistance in imipenem-resistant Acinetobacter baumannii. J. Clin. Lab. Anal. 2019, 33, e22976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, D.; Talukdar, A.D.; Choudhury, M.D.; Maurya, A.P.; Chanda, D.D.; Chakravorty, A.; Bhattacharjee, A. Carbapenem nonsusceptibility with modified OprD in clinical isolates of Pseudomonas aeruginosa from India. Indian J. Med. Microbiol. 2017, 35, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Estepa, V.; Rojo-Bezares, B.; Azcona-Gutierrez, J.M.; Olarte, I.; Torres, C.; Saenz, Y. Characterisation of carbapenem-resistance mechanisms in clinical Pseudomonas aeruginosa isolates recovered in a Spanish hospital. Enferm. Infecc. Microbiol. Clin. 2017, 35, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.; Marti, S.; Sanchez-Cespedes, J. Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii. J. Antimicrob. Chemother. 2007, 59, 1210–1215. [Google Scholar] [CrossRef] [Green Version]
- Yoon, E.J.; Courvalin, P.; Grillot-Courvalin, C. RND-type efflux pumps in multidrug-resistant clinical isolates of Acinetobacter baumannii: Major role for AdeABC overexpression and AdeRS mutations. Antimicrob. Agents Chemother. 2013, 57, 2989–2995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coyne, S.; Courvalin, P.; Perichon, B. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob. Agents Chemother. 2011, 55, 947–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damier-Piolle, L.; Magnet, S.; Bremont, S.; Lambert, T.; Courvalin, P. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob Agents Chemother 2008, 52, 557–562. [Google Scholar] [CrossRef] [Green Version]
- Coyne, S.; Rosenfeld, N.; Lambert, T.; Courvalin, P.; Perichon, B. Overexpression of resistance-nodulation-cell division pump AdeFGH confers multidrug resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2010, 54, 4389–4393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamorano, L.; Miro, E.; Juan, C.; Gomez, L.; Bou, G.; Gonzalez-Lopez, J.J.; Martinez-Martinez, L.; Aracil, B.; Conejo, M.C.; Oliver, A.; et al. Mobile genetic elements related to the diffusion of plasmid-mediated AmpC beta-lactamases or carbapenemases from Enterobacteriaceae: Findings from a multicenter study in Spain. Antimicrob. Agents Chemother. 2015, 59, 5260–5266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giles, W.P.; Benson, A.K.; Olson, M.E.; Hutkins, R.W.; Whichard, J.M.; Winokur, P.L.; Fey, P.D. DNA sequence analysis of regions surrounding blaCMY-2 from multiple Salmonella plasmid backbones. Antimicrob. Agents Chemother. 2004, 48, 2845–2852. [Google Scholar] [CrossRef] [Green Version]



| Carbapenemases | STP/OX | Additional β-Lactamases | Antibiotics (mg/L) a | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| COT | MER | TIG | AMI | GEN | COL | CIP | TOB | |||
| OXA-23, OXA-58, OXA-66 | 2/195 | ADC-73, TEM-1 | >128 | >16 | 0.25 | >64 | >32 | 2 | >16 | >32 |
| Antibiotics | Resistance Gene | Identity (%) | Query/Template Length (bp) | Product | Accession Number |
|---|---|---|---|---|---|
| β-lactam | blaADC-73 | 100 | 1152/1152 | β-lactamase (AmpC) | CP050390 |
| blaTEM-1 | 100 | 861/861 | β-lactamase (broad-spectrum) | CP050916 | |
| blaOXA-23 | 100 | 822/822 | β-lactamase (carbapenemase) | AY795964 | |
| blaOXA-58 | 100 | 843/843 | β-lactamase (carbapenemase) | AY665723 | |
| blaOXA-66 | 100 | 825/825 | β-lactamase (carbapenemase) | AY750909 | |
| Aminoglycoside | aph(3′)-Ia | 100 | 816/816 | Aminoglycoside phosphotransferase | X62115 |
| aph(3″)-Ib | 100 | 804/804 | Aminoglycoside phosphotransferase | MZ419555 | |
| aph(6)-Id | 100 | 837/837 | Streptomycin phosphotransferase | MW690133 | |
| armA | 100 | 774/774 | Aminoglycoside methyltransferase | AY220558 | |
| Macrolide | msr(E) | 100 | 1476/1476 | ABC-F type ribosomal protection protein | FR751518 |
| mph(E) | 100 | 885/885 | Macrolide 2′-phosphotransferase | CP077784 | |
| Sulphonamide | sul2 | 100 | 816/816 | Dihydropteroate synthase | AY034138 |
| Tetracycline | tet(B) | 100 | 1218/1218 | Tetracycline efflux pump | CP051474 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlynarcik, P.; Dolejska, M.; Vagnerova, I.; Petrzelova, J.; Sukkar, I.; Zdarska, V.; Kolar, M. Insights into the Resistome and Phylogenomics of a ST195 Multidrug-Resistant Acinetobacter baumannii Clinical Isolate from the Czech Republic. Life 2021, 11, 1079. https://doi.org/10.3390/life11101079
Mlynarcik P, Dolejska M, Vagnerova I, Petrzelova J, Sukkar I, Zdarska V, Kolar M. Insights into the Resistome and Phylogenomics of a ST195 Multidrug-Resistant Acinetobacter baumannii Clinical Isolate from the Czech Republic. Life. 2021; 11(10):1079. https://doi.org/10.3390/life11101079
Chicago/Turabian StyleMlynarcik, Patrik, Monika Dolejska, Iva Vagnerova, Jana Petrzelova, Iva Sukkar, Veronika Zdarska, and Milan Kolar. 2021. "Insights into the Resistome and Phylogenomics of a ST195 Multidrug-Resistant Acinetobacter baumannii Clinical Isolate from the Czech Republic" Life 11, no. 10: 1079. https://doi.org/10.3390/life11101079